Abstract
Natural killer (NK) cells play a vital role in xenotransplantation rejection. One approach to induce NK cell immune tolerance is to prevent the NK cell-mediated direct killing of porcine cells by targeting the interaction of the activating receptor NKG2D and its ligands. However, the identity of porcine ligands for the human NKG2D receptor has remained elusive. Previous studies on porcine UL-16 binding protein 1 (pULBP-1) as a ligand for human NKG2D have yielded contradictory results. The goal of the present study was to clarify the role of pULBP-1 in the immune response and its interaction with human NKG2D receptor. To accomplish this, the CRISPR/Cas9 gene editing tool was employed to disrupt the porcine ULBP-1 gene in a 5-gene knockout porcine endothelial cell line (GGTA1, CMAH, β4galNT2, SLA-I α chain, and β-2 microglobulin, 5GKO). A colony with two allele mutations in pULBP-1 was established as a 6-gene knockout pig cell line (6GKO). We found that pULBP-1-deficient pig cells exhibited a reduced binding capacity to human NKG2D-Fc, a recombinant chimera protein. However, the removal of ULBP-1 from porcine endothelial cells did not significantly impact human NK cell degranulation or cytotoxicity upon stimulation with the pig cells. These findings conclusively demonstrate that pULBP-1 is not a crucial ligand for initiating xenogeneic human NK cell activation.
1. Introduction
Xenotransplantation offers a promising solution to the critical shortage of human organs for transplantation. Previous studies suggest that the transplantation of pig organs into humans might provide a viable option for this purpose [1]. Indeed, recent progress in pig genetic engineering has significantly advanced the potential for incorporating xenotransplantation into clinical settings [2]. This approach involves the removal of xenoantigens from vital organs as well as introducing human immune regulatory proteins to these organs to limit the deleterious immune response. For instance, the transplantation of a genetically modified pig heart into a human supported a patient for two months, thereby marking a milestone in pig-to-human xenotransplantation in practice. Therefore, the prevention or elimination of hyperacute immune rejection is feasible [3]. However, further investigations revealed endothelial injuries in the xenograft, potentially resulting from antibody-mediated rejection [4]. Thus, the persistent challenge of immune rejection remains a significant hurdle due to the inherent immune-related incompatibilities between humans and pigs. Identifying the specific mechanisms that aim at extending the survival of xenografts is necessary to improve the viability of pig-to-human xenotransplantation.
Previous studies identified the infiltration and activation of natural killer (NK) cells in both allotransplantation and xenotransplantation [5,6]. NK cells are vital components of the innate immune system that play a central role in recognizing and eliminating non-self or stressed cells [7]. NK cells lead to the immune rejection of transplanted organs due to specific mechanisms which attack the endothelium. For instance, antibody-dependent cell-mediated cytotoxicity (ADCC) acts via FcγRIIIA (CD16) binding to the Fc region of human IgG on porcine endothelium [8]. In addition, direct cytotoxicity is mediated through receptor–ligand interactions [9,10]. Lastly, the secretion of pro-inflammatory molecules like interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) triggers T-cell activation, thereby evoking an adaptive immune response against the xenograft [11,12,13]. While the removal of major xenoantigens from genetically modified pig cells can prevent NK cell-mediated ADCC, the mediators of direct cytotoxicity of these genetically modified pig endothelial cells by human NK cells via receptor–ligand interactions remain largely unknown [14].
NK cells express a diverse array of inhibitory and activating receptors, which engage with their ligands on the target cells to distinguish self, non-self, and abnormal cells [15]. The equilibrium between these inhibitory and activating signals governs the function of NK cells. In xenotransplantation, the inhibitory signals are lacking because MHC class I molecules are not recognized by the inhibitory receptors on NK cells across species [14,16,17]. Therefore, the dominance of activating signals results in the killing of pig endothelial cells by human NK cells.
Human NKG2D has been identified as a pivotal activating receptor in xenogeneic human NK cell-mediated cytotoxicity against pig cells [9,10]. NKG2D ligands include ULBPs and MHC class I chain-related protein (MIC) A and B in humans [18,19], as well as Rae-1 and H-60 in mice [20,21].
To date, the functional role of porcine ligands for the human NKG2D receptor is not yet clear; however, previous studies have established a potential relationship for specific porcine ligands. For instance, porcine UL-16 binding protein 1 (pULBP-1) has been cloned and characterized as a homolog of human ULBP [22]. The sequence of porcine MIC2 (pMIC2) also confirmed that pMIC2 is a homologue of human MIC proteins [23]. A study by Lilienfeld et al. reported that pULBP-1, but not pMIC2, is the predominant functional ligand for human NKG2D [24]. Contradictory to these findings, Tran et al. revealed that NKG2D-dependent cytotoxicity persisted even after enzymatic removal of pULBP-1 from porcine renal epithelial and intestinal endothelial cells, thereby implicating the presence of other ligands capable of binding to human NKG2D receptor and inducing NK cell activation [25]. Currently, the role of pULBP-1 as the primary trigger for NK cell activation in the context of pig-to-human xenotransplantation has yet to be established.
The present study re-evaluated the involvement of porcine ULBP-1 in human NK cell activation. For this purpose, we employed CRISPR/Cas9 gene editing technology to disrupt the pULBP-1 gene in a 5-gene knockout porcine endothelial cell line (GGTA1, CMAH, β4galNT2, SLA-I α chain, and β-2 microglobulin, 5GKO). The 5GKO cells have significantly reduced their immunogenicity by eliminating three major carbohydrate xenoantigens (α Gal, HD, and Sda) as well as the swine leukocyte antigen I (SLA-I α chain and β-2 microglobulin). Those proteins are major xenoantigens for antibody-mediated immune rejection. However, the 5GKO cells retain the capability to activate human NK cells, similar to wild type (WT) pig cells [14]. Subsequently, we investigated the binding capability of pULBP-1-deficient pig cells with a human NKG2D-Fc chimera protein. Additionally, we determined the extent of activation and cytotoxicity exhibited by human NK cells in response to these pULBP-1-deficient pig cells. The findings from this study indicate that pULBP-1 is not a functional ligand for the human NKG2D receptor.
2. Materials and Methods
2.1. Maintenance of Immortalized Porcine Liver-Derived Endothelial Cells (ipLDEC)
ipLDEC with five-gene knockout (5GKO) were cultured on Biocoat collagen I-coated plates or dishes (Corning Incorporated, Corning, NY, USA) in media (a-MEM:EGM-MV 3:1) (Invitrogen/Lonza, Switzerland) supplemented with 10% FBS (Hyclone, Logan, UT, USA), 10% horse serum (Invitrogen, Carlsbad, CA, USA), 12 mM HEPES, and 1% pen/strep (Invitrogen), as described in our previous studies [14,26].
2.2. CRISPR/Cas9-Mediated Disruption of the Porcine ULBP-1 Gene
Two target sequences in exon 2 of the pULBP-1 gene (ATCCAATCCGTGAGTTCCCTGGG and ATCGAAACACTCAAAGACGTTGG) were selected for guide RNA (gRNA) recognition. For ULBP-1 gRNA production, two pairs of primers (forward 5′ CACCGATCCAATCCGTGAGTTCCCT 3′/reverse 5′ AAACAGGGAACTCACGGATTGGATC 3′; forward 5′ CACCGTCGAAACACTCAAAGACGT 3′/reverse 5′ AAACACGTCTTTGAGTGTTTCGAC 3′) were synthesized (IDT, Coralville, IA), dimerized, and cloned to the CRISPR/Cas9 vector pSpCas9(BB)-2A-GFP (pX458), a gift from Dr. Feng Zhang [27] (Addgene plasmid # 48138), resulting in the pX458-pULBP-1A and pX458-pULBP-1B gRNA plasmid constructs. 5GKO cells were co-transfected with pX458-pULBP-1A&B gRNAs as previously described [28]. Briefly, 1 µg of each plasmid was added to 1 × 106 of 5GKO cells. The cell mixture was electroporated at 1300 V, 30 ms, 1 pulse using Neon Transfection System (Thermo Fisher Scientific, Waltham, MA, USA). Two days after transfection, GFP-expressing 5GKO cells were enriched by flow sorting. Sorted GFP-positive cells were placed on three 10 cm dishes at low density (500 cells/dish) and cultured for 10 days in a 5% CO2 incubator at 37 °C. Twenty single-cell clones were isolated and expended. Cells from each clone were lysed for PCR analysis. A pair of primers to amplify the pULBP1A&B gRNAs targeted region was designed and synthesized (IDT): forward 5′ ACTTCATCATCATTCCAAAGCC 3′/reverse 5′ CACTTGGATTTCCTGACTCACC 3′. PCR product was purified and sequenced using a sequencing primer 5′ ACTTCATCATCATTCCAAAGCC 3′ (Azenta Life Sciences, South Plainfield, NJ, USA).
Once mutation was confirmed by Sanger sequencing, PCR fragments were cloned into Zero Blunt TOPO vector (Thermo Fisher Scientific, Waltham, MA, USA). The mutations in both alleles were further revealed by Sanger sequencing using M13F primer. A cell line bearing frame-shift mutations in both pULBP-1 alleles was selected as 6-gene knockout cell line (6GKO, GGTA1/CMAH/β4galNT2/SLA-I α chain/β-2 microglobulin/pULBP-1). The mutated pULBP-1 protein sequences were predicted using MacVector software (MacVector, Inc., Apex, NC, USA).
2.3. Binding of pULBP-1 Deficient Cells to Human NKG2D-Fc Chimera Protein
pULBP-1-deficient cells (6GKO) were harvested, washed in RPMI 1640 media with 0.1% NaN3, and incubated with recombinant human NKG2D-Fc chimera protein (R&D Systems, Minneapolis, MN, USA) at 5 µg/mL for one hour at room temperature. Cells were washed three times in RPMI 1640 media with 0.1% NaN3 and stained with Alexa Fluor® 488 AffiniPure F(ab’)2 Fragment Donkey Anti-Human IgG, Fcγ fragment specific (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at 7 μg/mL (1:200) for 45 min at room temperature. Cells were washed and subjected to flow cytometry using an LSRFortessa analyzer (X-20) (BD Bioscience, San Jose, CA, USA). 5GKO cells binding to NKG2D-Fc chimera protein was used as a positive control. 5GKO or 6GKO cells stained with Alexa Fluor® 488 AffiniPure F(ab’)2 Fragment Donkey Anti-Human IgG alone were used as a negative control (background). Three independent experiments were performed.
2.4. Human NK Cell Activation in Response to Porcine Endothelial Cell Stimulation
NK cell activation was assessed by CD107a degranulation assays as previously reported [29]. Buffy coats were purchased from Versiti Indiana Blood Center. Fresh whole blood was drawn from two volunteers according to the guidelines of the Institutional Review Board (IRB) of Indiana University, IRB#11013. Ficoll-Paque Plus (GE-Healthcare, Pittsburgh, PA, USA) and Lymphoprep (STEMCELL Technologies, Vancouver, Canada) gradient centrifugations were used to isolate human peripheral blood mononuclear cells (PBMCs) from buffy coats and fresh whole blood, respectively. PBMCs were cultured in the RPMI1640 medium supplemented with 10% FBS, 1% penicillin/streptomycin, and 20 ng/mL recombinant human IL-2 (rhIL-2) (BioLegend, San Diego, CA, USA) at 37 °C in a 5% CO2 incubator for 5 days. The day before co-culture, 5GKO and 6GKO cells were seeded at 5 × 104 per well in a Biocoat collagen I-coated 48-well plate (Corning Incorporated, Corning, NY, USA). PBMCs treated with rhIL-2 were added to pig endothelial cells at 5 × 105 per well and the cells were co-cultured for 2 h at 37 °C in a CO2 incubator. For antibody blocking studies, rhIL-2 treated PBMC were pre-incubated with anti-NKG2D antibody and isotype antibody at 10 µg/mL (BioLegend, San Diego, CA, USA) at 4 °C for 30 min, then co-cultured with pig cells for 2 h at 37 °C in a CO2 incubator. Co-cultured cells were collected and stained with fixable viability dye eFluor 780 (Thermo Fisher Scientific, Waltham, MA, USA) to distinguish between live and dead cells, followed by staining with fluorochrome-conjugated antibodies against human CD45, CD3, CD56, and CD107a (BioLegend, San Diego, CA, USA). After fixing with 2% paraformaldehyde (PFA), stained cells were then acquired using an LSRFortessa flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and analyzed using FlowJo v10 software (BD Biosciences, Franklin Lakes, NJ, USA). NK cell activation was assessed by the frequency of CD107a-expressing cells within the live singlet CD45+ CD3-CD56+ NK cell population.
2.5. Calcein-AM Release Assay
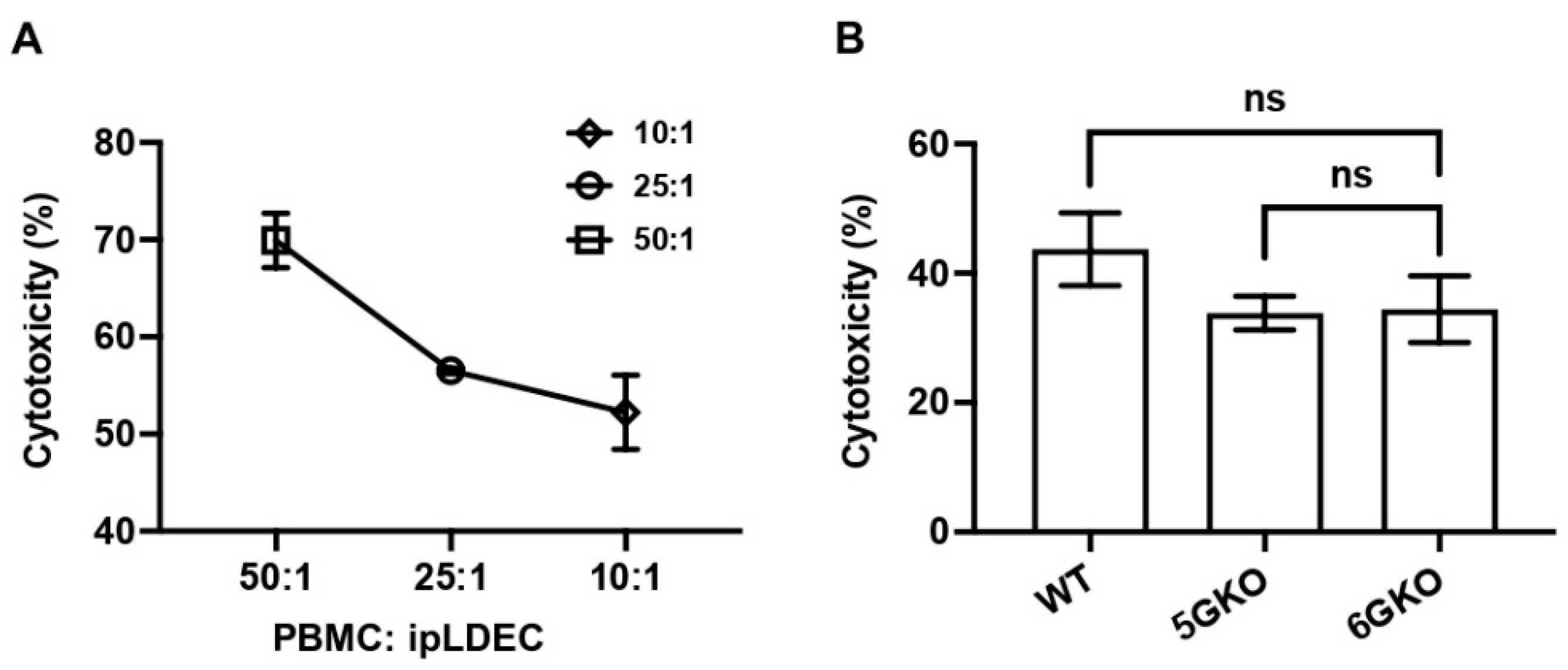
Human NK cell-mediated cytotoxicity of pig endothelial cells was measured by the Calcein-AM assay [30]. Pig endothelial cells were labeled with Calcein AM (BioLegend, San Diego, CA, USA) according to manufacturer’s protocol. The ratios of human PBMC and pig endothelial cells at 50:1, 25:1, and 10:1 were tested in a pilot experiment. A ratio of 10:1 (PBMC:pig endothelial cell) was used in subsequent experiments. A million WT, 5GKO, and 6GKO cells were labeled with 0.01 µM Calcein-AM for 30 min at 37 °C in the dark. Labeled cells were co-cultured with rhIL-2 activated human PBMCs (n = 6) in triplicate. After incubation at 37 °C in a 5% CO2 incubator for 4 h, 100 μL of each supernatant was harvested and transferred into a 96-well microplate for fluorescence-based assays (Thermo Fisher Scientific, Waltham, MA, USA). Samples were measured using a Cytation 5 imaging reader (BioTek Instruments, Inc., Winooski, VT, USA) at an excitation wavelength of 485 nm and emission wavelength of 530 nm. The fluorescence of culture media alone was subtracted from all samples. Specific lysis was calculated according to the formula [(test release − spontaneous release)/(maximum release − spontaneous release)] × 100. Spontaneous release represents Calcein-AM release from pig cells in medium alone, and maximum release is Calcein-AM release from pig cells lysed in medium with 0.4% Triton X-100. Each sample was assessed in a minimum of three replicate wells, ensuring that the data collected is more reliable and statistically meaningful.
2.6. Statistical Analysis
Statistical analyses were performed using GraphPad Prism 9 software (GraphPad Software, San Diego, CA, USA). Data are expressed as mean ± standard error of the mean (SEM). A one-way ANOVA was used to determine statistically significant difference among multiple groups. The difference between two groups was calculated with Student’s t-test. A p-value less than 0.05 was considered statistically significant. * p < 0.05; ** p < 0.01; ns: not significant.
3. Results
3.1. NKG2D Plays a Pivotal Role in Human NK Cell-to-Pig Endothelial Cell Xenogeneic Immune Response
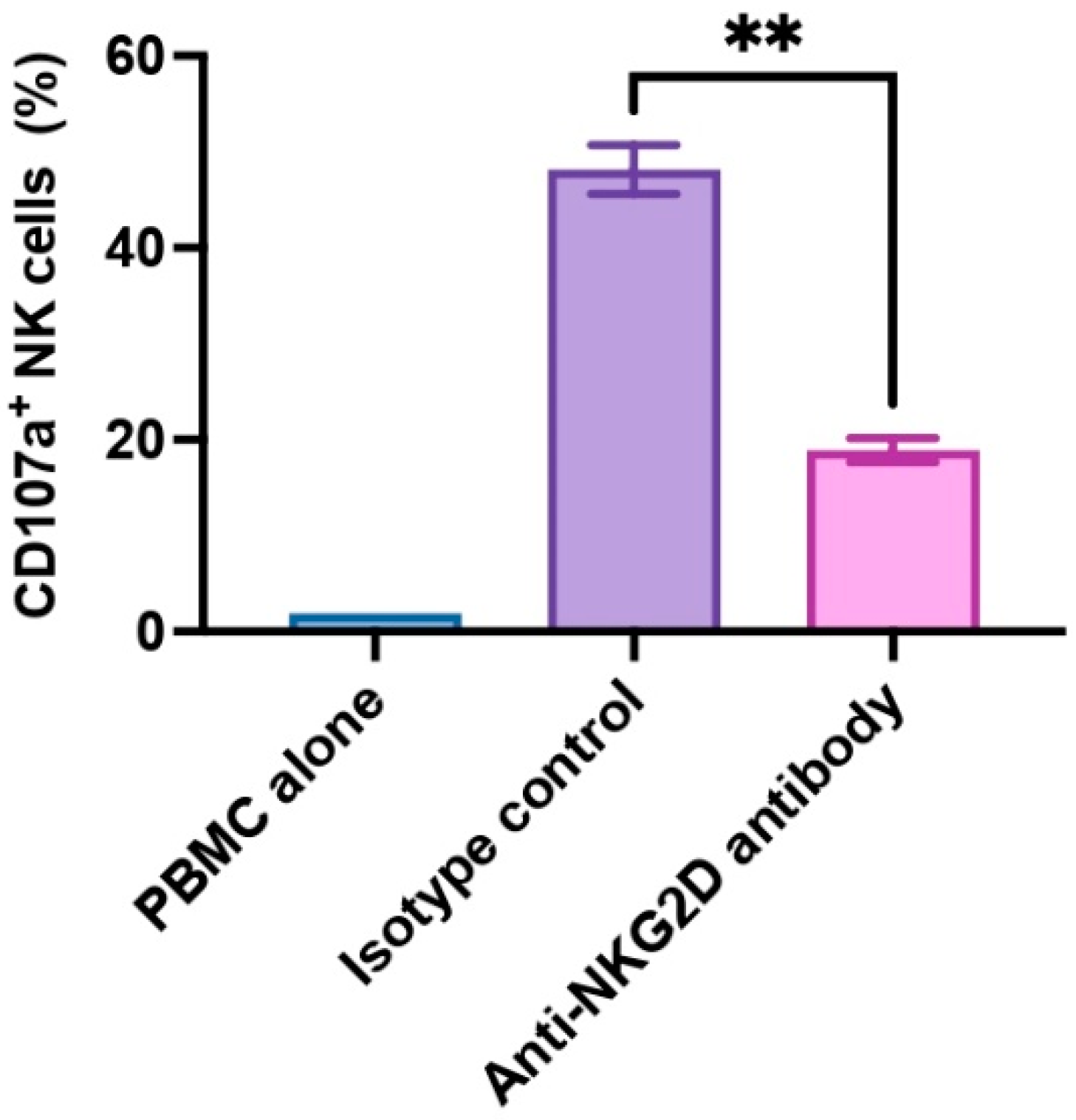
In the present study, we utilized PBMCs to allow for a more physiologically representative NK cell activation to account for interactions with other immune cells. In healthy individuals, NK cells constitute approximately 5–15% of the total PBMC population. NK cell activation often depends on cytokine signals, such as IL-2 and IL-15, and these signals originate from T cells or other immune cells [31]. We assessed human NK cell activation by monitoring CD107a expression within the CD3-CD56+ subset (NK cells). CD107a is also known as lysosomal-associated membrane protein-1 (LAMP-1), and CD107a serves as a functional indicator for NK cell activation in that CD107a surface expression correlates with cytokine production as well as NK cell-mediated cytotoxicity [32]. In our experiment, we examined PBMCs from three donors (n = 3), and we found that anti-NKG2D antibody exhibited strong inhibitory effects on NK cell activation. The presence of anti-NKG2D antibody led to a significant reduction in CD107a expression on NK cells compared to isotype control antibody (p < 0.01, 18.87% ± 1.24% vs. 48.13% ± 2.55%) upon WT pig cell stimulation (Figure 1). These findings confirm the pivotal role of NKG2D receptor and its ligands in activating NK cells.
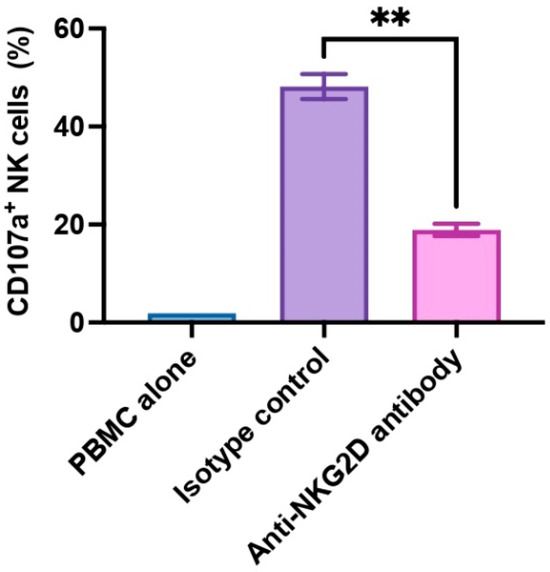
Figure 1.
NKG2D receptor-mediated xenogeneic human NK cell activation. Human PBMCs (n = 3) were treated with rhIL-2 for 5 days, then pre-incubated with anti-human NKG2D antibody or an isotype control antibody at 10 µg/mL for 30 min, and co-cultured with WT pig endothelial cells for 2 h. Human NK cell activation was assessed by the frequency of CD107a-expressing cells in the CD3−CD56+ NK cell population. PBMC alone was used as an unstimulated control. The isotype antibody-treated group was used as a control. Data were shown as mean ± SEM. Paired t-test was performed to analyze the differences between the two groups. ** p < 0.01.
3.2. Disruption of pULBP-1 Gene in 5GKO Cells
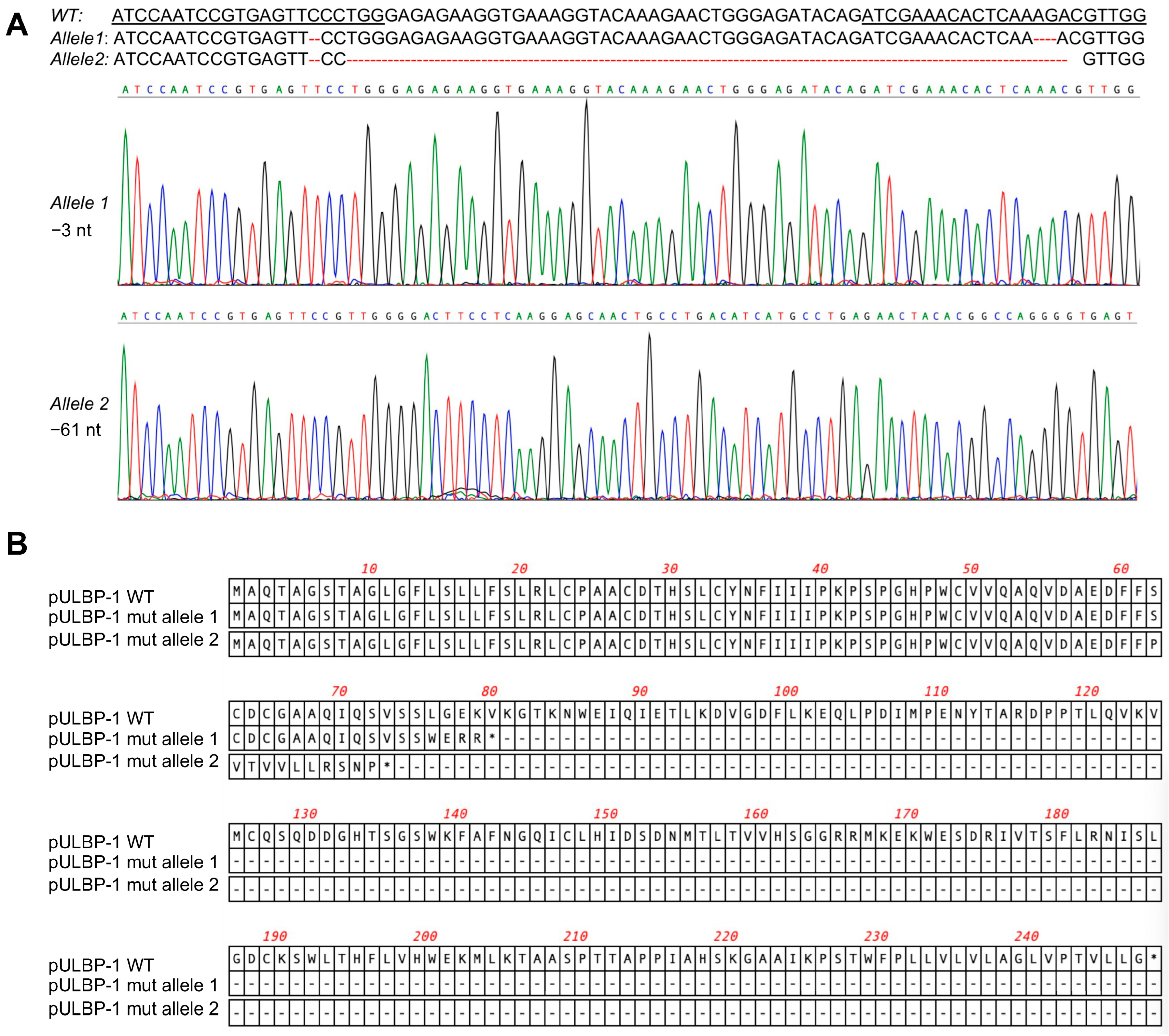
To disrupt the pULBP-1 gene in 5GKO cells, 5GKO cells were transfected with two pX458-pULBP-1 gRNAs targeting different regions in exon 2 of pULBP-1 gene. Transfected cells were enriched by flow sorting of green fluorescence protein (GFP)-expressing cells. To identify a cell line bearing mutations in both alleles of the pULBP-1 gene, single sorted cells were expanded and further screened by PCR and Sanger sequence. We ultimately identified a cell line with two allele mutations: one allele with 3 nucleotides deleted and another allele with 61 nucleotides deleted (Figure 2A). Mutations occurred in both gRNA-targeted regions. These mutations resulted in frameshifts, and predictive software indicated that the two mutated alleles encoded truncated pULBP-1 proteins with 72 and 79 amino acids, respectively (Figure 2B). Because these mutations led to the loss of glycosylphosphatidylinositol (GPI) anchor signal in pULBP-1, the truncated pULBP-1 proteins are no longer retained in the cell membrane.
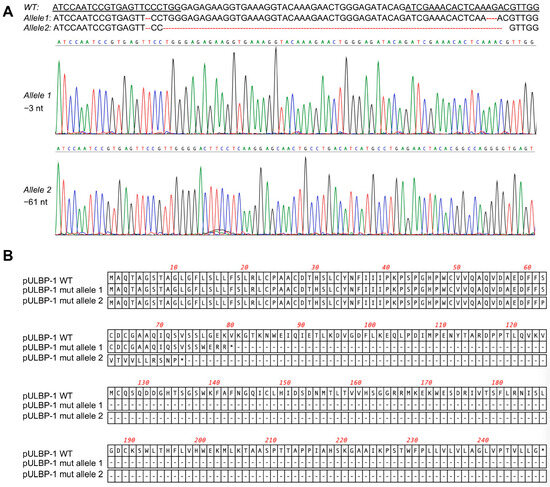
Figure 2.
Elimination of pULBP-1 in pig endothelial cells. (A) Disruption of pULBP-1 gene. CRISPR/Cas9 gRNAs targeted pULBP-1 DNA sequences were underlined. The missing nucleotides were marked using dash line in red. Sanger DNA sequencing chromatograms of the two pULBP-1 mutant alleles in 6GKO were shown. Three nucleotides were deleted in allele 1, and sixty-one nucleotides were missing in allele 2. (B) Translation of mutated pULBP-1. Deletion of 3 nucleotides in allele 1 led to a truncated pULBP-1 with 79 amino acids. Deletion of 61 nucleotides in allele 2 led to a truncated pULBP-1 with 72 amino acids.
3.3. pULBP-1-Deficient Cells Exhibit a Reduced Binding Potential to NKG2D-Fc Chimera Protein
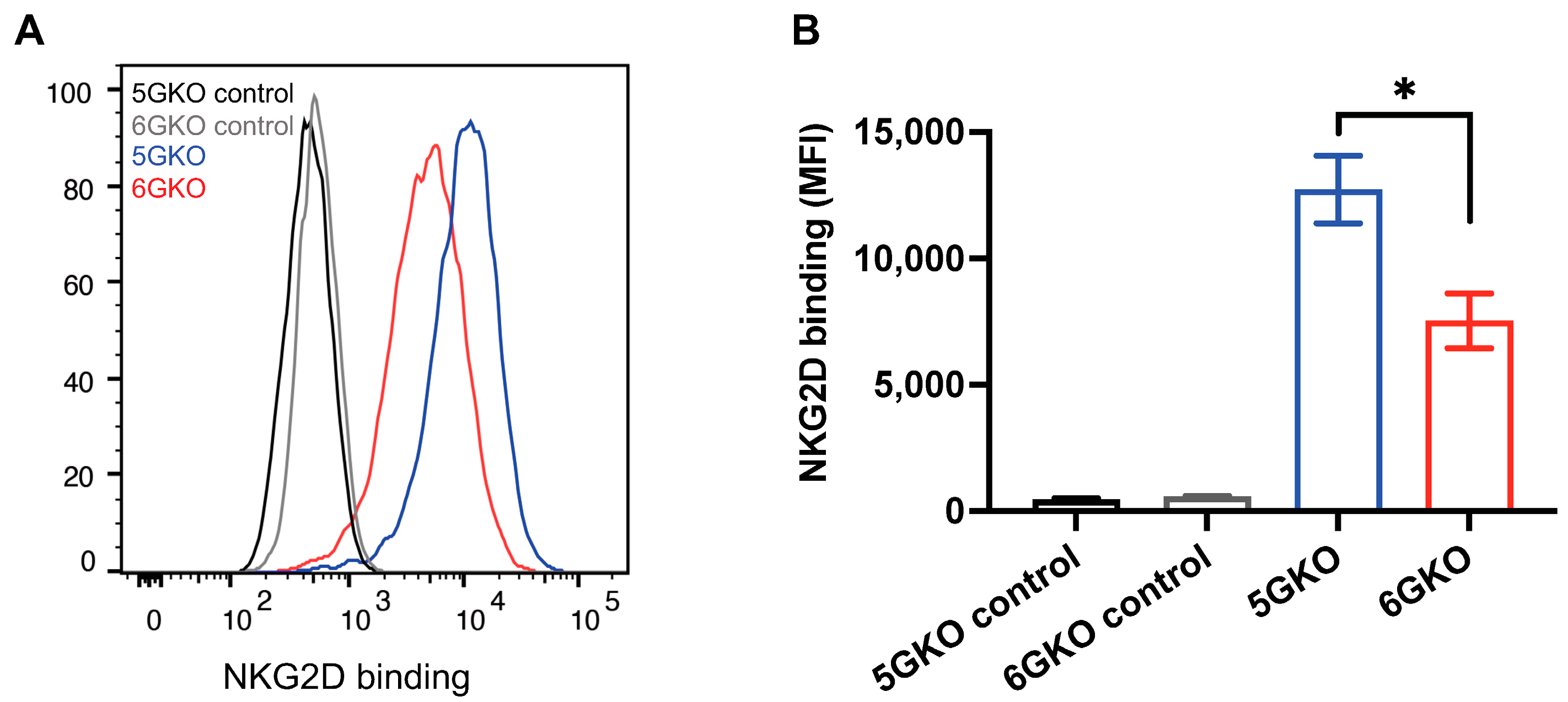
The interaction of pULBP-1 ligand and human NKG2D receptor has been previously reported [24,33]. To determine whether the binding of NKG2D to pULBP-1-deficient porcine cells was altered, 6GKO and 5GKO cells were incubated with a recombinant human NKG2D-Fc chimera protein, followed by the staining with Alexa Fluor® 488 AffiniPure F(ab’)2 Fragment Donkey Anti-Human IgG, Fcγ fragment-specific. A representative flow cytometry result showed that human NKG2D-Fc binding to 6GKO cells was reduced compared to the binding to 5GKO cells (Figure 3A). The mean fluorescent intensity (MFI) of NKG2D-Fc binding was 7527 ± 1081 for 6GKO and 12,729 ± 1331 for 5GKO (presented as mean ± SEM). 6GKO cells exhibited 40.9% reduction in binding to NKG2D-Fc protein compared to the parental 5GKO cells (p < 0.05, n = 3) (Figure 3B). This result demonstrated the absence of the pULBP-1 protein on 6GKO cells as well as the presence of unidentified ligands on pig cells that can bind to the human NKG2D-Fc protein.
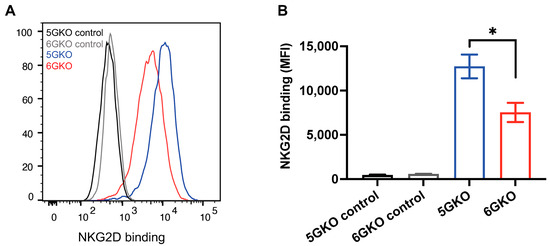
Figure 3.
pULBP-1 deficient cells exhibited a reduced binding capability to human NKG2D-Fc. (A) Representative binding results of NKG2D-Fc to pig cells. pULBP-1 deficient 5GKO cells (6GKO) were incubated with recombinant human NKG2D-Fc at 5 µg/mL followed by staining with Alexa Fluor 488-AffiniPure F(ab’)2 Fragment Donkey anti-Human IgG shown in red. 5GKO cells binding to human NKG2D-Fc was used as a positive control shown in blue. 5GKO and 6GKO cells stained with Alexa Fluor 488-AffiniPure F(ab’)2 Fragment Donkey anti-Human IgG alone were used as negative controls, shown in black and grey, respectively. (B) 6GKO cell binding to NKG2D-Fc chimera protein was significantly reduced compared to 5GKO cell binding to NKG2D-Fc chimera protein. Data were shown as mean ± SEM. Student’s t-test was performed to analyze the difference between the two groups. * p < 0.05.
3.4. pULBP-1 Does Not Contribute to Xenogeneic Human NK Cell Activation
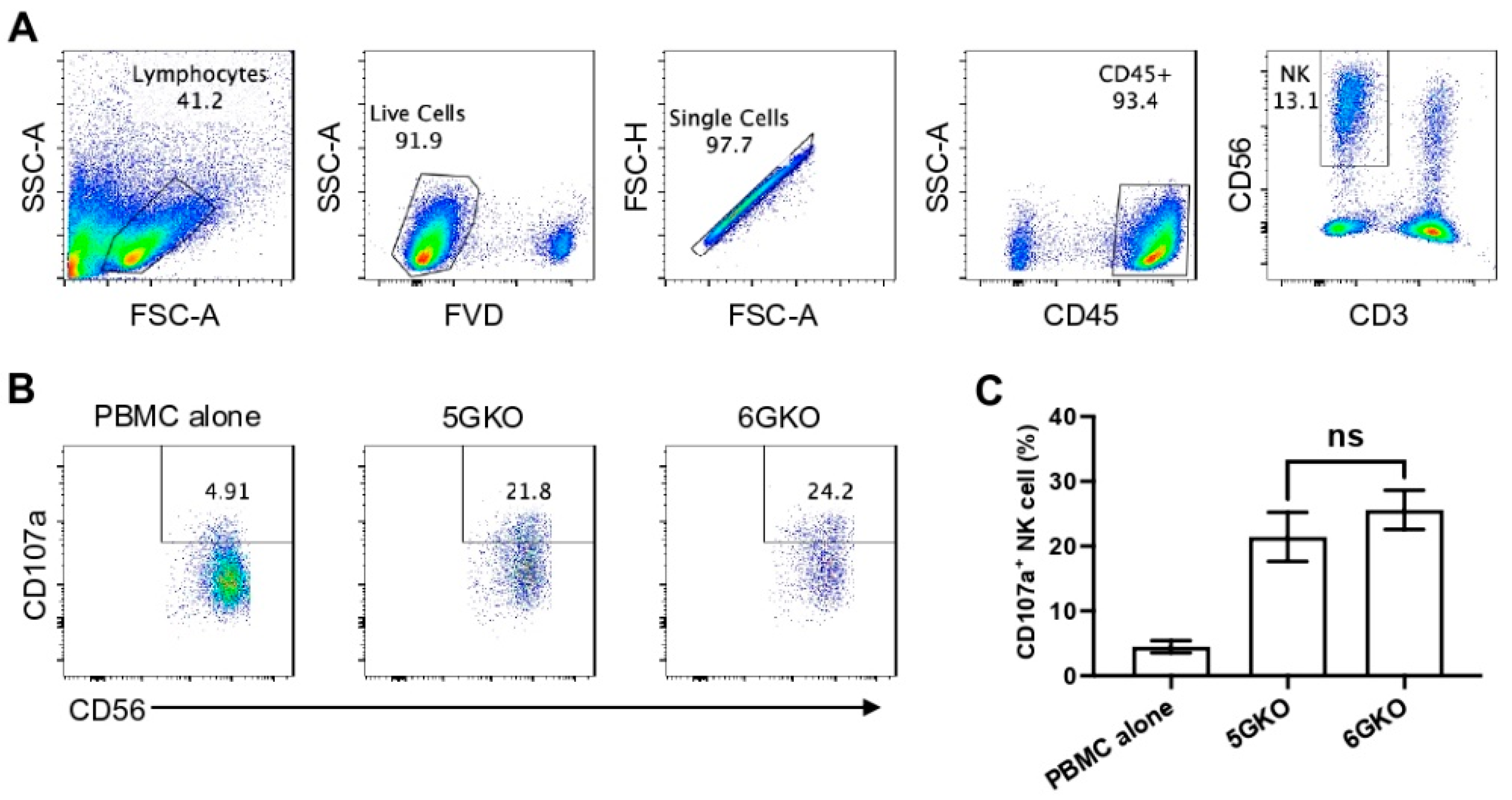
Because previous studies examining the role of pULBP-1 in human NK cell activation led to contradictory findings [24,25], we directly compared human NK cell activation in response to the stimulation of 5GKO and 6GKO cells. Our previous study showed that 5GKO cells induced human NK cell activation similar to WT and TKO (triple-gene knockout, GGTA1/CMAH/β4galNT2) cells [14]. The flow cytometry gating strategy to identify live human CD3-CD56+ NK cells is shown in Figure 4A. Representative flow plots showing human NK cell degranulation upon the stimulation of 5GKO or 6GKO cells are shown in Figure 4B. Human NK cell activation was measured by the percentage of CD107a positive cells in NK cell population. Student’s t-test indicated no significant difference between 5GKO and 6GKO cells in stimulating human NK cell degranulation (n = 8, p = 0.4962).
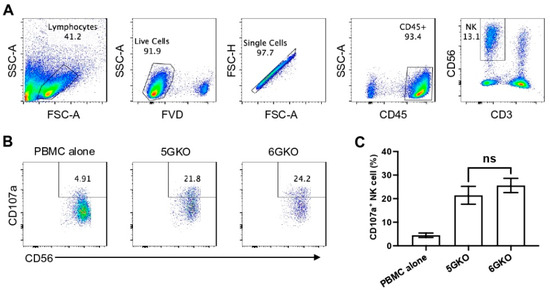
Figure 4.
Human NK cell activation in response to the stimulation of pULBP-1-deficient pig cells. 5GKO and 6GKO cells were co-cultured with rhIL-2 treated PBMCs (n = 8) for 2 h. (A) The flow cytometry gating strategies to show the live NK cell population. (B) Representative flow plots of NK cell activation to the modified porcine cells simulation assessed by the percentage of CD107a positive cells in the CD3-CD56+ population. PBMC alone was used as a control. (C) pULBP-1 deficiency had no impact on human NK cell degranulation. Data presented as mean ± SEM. The differences between the two groups were analyzed by Student’s t-test. ns, not significant.
3.5. pULBP-1 on Porcine Cells Has No Effect on Human NK Cell-Mediated Cytotoxicity
Human NK cell cytotoxicity was measured by the Calcein-AM assay [30]. A pilot study was conducted to test the ratio of human PBMCs and porcine endothelial cells. The killing activity was at 70.3%, 56.5%, and 52.7%, respectively, with human PBMCs to pig cells at ratios of 50:1, 25:1, and 10:1 (Figure 5A). Therefore, based on these findings, a ratio of 10:1 was selected for subsequent experiments. The result indicated no statistically significant difference in NK cell cytotoxicity between human NK cells stimulated by 5GKO and 6GKO porcine cells (p = 0.9941). Therefore, the pULBP-1 on porcine cells is not a functional ligand for human NK cell-mediated cytotoxicity (Figure 5B). WT porcine cells were also tested in this experiment, and there was no significant difference in NK cell cytotoxicity between human NK cells stimulated by WT and 6GKO porcine cells (p = 0.2946).
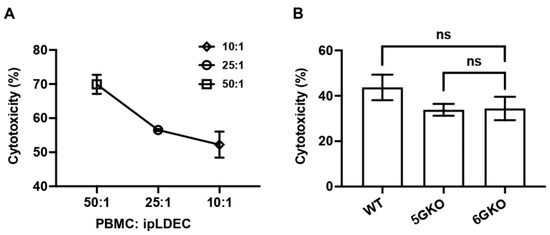
Figure 5.
pULBP-1 is dispensable for pig endothelial cells triggered human NK cell cytotoxicity. (A) The ratios of human PBMCs to pig endothelial cells at 50:1, 25:1, and 10:1 were tested in the Calcein-AM assay. A ratio of 10:1 was chosen in subsequent cytotoxicity assays. (B) Human PBMCs (n = 6) were co-cultured with Calcein-AM labeled WT, 5GKO, and 6GKO cells for 4 h. Fluorescence of the co-culture supernatant was measured by a Cytation 5 imaging reader. Cytotoxicity was calculated as described in Materials and Methods. Data were presented as mean ± SEM. One-way ANOVA was used to analyze the differences among the groups. ns: not significant.
4. Discussion
The goal of this study was to re-evaluate the involvement of porcine ULBP-1 in human NK cells activation and its role as a primary trigger for NK cell activation in the context of pig-to-human xenotransplantation. Using CRISPR/Cas9-mediated gene editing, we assessed whether the pULBP-1 is a potential NKG2D ligand. We successfully eliminated the pULBP-1 protein from pig endothelial cells. The wildtype pULBP-1 gene encodes 247 amino acids. We introduced the mutations in both alleles of the pULBP-1 gene, thereby resulting in the production of truncated ULBP-1 proteins encoding 72 and 79 amino acids. These truncated pULBP-1 were no longer retained in the cell membrane due to the lack of the GPI anchor [18,25]. In addition, we found that pULBP-1-deficient pig cells showed approximately a 40.9% reduction in binding capacity to human NKG2D-Fc compared to the control cells. These findings are consistent with the absence of ULBP-1 protein. Next, we compared human NK cell activation stimulated by pULBP-1-deficient pig cells as well as control pig cells. Surprisingly, despite a significant reduction in binding, we observed no significant difference in NK cell degranulation or cytotoxicity. Therefore, these findings suggest that pULBP-1 is not a functional ligand for the human NKG2D receptor.
These findings from the present study are inconsistent with a previous report suggesting that pULBP-1 is a critical ligand for human NKG2D receptor, which leads to NK cell-mediated cytotoxicity [24]. For instance, by reducing pULBP-1 mRNA expression or introducing anti-pULBP-1 polyclonal antibody, Lilienfeld et al. found that human NK cell-mediated cytotoxicity was partially reduced or completely blocked in response to pig endothelial cells. In addition, a pULBP-1 knockout pig was previously generated, and the findings indicated that porcine aortic endothelial cells deficient in pULBP-1 exhibited higher cell viability (85.36%) compared to the control group (69.58%) in a cytotoxicity assay using a human NK cell line NK92MI (p = 0.0565) [34].
To shed further light on the role of pULBP-1 in initiating NK cell activation, we used pULBP-1-deficient endothelial cells to stimulate human NK cell responses. We observed reduced, but significant NKG2D-Fc binding on pULBP-1-deficient pig cells, which indicated the presence of unidentified NKG2D ligands. This observation is consistent with previous research [25]. Indeed, these unidentified ligands on pig cells likely interact with the NKG2D receptor to initiate NK cell activation, thereby leading to the destruction of pig cells. Identifying these unknown porcine NKG2D ligands warrants future studies, as those ligands can potentially be used to manipulate NKG2D signals and block human NK cell activation in xenotransplantation.
An alternative strategy to regulate xenoreactive human NK cells is to introduce HLA class I molecules into pig cells, providing inhibitory signals to mitigate NK cell activation. Pig cells are susceptible to destruction by human NK cells due to imbalanced inhibitory and activating signals. Unlike human leukocyte antigen (HLA) class I molecules, swine leukocyte antigen class I (SLA-I) molecules cannot be recognized by inhibitory receptors on human NK cells [14,16]. Therefore, the dominance of activating signals initiates human NK cell activation. Several studies have shown that porcine cells expressing non-classical HLA class I molecules, including HLA-E and/or HLA-G, can protect porcine cells from human NK cell cytotoxicity [35,36,37]. Further, our recent study indicated that co-expressing HLA-E and HLA-G on porcine cells effectively inhibits human NK cell activation [29].
5. Conclusions
Our study confirmed that the human NK cell activating receptor NKG2D played a crucial role in the human NK cell-to-pig endothelial cell xenogeneic immune response. We further demonstrated that pULBP-1 could bind to the NKG2D chimera protein, but it was dispensable for NKG2D-mediated NK cell activation. Future studies will focus on identifying and eliminating porcine ligands for human NKG2D receptor from porcine cells, as well as introducing HLA-E and HLA-G inhibitory ligands into porcine cells through genetic engineering. These strategies have great potential to block human NK cell activation and NK cell-mediated rejection [38]. Additionally, investigating the interplay between NK cells and other immune cell populations, such as macrophages and T cells, could provide a more comprehensive understanding of the immune response to xenogeneic cells. Together, our study underscores the necessity for comprehensive investigations into the ligand–receptor interactions involved in NK cell activation and emphasizes the importance of considering the broader context of immune recognition in xenotransplantation.
Author Contributions
Conceptualization, P.L. and B.E.; methodology, K.J.L. and P.L.; investigation, K.J.L. and P.L.; resources, B.E. and P.L.; data curation, K.J.L. and P.L.; writing—original draft preparation, P.L. and B.W.; writing—review and editing, K.J.L., J.P.S., W.L., W.Z., B.W., A.A.C.-N., J.R.B., D.K.C.C., B.E. and P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by NIH NIAID R21AI164002 (PL). The Xenotransplantation research program has been supported by internal funds of the Department of Surgery, in part, with support by the Board of Directors of the Indiana University Health Values Fund for Research Award (VFR-457-Ekser).
Institutional Review Board Statement
This study involving human subjects was approved by the Indiana University Institutional Review Board (IRB#11013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Sus scrofa UL16 binding protein 1 (ULBP1) mRNA, NCBI reference sequence: NM_001004035.1.
Acknowledgments
The authors thank the members of the Indiana University Melvin and Bren Simon Comprehensive Cancer Center Flow Cytometry Core for their outstanding technical support. The Indiana University Melvin and Bren Simon Comprehensive Cancer Center Flow Cytometry Core is funded in part by NIH, National Cancer Institute (NCI) grant P30 CA082709 and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant U54 DK106846. The Flow Cytometry Core is supported in part by NIH instrumentation grant 1S10D012270.
Conflicts of Interest
D.K.C.C. is a consultant to eGenesis Bio, Cambridge, MA, USA. Other authors declare no potential conflict of interest.
References
- Cooper, D.K.; Ekser, B.; Ramsoondar, J.; Phelps, C.; Ayares, D. The role of genetically engineered pigs in xenotransplantation research. J. Pathol. 2016, 238, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Yang, B.; Wang, R.; Qin, C. Xenotransplantation: Current Status in Preclinical Research. Front. Immunol. 2019, 10, 3060. [Google Scholar] [CrossRef] [PubMed]
- Griffith, B.P.; Goerlich, C.E.; Singh, A.K.; Rothblatt, M.; Lau, C.L.; Shah, A.; Lorber, M.; Grazioli, A.; Saharia, K.K.; Hong, S.N.; et al. Genetically Modified Porcine-to-Human Cardiac Xenotransplantation. N. Engl. J. Med. 2022, 387, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, M.M.; Singh, A.K.; Scobie, L.; Goerlich, C.E.; Grazioli, A.; Saharia, K.; Crossan, C.; Burke, A.; Drachenberg, C.; Oguz, C.; et al. Graft dysfunction in compassionate use of genetically engineered pig-to-human cardiac xenotransplantation: A case report. Lancet 2023, 402, 397–410. [Google Scholar] [CrossRef]
- Yazdani, S.; Callemeyn, J.; Gazut, S.; Lerut, E.; de Loor, H.; Wevers, M.; Heylen, L.; Saison, C.; Koenig, A.; Thaunat, O.; et al. Natural killer cell infiltration is discriminative for antibody-mediated rejection and predicts outcome after kidney transplantation. Kidney Int. 2019, 95, 188–198. [Google Scholar] [CrossRef]
- Quan, D.; Bravery, C.; Chavez, G.; Richards, A.; Cruz, G.; Copeman, L.; Atkinson, C.; Holmes, B.; Davies, H.; Cozzi, E.; et al. Identification, detection, and in vitro characterization of cynomolgus monkey natural killer cells in delayed xenograft rejection of hDAF transgenic porcine renal xenografts. Transplant. Proc. 2000, 32, 936–937. [Google Scholar] [CrossRef]
- Bancroft, G.J. The role of natural killer cells in innate resistance to infection. Curr. Opin. Immunol. 1993, 5, 503–510. [Google Scholar] [CrossRef]
- Yin, D.; Zeng, H.; Ma, L.; Shen, J.; Xu, H.; Byrne, G.W.; Chong, A.S. Cutting Edge: NK cells mediate IgG1-dependent hyperacute rejection of xenografts. J. Immunol. 2004, 172, 7235–7238. [Google Scholar] [CrossRef]
- Forte, P.; Lilienfeld, B.G.; Baumann, B.C.; Seebach, J.D. Human NK cytotoxicity against porcine cells is triggered by NKp44 and NKG2D. J. Immunol. 2005, 175, 5463–5470. [Google Scholar] [CrossRef]
- Kim, T.J.; Kim, N.; Kim, E.O.; Choi, J.R.; Bluestone, J.A.; Lee, K.M. Suppression of human anti-porcine natural killer cell xenogeneic responses by combinations of monoclonal antibodies specific to CD2 and NKG2D and extracellular signal-regulated kinase kinase inhibitor. Immunology 2010, 130, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.C.; Goodman, J.; Sasaki, H.; Lowell, J.; Mohanakumar, T. Activation of natural killer cells and macrophages by porcine endothelial cells augments specific T-cell xenoresponse. Am. J. Transplant. 2002, 2, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.C.; Campos, E.F.; Saraiva Camara, N.O.; David, D.S.; Malheiros, D.M. Compartment-specific expression of natural killer cell markers in renal transplantation: Immune profile in acute rejection. Transpl. Int. 2016, 29, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Birmele, B.; Thibault, G.; Nivet, H.; Gruel, Y.; Bardos, P.; Lebranchu, Y. Human lymphocyte adhesion to xenogeneic porcine endothelial cells: Modulation by human TNF-alpha and involvement of VLA-4 and LFA-1. Transpl. Immunol. 1996, 4, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Walsh, J.R.; Lopez, K.; Isidan, A.; Zhang, W.; Chen, A.M.; Goggins, W.C.; Higgins, N.G.; Liu, J.; Brutkiewicz, R.R.; et al. Genetic engineering of porcine endothelial cell lines for evaluation of human-to-pig xenoreactive immune responses. Sci. Rep. 2021, 11, 13131. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Oettinger, H.F.; Sachs, D.H.; Edge, A.S. Analysis of polymorphism in porcine MHC class I genes: Alterations in signals recognized by human cytotoxic lymphocytes. J. Immunol. 1997, 159, 2318–2326. [Google Scholar] [CrossRef]
- Seebach, J.D.; Comrack, C.; Germana, S.; LeGuern, C.; Sachs, D.H.; DerSimonian, H. HLA-Cw3 expression on porcine endothelial cells protects against xenogeneic cytotoxicity mediated by a subset of human NK cells. J. Immunol. 1997, 159, 3655–3661. [Google Scholar] [CrossRef]
- Cosman, D.; Mullberg, J.; Sutherland, C.L.; Chin, W.; Armitage, R.; Fanslow, W.; Kubin, M.; Chalupny, N.J. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 2001, 14, 123–133. [Google Scholar] [CrossRef]
- Bauer, S.; Groh, V.; Wu, J.; Steinle, A.; Phillips, J.H.; Lanier, L.L.; Spies, T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 1999, 285, 727–729. [Google Scholar] [CrossRef]
- Cerwenka, A.; Bakker, A.B.; McClanahan, T.; Wagner, J.; Wu, J.; Phillips, J.H.; Lanier, L.L. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity 2000, 12, 721–727. [Google Scholar] [CrossRef]
- Diefenbach, A.; Jamieson, A.M.; Liu, S.D.; Shastri, N.; Raulet, D.H. Ligands for the murine NKG2D receptor: Expression by tumor cells and activation of NK cells and macrophages. Nat. Immunol. 2000, 1, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Borges, C.N.; Phanavanh, B.; Saraswati, S.; Dennis, R.A.; Crew, M.D. Molecular cloning and characterization of a porcine UL16 binding protein (ULBP)-like cDNA. Mol. Immunol. 2005, 42, 665–671. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chardon, P.; Rogel-Gaillard, C.; Cattolico, L.; Duprat, S.; Vaiman, M.; Renard, C. Sequence of the swine major histocompatibility complex region containing all non-classical class I genes. Tissue Antigens 2001, 57, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Lilienfeld, B.G.; Garcia-Borges, C.; Crew, M.D.; Seebach, J.D. Porcine UL16-binding protein 1 expressed on the surface of endothelial cells triggers human NK cytotoxicity through NKG2D. J. Immunol. 2006, 177, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.D.; Christiansen, D.; Winterhalter, A.; Brooks, A.; Gorrell, M.; Lilienfeld, B.G.; Seebach, J.D.; Sandrin, M.; Sharland, A. Porcine cells express more than one functional ligand for the human lymphocyte activating receptor NKG2D. Xenotransplantation 2008, 15, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Estrada, J.; Zhang, F.; Waghmare, S.K.; Mir, B. Isolation, characterization, and nuclear reprogramming of cell lines derived from porcine adult liver and fat. Cell Reprogram 2010, 12, 599–607. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Li, P.; Estrada, J.L.; Burlak, C.; Montgomery, J.; Butler, J.R.; Santos, R.M.; Wang, Z.Y.; Paris, L.L.; Blankenship, R.L.; Downey, S.M.; et al. Efficient generation of genetically distinct pigs in a single pregnancy using multiplexed single-guide RNA and carbohydrate selection. Xenotransplantation 2015, 22, 20–31. [Google Scholar] [CrossRef]
- Cross-Najafi, A.A.; Farag, K.; Isidan, A.; Li, W.; Zhang, W.; Lin, Z.; Walsh, J.R.; Lopez, K.; Park, Y.; Higgins, N.G.; et al. Co-expression of HLA-E and HLA-G on genetically modified porcine endothelial cells attenuates human NK cell-mediated degranulation. Front. Immunol. 2023, 14, 1217809. [Google Scholar] [CrossRef]
- Neri, S.; Mariani, E.; Meneghetti, A.; Cattini, L.; Facchini, A. Calcein-acetyoxymethyl cytotoxicity assay: Standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin. Diagn. Lab. Immunol. 2001, 8, 1131–1135. [Google Scholar] [CrossRef]
- Kerdiles, Y.; Ugolini, S.; Vivier, E. T cell regulation of natural killer cells. J. Exp. Med. 2013, 210, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Alter, G.; Malenfant, J.M.; Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 2004, 294, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Lilienfeld, B.G.; Schildknecht, A.; Imbach, L.L.; Mueller, N.J.; Schneider, M.K.; Seebach, J.D. Characterization of porcine UL16-binding protein 1 endothelial cell surface expression. Xenotransplantation 2008, 15, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Joanna, Z.; Magdalena, H.; Agnieszka, N.T.; Jacek, J.; Ryszard, S.; Zdzislaw, S.; Barbara, G.; Daniel, L. The production of UL16-binding protein 1 targeted pigs using CRISPR technology. 3 Biotech. 2018, 8, 70. [Google Scholar] [CrossRef]
- Forte, P.; Matter-Reissmann, U.B.; Strasser, M.; Schneider, M.K.; Seebach, J.D. Porcine aortic endothelial cells transfected with HLA-G are partially protected from xenogeneic human NK cytotoxicity. Hum. Immunol. 2000, 61, 1066–1073. [Google Scholar] [CrossRef]
- Weiss, E.H.; Lilienfeld, B.G.; Muller, S.; Muller, E.; Herbach, N.; Kessler, B.; Wanke, R.; Schwinzer, R.; Seebach, J.D.; Wolf, E.; et al. HLA-E/human beta2-microglobulin transgenic pigs: Protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation 2009, 87, 35–43. [Google Scholar] [CrossRef]
- Lilienfeld, B.G.; Crew, M.D.; Forte, P.; Baumann, B.C.; Seebach, J.D. Transgenic expression of HLA-E single chain trimer protects porcine endothelial cells against human natural killer cell-mediated cytotoxicity. Xenotransplantation 2007, 14, 126–134. [Google Scholar] [CrossRef]
- Lopez, K.J.; Cross-Najafi, A.A.; Farag, K.; Obando, B.; Thadasina, D.; Isidan, A.; Park, Y.; Zhang, W.; Ekser, B.; Li, P. Strategies to induce natural killer cell tolerance in xenotransplantation. Front. Immunol. 2022, 13, 941880. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).