Chemical and Genetic Modulation of Complex I of the Electron Transport Chain Enhances the Biotherapeutic Protein Production Capacity of CHO Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. LOPAC®1280 Chemical Library Screen
2.2. Transient Transfection
2.3. Batch Assay
2.4. Simulated Perfusion Assay
2.5. RNA Sequencing and Analysis
2.6. Mitochondrial Mass
2.7. Mitochondrial Membrane Potential (MMP)
2.8. Real-Time ATP Rate Assay
2.9. Ndufa13 and Ndufa5 Cell Line Engineering and Simulated Perfusion Assay
2.10. Perfusion Culture in 2 mL Mobius® Breez Microbioreactor Systems
3. Results
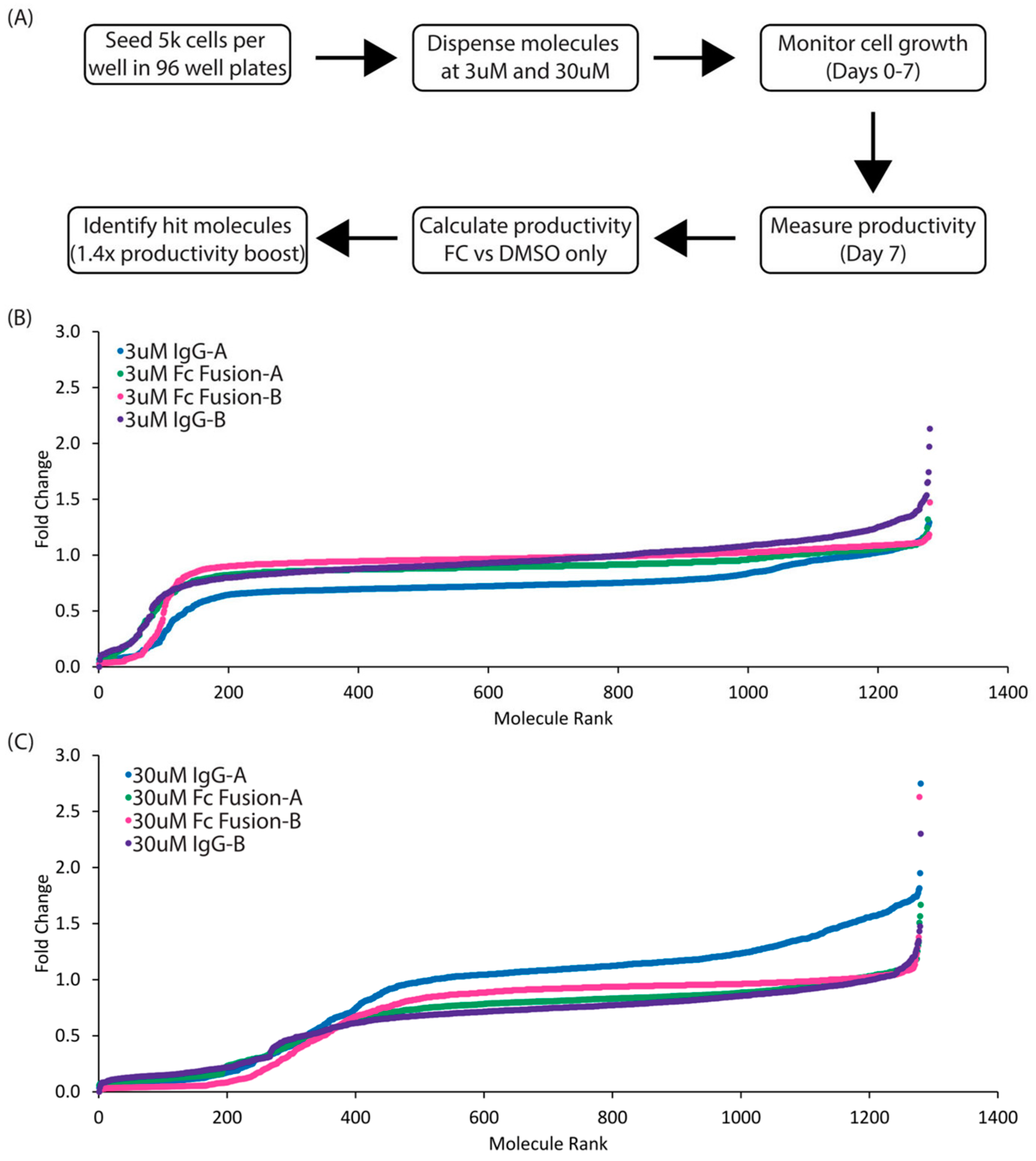
3.1. Identification and Validation of Pharmacologically Active Chemicals That Enhance Therapeutic Protein Production
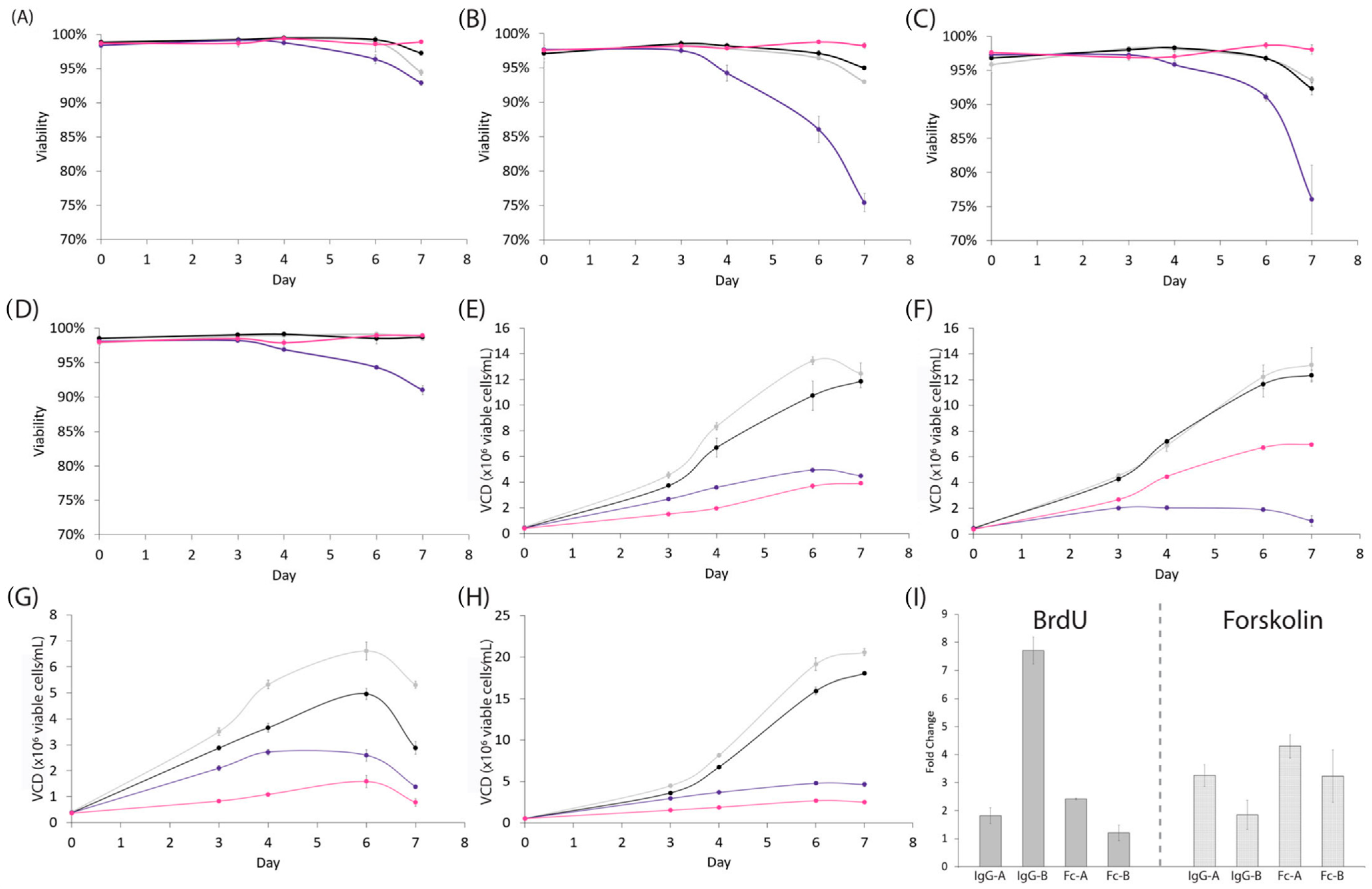
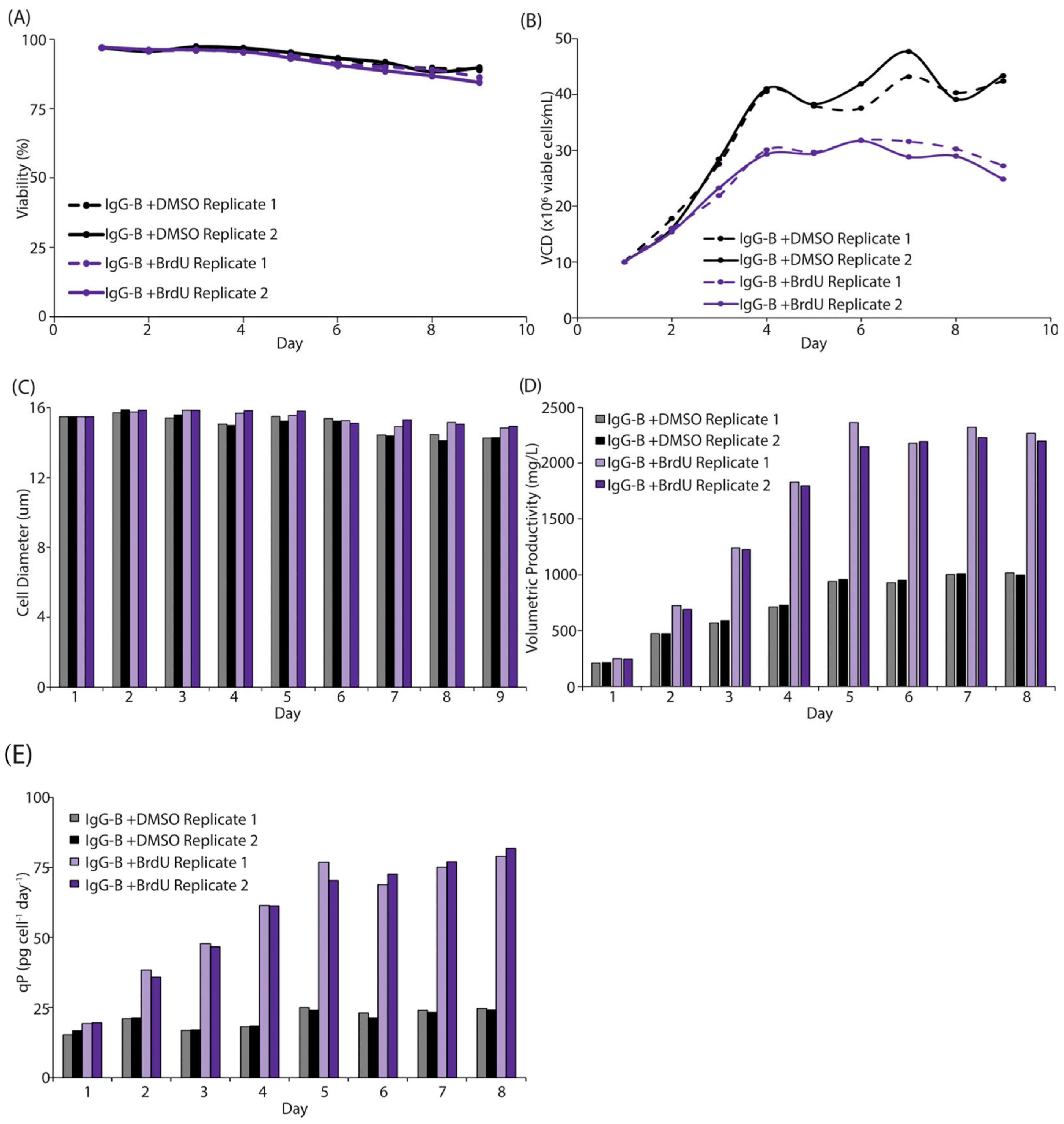
3.2. BrdU Enhances the Efficiency of Recombinant Protein Production in Intensified Processes
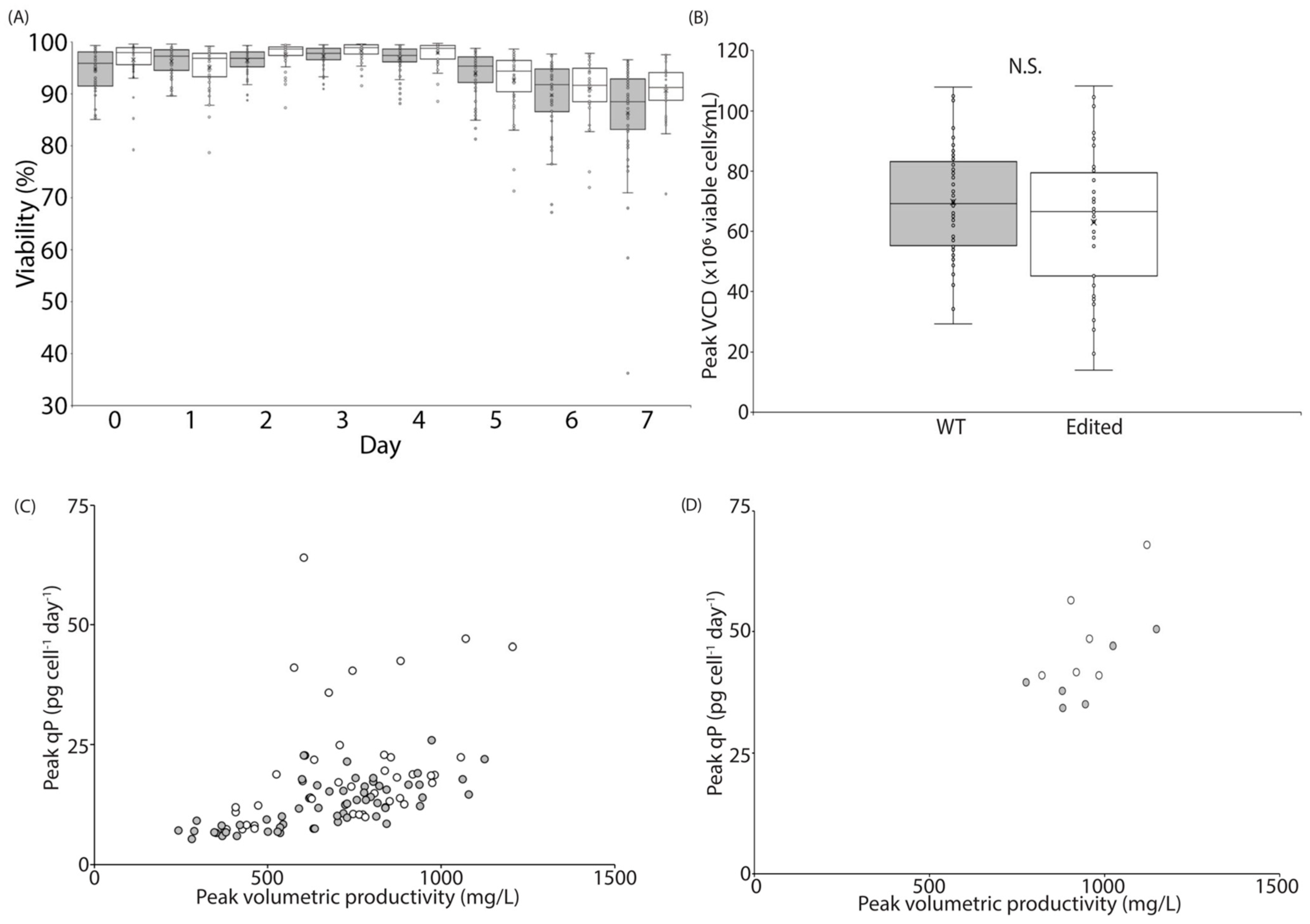
3.3. BrdU Invokes Changes in the CHO Transcriptome Which Can Be Partially Recapitulated via Targeted Genome Engineering
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, P.-C.; Chan, K.F.; Kiess, I.A.; Tan, J.; Shahreel, W.; Wong, S.-Y.; Song, Z. Attenuated glutamine synthetase as a selection marker in CHO cells to efficiently isolate highly productive stable cells for the production of antibodies and other biologics. MAbs 2019, 11, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, Y.-G.; Lee, G.M. CHO cells in biotechnology for production of recombinant proteins: Current state and further potential. Appl. Microbiol. Biotechnol. 2012, 93, 917–930. [Google Scholar] [CrossRef]
- Chang, J.; Chen, X.; Wang, R.; Shi, R.; Wang, X.; Lu, W.; Ma, S.; Xia, Q. High-Throughput Screening Identifies Two Novel Small Molecule Enhancers of Recombinant Protein Expression. Molecules 2020, 25, 353. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.R.; Jinger, X.; Hermiston, T.W. Effect of different UCOE-promoter combinations in creation of engineered cell lines for the production of Factor VIII. BMC Res. Notes 2011, 4, 178. [Google Scholar] [CrossRef]
- Jalah, R.; Rosati, M.; Kulkarni, V.; Patel, V.; Bergamaschi, C.; Valentin, A.; Zhang, G.-M.; Sidhu, M.K.; Eldredige, J.H.; Weiner, D.B.; et al. Efficient systemic expression of bioactive IL-15 in mice upon delivery of optimized DNA expression plasmids. DNA Cell Biol. 2007, 26, 827–840. [Google Scholar] [CrossRef]
- Matasci, M.; Baldi, L.; Hacker, D.L.; Wurm, F.M. The PiggyBac transposon enhances the frequency of CHO stable cell line generation and yields recombinant lines with superior productivity and stability. Biotechnol. Bioeng. 2011, 108, 2141–2150. [Google Scholar] [CrossRef]
- Dalton, A.C.; Barton, W.A. Over-expression of secreted proteins from mammalian cell lines. Protein Sci. 2014, 23, 517–525. [Google Scholar] [CrossRef]
- Allen, M.J.; Boyce, J.P.; Trentalange, M.T.; Treiber, D.L.; Rasmussen, B.; Tillotson, B.; Davis, R.; Reddy, P. Identification of novel small molecule enhancers of protein production by cultured mammalian cells. Biotechnol. Bioeng. 2008, 100, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.-J.; Turincio, R.; Ng, S.; Li, J.; Wilson, B.; Chan, P.; Zak, M.; Reilly, D.; Beresini, M.H.; Wong, A.W. High throughput screening identifies novel, cell cycle-arresting small molecule enhancers of transient protein expression. Biotechnol. Prog. 2017, 33, 1579–1588. [Google Scholar] [CrossRef]
- Yoon, C.; Kim, D.; Lim, J.H.; Lee, G.M. Forskolin Increases cAMP Levels and Enhances Recombinant Antibody Production in CHO Cell Cultures. Biotechnol. J. 2020, 15, 2000264. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Li, W. RSeQC: Quality control of RNA-seq experiments. Bioinformatics 2012, 28, 2184–2185. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Liu, R.; Holik, A.Z.; Su, S.; Jansz, N.; Chen, K.; Leong, H.S.; Blewitt, M.E.; Asselin-Labat, M.-L.; Smyth, G.K.; Ritchie, M.E. Why weight? Modelling sample and observational level variability improves power in RNA-seq analysis. Nucleic Acids Res. 2015, 43, e97. [Google Scholar] [CrossRef] [PubMed]
- Kretzmer, C.; Narasimhan, R.L.; Lal, R.D.; Balassi, V.; Ravellette, J.; Manjunath, A.K.K.; Koshy, J.J.; Viano, M.; Torre, S.; Zanda, V.M.; et al. De novo assembly and annotation of the CHOZN GS−/− genome supports high-throughput genome-scale screening. Biotechnol. Bioeng. 2022, 119, 3632–3646. [Google Scholar] [CrossRef]
- Lin, D.; Yalamanchili, H.B.; Zhang, X.; Lewis, N.E.; Alves, C.S.; Groot, J.; Arnsdorf, J.; Bjorn, S.P.; Wulff, T.; Voldborg, B.G.; et al. CHOmics: A web-based tool for multi-omics data analysis and interactive visualization in CHO cell lines. PLoS Comput. Biol. 2020, 16, e1008498. [Google Scholar] [CrossRef]
- Hillard, W.; MacDonald, M.L.; Lee, K.H. Chromosome-scale scaffolds for the Chinese hamster reference genome assembly to facilitate the study of the CHO epigenome. Biotechnol. Bioeng. 2020, 117, 2331–2339. [Google Scholar] [CrossRef]
- Xu, X.; Nagarajan, H.; Lewis, N.E.; Pan, S.; Cai, Z.; Liu, X.; Chen, W.; Xie, M.; Wang, W.; Hammond, S.; et al. The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line. Nat. Biotechnol. 2011, 29, 735–741. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. GigaScience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, H.; Lee, K.; Castan, A.; Chotteau, V. Optimization of medium with perfusion microbioreactors for high density CHO cell cultures at very low renewal rate aided by design of experiments. Biotechnol. Bioeng. 2023, 120, 2523–2541. [Google Scholar] [CrossRef]
- Taupin, P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res. Rev. 2007, 53, 198–214. [Google Scholar] [CrossRef]
- Brandt, U. Energy converting NADH: Quinone oxioreductase (complex I). Annu. Rev. Biochem. 2006, 75, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 1, 50–59. [Google Scholar] [CrossRef]
- Chakrabatri, L.; Chaerkady, R.; Wang, J.; Weng, S.H.S.; Wang, C.; Qian, C.; Cazares, L.; Hess, S.; Amaya, P.; Zhu, J.; et al. Mitochondrial membrane potential-enriched CHO host: A novel and powerful tool for improving biomanufacturing capability. MAbs 2022, 14, 2020081. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, L.; Mathew, A.; Li, L.; Han, S.; Klover, J.; Albanetti, T.; Nelson-Hawley, P. Mitochondrial membrane potential identifies cells with high recombinant protein productivity. J. Immunol. Methods 2019, 464, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Sazanov, L.A. Respiratory complex I: Mechanistic and structural insights provided by the crystal structure of the hydrophilic domain. Biochemistry 2007, 46, 2275–2288. [Google Scholar] [CrossRef]
- Guerrero-Castillo, S.; Baertling, F.; Kownatzki, D.; Wessels, H.J.; Arnold, S.; Brandt, U.; Lijtmans, L. The assembly pathway of mitochondrial respiratory chain Complex I. Cell Metab. 2017, 25, 128–139. [Google Scholar] [CrossRef]
- Gonzalez-Quintana, A.; Garcia-Consuegra, I.; Belanger-Quintana, A.; Serrano-Lorenzo, P.; Lucia, A.; Blazquez, A.; Docampo, J.; Ugalde, C.; Moran, M.; Arenas, J.; et al. Novel NDUFA13 Mutations Associated with OXPHOS Deficiency and Leigh Syndrome: A Second Family Report. Genes 2020, 11, 855. [Google Scholar] [CrossRef] [PubMed]


| Regulation | Reactome Pathway | p-Value | Number of Genes Up- or Down-Regulated | Number of Genes in Pathway | Percent of Genes Up- or Down-Regulated |
|---|---|---|---|---|---|
| Up | Assembly of collagen fibrils and other multimeric structures | −11.41 | 21 | 25 | 84.0% |
| Up | Extracellular matrix organization | −10.62 | 77 | 116 | 66.4% |
| Up | Muscle contraction | −9.98 | 60 | 79 | 75.9% |
| Up | Striated Muscle Contraction | −9.61 | 15 | 17 | 88.2% |
| Up | Collagen chain trimerization | −9.29 | 19 | 19 | 100.0% |
| Up | Collagen formation | −8.93 | 26 | 40 | 65.0% |
| Up | Platelet homeostasis | −8.35 | 21 | 28 | 75.0% |
| Up | Cross-presentation of particulate exogenous antigens (phagosomes) | −8.15 | 6 | 6 | 100.0% |
| Up | Nitric oxide stimulates guanylate cyclase | −7.37 | 8 | 8 | 100.0% |
| Up | Class A/1 (Rhodopsin-like receptors) | −7.13 | 54 | 74 | 73.0% |
| Up | SLC-mediated transmembrane transport | −6.83 | 91 | 139 | 65.5% |
| Up | Peptide ligand-binding receptors | −6.72 | 29 | 43 | 67.4% |
| Up | Transport of inorganic cations/anions and amino acids/oligopeptides | −6.63 | 43 | 59 | 72.9% |
| Up | Collagen degradation | −6.59 | 10 | 15 | 66.7% |
| Up | Dopamine Neurotransmitter Release Cycle | −6.29 | 5 | 6 | 83.3% |
| Up | Hemostasis | −6.24 | 169 | 252 | 67.1% |
| Up | Transmembrane transport of small molecules | −6.11 | 194 | 313 | 62.0% |
| Up | Collagen biosynthesis and modifying enzymes | −6.10 | 22 | 32 | 68.8% |
| Up | cGMP effects | −6.01 | 7 | 7 | 100.0% |
| Up | Li1CAM interactions | −5.56 | 34 | 52 | 65.4% |
| Down | Mitochondrial translation termination | −56.18 | 58 | 63 | 92.1% |
| Down | Mitochondrial translation | −55.45 | 58 | 63 | 92.1% |
| Down | Mitochondrial translation elongation | −52.86 | 55 | 60 | 91.7% |
| Down | Organelle biogenesis and maintenance | −48.58 | 143 | 205 | 69.8% |
| Down | Gene Expression | −32.19 | 444 | 721 | 61.6% |
| Down | Separation of Sister Chromatids | −25.58 | 85 | 115 | 73.9% |
| Down | Mitotic Anaphase | −25.37 | 89 | 121 | 73.6% |
| Down | Mitotic Metaphase and Anaphase | −25.12 | 90 | 122 | 73.8% |
| Down | M Phase | −22.21 | 119 | 166 | 71.7% |
| Down | Cell Cycle, Mitotic | −22.16 | 193 | 312 | 61.9% |
| Down | Respiratory electron transport | −20.17 | 37 | 41 | 90.2% |
| Down | Repiratory electron transport, ATP synthesis by chemiosmotic coupling, and heat | −19.06 | 46 | 53 | 86.8% |
| Down | Metabolism of proteins | −18.39 | 344 | 630 | 54.6% |
| Down | Complex I biogenesis | −17.69 | 28 | 31 | 90.3% |
| Down | Processing of Capped Intron-Containing Pre-mRNA | −17.27 | 110 | 157 | 70.1% |
| Down | Cell Cycle | −16.08 | 45 | 89 | 50.6% |
| Down | Post-translational protein modification | −15.93 | 272 | 516 | 52.7% |
| Down | DNA Repair | −15.38 | 115 | 187 | 61.5% |
| Down | The citric acid (TCA) cycle and respiratory electron transport | −15.12 | 62 | 80 | 77.5% |
| Down | Mitotic Prometaphase | −15.00 | 54 | 76 | 71.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kretzmer, C.; Reger, K.; Balassi, V.; Pham, Q.L.; Johns, M.; Peters, S.T.; Petersen, A.; Mahadevan, J.; Gustin, J.; Borgschulte, T.; et al. Chemical and Genetic Modulation of Complex I of the Electron Transport Chain Enhances the Biotherapeutic Protein Production Capacity of CHO Cells. Cells 2023, 12, 2661. https://doi.org/10.3390/cells12222661
Kretzmer C, Reger K, Balassi V, Pham QL, Johns M, Peters ST, Petersen A, Mahadevan J, Gustin J, Borgschulte T, et al. Chemical and Genetic Modulation of Complex I of the Electron Transport Chain Enhances the Biotherapeutic Protein Production Capacity of CHO Cells. Cells. 2023; 12(22):2661. https://doi.org/10.3390/cells12222661
Chicago/Turabian StyleKretzmer, Corey, Kelsey Reger, Vincent Balassi, Quang Long Pham, Michael Johns, Samuel T. Peters, Amber Petersen, Jana Mahadevan, Jason Gustin, Trissa Borgschulte, and et al. 2023. "Chemical and Genetic Modulation of Complex I of the Electron Transport Chain Enhances the Biotherapeutic Protein Production Capacity of CHO Cells" Cells 12, no. 22: 2661. https://doi.org/10.3390/cells12222661
APA StyleKretzmer, C., Reger, K., Balassi, V., Pham, Q. L., Johns, M., Peters, S. T., Petersen, A., Mahadevan, J., Gustin, J., Borgschulte, T., & Razafsky, D. (2023). Chemical and Genetic Modulation of Complex I of the Electron Transport Chain Enhances the Biotherapeutic Protein Production Capacity of CHO Cells. Cells, 12(22), 2661. https://doi.org/10.3390/cells12222661







