Sex-Specific Whole-Transcriptome Analysis in the Cerebral Cortex of FAE Offspring
Abstract
1. Introduction
2. Materials and Methods
2.1. Fetal Alcohol Exposure
2.2. Plasma EtOH Concentration Analysis
2.3. RNA Isolation and Next-Generation Sequencing
2.4. RNA-Seq Analysis
2.5. GSEA Pathway Analysis
2.6. Ingenuity Pathway Analysis
2.7. Statistical Analysis
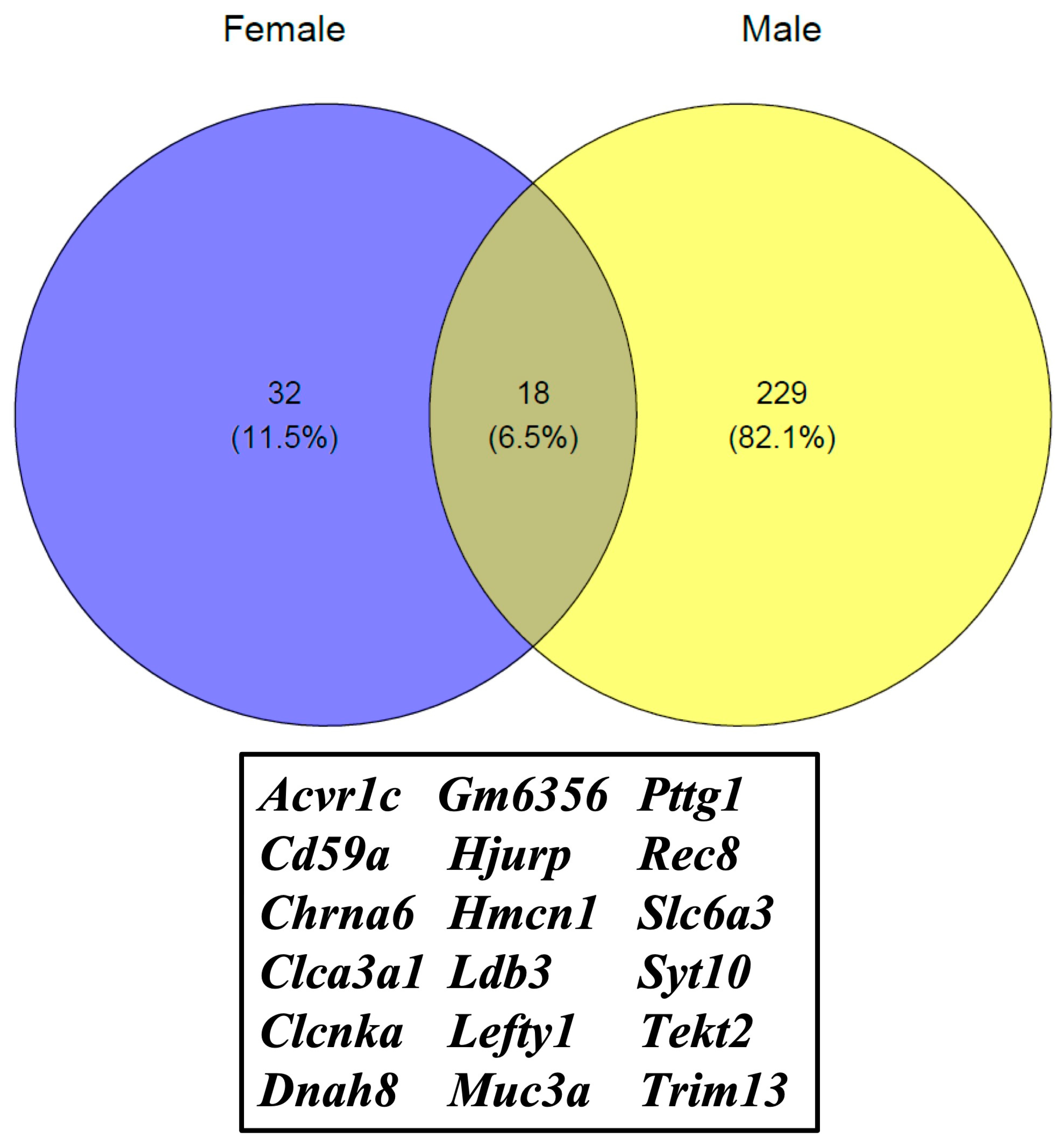
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vorgias, D.; Bernstein, B. Fetal Alcohol Syndrome; StatPearls: Treasure Island, CA, USA, 2022. [Google Scholar]
- Moore, E.M.; Xia, Y. Neurodevelopmental Trajectories Following Prenatal Alcohol Exposure. Front. Hum. Neurosci. 2021, 15, 695855. [Google Scholar] [CrossRef]
- Tunc-Ozcan, E.; Ullmann, T.M.; Shukla, P.K.; Redei, E.E. Low-dose thyroxine attenuates autism-associated adverse effects of fetal alcohol in male offspring’s social behavior and hippocampal gene expression. Alcohol. Clin. Exp. Res. 2013, 37, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.K.; Meena, A.S.; Rao, R.; Rao, R. Deletion of TLR-4 attenuates fetal alcohol exposure-induced gene expression and social interaction deficits. Alcohol 2018, 73, 73–78. [Google Scholar] [CrossRef]
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e290–e299. [Google Scholar] [CrossRef]
- Popova, S.; Dozet, D.; Akhand Laboni, S.; Brower, K.; Temple, V. Why do women consume alcohol during pregnancy or while breastfeeding? Drug Alcohol Rev. 2022, 41, 759–777. [Google Scholar] [CrossRef] [PubMed]
- Sher, J. Fetal alcohol spectrum disorders: Preventing collateral damage from COVID-19. Lancet Public Health 2020, 5, e424. [Google Scholar] [CrossRef] [PubMed]
- Klintsova, A.Y.; Hamilton, G.F.; Boschen, K.E. Long-term consequences of developmental alcohol exposure on brain structure and function: Therapeutic benefits of physical activity. Brain Sci. 2012, 3, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Cosín-Tomás, M.; Leclerc, D.; Malysheva, O.V.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Altered Sex-Specific Gene Expression in Brain of Young Mice and Embryos. Nutrients 2022, 14, 1051. [Google Scholar] [CrossRef] [PubMed]
- Nakhid, D.; McMorris, C.; Sun, H.; Gibbard, W.B.; Tortorelli, C.; Lebel, C. Brain volume and magnetic susceptibility differences in children and adolescents with prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 2022, 46, 1797–1807. [Google Scholar] [CrossRef]
- Gautam, P.; Lebel, C.; Narr, K.L.; Mattson, S.N.; May, P.A.; Adnams, C.M.; Riley, E.P.; Jones, K.L.; Kan, E.C.; Sowell, E.R. Volume changes and brain-behavior relationships in white matter and subcortical gray matter in children with prenatal alcohol exposure. Hum. Brain Mapp. 2015, 36, 2318–2329. [Google Scholar] [CrossRef]
- Luo, J. Autophagy and ethanol neurotoxicity. Autophagy 2014, 10, 2099–2108. [Google Scholar] [CrossRef] [PubMed]
- Darbinian, N.; Selzer, M. Oligodendrocyte pathology in fetal alcohol spectrum disorders. Neural Regen. Res. 2022, 17, 497–502. [Google Scholar]
- Zhang, K.; Luo, J. Role of MCP-1 and CCR2 in alcohol neurotoxicity. Pharmacol. Res. 2019, 139, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaer, A.E.; Flentke, G.R.; Berres, M.E.; Garic, A.; Smith, S.M. Exon level machine learning analyses elucidate novel candidate miRNA targets in an avian model of fetal alcohol spectrum disorder. PLoS Comput. Biol. 2019, 15, e1006937. [Google Scholar] [CrossRef] [PubMed]
- Wallén, E.; Auvinen, P.; Kaminen-Ahola, N. The Effects of Early Prenatal Alcohol Exposure on Epigenome and Embryonic Development. Genes 2021, 12, 1095. [Google Scholar] [CrossRef]
- Alberry, B.L.; Castellani, C.A.; Singh, S.M. Hippocampal transcriptome analysis following maternal separation implicates altered RNA processing in a mouse model of fetal alcohol spectrum disorder. J. Neurodev. Disord. 2020, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Tunc-Ozcan, E.; Ferreira, A.B.; Redei, E.E. Modeling Fetal Alcohol Spectrum Disorder: Validating an Ex Vivo Primary Hippocampal Cell Culture System. Alcohol. Clin. Exp. Res. 2016, 40, 1273–1282. [Google Scholar] [CrossRef]
- Morozova, T.V.; Shankar, V.; MacPherson, R.A.; Mackay, T.F.C.; Anholt, R.R.H. Modulation of the Drosophila transcriptome by developmental exposure to alcohol. BMC Genom. 2022, 23, 347. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Belinda, P.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. Voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Pinson, M.R.; Tseng, A.M.; Adams, A.; Lehman, T.E.; Chung, K.; Gutierrez, J.; Larin, K.V.; Chambers, C.; Miranda, R.C. Prenatal alcohol exposure contributes to negative pregnancy outcomes by altering fetal vascular dynamics and the placental transcriptome. Alcohol. Clin. Exp. Res. 2022, 46, 1036–1049. [Google Scholar] [CrossRef] [PubMed]
- Resendiz, M.; Mason, S.; Lo, C.-L.; Zhou, F.C. Epigenetic regulation of the neural transcriptome and alcohol interference during development. Front. Genet. 2014, 5, 285. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.K.; Sittig, L.J.; Ullmann, T.M.; Redei, E.E. Candidate placental biomarkers for intrauterine alcohol exposure. Alcohol. Clin. Exp. Res. 2011, 35, 559–565. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, X.; You, C.; Ma, S.; Luo, Y.; Peng, S.; Tang, F.; Tian, X.; Wang, F.; Huang, Z. MUC3A promotes non-small cell lung cancer progression via activating the NFkappaB pathway and attenuates radiosensitivity. Int. J. Biol. Sci. 2021, 17, 2523–2536. [Google Scholar] [CrossRef] [PubMed]
- Breugelmans, T.; Arras, W.; Boen, L.E.; Borms, E.; Kamperdijk, L.; De Man, J.; van de Vijver, E.; van Gils, A.; de Winter, B.Y.; Moes, N.; et al. Aberrant Mucin Expression Profiles Associate With Pediatric Inflammatory Bowel Disease Presentation and Activity. Inflamm. Bowel Dis. 2022. print. [Google Scholar] [CrossRef]
- Gui, Y.; Thomas, M.H.; Garcia, P.; Karout, M.; Halder, R.; Michelucci, A.; Kollmus, H.; Zhou, C.; Melmed, S.; Schughart, K.; et al. Pituitary Tumor Transforming Gene 1 Orchestrates Gene Regulatory Variation in Mouse Ventral Midbrain During Aging. Front. Genet. 2020, 11, 566734. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhou, J.; Chen, J.; Carr, A.M.; Watanabe, Y. Meikin synergizes with shugoshin to protect cohesin Rec8 during meiosis I. Genes Dev. 2021, 35, 692–697. [Google Scholar] [CrossRef]
- Nozu, K.; Inagaki, T.; Fu, X.J.; Nozu, Y.; Kaito, H.; Kanda, K.; Sekine, T.; Igarashi, T.; Nakanishi, K.; Yoshikawa, N.; et al. Molecular analysis of digenic inheritance in Bartter syndrome with sensorineural deafness. J. Med. Genet. 2008, 45, 182–186. [Google Scholar] [CrossRef]
- Stevenson, M.; Pagnamenta, A.T.; Mack, H.G.; Savige, J.; Giacopuzzi, E.; E Lines, K.; Taylor, J.C.; Thakker, R.V. The Bartter-Gitelman Spectrum: 50-Year Follow-up With Revision of Diagnosis After Whole-Genome Sequencing. J. Endocr. Soc. 2022, 6, bvac079. [Google Scholar] [CrossRef] [PubMed]
- Dear, T.; Möller, A.; Boehm, T. CAPN11: A calpain with high mRNA levels in testis and located on chromosome 6. Genomics 1999, 59, 243–247. [Google Scholar] [CrossRef]
- Li, X.; Xu, L.-S.; Xu, Y.-F.; Yang, Q.; Fang, Z.-X.; Yao, M.; Chen, W.-Y. The gene regulatory network in different brain regions of neuropathic pain mouse models. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5053–5061. [Google Scholar]
- Rashkin, S.R.; Cleves, M.; Shaw, G.M.; Nembhard, W.N.; Nestoridi, E.; Jenkins, M.M.; Romitti, P.A.; Lou, X.; Browne, M.L.; Mitchell, L.E.; et al. A genome-wide association study of obstructive heart defects among participants in the National Birth Defects Prevention Study. Am. J. Med. Genet. A 2022, 188, 2303–2314. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.B.; Karet, F.E.; Hamdan, J.M.; Pietro, A.D.; Sanjad, S.A.; Lifton, R.P. Bartter’s syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat. Genet. 1996, 13, 183–188. [Google Scholar] [CrossRef]
- Fremont, O.T.; Chan, J.C.M. Understanding Bartter syndrome and Gitelman syndrome. World J. Pediatr. 2012, 8, 25–30. [Google Scholar] [CrossRef]
- Walker, P.L.C.; Corrigan, A.; Arenas, M.; Escuredo, E.; Fairbanks, L.; Marinaki, A. Purine nucleoside phosphorylase deficiency: A mutation update. Nucleosides Nucleotides Nucleic Acids 2011, 30, 1243–1247. [Google Scholar] [CrossRef]
- Song, Y.; Luo, Q.; Long, H.; Hu, Z.; Que, T.; Zhang, X.A.; Li, Z.; Wang, G.; Yi, L.; Liu, Z.; et al. Alpha-enolase as a potential cancer prognostic marker promotes cell growth, migration, and invasion in glioma. Mol. Cancer 2014, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Guven, K.; Gunning, P.; Fath, T. TPM3 and TPM4 gene products segregate to the postsynaptic region of central nervous system synapses. Bioarchitecture 2011, 1, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Licheri, V.; Brigman, J.L. Altering Cell-Cell Interaction in Prenatal Alcohol Exposure Models: Insight on Cell-Adhesion Molecules During Brain Development. Front. Mol. Neurosci. 2021, 14, 753537. [Google Scholar] [CrossRef]
- Wu, Q.; Maniatis, T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell 1999, 97, 779–790. [Google Scholar] [CrossRef] [PubMed]



| Term | p-Value |
|---|---|
| IPA Pathways | |
| EIF2 signaling | 2.75 × 10−23 |
| Huntington’s disease signaling | 1.98 × 10−20 |
| Synaptogenesis signaling pathway | 2.93 × 10−16 |
| Sirtuin signaling pathway | 9.75 × 10−16 |
| Estrogen receptor signaling | 2.02 × 10−14 |
| KEGG Pathways | |
| Oxidative phosphorylation | 1.86 × 10−08 |
| Parkinson’s disease | 1.57 × 10−07 |
| Alzheimer’s disease | 0.000163 |
| Huntington’s disease | 0.000163 |
| Type II diabetes mellitus | 0.019792 |
| IPA Diseases and Disorders | |
| Organismal injury and abnormalities | 1.07 × 10−03 |
| Neurological diseases | 1.07 × 10−03 |
| Hereditary disorders | 6.39 × 10−04 |
| Psychological disorders | 6.39 × 10−04 |
| Skeletal and muscular disorders | 6.39 × 10−04 |
| IPA Molecular and Cellular Functions | |
| Cellular development | 1.05 × 10−03 |
| Cellular growth and proliferation | 1.05 × 10−03 |
| Cell morphology | 1.07 × 10−03 |
| Cellular assembly and organization | 1.08 × 10−03 |
| Cellular function and maintenance | 1.02 × 10−03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, N.K.; Shrinath, P.; Rao, R.; Shukla, P.K. Sex-Specific Whole-Transcriptome Analysis in the Cerebral Cortex of FAE Offspring. Cells 2023, 12, 328. https://doi.org/10.3390/cells12020328
Mishra NK, Shrinath P, Rao R, Shukla PK. Sex-Specific Whole-Transcriptome Analysis in the Cerebral Cortex of FAE Offspring. Cells. 2023; 12(2):328. https://doi.org/10.3390/cells12020328
Chicago/Turabian StyleMishra, Nitish K., Pulastya Shrinath, Radhakrishna Rao, and Pradeep K. Shukla. 2023. "Sex-Specific Whole-Transcriptome Analysis in the Cerebral Cortex of FAE Offspring" Cells 12, no. 2: 328. https://doi.org/10.3390/cells12020328
APA StyleMishra, N. K., Shrinath, P., Rao, R., & Shukla, P. K. (2023). Sex-Specific Whole-Transcriptome Analysis in the Cerebral Cortex of FAE Offspring. Cells, 12(2), 328. https://doi.org/10.3390/cells12020328









