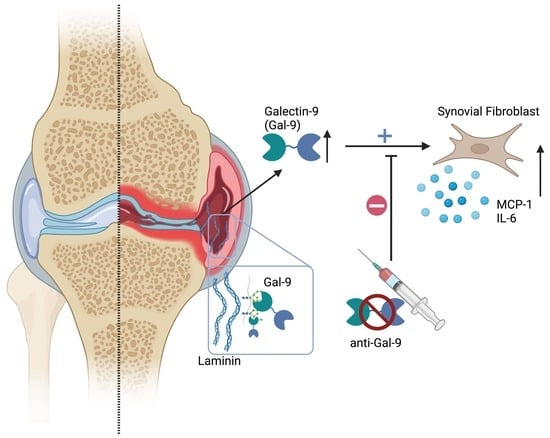
Increased Galectin-9 Levels Correlate with Disease Activity in Patients with DMARD-Naïve Rheumatoid Arthritis and Modulate the Secretion of MCP-1 and IL-6 from Synovial Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Material
2.2. Sample Handling
2.3. Culture Conditions
2.4. RA SFMCs In Vitro Cultures
2.5. RA and OA FLSs In Vitro Cultures
2.6. Anti-Gal-9 Antibody Treatment of FLS Cultures
2.7. RA Autologous FLS and PBMC In Vitro Co-Cultures
2.8. ELISA
2.9. Flow Cytometry
2.10. Immunofluorescence of RA Synovial Tissue
2.11. Cell Viability of In Vitro Co-Cultures
2.12. Microarray Analysis
2.13. Statistics
2.14. Ethics
3. Results
3.1. Increased Gal-9 Levels in Plasma and Synovial Fluids in Rheumatoid Arthritis
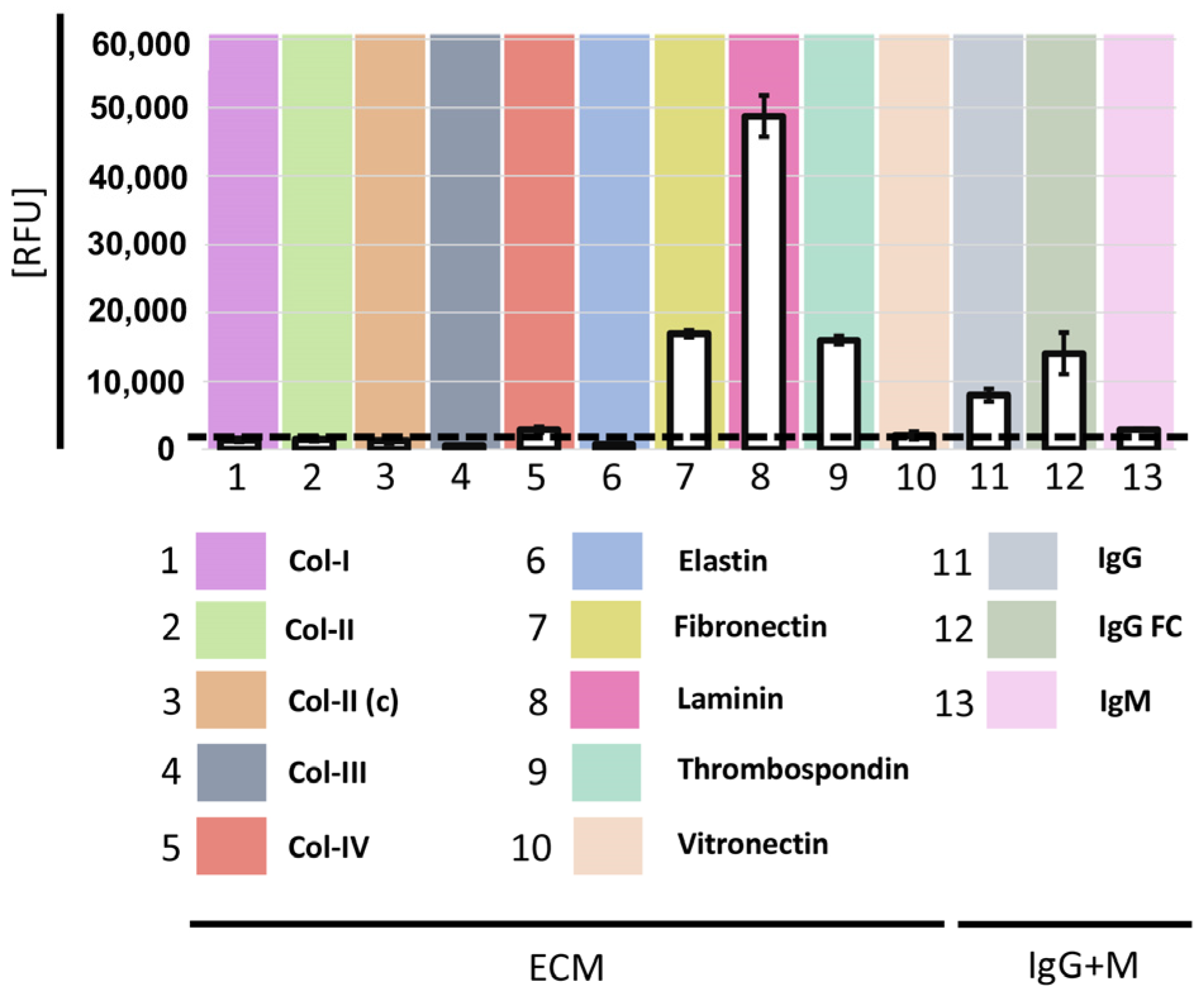
3.2. Gal-9 Binds to Human Laminin, Fibronectin, and Thrombospondin and IgG
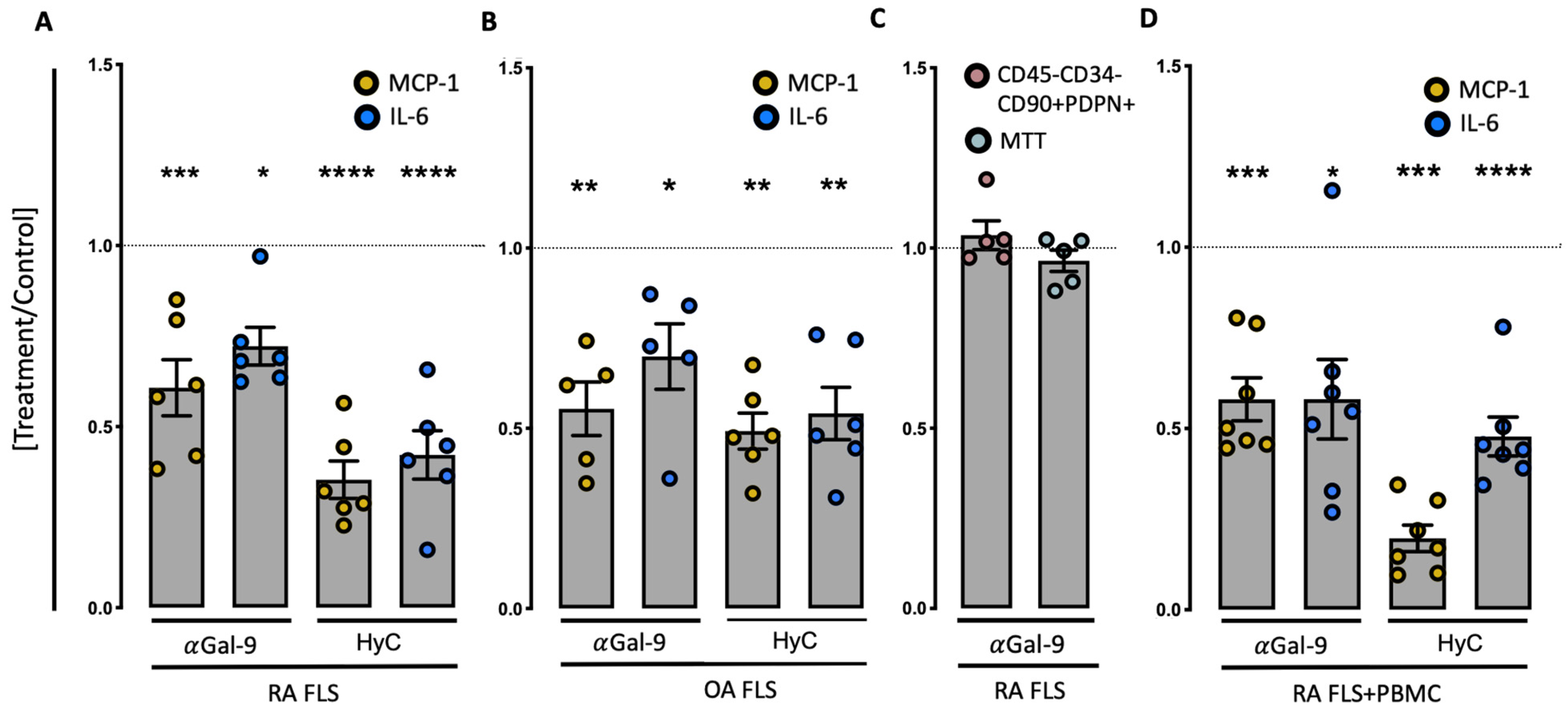
3.3. Anti-Gal-9 Antibodies Inhibit Cytokine Production by Fibroblast-Like Synoviocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, F.; Wei, K.; Slowikowski, K.; Fonseka, C.Y.; Rao, D.A.; Kelly, S.; Goodman, S.M.; Tabechian, D.; Hughes, L.B.; Sa-lomon-Escoto, K.; et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat Immunol. 2019, 20, 928–942. [Google Scholar] [CrossRef]
- Taylor, P.C. Developing anti-TNF and biologic agents. Rheumatology 2011, 50, 1351–1353. [Google Scholar] [CrossRef] [Green Version]
- Smolen, J.S.; Aletaha, D. Rheumatoid arthritis therapy reappraisal: Strategies, opportunities and challenges. Nat. Rev. Rheumatol. 2015, 11, 276–289. [Google Scholar] [CrossRef]
- Feldmann, M.; Maini, R.N. Perspectives from Masters in Rheumatology and Autoimmunity: Can We Get Closer to a Cure for Rheumatoid Arthritis? Arthritis Rheumatol. 2015, 67, 2283–2291. [Google Scholar] [CrossRef]
- Nam, J.L.; Takase-Minegishi, K.; Ramiro, S.; Chatzidionysiou, K.; Smolen, J.S.; Van Der Heijde, D.; Bijlsma, J.W.; Burmester, G.R.; Dougados, M.; Scholte-Voshaar, M.; et al. Efficacy of biological disease-modifying antirheumatic drugs: A systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 1113–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Compán, V.; Plasencia-Rodríguez, C.; de Miguel, E.; Diaz del Campo, P.; Balsa, A.; Gratacós, J. Switching biological disease-modifying antirheumatic drugs in patients with axial spondyloarthritis: Results from a systematic literature review. RMD Open 2017, 3, e000524-8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Prim. 2018, 4, 18001. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M.J. Molecular and cellular heterogeneity in the Rheumatoid Arthritis synovium: Clinical correlates of synovitis. Best Pract. Res. Clin. Rheumatol. 2014, 28, 539–549. [Google Scholar] [CrossRef]
- Pitzalis, C.; Kelly, S.; Humby, F. New learnings on the pathophysiology of RA from synovial biopsies. Curr. Opin. Rheumatol. 2013, 25, 334–344. [Google Scholar] [CrossRef]
- Humby, F.; Lewis, M.; Ramamoorthi, N.; Hackney, J.A.; Barnes, M.R.; Bombardieri, M.; Setiadi, A.F.; Kelly, S.; Bene, F.; DiCicco, M.; et al. Synovial cellular and molecular signatures stratify clinical response to csDMARD therapy and predict radiographic progression in early rheumatoid arthritis patients. Ann. Rheum. Dis. 2019, 78, 761–772. [Google Scholar] [CrossRef]
- Turner, J.D.; Filer, A. The role of the synovial fibroblast in rheumatoid arthritis pathogenesis. Curr. Opin. Rheumatol. 2015, 27, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.F.; Garcia-Carbonell, R.; Whisenant, K.D.; Guma, M. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, F.; Slowikowski, K.; Wei, K.; Marshall, J.L.; Rao, D.A.; Chang, S.K.; Nguyen, H.N.; Noss, E.H.; Turner, J.D.; Earp, B.E.; et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 2018, 9, 789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croft, A.P.; Campos, J.; Jansen, K.; Turner, J.D.; Marshall, J.; Attar, M.; Savary, L.; Wehmeyer, C.; Naylor, A.J.; Kemble, S.; et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 2019, 570, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.; Khatri, P.; Utz, P.J.; Kuo, A.J. Single-cell technologies—Studying rheumatic diseases one cell at a time. Nat. Rev. Rheumatol. 2019, 15, 340–354. [Google Scholar] [CrossRef]
- Vilar, K.D.; Pereira, M.C.; Tavares Dantas, A.; de Melo Rêgo, M.J.; Pitta, I.D.; Pinto Duarte, Â.L.; da Rocha Pitta, M.G. Galectin-9 gene (LGALS9) polymorphisms are associated with rheumatoid arthritis in Brazilian patients. PLoS ONE 2019, 14, e0223191. [Google Scholar] [CrossRef] [Green Version]
- Wiersma, V.R.; Clarke, A.; Pouwels, S.D.; Perry, E.; Abdullah, T.M.; Kelly, C.; Soyza, A.D.; Hutchinson, D.; Eggleton, P.; Bremer, E. Galectin-9 Is a Possible Promoter of Immunopathology in Rheumatoid Arthritis by Activation of Peptidyl Arginine Deiminase 4 (PAD-4) in Granulocytes. Int. J. Mol. Sci. 2019, 20, 4046. [Google Scholar] [CrossRef] [Green Version]
- Seki, M.; Sakata, K.-M.; Oomizu, S.; Arikawa, T.; Sakata, A.; Ueno, M.; Nobumoto, A.; Niki, T.; Saita, N.; Ito, K.; et al. Beneficial effect of galectin 9 on rheumatoid arthritis by induction of apoptosis of synovial fibroblasts. Arthritis Rheum. 2007, 56, 3968–3976. [Google Scholar] [CrossRef]
- Pearson, M.J.; Bik, M.A.; Ospelt, C.; Naylor, A.J.; Wehmeyer, C.; Jones, S.W.; Buckley, C.D.; Gay, S.; Filer, A.; Lord, J.M. Endogenous Galectin-9 Suppresses Apoptosis in Human Rheumatoid Arthritis Synovial Fibroblasts. Sci. Rep. 2018, 8, 12887. [Google Scholar] [CrossRef]
- Scott, D.L.; Salmon, M.; Morris, C.J.; Wainwright, A.C.; Walton, K.W. Laminin and vascular proliferation in rheumatoid arthritis. Ann. Rheum. Dis. 1984, 43, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, S.A.; Bracho-Sanchez, E.; Fettis, M.M.; Seroski, D.T.; Freeman, S.L.; Restuccia, A.; Keselowsky, B.G.; Hudalla, G.A. Locally anchoring enzymes to tissues via extracellular glycan recognition. Nat. Commun. 2018, 9, 4943. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Kasai, K. The family of metazoan metal-independent beta-galactoside-binding lectins: Structure, function and molecular evolution. Glycobiology 1993, 3, 297–304. [Google Scholar] [CrossRef]
- Houzelstein, D.; Gonçalves, I.R.; Fadden, A.J.; Sidhu, S.S.; Cooper, D.N.W.; Drickamer, K.; Leffler, H.; Poirier, F. Phylogenetic Analysis of the Vertebrate Galectin Family. Mol. Biol. Evol. 2004, 21, 1177–1187. [Google Scholar] [CrossRef] [Green Version]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. Essentials of Glycobiology, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2017; 1p. [Google Scholar]
- Hørslev-Petersen, K.; Hetland, M.L.; Junker, P.; Pødenphant, J.; Ellingsen, T.; Ahlquist, P.; Lindegaard, H.M.; Linauskas, A.; Schlemmer, A.; Dam, M.Y.; et al. Adalimumab added to a treat-to-target strategy with methotrexate and intra-articular triamcinolone in early rheumatoid arthritis increased remission rates, function and quality of life. The OPERA Study: An investigator-initiated, randomised, double-blind, parallel-group, placebo-controlled trial. Ann. Rheum. Dis. 2014, 73, 654–661. [Google Scholar] [PubMed] [Green Version]
- Hørslev-Petersen, K.; Hetland, M.L.; Ørnbjerg, L.M.; Junker, P.; Pødenphant, J.; Ellingsen, T.; Ahlquist, P.; Lindegaard, H.M.; Linauskas, A.; Schlemmer, A.; et al. Clinical and radiographic outcome of a treat-to-target strategy using methotrexate and intra-articular glucocorticoids with or without adalimumab induction: A 2-year investigator-initiated, double-blinded, randomised, controlled trial (OPERA). Ann. Rheum. Dis. 2016, 75, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Saag, K.G.; Bridges, S.L., Jr.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 68, 1–26. [Google Scholar] [CrossRef]
- Brennan, F.M.; Jackson, A.; Chantry, D.; Maini, R.; Feldmann, M. Inhibitory effect of TNFα antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet 1989, 334, 244–247. [Google Scholar] [CrossRef]
- Kragstrup, T.W.; Adams, M.; Lomholt, S.; Nielsen, M.A.; Heftdal, L.D.; Schafer, P.; Deleuran, B. IL-12/IL-23p40 identified as a downstream target of apremilast in ex vivomodels of arthritis. Ther. Adv. Musculoskelet. 2019, 11, 1759720X19828669. [Google Scholar]
- Nielsen, M.A.; Lomholt, S.; Mellemkjær, A.; Andersen, M.N.; Buckley, C.D.; Kragstrup, T.W. Responses to Cytokine Inhibitors Associated with Cellular Composition in Models of Immune-Mediated Inflammatory Arthritis. ACR Open Rheumatol. 2020, 2, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kragstrup, T.W.; Sohn, D.H.; Lepus, C.M.; Onuma, K.; Wang, Q.; Robinson, W.H.; Sokolove, J. Fibroblast-like synovial cell production of extra domain A fibronectin associates with inflammation in osteoarthritis. BMC Rheumatol. 2019, 3, 46–48. [Google Scholar] [CrossRef] [Green Version]
- Lhuillier, C.; Barjon, C.; Baloche, V.; Niki, T.; Gelin, A.; Mustapha, R.; Claër, L.; Hoos, S.; Chiba, Y.; Ueno, M.; et al. Characterization of neutralizing antibodies reacting with the 213–224 amino-acid segment of human galectin-9. PLoS ONE 2018, 13, e0202512. [Google Scholar] [CrossRef]
- Kragstrup, T.W.; Vorup-Jensen, T.; Deleuran, B.; Hvid, M. A simple set of validation steps identifies and removes false results in a sandwich enzyme- linked immunosorbent assay caused by anti- animal IgG antibodies in plasma from arthritis patients. SpringerPlus 2013, 2, 263. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.A.; Juul-Madsen, K.; Stegmayr, J.; Gao, C.; Mehta, A.Y.; Greisen, S.R.; Kragstrup, T.W.; Hvid, M.; Vorup-Jensen, T.; Cummings, R.D.; et al. Galectin-3 Decreases 4-1BBL Bioactivity by Crosslinking Soluble and Membrane Expressed 4-1BB. Front. Immunol. 2022, 13, 915890. [Google Scholar] [CrossRef]
- Nielsen, M.A.; Andersen, T.; Etzerodt, A.; Kragstrup, T.W.; Rasmussen, T.K.; Stengaard-Pedersen, K.; Hetland, M.L.; Hørslev-Petersen, K.; Junker, P.; Østergaard, M.; et al. A disintegrin and metalloprotease-17 and galectin-9 are important regulators of local 4-1BB activity and disease outcome in rheumatoid arthritis. Rheumatology 2016, 55, 1871–1879. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, H.; Fujita, Y.; Asano, T.; Matsuoka, N.; Temmoku, J.; Sato, S.; Yashiro–Furuya, M.; Yokose, K.; Yoshida, S.; Suzuki, E.; et al. Association between inflammatory cytokines and immune-checkpoint molecule in rheumatoid arthritis. PLoS ONE 2021, 16, e0260254. [Google Scholar] [CrossRef]
- Wei, K.; Korsunsky, I.; Marshall, J.L.; Gao, A.; Watts, G.F.M.; Major, T.; Croft, A.P.; Watts, J.; Blazar, P.E.; Lange, J.K.; et al. Notch signalling drives synovial fibroblast identity and arthritis pathology. Nature 2020, 582, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, S.; Knedla, A.; Tennie, C.; Kampmann, A.; Wunrau, C.; Dinser, R.; Korb, A.; Schnäker, E.-M.; Tarner, I.H.; Robbins, P.D.; et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat Med. 2009, 15, 1414–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, M.; Køster, D.; Greisen, S.; Troldborg, A.; Stengaard-Pedersen, K.; Junker, P.; Hørslev-Petersen, K.; Hetland, M.; Østergaard, M.; Hvid, M.; et al. Increased synovial galectin-3 induce inflammatory fibroblast activation and osteoclastogenesis in patients with rheumatoid arthritis. Scand. J. Rheumatol. 2022, 52, 33–41. [Google Scholar] [CrossRef]
- Woo, H.J.; Shaw, L.M.; Messier, J.M.; Mercurio, A.M. The major non-integrin laminin binding protein of macrophages is identical to carbohydrate binding protein 35 (Mac-2). J. Biol. Chem. Am. Soc. Biochem. Mol. Biol. 1990, 265, 7097–7099. [Google Scholar] [CrossRef]
- Sofat, N.; Wait, R.; Robertson, S.D.; Baines, D.L.; Baker, E.H. Interaction between extracellular matrix molecules and microbial pathogens: Evidence for the missing link in autoimmunity with rheumatoid arthritis as a disease model. Front. Microbiol. 2015, 5, 783. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Veronesi, F.; Frizziero, A.; Fini, M. Role and translational implication of galectins in arthritis pathophysiology and treatment: A systematic literature review. J. Cell. Physiol. 2018, 234, 1588–1605. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yéléhé-Okouma, M.; Ea, H.-K.; Jouzeau, J.-Y.; Reboul, P. Galectin-3: A key player in arthritis. Jt. Bone Spine 2017, 84, 15–20. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Toscano, M.A. Turning “sweet” on immunity: Galectin–glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. [Google Scholar] [CrossRef]
- Oehler, S.; Neureiter, D.; Meyer-Scholten, C.; Aigner, T. Subtyping of osteoarthritic synoviopathy. Clin. Exp. 2002, 20, 633–640. [Google Scholar]
- Pap, T.; Dankbar, B.; Wehmeyer, C.; Korb-Pap, A.; Sherwood, J. Synovial fibroblasts and articular tissue remodelling: Role and mechanisms. Semin. Cell Dev. Biol. 2020, 101, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.N.D.; Zoccheddu, M.; Yang, S.; Nygaard, G.; Secchi, C.; Doody, K.M.; Slowikowski, K.; Mizoguchi, F.; Humby, F.; Hands, R.; et al. Synoviocyte-targeted therapy synergizes with TNF inhibition in arthritis reversal. Sci. Adv. 2020, 6, eaba4353. [Google Scholar] [CrossRef]
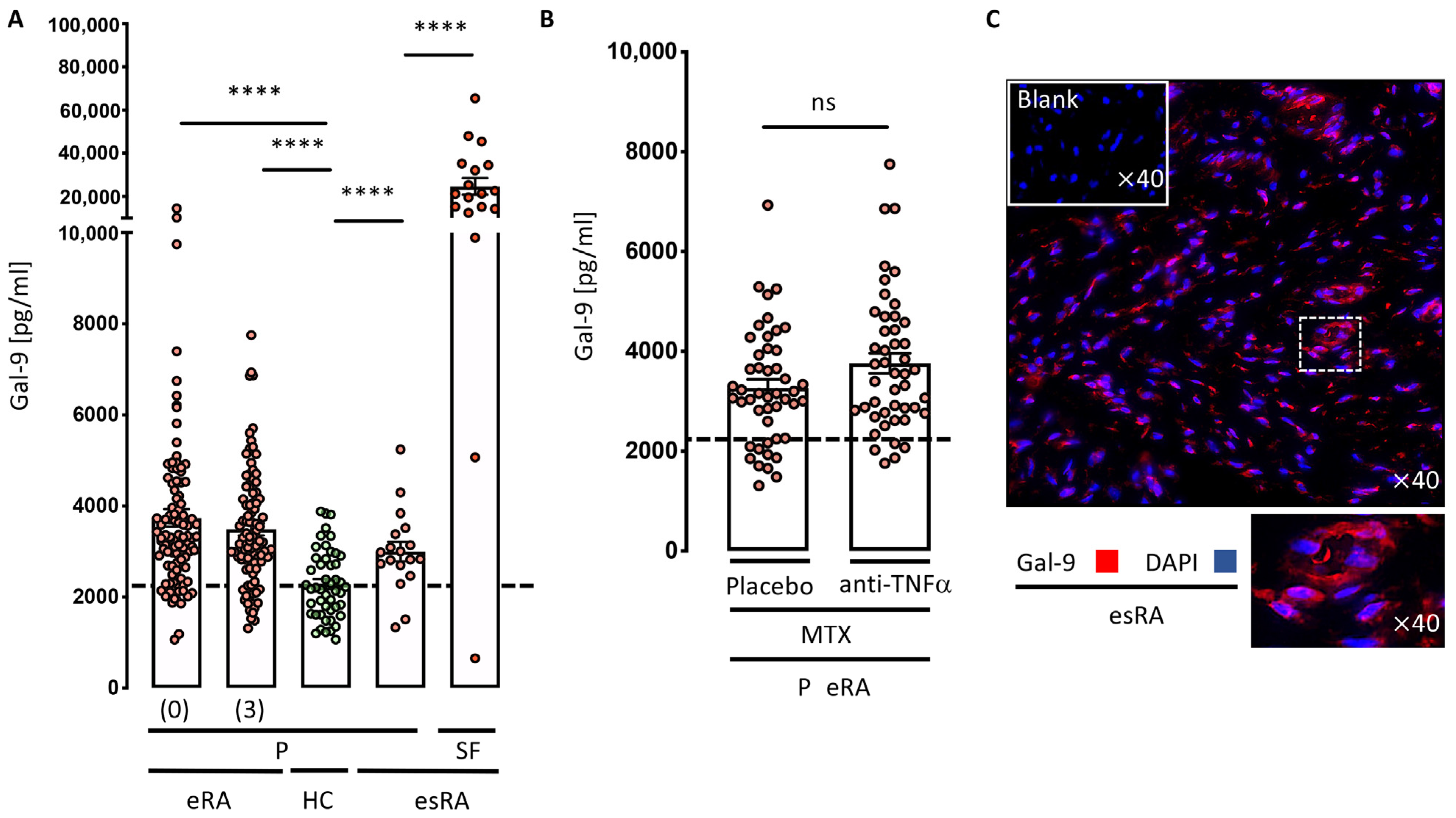
| Patient Characteristics | Early RA (n = 98) | Established RA (n = 18) | HC (n = 48) | ||
|---|---|---|---|---|---|
| Time since diagnosis (months) | 0 | 3 | 24 | 84 (12–288) | |
| Age (years) | 56 (46–65) | - | - | 47 (37–61) | 49 (45–58) |
| Gender (% female) | 73 | - | - | 70 | 57 |
| Baseline characteristics | |||||
| IgM-RF (% positive) | 68 | - | - | 39 | - |
| Anti-CCP antibody (% positive) | 57 | - | - | 46 | - |
| Treated with ADA (%) | 49 | - | - | 8 | - |
| Disease activity | |||||
| DAS28CRP (0–10) | 5.7 (5.1–6.4) | 2.1 (1.8–3.2) | 2.0 (1.8–2.7) | 5.0 (3.2–5.5) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, M.A.; Køster, D.; Mehta, A.Y.; Stengaard-Pedersen, K.; Busson, P.; Junker, P.; Hørslev-Petersen, K.; Hetland, M.L.; Østergaard, M.; Hvid, M.; et al. Increased Galectin-9 Levels Correlate with Disease Activity in Patients with DMARD-Naïve Rheumatoid Arthritis and Modulate the Secretion of MCP-1 and IL-6 from Synovial Fibroblasts. Cells 2023, 12, 327. https://doi.org/10.3390/cells12020327
Nielsen MA, Køster D, Mehta AY, Stengaard-Pedersen K, Busson P, Junker P, Hørslev-Petersen K, Hetland ML, Østergaard M, Hvid M, et al. Increased Galectin-9 Levels Correlate with Disease Activity in Patients with DMARD-Naïve Rheumatoid Arthritis and Modulate the Secretion of MCP-1 and IL-6 from Synovial Fibroblasts. Cells. 2023; 12(2):327. https://doi.org/10.3390/cells12020327
Chicago/Turabian StyleNielsen, Morten A., Ditte Køster, Akul Y. Mehta, Kristian Stengaard-Pedersen, Pierre Busson, Peter Junker, Kim Hørslev-Petersen, Merete Lund Hetland, Mikkel Østergaard, Malene Hvid, and et al. 2023. "Increased Galectin-9 Levels Correlate with Disease Activity in Patients with DMARD-Naïve Rheumatoid Arthritis and Modulate the Secretion of MCP-1 and IL-6 from Synovial Fibroblasts" Cells 12, no. 2: 327. https://doi.org/10.3390/cells12020327
APA StyleNielsen, M. A., Køster, D., Mehta, A. Y., Stengaard-Pedersen, K., Busson, P., Junker, P., Hørslev-Petersen, K., Hetland, M. L., Østergaard, M., Hvid, M., Leffler, H., Kragstrup, T. W., Cummings, R. D., & Deleuran, B. (2023). Increased Galectin-9 Levels Correlate with Disease Activity in Patients with DMARD-Naïve Rheumatoid Arthritis and Modulate the Secretion of MCP-1 and IL-6 from Synovial Fibroblasts. Cells, 12(2), 327. https://doi.org/10.3390/cells12020327