mTORC1 Signaling in AgRP Neurons Is Not Required to Induce Major Neuroendocrine Adaptations to Food Restriction
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Immunofluorescence
2.3. Metabolic Measurements
2.4. Food Restriction Protocol
2.5. Tissue Analysis
2.6. Statistical Analysis
3. Results
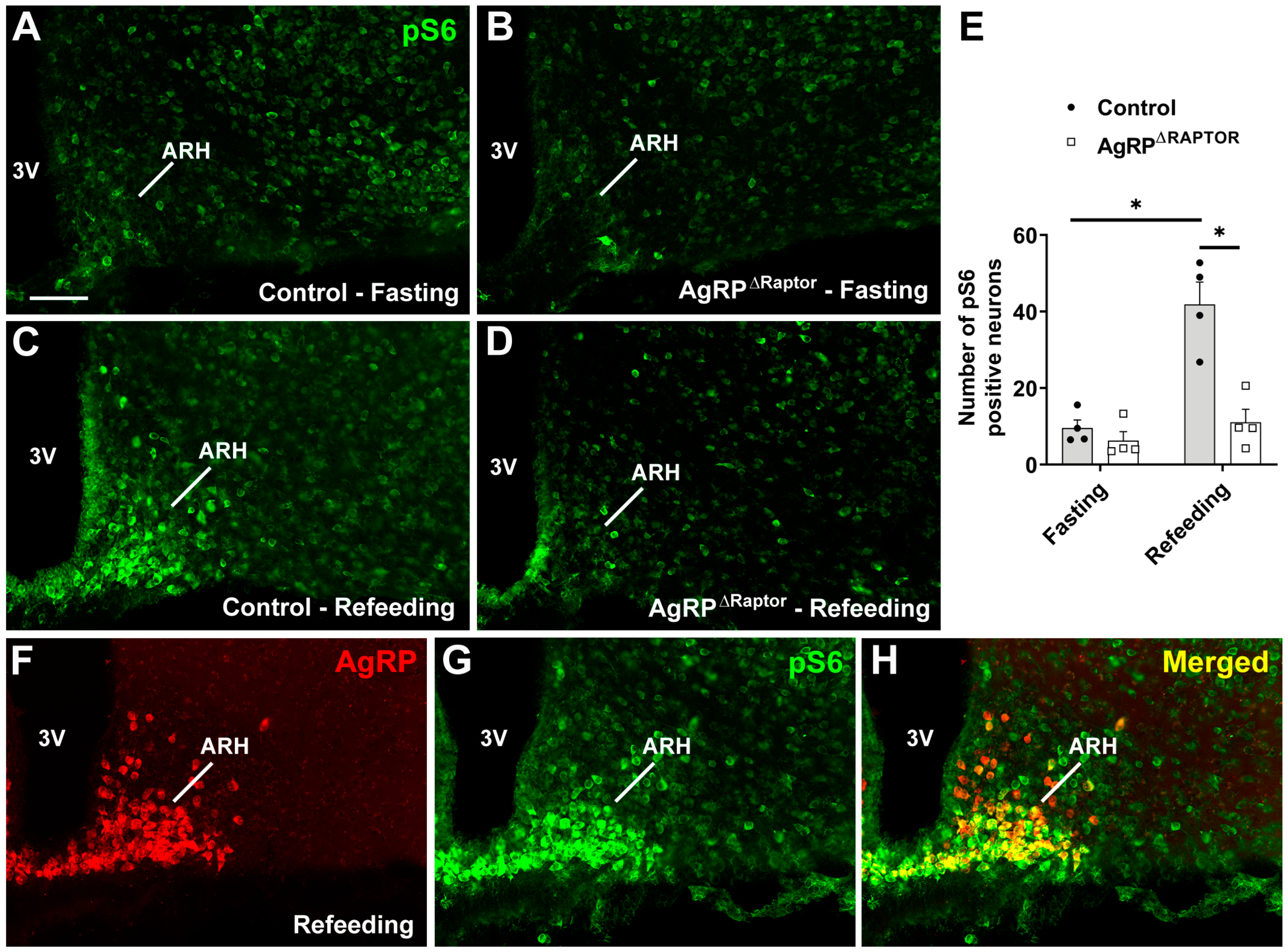
3.1. Generation of Mice Carrying Ablation of the mTORC1 Signaling in ARHAgRP Neurons
3.2. AgRPΔRaptor Mice Show Normal Body Weight but a Slight Improvement in Glucose Homeostasis
3.3. mTORC1 Signaling in AgRP Neurons Does Not Regulate Body Weight during Food Restriction and Refeeding
3.4. Absence of mTORC1 Signaling in AgRP Neurons Partially Blunts the Reduction in Energy Expenditure Caused by Food Restriction
3.5. Neuroendocrine Responses to Food Restriction Are Mildly Attenuated in AgRPΔRaptor Mice
3.6. AgRPΔRaptor Mice Show Increased Hyperphagia during Refeeding after an Acute Fasting
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andermann, M.L.; Lowell, B.B. Toward a wiring diagram understanding of appetite control. Neuron 2017, 95, 757–778. [Google Scholar] [CrossRef] [PubMed]
- Krashes, M.J.; Koda, S.; Ye, C.; Rogan, S.C.; Adams, A.C.; Cusher, D.S.; Maratos-Flier, E.; Roth, B.L.; Lowell, B.B. Rapid, reversible activation of agrp neurons drives feeding behavior in mice. J. Clin. Investig. 2011, 121, 1424–1428. [Google Scholar] [CrossRef]
- Gong, L.; Yao, F.; Hockman, K.; Heng, H.H.; Morton, G.J.; Takeda, K.; Akira, S.; Low, M.J.; Rubinstein, M.; Mackenzie, R.G. Signal transducer and activator of transcription-3 is required in hypothalamic agouti-related protein/neuropeptide y neurons for normal energy homeostasis. Endocrinology 2008, 149, 3346–3354. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, J.A.; Silveira, M.A.; Lima, L.B.; Furigo, I.C.; Zampieri, T.T.; Ramos-Lobo, A.M.; Buonfiglio, D.C.; Teixeira, P.D.; Frazao, R.; Donato, J., Jr. Changes in leptin signaling by socs3 modulate fasting-induced hyperphagia and weight regain in mice. Endocrinology 2016, 157, 3901–3914. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lobo, A.M.; Furigo, I.C.; Teixeira, P.D.S.; Zampieri, T.T.; Wasinski, F.; Buonfiglio, D.C.; Donato, J., Jr. Maternal metabolic adaptations are necessary for normal offspring growth and brain development. Physiol. Rep. 2018, 6, e13643. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.M.; Belsham, D.D. Insulin directly regulates npy and agrp gene expression via the mapk mek/erk signal transduction pathway in mhypoe-46 hypothalamic neurons. Mol. Cell. Endocrinol. 2009, 307, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.W.; Kaelin, C.B.; Takeda, K.; Akira, S.; Schwartz, M.W.; Barsh, G.S. Pi3k integrates the action of insulin and leptin on hypothalamic neurons. J. Clin. Investig. 2005, 115, 951–958. [Google Scholar] [CrossRef]
- Morrison, C.D.; Morton, G.J.; Niswender, K.D.; Gelling, R.W.; Schwartz, M.W. Leptin inhibits hypothalamic npy and agrp gene expression via a mechanism that requires phosphatidylinositol 3-oh-kinase signaling. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E1051–E1057. [Google Scholar] [CrossRef]
- Al-Qassab, H.; Smith, M.A.; Irvine, E.E.; Guillermet-Guibert, J.; Claret, M.; Choudhury, A.I.; Selman, C.; Piipari, K.; Clements, M.; Lingard, S.; et al. Dominant role of the p110beta isoform of pi3k over p110alpha in energy homeostasis regulation by pomc and agrp neurons. Cell Metab. 2009, 10, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Furigo, I.C.; Teixeira, P.D.; Quaresma, P.G.F.; Mansano, N.S.; Frazao, R.; Donato, J. Stat5 ablation in agrp neurons increases female adiposity and blunts food restriction adaptations. J. Mol. Endocrinol. 2020, 64, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.J.; Manning, B.D. Mtor couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol. Metab. 2011, 22, 94–102. [Google Scholar] [CrossRef]
- Kim, D.H.; Sarbassov, D.D.; Ali, S.M.; King, J.E.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. Mtor interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 2002, 110, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Peterson, T.R.; Laplante, M.; Oh, S.; Sabatini, D.M. Mtorc1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 2010, 468, 1100–1104. [Google Scholar] [CrossRef]
- Cota, D.; Proulx, K.; Smith, K.A.; Kozma, S.C.; Thomas, G.; Woods, S.C.; Seeley, R.J. Hypothalamic mtor signaling regulates food intake. Science 2006, 312, 927–930. [Google Scholar] [CrossRef]
- Blouet, C.; Ono, H.; Schwartz, G.J. Mediobasal hypothalamic p70 s6 kinase 1 modulates the control of energy homeostasis. Cell Metab 2008, 8, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, D.; Trajkovic, V.; Muller-Luhlhoff, S.; Brandt, E.; Abplanalp, W.; Bumke-Vogt, C.; Liehl, B.; Wiedmer, P.; Janjetovic, K.; Starcevic, V.; et al. Ghrelin-induced food intake and adiposity depend on central mtorc1/s6k1 signaling. Mol. Cell. Endocrinol. 2013, 381, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Inoki, K.; Munzberg, H.; Opland, D.; Faouzi, M.; Villanueva, E.C.; Ikenoue, T.; Kwiatkowski, D.; MacDougald, O.A.; Myers, M.G., Jr.; et al. Critical role for hypothalamic mtor activity in energy balance. Cell Metab. 2009, 9, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.D.; Xi, X.; White, C.L.; Ye, J.; Martin, R.J. Amino acids inhibit agrp gene expression via an mtor-dependent mechanism. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E165–E171. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.; Fernandez-Mallo, D.; Novelle, M.G.; Vazquez, M.J.; Tena-Sempere, M.; Nogueiras, R.; Lopez, M.; Dieguez, C. Hypothalamic mtor signaling mediates the orexigenic action of ghrelin. PLoS ONE 2012, 7, e46923. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.; Uchida, A.; Chuang, J.C.; Walker, A.; Liu, T.; Osborne-Lawrence, S.; Mason, B.L.; Mosher, C.; Berglund, E.D.; et al. Arcuate agrp neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol. Metab. 2014, 3, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Bongmba, O.Y.N.; Yue, J.; Lee, J.H.; Lin, L.; Saito, K.; Pradhan, G.; Li, D.P.; Pan, H.L.; Xu, A.; et al. Suppression of ghs-r in agrp neurons mitigates diet-induced obesity by activating thermogenesis. Int. J. Mol. Sci. 2017, 18, 832. [Google Scholar] [CrossRef] [PubMed]
- Albert, V.; Cornu, M.; Hall, M.N. Mtorc1 signaling in agrp neurons mediates circadian expression of agrp and npy but is dispensable for regulation of feeding behavior. Biochem. Biophys. Res. Commun. 2015, 464, 480–486. [Google Scholar] [CrossRef]
- Burke, L.K.; Darwish, T.; Cavanaugh, A.R.; Virtue, S.; Roth, E.; Morro, J.; Liu, S.M.; Xia, J.; Dalley, J.W.; Burling, K.; et al. Mtorc1 in agrp neurons integrates exteroceptive and interoceptive food-related cues in the modulation of adaptive energy expenditure in mice. eLife 2017, 6, e22848. [Google Scholar] [CrossRef]
- de Souza, G.O.; Wasinski, F.; Donato, J., Jr. Characterization of the metabolic differences between male and female c57bl/6 mice. Life Sci. 2022, 301, 120636. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lemus, M.B.; Stark, R.; Bayliss, J.A.; Reichenbach, A.; Lockie, S.H.; Andrews, Z.B. The temporal pattern of cfos activation in hypothalamic, cortical, and brainstem nuclei in response to fasting and refeeding in male mice. Endocrinology 2014, 155, 840–853. [Google Scholar] [CrossRef]
- Furigo, I.C.; Teixeira, P.D.S.; de Souza, G.O.; Couto, G.C.L.; Romero, G.G.; Perello, M.; Frazao, R.; Elias, L.L.; Metzger, M.; List, E.O.; et al. Growth hormone regulates neuroendocrine responses to weight loss via agrp neurons. Nat. Commun. 2019, 10, 662. [Google Scholar] [CrossRef] [PubMed]
- Haspula, D.; Cui, Z. Neurochemical basis of inter-organ crosstalk in health and obesity: Focus on the hypothalamus and the brainstem. Cells 2023, 12, 1801. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Frank, A.P.; Palmer, B.F.; Rodriguez-Navas, C.; Criollo, A.; Clegg, D.J. A sexually dimorphic hypothalamic response to chronic high-fat diet consumption. Int. J. Obes. 2016, 40, 206–209. [Google Scholar] [CrossRef]
- Morselli, E.; Frank, A.P.; Santos, R.S.; Fatima, L.A.; Palmer, B.F.; Clegg, D.J. Sex and gender: Critical variables in pre-clinical and clinical medical research. Cell Metab. 2016, 24, 203–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palmer, B.F.; Clegg, D.J. The sexual dimorphism of obesity. Mol. Cell. Endocrinol. 2015, 402, 113–119. [Google Scholar] [CrossRef] [PubMed]
- de Souza, G.O.; Chaves, F.M.; Silva, J.N.; Pedroso, J.A.B.; Metzger, M.; Frazao, R.; Donato, J. Gap junctions regulate the activity of agrp neurons and diet-induced obesity in male mice. J. Endocrinol. 2022, 255, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, J.A.B.; Wasinski, F.; Donato, J., Jr. Prolonged fasting induces long-lasting metabolic consequences in mice. J. Nutr. Biochem. 2020, 84, 108457. [Google Scholar] [CrossRef] [PubMed]
- Heeley, N.; Kirwan, P.; Darwish, T.; Arnaud, M.; Evans, M.L.; Merkle, F.T.; Reimann, F.; Gribble, F.M.; Blouet, C. Rapid sensing of l-leucine by human and murine hypothalamic neurons: Neurochemical and mechanistic insights. Mol. Metab. 2018, 10, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Varela, L.; Martinez-Sanchez, N.; Gallego, R.; Vazquez, M.J.; Roa, J.; Gandara, M.; Schoenmakers, E.; Nogueiras, R.; Chatterjee, K.; Tena-Sempere, M.; et al. Hypothalamic mtor pathway mediates thyroid hormone-induced hyperphagia in hyperthyroidism. J. Pathol. 2012, 227, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.B.; Tien, A.C.; Boddupalli, G.; Xu, A.W.; Jan, Y.N.; Jan, L.Y. Rapamycin ameliorates age-dependent obesity associated with increased mtor signaling in hypothalamic pomc neurons. Neuron 2012, 75, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Stilgenbauer, L.; de Lima, J.B.M.; Debarba, L.K.; Khan, M.; Koshko, L.; Kopchick, J.J.; Bartke, A.; Schneider, A.; Sadagurski, M. Growth hormone receptor (ghr) in agrp neurons regulates thermogenesis in a sex-specific manner. Geroscience 2023, 45, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti-de-Albuquerque, J.P.; Bober, J.; Zimmer, M.R.; Dietrich, M.O. Regulation of substrate utilization and adiposity by agrp neurons. Nat. Commun. 2019, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Mehlkop, O.; Scharn, A.; Nolte, H.; Klemm, P.; Henschke, S.; Steuernagel, L.; Sotelo-Hitschfeld, T.; Kaya, E.; Wunderlich, C.M.; et al. Nutrient-sensing agrp neurons relay control of liver autophagy during energy deprivation. Cell Metab. 2023, 35, 786–806.e713. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.C.A.; de Oliveira, F.P.; Fernandez, G.; da Guia Vieira, L.; Rosa, C.G.; do Nascimento, T.; de Castro Franca, S.; Donato, J., Jr.; Vella, K.R.; Antunes-Rodrigues, J.; et al. Arcuate agrp, but not pomc neurons, modulate paraventricular crf synthesis and release in response to fasting. Cell Biosci. 2022, 12, 118. [Google Scholar]
- van de Wall, E.; Leshan, R.; Xu, A.W.; Balthasar, N.; Coppari, R.; Liu, S.M.; Jo, Y.H.; MacKenzie, R.G.; Allison, D.B.; Dun, N.J.; et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 2008, 149, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bartolome, C.L.; Low, C.S.; Yi, X.; Chien, C.H.; Wang, P.; Kong, D. Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature 2018, 556, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Luquet, S.; Perez, F.A.; Hnasko, T.S.; Palmiter, R.D. Npy/agrp neurons are essential for feeding in adult mice but can be ablated in neonates. Science 2005, 310, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.R.; Lemes, S.F.; de Fante, T.; Saenz de Miera, C.; Pavan, I.C.B.; Bezerra, R.M.N.; Prada, P.O.; Torsoni, M.A.; Elias, C.F.; Simabuco, F.M. Modulation of hypothalamic s6k1 and s6k2 alters feeding behavior and systemic glucose metabolism. J. Endocrinol. 2020, 244, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Leibel, R.L.; Rosenbaum, M.; Hirsch, J. Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med. 1995, 332, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.L.; Heist, K.; DePaoli, A.M.; Veldhuis, J.D.; Mantzoros, C.S. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J. Clin. Investig. 2003, 111, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, L.E.; Pierce, A.A.; Xu, A.W. Functional requirement of agrp and npy neurons in ovarian cycle-dependent regulation of food intake. Proc. Natl. Acad. Sci. USA 2009, 106, 15932–15937. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Furlow, J.D. Glucocorticoids and skeletal muscle. Adv. Exp. Med. Biol. 2015, 872, 145–176. [Google Scholar] [PubMed]
- Hasselgren, P.O. Glucocorticoids and muscle catabolism. Curr. Opin. Clin. Nutr. Metab. Care 1999, 2, 201–205. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, G.O.; Teixeira, P.D.S.; Câmara, N.O.S.; Donato, J., Jr. mTORC1 Signaling in AgRP Neurons Is Not Required to Induce Major Neuroendocrine Adaptations to Food Restriction. Cells 2023, 12, 2442. https://doi.org/10.3390/cells12202442
de Souza GO, Teixeira PDS, Câmara NOS, Donato J Jr. mTORC1 Signaling in AgRP Neurons Is Not Required to Induce Major Neuroendocrine Adaptations to Food Restriction. Cells. 2023; 12(20):2442. https://doi.org/10.3390/cells12202442
Chicago/Turabian Stylede Souza, Gabriel O., Pryscila D. S. Teixeira, Niels O. S. Câmara, and Jose Donato, Jr. 2023. "mTORC1 Signaling in AgRP Neurons Is Not Required to Induce Major Neuroendocrine Adaptations to Food Restriction" Cells 12, no. 20: 2442. https://doi.org/10.3390/cells12202442
APA Stylede Souza, G. O., Teixeira, P. D. S., Câmara, N. O. S., & Donato, J., Jr. (2023). mTORC1 Signaling in AgRP Neurons Is Not Required to Induce Major Neuroendocrine Adaptations to Food Restriction. Cells, 12(20), 2442. https://doi.org/10.3390/cells12202442






