Ciliary Ultrastructure Assessed by Transmission Electron Microscopy in Adults with Bronchiectasis and Suspected Primary Ciliary Dyskinesia but Inconclusive Genotype
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Whole-Exome Sequencing
2.3. Transmission Electron Microscopy and PCD Detect Software
2.4. Statistical Analysis and Ethical Approval
3. Results
3.1. Patient Characteristics
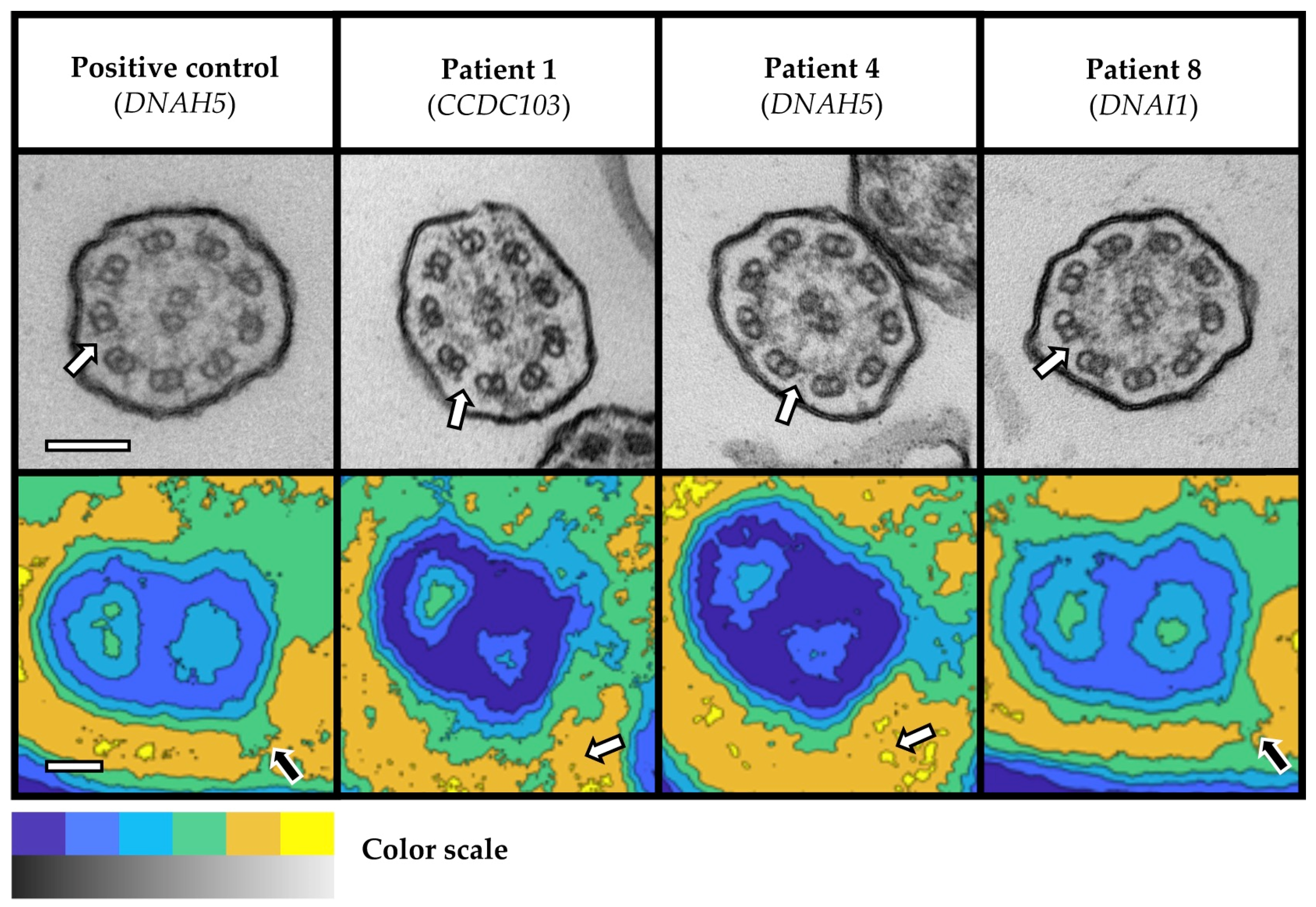
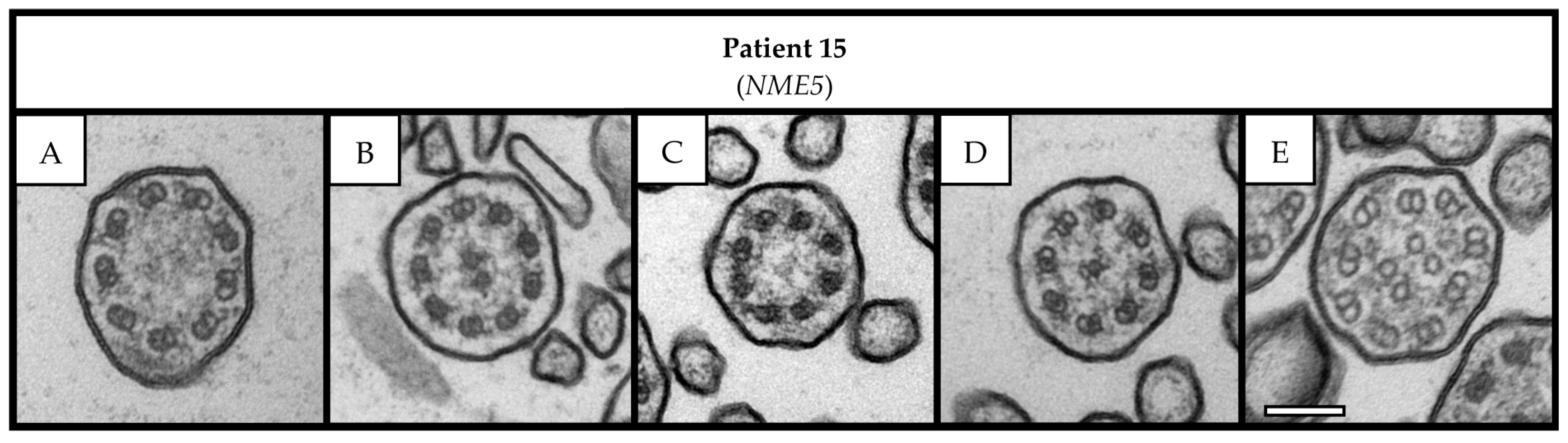
3.2. Transmission Electron Microscopy Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallmeier, J.; Nielsen, K.G.; Kuehni, C.E.; Lucas, J.S.; Leigh, M.W.; Zariwala, M.A.; Omran, H. Motile ciliopathies. Nat. Rev. Dis. Primers 2020, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Ringshausen, F.C.; Shapiro, A.J.; Nielsen, K.G.; Mazurek, H.; Pifferi, M.; Donn, K.H.; van der Eerden, M.M.; Loebinger, M.R.; Zariwala, M.A.; Leigh, M.W.; et al. Safety and efficacy of the epithelial sodium channel blocker idrevloride in people with primary ciliary dyskinesia (CLEAN-PCD): A multinational, phase 2, randomised, double-blind, placebo-controlled crossover trial. Lancet Respir. Med. 2023. [Google Scholar] [CrossRef]
- Hannah, W.B.; Seifert, B.A.; Truty, R.; Zariwala, M.A.; Ameel, K.; Zhao, Y.; Nykamp, K.; Gaston, B. The global prevalence and ethnic heterogeneity of primary ciliary dyskinesia gene variants: A genetic database analysis. Lancet Respir. Med. 2022, 10, 459–468. [Google Scholar] [CrossRef]
- Shoemark, A.; Griffin, H.; Wheway, G.; Hogg, C.; Lucas, J.S.; Genomics England Research Consortium; Camps, C.; Taylor, J.; Carroll, M.; Loebinger, M.R.; et al. Genome sequencing reveals underdiagnosis of primary ciliary dyskinesia in bronchiectasis. Eur. Respir. J. 2022, 60, 2200176. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.S.; Barbato, A.; Collins, S.A.; Goutaki, M.; Behan, L.; Caudri, D.; Dell, S.; Eber, E.; Escudier, E.; Hirst, R.A.; et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur. Respir. J. 2017, 49, 1601090. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.J.; Davis, S.D.; Polineni, D.; Manion, M.; Rosenfeld, M.; Dell, S.D.; Chilvers, M.A.; Ferkol, T.W.; Zariwala, M.A.; Sagel, S.D.; et al. Diagnosis of Primary Ciliary Dyskinesia. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 197, e24–e39. [Google Scholar] [CrossRef]
- Shoemark, A.; Boon, M.; Brochhausen, C.; Bukowy-Bieryllo, Z.; De Santi, M.M.; Goggin, P.; Griffin, P.; Hegele, R.G.; Hirst, R.A.; Leigh, M.W.; et al. International consensus guideline for reporting transmission electron microscopy results in the diagnosis of primary ciliary dyskinesia (BEAT PCD TEM Criteria). Eur. Respir. J. 2020, 55, 1900725. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Shoemark, A.; Rubbo, B.; Legendre, M.; Fassad, M.R.; Haarman, E.G.; Best, S.; Bon, I.C.M.; Brandsma, J.; Burgel, P.R.; Carlsson, G.; et al. Topological data analysis reveals genotype-phenotype relationships in primary ciliary dyskinesia. Eur. Respir. J. 2021, 58, 2002359. [Google Scholar] [CrossRef]
- von Hardenberg, S.; Wallaschek, H.; Du, C.; Schmidt, G.; Auber, B. A holistic approach to maximise diagnostic output in trio exome sequencing. Front. Pediatr. 2023, 11, 1183891. [Google Scholar] [CrossRef]
- Pereira, R.; Barbosa, T.; Gales, L.; Oliveira, E.; Santos, R.; Oliveira, J.; Sousa, M. Clinical and Genetic Analysis of Children with Kartagener Syndrome. Cells 2019, 8, 900. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.H.; Huh, H.J.; Jeong, I.; Lee, N.Y.; Koh, W.J.; Park, H.C.; Ki, C.S. A nonsense variant in NME5 causes human primary ciliary dyskinesia with radial spoke defects. Clin. Genet. 2020, 98, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Al Mutairi, F.; Alkhalaf, R.; Alkhorayyef, A.; Alroqi, F.; Yusra, A.; Umair, M.; Nouf, F.; Khan, A.; Meshael, A.; Hamad, A.; et al. Homozygous truncating NEK10 mutation, associated with primary ciliary dyskinesia: A case report. BMC Pulm. Med. 2020, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Lablans, M.; Ataian, M.; Raidt, J.; Wallmeier, J.; Grosse-Onnebrink, J.; Kuehni, C.E.; Haarman, E.G.; Leigh, M.W.; Quittner, A.L.; et al. An international registry for primary ciliary dyskinesia. Eur. Respir. J. 2016, 47, 849–859. [Google Scholar] [CrossRef]
- Paff, T.; Kooi, I.E.; Moutaouakil, Y.; Riesebos, E.; Sistermans, E.A.; Daniels, H.; Weiss, J.M.M.; Niessen, H.; Haarman, E.G.; Pals, G.; et al. Diagnostic yield of a targeted gene panel in primary ciliary dyskinesia patients. Hum. Mutat. 2018, 39, 653–665. [Google Scholar] [CrossRef]
- Mariani, S.; Li, T.; Hegermann, J.; Bounader, K.; Hanke, J.; Meyer, T.; Jannsen-Peters, H.; Haverich, A.; Schmitto, J.D.; Dogan, G. Biocompatibility of an apical ring plug for left ventricular assist device explantation: Results of a feasibility pre-clinical study. Artif. Organs 2022, 46, 827–837. [Google Scholar] [CrossRef]
- Shoemark, A.; Pinto, A.L.; Patel, M.P.; Daudvohra, F.; Hogg, C.; Mitchison, H.M.; Burgoyne, T. PCD Detect: Enhancing ciliary features through image averaging and classification. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L1048–L1060. [Google Scholar] [CrossRef]
- Kuehni, C.E.; Frischer, T.; Strippoli, M.P.; Maurer, E.; Bush, A.; Nielsen, K.G.; Escribano, A.; Lucas, J.S.; Yiallouros, P.; Omran, H.; et al. Factors influencing age at diagnosis of primary ciliary dyskinesia in european children. Eur. Respir. J. 2010, 36, 1248–1258. [Google Scholar] [CrossRef]
- Contarini, M.; Shoemark, A.; Rademacher, J.; Finch, S.; Gramegna, A.; Gaffuri, M.; Roncoroni, L.; Seia, M.; Ringshausen, F.C.; Welte, T.; et al. Why, when and how to investigate primary ciliary dyskinesia in adult patients with bronchiectasis. Multidiscip. Respir. Med. 2018, 13, 26. [Google Scholar] [CrossRef]
- Goutaki, M.; Meier, A.B.; Halbeisen, F.S.; Lucas, J.S.; Dell, S.D.; Maurer, E.; Casaulta, C.; Jurca, M.; Spycher, B.D.; Kuehni, C.E. Clinical manifestations in primary ciliary dyskinesia: Systematic review and meta-analysis. Eur. Respir. J. 2016, 48, 1081–1095. [Google Scholar] [CrossRef]
- Davis, S.D.; Rosenfeld, M.; Lee, H.S.; Ferkol, T.W.; Sagel, S.D.; Dell, S.D.; Milla, C.; Pittman, J.E.; Shapiro, A.J.; Sullivan, K.M.; et al. Primary ciliary dyskinesia: Longitudinal study of lung disease by ultrastructure defect and genotype. Am. J. Respir. Crit. Care Med. 2019, 199, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.J.; Josephson, M.; Rosenfeld, M.; Yilmaz, O.; Davis, S.D.; Polineni, D.; Guadagno, E.; Leigh, M.W.; Lavergne, V. Accuracy of Nasal Nitric Oxide Measurement as a Diagnostic Test for Primary Ciliary Dyskinesia. A Systematic Review and Meta-analysis. Ann. Am. Thorac. Soc. 2017, 14, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Shoemark, A.; Burgoyne, T.; Kwan, R.; Dixon, M.; Patel, M.P.; Rogers, A.V.; Onoufriadis, A.; Scully, J.; Daudvohra, F.; Cullup, T.; et al. Primary ciliary dyskinesia with normal ultrastructure: Three-dimensional tomography detects absence of DNAH11. Eur. Respir. J. 2018, 51, 1701809. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.M.; Crinnion, L.A.; Morgan, J.E.; Harrison, S.M.; Diggle, C.P.; Adlard, J.; Lindsay, H.A.; Camm, N.; Charlton, R.; Sheridan, E.; et al. Robust diagnostic genetic testing using solution capture enrichment and a novel variant-filtering interface. Hum. Mutat. 2014, 35, 434–441. [Google Scholar] [CrossRef][Green Version]
- Liu, C.; Miyata, H.; Gao, Y.; Sha, Y.; Tang, S.; Xu, Z.; Whitfield, M.; Patrat, C.; Wu, H.; Dulioust, E.; et al. Bi-allelic DNAH8 Variants Lead to Multiple Morphological Abnormalities of the Sperm Flagella and Primary Male Infertility. Am. J. Hum. Genet. 2020, 107, 330–341. [Google Scholar] [CrossRef]
- Guichard, C.; Harricane, M.C.; Lafitte, J.J.; Godard, P.; Zaegel, M.; Tack, V.; Lalau, G.; Bouvagnet, P. Axonemal dynein intermediate-chain gene (DNAI1) mutations result in situs inversus and primary ciliary dyskinesia (Kartagener syndrome). Am. J. Hum. Genet. 2001, 68, 1030–1035. [Google Scholar] [CrossRef]
- Hornef, N.; Olbrich, H.; Horvath, J.; Zariwala, M.A.; Fliegauf, M.; Loges, N.T.; Wildhaber, J.; Noone, P.G.; Kennedy, M.; Antonarakis, S.E.; et al. DNAH5 mutations are a common cause of primary ciliary dyskinesia with outer dynein arm defects. Am. J. Respir. Crit. Care Med. 2006, 174, 120–126. [Google Scholar] [CrossRef]
- Panizzi, J.R.; Becker-Heck, A.; Castleman, V.H.; Al-Mutairi, D.A.; Liu, Y.; Loges, N.T.; Pathak, N.; Austin-Tse, C.; Sheridan, E.; Schmidts, M.; et al. CCDC103 mutations cause primary ciliary dyskinesia by disrupting assembly of ciliary dynein arms. Nat. Genet. 2012, 44, 714–719. [Google Scholar] [CrossRef]
- Becker-Heck, A.; Zohn, I.E.; Okabe, N.; Pollock, A.; Lenhart, K.B.; Sullivan-Brown, J.; McSheene, J.; Loges, N.T.; Olbrich, H.; Haeffner, K.; et al. The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat. Genet. 2011, 43, 79–84. [Google Scholar] [CrossRef]
- Vanaken, G.J.; Bassinet, L.; Boon, M.; Mani, R.; Honore, I.; Papon, J.F.; Cuppens, H.; Jaspers, M.; Lorent, N.; Coste, A.; et al. Infertility in an adult cohort with primary ciliary dyskinesia: Phenotype-gene association. Eur. Respir. J. 2017, 50, 1700314. [Google Scholar] [CrossRef]
- Castleman, V.H.; Romio, L.; Chodhari, R.; Hirst, R.A.; de Castro, S.C.; Parker, K.A.; Ybot-Gonzalez, P.; Emes, R.D.; Wilson, S.W.; Wallis, C.; et al. Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities. Am. J. Hum. Genet. 2009, 84, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Pennekamp, P.; Menchen, T.; Dworniczak, B.; Hamada, H. Situs inversus and ciliary abnormalities: 20 years later, what is the connection? Cilia 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Chivukula, R.R.; Montoro, D.T.; Leung, H.M.; Yang, J.; Shamseldin, H.E.; Taylor, M.S.; Dougherty, G.W.; Zariwala, M.A.; Carson, J.; Daniels, M.L.A.; et al. A human ciliopathy reveals essential functions for NEK10 in airway mucociliary clearance. Nat. Med. 2020, 26, 244–251. [Google Scholar] [CrossRef] [PubMed]
- von Schledorn, L.; Puertollano Martin, D.; Cleve, N.; Zollner, J.; Roth, D.; Staar, B.O.; Hegermann, J.; Ringshausen, F.C.; Nawroth, J.; Martin, U.; et al. Primary Ciliary Dyskinesia Patient-Specific hiPSC-Derived Airway Epithelium in Air-Liquid Interface Culture Recapitulates Disease Specific Phenotypes In Vitro. Cells 2023, 12, 1467. [Google Scholar] [CrossRef] [PubMed]


| Subject | Age | Sex | Origin | Initial Etiology | Etiology after TEM | Nasal NO (nL/min) | ppFEV1 | Patient History (Signs, Symptoms, and Findings) a | Gene b | cDNA Change | Protein Change | ACMG Class | TEM Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 29 | F | Turkey | Probable PCD | Definite PCD | 11 | 69 | NRD, early onset of chronic wet cough and CRSwNP, repeat sinus surgery, chronic otitis media, and history of grommet insertions | CCDC103 | c.548T>C c.548T>C | p.(Leu183Pro) p.(Leu183Pro) | 3 3 | ODA |
| 2 | 61 | F | Russia | Idiopathic bronchiectasis | Idiopathic bronchiectasis | 251 | 37 | School-age onset of chronic wet cough, and P. aeruginosa infection | DNAH1 | c.5639C>T c.6925C>A | p.(Thr1880Met) p.(Pro2309Thr) | 3 3 | Normal |
| 3 | 63 | M | Germany | Possible PCD | Possible PCD | 170 | 33 | Pre-school onset of chronic wet cough and CRSwNP, positive family history, history of infertility, and P. aeruginosa infection | DNAH1 | c.2995C>T c.7633A>G | p.(Arg999Cys) p.(Ile2545Val) | 3 3 | Normal |
| 4 | 38 | F | Germany | Probable PCD | Definite PCD | 27 | 109 | NRD, early onset of chronic wet cough, CRSwNP, repeat sinus surgery, chronic otitis media, history of grommet insertions, P. aeruginosa infection, and abnormal HSVM (immotile cilia) | DNAH5 | c.10815delT c.11212-4A>G (intronic) | p.(Pro3606Hisfs*23) - | 5 3 | ODA |
| 5 | 58 | F | Kenya | Probable PCD | Probable PCD | 46 | 77 | Young-adult onset of chronic wet cough, CRSwNP, repeat sinus surgery, and P. aeruginosa infection | DNAH8 HYDIN | c.3215G>A c.1169T>C c.12401T>C c.2943T>C c.1043+5G>C | p.(Arg1072Gln) p.(Leu3723Leu) p.(Leu4134Pro) p.(Asp981=) p.? | 3 3 3 3 3 | Normal |
| 6 | 18 | M | Germany | Asthma | Asthma | 240 | 59 | Adolescent onset of chronic wet cough, and P. aeruginosa infection | DNAH11 | c.5924+12G>A c.6226G>A | p.? p.(Val2076Met) | 3 3 | Normal |
| 7 | 47 | M | Germany | Probable PCD | Probable PCD | 37 | 69 | NRD, early onset of chronic wet cough and CRSwNP, repeat sinus surgery, history of infertility, and abnormal HSVM (static stroke) | DNAH11 | c.7456A>G c.9815>G | p.(Thr2486AIa) p.(Asn3272Ser) | 3 3 | Normal |
| 8 | 46 | F | Turkey | Probable KS | Definite KS | - | 30 | Situs inversus, early onset of chronic wet cough and CRSwNP, history of lower lobe resection, middle lobe atelectasis, and M. abscessus infection | DNAI1 | c.1168G>A c.1168G>A | p.(Asp390Asn) p.(Asp390Asn) | 3 3 | ODA |
| 9 | 23 | M | Tunisia | Possible PCD | Possible PCD | 290 | 36 | NRD, early onset chronic wet cough and CRSwNP | CCDC39 | c.1363-3delC c.1781C>T | p.? p.(Thr594Ile) | 3 3 | Normal |
| 10 | 53 | M | Germany | Probable PCD | Definite PCD | 8 | 56 | NRD, early onset of chronic wet cough and CRSwNP, repeat sinus surgery, chronic otitis media, hearing loss and aid, history of middle lobe resection, and atrial septal defect | CCDC40 | c.3129delC c.3354C>A | p.(Phe1004Serfs*35) p.(Tyr1118*) | 5 3 | MTD + IDA |
| 11 | 19 | M | Germany | Idiopathic bronchiectasis | Idiopathic bronchiectasis | 236 | 82 | Adolescent onset of chronic wet cough | DNAI1 | c.1055A>G c.1207C>T | p.(Tyr352Cys) p.(His403Tyr) | 3 3 | Normal |
| 12 | 44 | F | Germany | Probable PCD | Definite PCD | 21 | 40 | Early onset chronic wet cough and CRSwNP, repeat sinus surgery, history of infertility, and P. aeruginosa infection | CCDC40 | c.1345C>T c.2597A>G | p.(Arg449*) p.(Asn866Ser) | 5 3 | MTD + IDA |
| 13 | 24 | M | Turkey | Probable KS | Definite KS | 17 | 51 | Situs inversus, NRD, early onset of chronic wet cough and CRSwNP, repeat sinus surgery, chronic otitis media, P. aeruginosa infection, and abnormal HSVM (reduced amplitude, rigid stroke) | DNAH5 CCDC40 | c.358G>A c.3656G>A c.615G>C c.615G>C | p.(Asp120Asn) p.(Arg1219His) p.= p.= | 3 3 3 | MTD + IDA |
| 14 | 51 | M | Turkey | Possible PCD | Possible PCD | 112 | 32 | Early onset of chronic wet cough and CRSwNP, parental consanguinity, and P. aeruginosa infection | DNAH7 | c.12056_12060delTATGT c.12056_12060delTATGT | p.(Leu4019Serfs*3) p.(Leu4019Serfs*3) | 3 3 | Normal |
| 15 | 23 | F | Turkey | Probable PCD | Probable PCD | 4 | 70 | Early onset of chronic wet cough and CRSwNP, repeat sinus surgery, history of middle and lower lobe resection, parental consanguinity, positive familial segregation analysis, P. aeruginosa infection, and abnormal HSVM (uncoordinated and circular beating) | NME5 | c.415delA c.415delA | p.(Ile139Tyrfs*8) p.(Ile139Tyrfs*8) | 4 4 | CC |
| 16 | 27 | M | Syria | Probable PCD | Probable PCD | 238 | 42 | NRD, early onset of chronic wet cough and CRSwNP, chronic otitis media, lower lobe atelectasis, affected sibling / positive familial segregation analysis, parental consanguinity, and P. aeruginosa infection | NEK10 | c.1389C>A c.1389C>A | p.(Tyr463*) p.(Tyr463*) | 4 4 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staar, B.O.; Hegermann, J.; Auber, B.; Ewen, R.; von Hardenberg, S.; Olmer, R.; Pink, I.; Rademacher, J.; Wetzke, M.; Ringshausen, F.C. Ciliary Ultrastructure Assessed by Transmission Electron Microscopy in Adults with Bronchiectasis and Suspected Primary Ciliary Dyskinesia but Inconclusive Genotype. Cells 2023, 12, 2651. https://doi.org/10.3390/cells12222651
Staar BO, Hegermann J, Auber B, Ewen R, von Hardenberg S, Olmer R, Pink I, Rademacher J, Wetzke M, Ringshausen FC. Ciliary Ultrastructure Assessed by Transmission Electron Microscopy in Adults with Bronchiectasis and Suspected Primary Ciliary Dyskinesia but Inconclusive Genotype. Cells. 2023; 12(22):2651. https://doi.org/10.3390/cells12222651
Chicago/Turabian StyleStaar, Ben O., Jan Hegermann, Bernd Auber, Raphael Ewen, Sandra von Hardenberg, Ruth Olmer, Isabell Pink, Jessica Rademacher, Martin Wetzke, and Felix C. Ringshausen. 2023. "Ciliary Ultrastructure Assessed by Transmission Electron Microscopy in Adults with Bronchiectasis and Suspected Primary Ciliary Dyskinesia but Inconclusive Genotype" Cells 12, no. 22: 2651. https://doi.org/10.3390/cells12222651
APA StyleStaar, B. O., Hegermann, J., Auber, B., Ewen, R., von Hardenberg, S., Olmer, R., Pink, I., Rademacher, J., Wetzke, M., & Ringshausen, F. C. (2023). Ciliary Ultrastructure Assessed by Transmission Electron Microscopy in Adults with Bronchiectasis and Suspected Primary Ciliary Dyskinesia but Inconclusive Genotype. Cells, 12(22), 2651. https://doi.org/10.3390/cells12222651







