Denosumab and Zoledronic Acid Differently Affect Circulating Immune Subsets: A Possible Role in the Onset of MRONJ
Abstract
:1. Introduction
2. Material and Methods
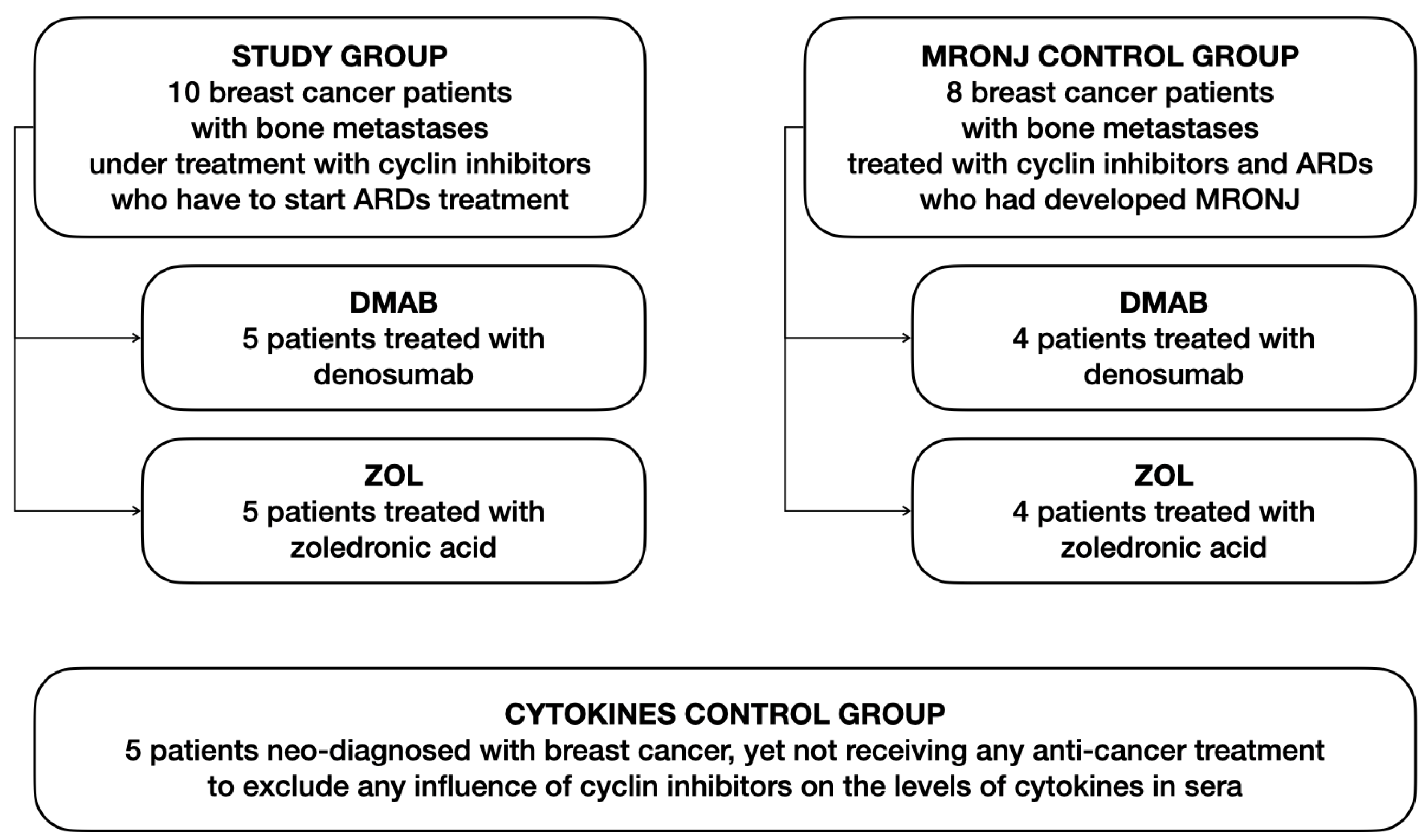
2.1. Patients and Study Design
2.2. Flow Cytometry
2.3. Cell Cultures
2.4. Cytokine Dosage
2.5. Statistical Analyses
3. Results
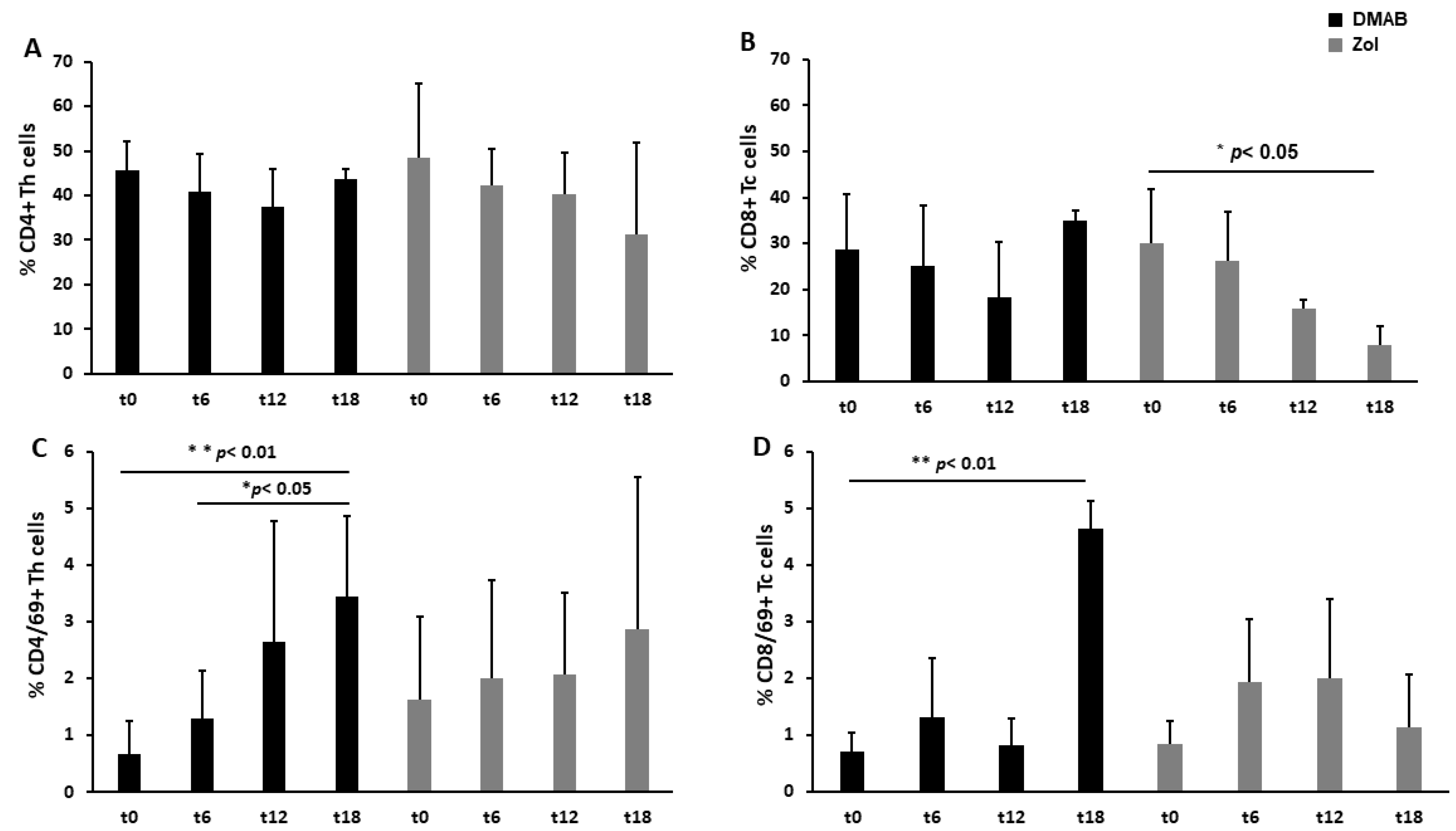
3.1. Dmab-Treated Patients Show an Increased Level of CD69, a T Cell Activation Marker
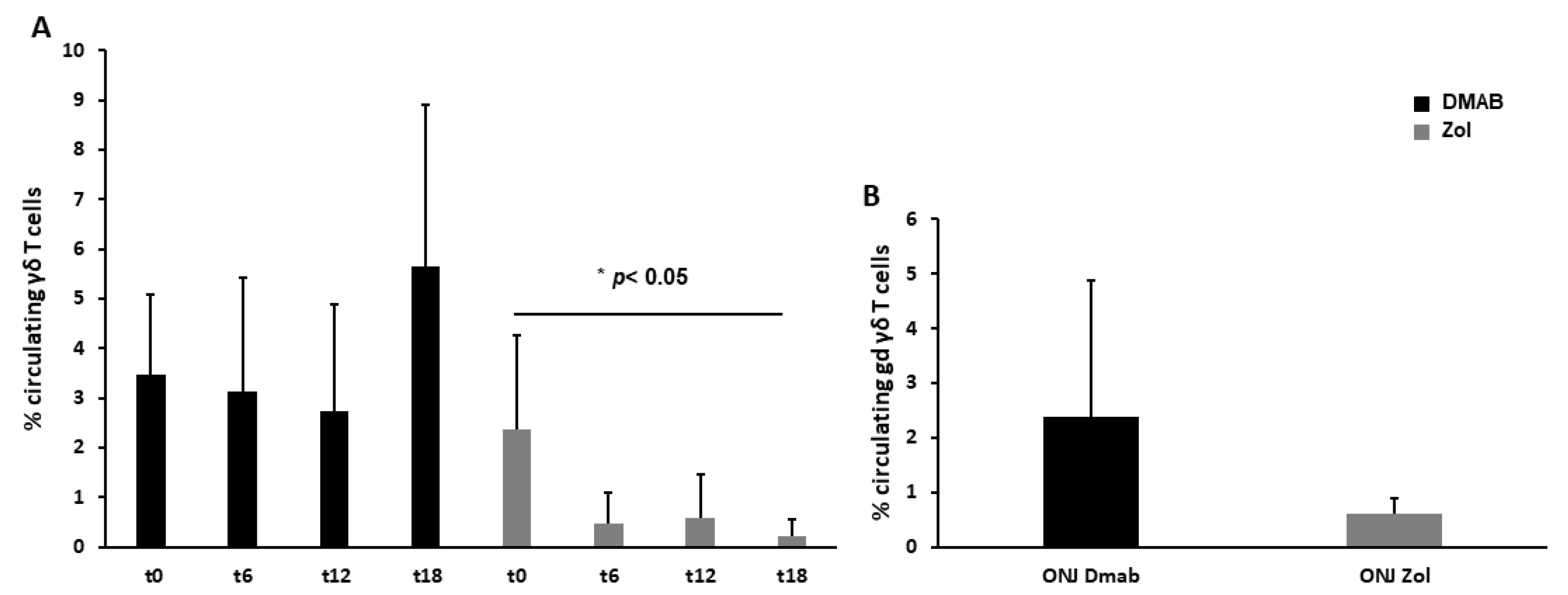
3.2. Dmab-Treated Patients Show a Normal Level of Circulating γδ T Cells
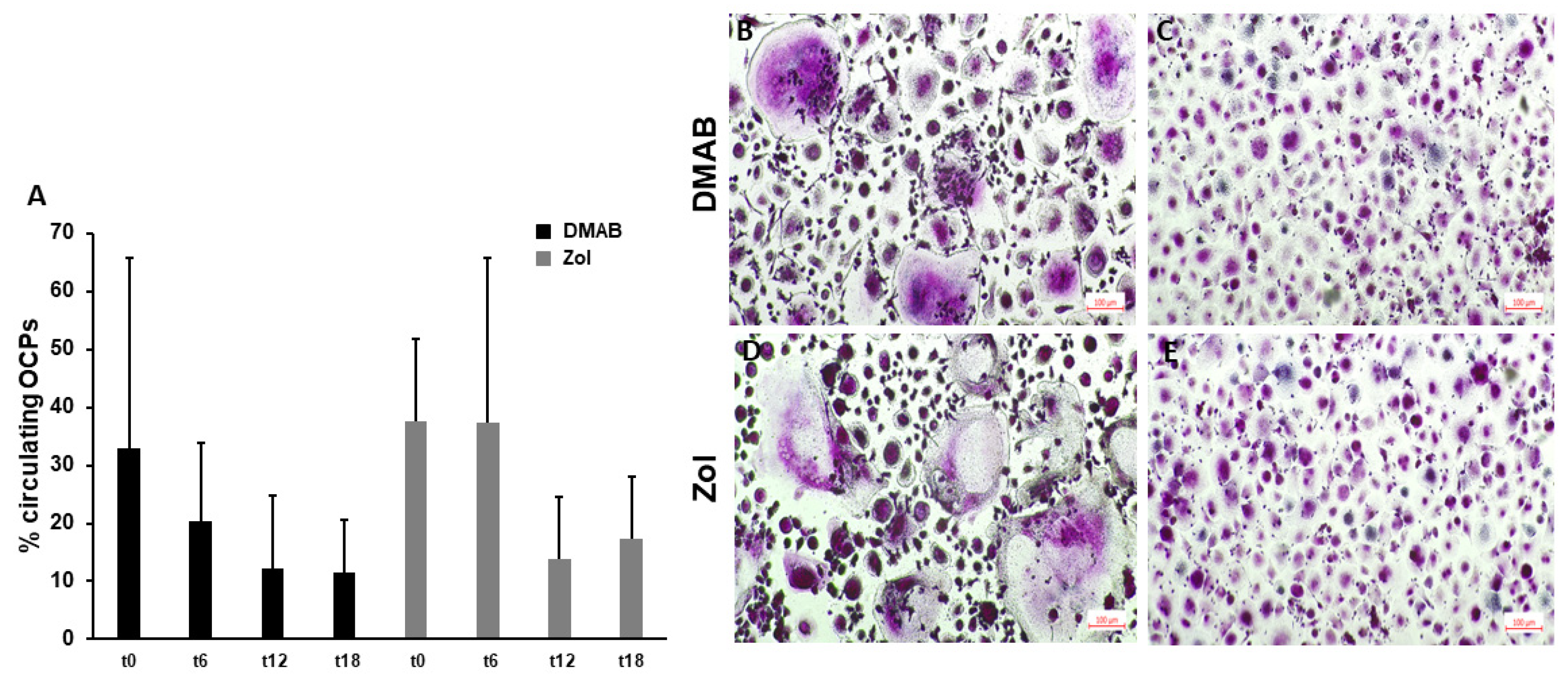
3.3. Circulating OCPs and OC Formation Decrease after 12 Months of Treatment with ARDs
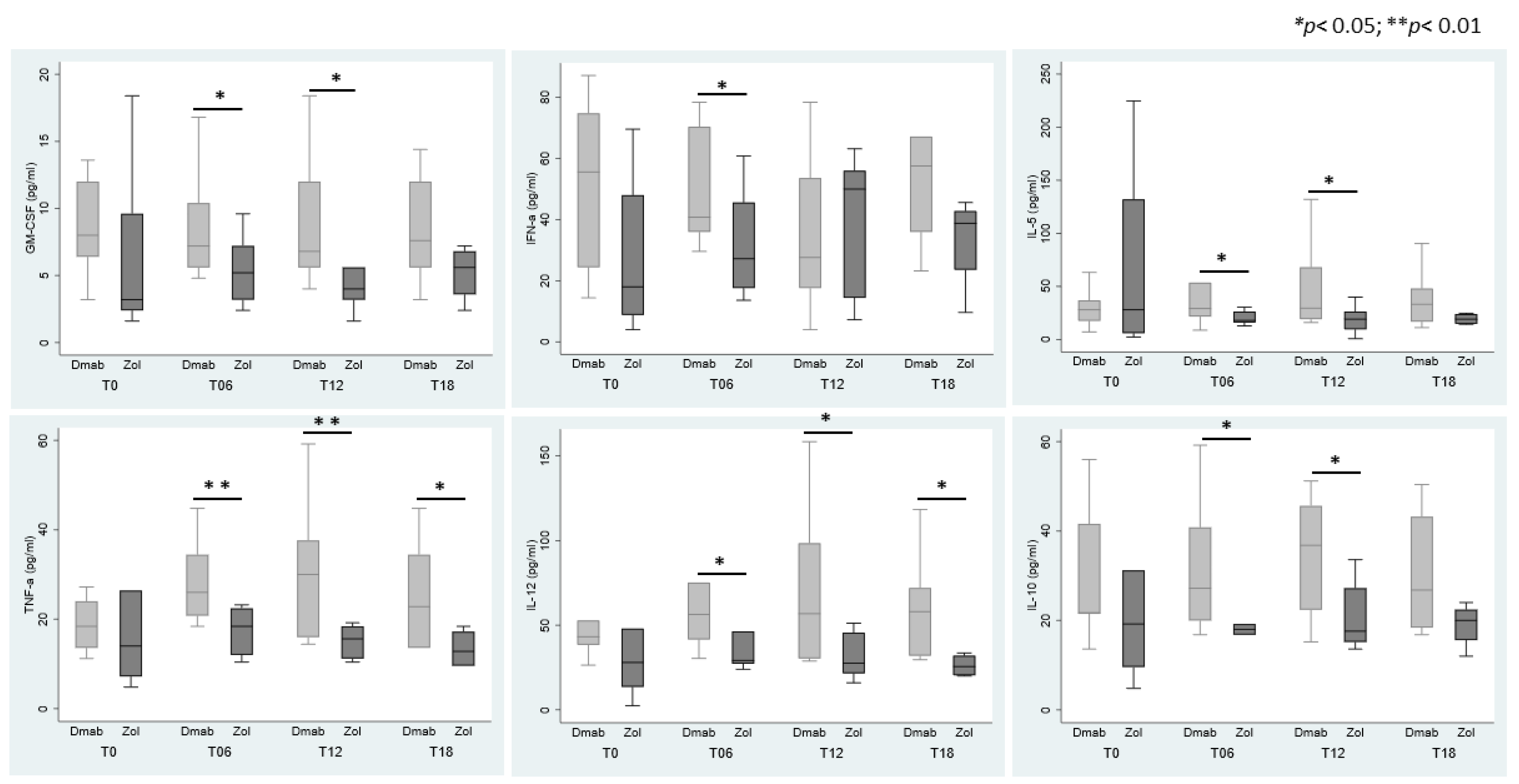
3.4. Cytokines Modulation in Sera According to ARDs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. Osteonecrosis of the jaw: Who gets it, and why? Bone 2009, 44, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Soma, T.; Iwasaki, R.; Sato, Y.; Kobayashi, T.; Nakamura, S.; Kaneko, Y.; Ito, E.; Okada, H.; Watanabe, H.; Miyamoto, K.; et al. Tooth extraction in mice administered zoledronate increases inflammatory cytokine levels and promotes osteonecrosis of the jaw. J. Bone Miner. Metab. 2021, 39, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Sawatari, Y.; Fortin, M.; Broumand, V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: Risk factors, recognition, prevention, and treatment. J. Oral Maxillofac. Surg. 2005, 63, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Fusco, V.; Santini, D.; Armento, G.; Tonini, G.; Campisi, G. Osteonecrosis of jaw beyond antiresorptive (bone-targeted) agents: New horizons in oncology. Expert. Opin. Drug Saf. 2016, 15, 925–935. [Google Scholar] [CrossRef]
- Eguia, A.; Bagan-Debon, L.; Cardona, F. Review and update on drugs related to the development of osteonecrosis of the jaw. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e71–e83. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Vardas, E.; Tziveleka, S.; Georgaki, M.; Kouri, M.; Katoumas, K.; Piperi, E.; Nikitakis, N.G. Oral Side Effects in Patients with Metastatic Renal Cell Carcinoma Receiving the Antiangiogenic Agent Pazopanib-Report of Three Cases. Dent. J. 2022, 10, 232. [Google Scholar] [CrossRef]
- Huja, S.S.; Fernandez, S.A.; Hill, K.J.; Li, Y. Remodeling dynamics in the alveolar process in skeletally mature dogs. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2006, 288, 1243–1249. [Google Scholar] [CrossRef]
- Allen, M.R.; Burr, D.B. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: So many hypotheses, so few data. J. Oral Maxillofac. Surg. 2009, 67, 61–70. [Google Scholar] [CrossRef]
- Ewald, F.; Wuesthoff, F.; Koehnke, R.; Friedrich, R.E.; Gosau, M.; Smeets, R.; Rohde, H.; Assaf, A.T. Retrospective analysis of bacterial colonization of necrotic bone and antibiotic resistance in 98 patients with medication-related osteonecrosis of the jaw (MRONJ). Clin. Oral Investig. 2021, 25, 2801–2809. [Google Scholar] [CrossRef]
- Nair, S.P.; Meghji, S.; Wilson, M.; Reddi, K.; White, P.; Henderson, B. Bacterially induced bone destruction: Mechanisms and misconceptions. Infect. Immun. 1996, 64, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Meghji, S.; Crean, S.J.; Hill, P.A.; Sheikh, M.; Nair, S.P.; Heron, K.; Henderson, B.; Mawer, E.B.; Harris, M. Surface-associated protein from Staphylococcus aureus stimulates osteoclastogenesis: Possible role in S. aureus-induced bone pathology. Br. J. Rheumatol. 1998, 37, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Yang, M.; Kim, T.; Kim, S.; Williams, D.W.; Esmaeili, M.; Hong, C.; Shin, K.H.; Kang, M.K.; Park, N.H.; et al. Indigenous microbiota protects development of medication-related osteonecrosis induced by periapical disease in mice. Int. J. Oral Sci. 2022, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Nicolatou-Galitis, O.; Schiodt, M.; Mendes, R.A.; Ripamonti, C.; Hope, S.; Drudge-Coates, L.; Niepel, D.; Van den Wyngaert, T. Medication-related osteonecrosis of the jaw: Definition and best practice for prevention, diagnosis, and treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Messer, J.G.; Mendieta Calle, J.L.; Jiron, J.M.; Castillo, E.J.; Van Poznak, C.; Bhattacharyya, N.; Kimmel, D.B.; Aguirre, J.I. Zoledronic acid increases the prevalence of medication-related osteonecrosis of the jaw in a dose dependent manner in rice rats (Oryzomys palustris) with localized periodontitis. Bone 2018, 108, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wang, H.; Liu, Z.; Xu, Z.L.; Wang, M.X.; Chen, Q.M.; Wu, M.L.; Ren, X.L.; Liang, Q.H.; Liu, F.P.; et al. Real-world study of antiresorptive-related osteonecrosis of jaw based on the US food and drug administration adverse event reporting system database. Front. Pharmacol. 2022, 13, 1017391. [Google Scholar] [CrossRef]
- Hanley, D.A.; Adachi, J.D.; Bell, A.; Brown, V. Denosumab: Mechanism of action and clinical outcomes. Int. J. Clin. Pract. 2012, 66, 1139–1146. [Google Scholar] [CrossRef]
- Francisconi, C.F.; Vieira, A.E.; Azevedo, M.C.S.; Tabanez, A.P.; Fonseca, A.C.; Trombone, A.P.F.; Letra, A.; Silva, R.M.; Sfeir, C.S.; Little, S.R.; et al. RANKL Triggers Treg-Mediated Immunoregulation in Inflammatory Osteolysis. J. Dent. Res. 2018, 97, 917–927. [Google Scholar] [CrossRef]
- Park, S.; Kanayama, K.; Kaur, K.; Tseng, H.C.; Banankhah, S.; Quje, D.T.; Sayre, J.W.; Jewett, A.; Nishimura, I. Osteonecrosis of the jaw developed in mice: Disease variants regulated by gammadelta T cells in oral mucosal barrier immunity. J. Biol. Chem. 2015, 290, 17349–17366. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, R.; Du, J.; Fu, Y.; Li, S.; Zhang, P.; Liu, L.; Jiang, H. Zoledronic acid promotes TLR-4-mediated M1 macrophage polarization in bisphosphonate-related osteonecrosis of the jaw. FASEB J. 2019, 33, 5208–5219. [Google Scholar] [CrossRef]
- Kalyan, S.; Wang, J.; Quabius, E.S.; Huck, J.; Wiltfang, J.; Baines, J.F.; Kabelitz, D. Systemic immunity shapes the oral microbiome and susceptibility to bisphosphonate-associated osteonecrosis of the jaw. J. Transl. Med. 2015, 13, 212. [Google Scholar] [CrossRef] [PubMed]
- Kalyan, S.; Kabelitz, D. Defining the nature of human gammadelta T cells: A biographical sketch of the highly empathetic. Cell Mol. Immunol. 2013, 10, 21–29. [Google Scholar] [CrossRef]
- Soma, T.; Iwasaki, R.; Sato, Y.; Kobayashi, T.; Ito, E.; Matsumoto, T.; Kimura, A.; Miyamoto, K.; Matsumoto, M.; Nakamura, M.; et al. Osteonecrosis development by tooth extraction in zoledronate treated mice is inhibited by active vitamin D analogues, anti-inflammatory agents or antibiotics. Sci. Rep. 2022, 12, 19. [Google Scholar] [CrossRef]
- Zhang, Q.; Atsuta, I.; Liu, S.; Chen, C.; Shi, S.; Shi, S.; Le, A.D. IL-17-mediated M1/M2 macrophage alteration contributes to pathogenesis of bisphosphonate-related osteonecrosis of the jaws. Clin. Cancer Res. 2013, 19, 3176–3188. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Sun, Y.; Kanayama, K.; Morinaga, K.; Hokugo, A.; Nishimura, I.; Jewett, A. Augmentation of IFN-gamma by bone marrow derived immune cells in the presence of severe suppression of IFN-gamma in gingivae induced by zoledronic acid and denosumab in Hu-BLT mice model of ONJ. Front. Endocrinol. 2023, 14, 1111627. [Google Scholar] [CrossRef]
- Elsayed, R.; Kurago, Z.; Cutler, C.W.; Arce, R.M.; Gerber, J.; Celis, E.; Sultan, H.; Elashiry, M.; Meghil, M.; Sun, C.; et al. Role of dendritic cell-mediated immune response in oral homeostasis: A new mechanism of osteonecrosis of the jaw. FASEB J. 2020, 34, 2595–2608. [Google Scholar] [CrossRef]
- Hagelauer, N.; Pabst, A.M.; Ziebart, T.; Ulbrich, H.; Walter, C. In vitro effects of bisphosphonates on chemotaxis, phagocytosis, and oxidative burst of neutrophil granulocytes. Clin. Oral Investig. 2015, 19, 139–148. [Google Scholar] [CrossRef]
- Gkouveris, I.; Soundia, A.; Gouveris, P.; Zouki, D.; Hadaya, D.; Tetradis, S. Macrophage Involvement in Medication-Related Osteonecrosis of the Jaw (MRONJ): A Comprehensive, Short Review. Cancers 2022, 14, 330. [Google Scholar] [CrossRef]
- Roato, I.; Mauceri, R.; Notaro, V.; Genova, T.; Fusco, V.; Mussano, F. Immune Dysfunction in Medication-Related Osteonecrosis of the Jaw. Int. J. Mol. Sci. 2023, 24, 7948. [Google Scholar] [CrossRef]
- Prager, I.; Watzl, C. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 2019, 105, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Idrees, A.S.; Sugie, T.; Inoue, C.; Murata-Hirai, K.; Okamura, H.; Morita, C.T.; Minato, N.; Toi, M.; Tanaka, Y. Comparison of gammadelta T cell responses and farnesyl diphosphate synthase inhibition in tumor cells pretreated with zoledronic acid. Cancer Sci. 2013, 104, 536–542. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Jauhiainen, M.; Monkkonen, H.; Rogers, M.J.; Monkkonen, J.; Thompson, K. Peripheral blood monocytes are responsible for gammadelta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br. J. Haematol. 2009, 144, 245–250. [Google Scholar] [CrossRef]
- Rossini, M.; Adami, S.; Viapiana, O.; Fracassi, E.; Ortolani, R.; Vella, A.; Zanotti, R.; Tripi, G.; Idolazzi, L.; Gatti, D. Long-term effects of amino-bisphosphonates on circulating gammadelta T cells. Calcif. Tissue Int. 2012, 91, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Salesi, N.; Pistilli, R.; Marcelli, V.; Govoni, F.A.; Bozza, F.; Bossone, G.; Venturelli, V.; Di Cocco, B.; Pacetti, U.; Ciorra, A.; et al. Bisphosphonates and oral cavity avascular bone necrosis: A review of twelve cases. Anticancer Res. 2006, 26, 3111–3115. [Google Scholar] [CrossRef] [PubMed]
- Kikuiri, T.; Kim, I.; Yamaza, T.; Akiyama, K.; Zhang, Q.; Li, Y.; Chen, C.; Chen, W.; Wang, S.; Le, A.D.; et al. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J. Bone Miner. Res. 2010, 25, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Ullrich, K.A.; Schulze, L.L.; Paap, E.M.; Muller, T.M.; Neurath, M.F.; Zundler, S. Immunology of IL-12: An update on functional activities and implications for disease. EXCLI J. 2020, 19, 1563–1589. [Google Scholar] [CrossRef]
- Dai, J.; Lu, Y.; Yu, C.; Keller, J.M.; Mizokami, A.; Zhang, J.; Keller, E.T. Reversal of chemotherapy-induced leukopenia using granulocyte macrophage colony-stimulating factor promotes bone metastasis that can be blocked with osteoclast inhibitors. Cancer Res. 2010, 70, 5014–5023. [Google Scholar] [CrossRef]
- Abiko, Y.; Saitoh, M.; Nishimura, M.; Yamazaki, M.; Sawamura, D.; Kaku, T. Role of beta-defensins in oral epithelial health and disease. Med. Mol. Morphol. 2007, 40, 179–184. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Kimura, M.Y.; Koyama-Nasu, R.; Yagi, R.; Nakayama, T. A new therapeutic target: The CD69-Myl9 system in immune responses. Semin. Immunopathol. 2019, 41, 349–358. [Google Scholar] [CrossRef]
- Koyama-Nasu, R.; Wang, Y.; Hasegawa, I.; Endo, Y.; Nakayama, T.; Kimura, M.Y. The cellular and molecular basis of CD69 function in anti-tumor immunity. Int. Immunol. 2022, 34, 555–561. [Google Scholar] [CrossRef]
- Movila, A.; Mawardi, H.; Nishimura, K.; Kiyama, T.; Egashira, K.; Kim, J.Y.; Villa, A.; Sasaki, H.; Woo, S.B.; Kawai, T. Possible pathogenic engagement of soluble Semaphorin 4D produced by gammadeltaT cells in medication-related osteonecrosis of the jaw (MRONJ). Biochem. Biophys. Res. Commun. 2016, 480, 42–47. [Google Scholar] [CrossRef]
- Maleki, K.T.; Cornillet, M.; Bjorkstrom, N.K. Soluble SEMA4D/CD100: A novel immunoregulator in infectious and inflammatory diseases. Clin. Immunol. 2016, 163, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; White, G.; Romeril, K.; Buyck, H.; Stephens, M.; Brooks, C.; Weinkove, R. Innate-like T cell profile in myeloma: Severe deficiency of Vgamma9Vdelta2 T cells in aminobisphosphonate-treated patients. Leuk. Lymphoma 2016, 57, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Gober, H.J.; Kistowska, M.; Angman, L.; Jeno, P.; Mori, L.; De Libero, G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.H.; Wallace, M.; Malkovsky, M. Fas-dependent, activation-induced cell death of gammadelta cells. J. Biol. Regul. Homeost. Agents 2001, 15, 277–285. [Google Scholar] [PubMed]
- Green, D.R.; Droin, N.; Pinkoski, M. Activation-induced cell death in T cells. Immunol. Rev. 2003, 193, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.A.; White, A.J.; Jenkinson, W.E.; Turchinovich, G.; Nakamura, K.; Withers, D.R.; McConnell, F.M.; Desanti, G.E.; Benezech, C.; Parnell, S.M.; et al. Rank signaling links the development of invariant gammadelta T cell progenitors and Aire(+) medullary epithelium. Immunity 2012, 36, 427–437. [Google Scholar] [CrossRef]
- Kalyan, S. It May Seem Inflammatory, but Some T Cells Are Innately Healing to the Bone. J. Bone Miner. Res. 2016, 31, 1997–2000. [Google Scholar] [CrossRef]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.R.; Josien, R.; Lee, S.Y.; Sauter, B.; Li, H.L.; Steinman, R.M.; Choi, Y. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J. Exp. Med. 1997, 186, 2075–2080. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, A.; Thompson, K. Novel immunostimulatory effects of osteoclasts and macrophages on human gammadelta T cells. Bone 2015, 71, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Roato, I.; Grano, M.; Brunetti, G.; Colucci, S.; Mussa, A.; Bertetto, O.; Ferracini, R. Mechanisms of spontaneous osteoclastogenesis in cancer with bone involvement. FASEB J. 2005, 19, 228–230. [Google Scholar] [CrossRef]
- Roato, I.; Gorassini, E.; Buffoni, L.; Lyberis, P.; Ruffini, E.; Bonello, L.; Baldi, I.; Ciuffreda, L.; Mussa, A.; Ferracini, R. Spontaneous osteoclastogenesis is a predictive factor for bone metastases from non-small cell lung cancer. Lung Cancer 2008, 61, 109–116. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef]
- Panebianco, C.; Andriulli, A.; Pazienza, V. Pharmacomicrobiomics: Exploiting the drug-microbiota interactions in anticancer therapies. Microbiome 2018, 6, 92. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

| DMAB | ZOL | |||||||
|---|---|---|---|---|---|---|---|---|
| T0 | T6 | T12 | T18 | T0 | T6 | T12 | T18 | |
| GM-CSF | 8.6 ± 1.3 | 8.6 ± 1.4 | 8.6 ± 1.4 | 8.4 ± 1.8 | 6.8 ± 2.0 | 5.5 ± 1.1 | 4.0 ± 0.6 | 5.2 ± 1.1 |
| IFNα | 51.4 ± 9.8 | 50.0 ± 6.2 | 35.8 ± 7.6 | 59.9 ± 13.4 | 29.7 ± 7.9 | 32.0 ± 7.4 | 40.1 ± 9.5 | 33.2 ± 8.0 |
| IFNγ | 13.4 ± 7.0 | 32.9 ± 13.4 | 41.9 ± 14.7 | 24.3 ± 20.7 | 114.9 ± 55.1 | 10.4 ± 7.2 | not detectable | not detectable |
| IL-4 | 395.8 ± 43.1 | 419.8 ± 55.1 | 419.0 ± 53.0 | 467.2 ± 70.9 | 328.6 ± 34.7 | 412.1 ± 25.7 | 446.9 ± 24.4 | 380.6 ± 43.9 |
| IL-5 | 29.6 ± 6.1 | 45.0 ± 12.1 | 49.8 ± 12.8 | 38.8 ± 11.6 | 69.9 ± 33.8 | 20.3 ± 2.7 | 19.2 ± 5.5 | 19.4 ± 2.7 |
| IL-6 | 9.8 ± 0.9 | 10.6 ± 1.0 | 11.8 ± 2.3 | 9.6 ± 2.5 | 8.1 ± 1.3 | 10.0 ± 1.8 | 9.5 ± 1.4 | 10.2 ± 2.0 |
| IL-10 | 30.5 ± 4.9 | 32.8 ± 4.9 | 34.0 ± 4.2 | 30.4 ± 5.9 | 32.6 ± 10.8 | 20.1 ± 4.5 | 20.8 ± 3.2 | 19.0 ± 2.5 |
| IL-12 | 52.5 ± 9.0 | 69.2 ± 13.5 | 73.6 ± 16.2 | 61.3 ± 13.3 | 72.2 ± 32.6 | 38.7 ± 8.1 | 31.6 ± 5.7 | 26.2 ± 3.4 |
| IL-17A | 17.9 ± 4.9 | 28.3 ± 5.6 | 27.3 ± 7.1 | 29.6 ± 13.5 | 35.2 ± 11.5 | 19.7 ± 5.7 | 13.2 ± 3.1 | 16.4 ± 6.1 |
| TNFα | 21.8 ± 3.7 | 27.8 ± 2.8 | 29.6 ± 4.4 | 25.3 ± 5.1 | 23.8 ± 7.7 | 17.5 ± 2.2 | 15.1 ± 1.5 | 13.4 ± 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roato, I.; Pavone, L.; Pedraza, R.; Bosso, I.; Baima, G.; Erovigni, F.; Mussano, F. Denosumab and Zoledronic Acid Differently Affect Circulating Immune Subsets: A Possible Role in the Onset of MRONJ. Cells 2023, 12, 2430. https://doi.org/10.3390/cells12202430
Roato I, Pavone L, Pedraza R, Bosso I, Baima G, Erovigni F, Mussano F. Denosumab and Zoledronic Acid Differently Affect Circulating Immune Subsets: A Possible Role in the Onset of MRONJ. Cells. 2023; 12(20):2430. https://doi.org/10.3390/cells12202430
Chicago/Turabian StyleRoato, Ilaria, Lorenzo Pavone, Riccardo Pedraza, Ilaria Bosso, Giacomo Baima, Francesco Erovigni, and Federico Mussano. 2023. "Denosumab and Zoledronic Acid Differently Affect Circulating Immune Subsets: A Possible Role in the Onset of MRONJ" Cells 12, no. 20: 2430. https://doi.org/10.3390/cells12202430
APA StyleRoato, I., Pavone, L., Pedraza, R., Bosso, I., Baima, G., Erovigni, F., & Mussano, F. (2023). Denosumab and Zoledronic Acid Differently Affect Circulating Immune Subsets: A Possible Role in the Onset of MRONJ. Cells, 12(20), 2430. https://doi.org/10.3390/cells12202430







