Mechanisms of Activation of Brain’s Drainage during Sleep: The Nightlife of Astrocytes
Abstract
:1. Introduction
2. Mechanisms of Modulation of Sleep by Astrocytes
2.1. Astroglial Calcium
2.2. Adenosine-Mediated Pathway
2.3. Non-Adenosine Pathways
2.4. Astrocytes Participate in Circadian Timekeeping
3. Neuro-Glia-Vascular Unit Is also Local-Sleep Unit and Local Brain-Drainage Unit
3.1. Neuro-Glia-Vascular Unit
3.2. NGVU as Sleep Unit
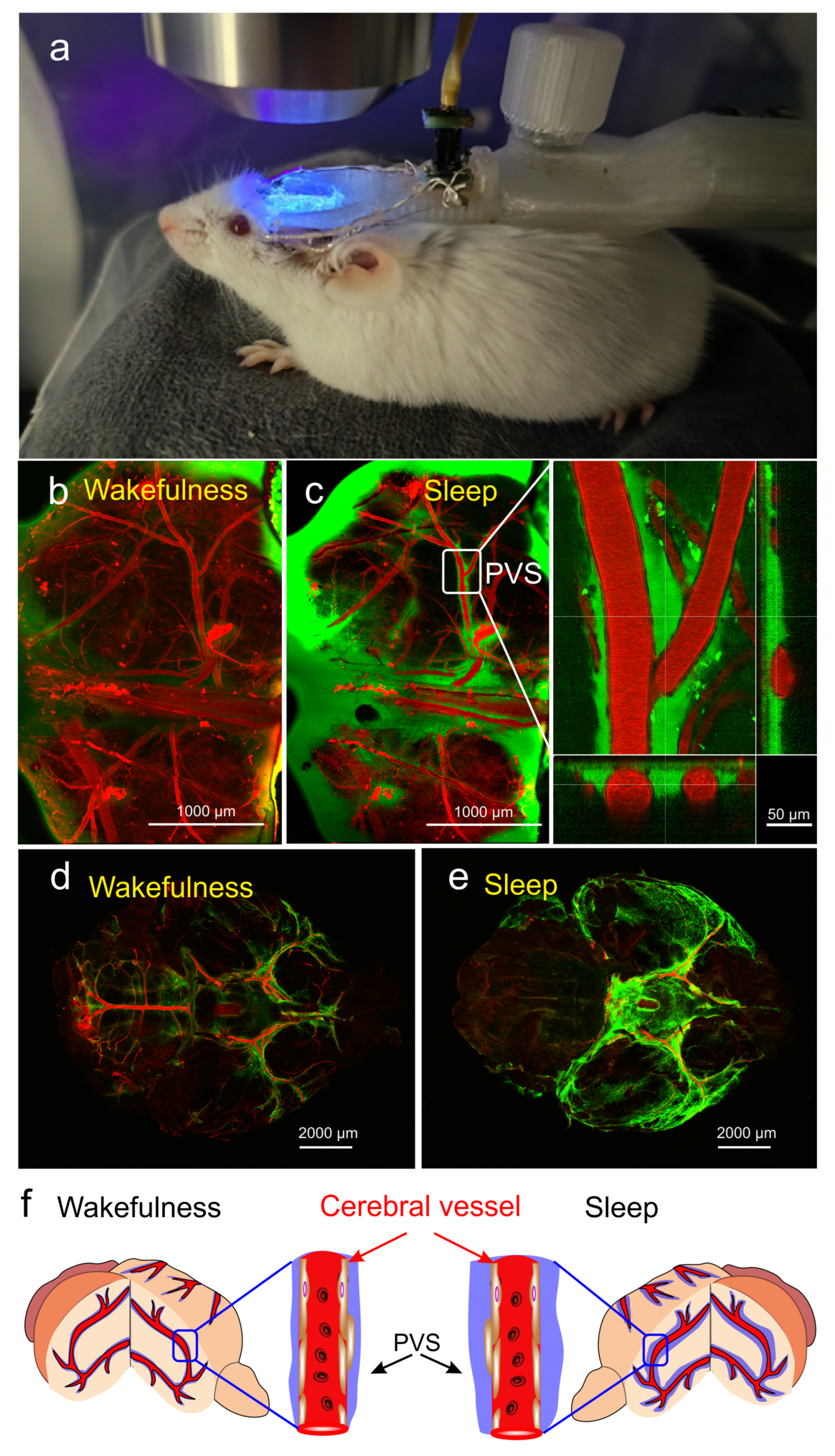
3.3. Why NGVU Is Also a Drainage Unit
4. Astrocyte Volume Regulation
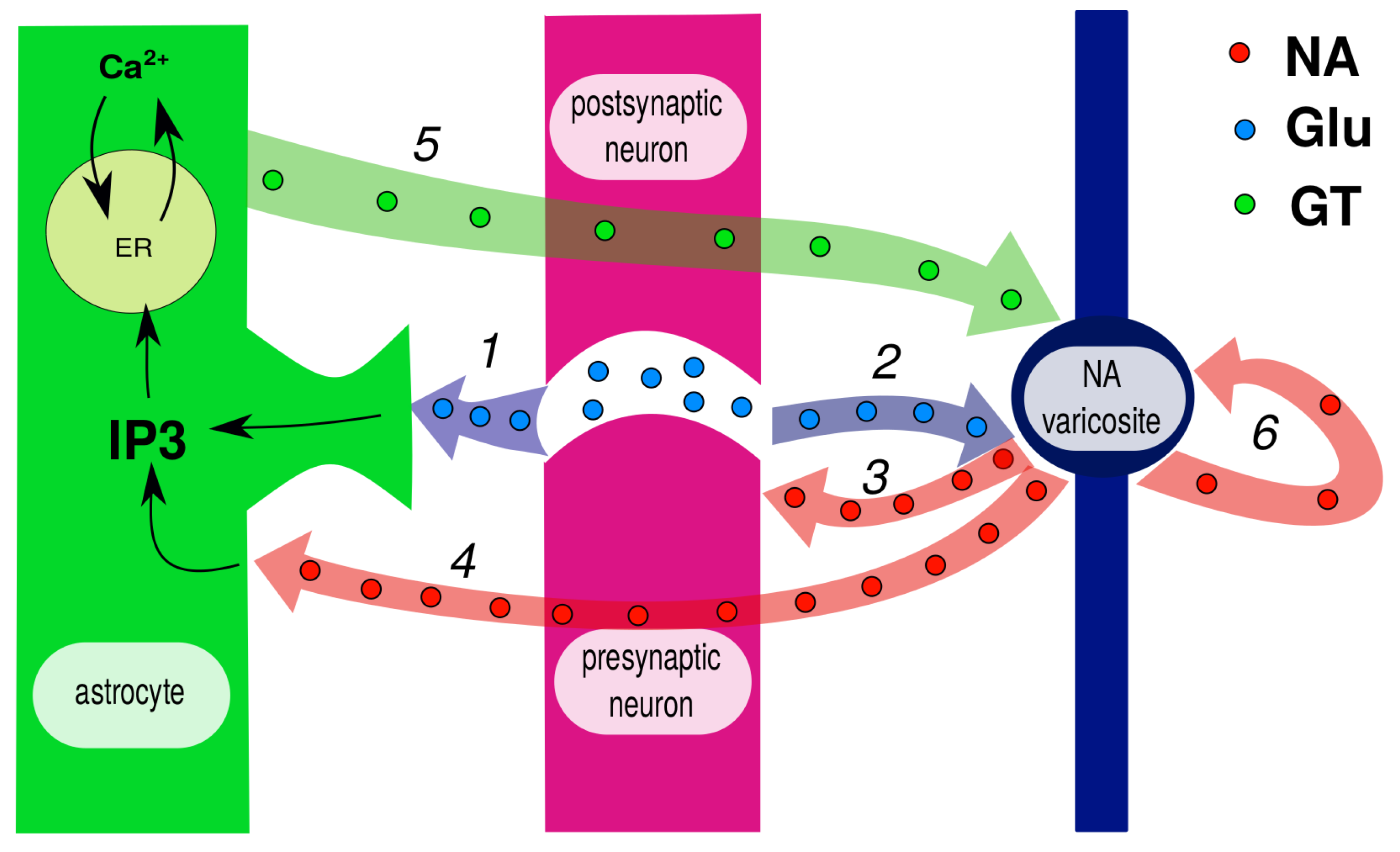
5. Noradrenaline: Master Regulator or Trigger?
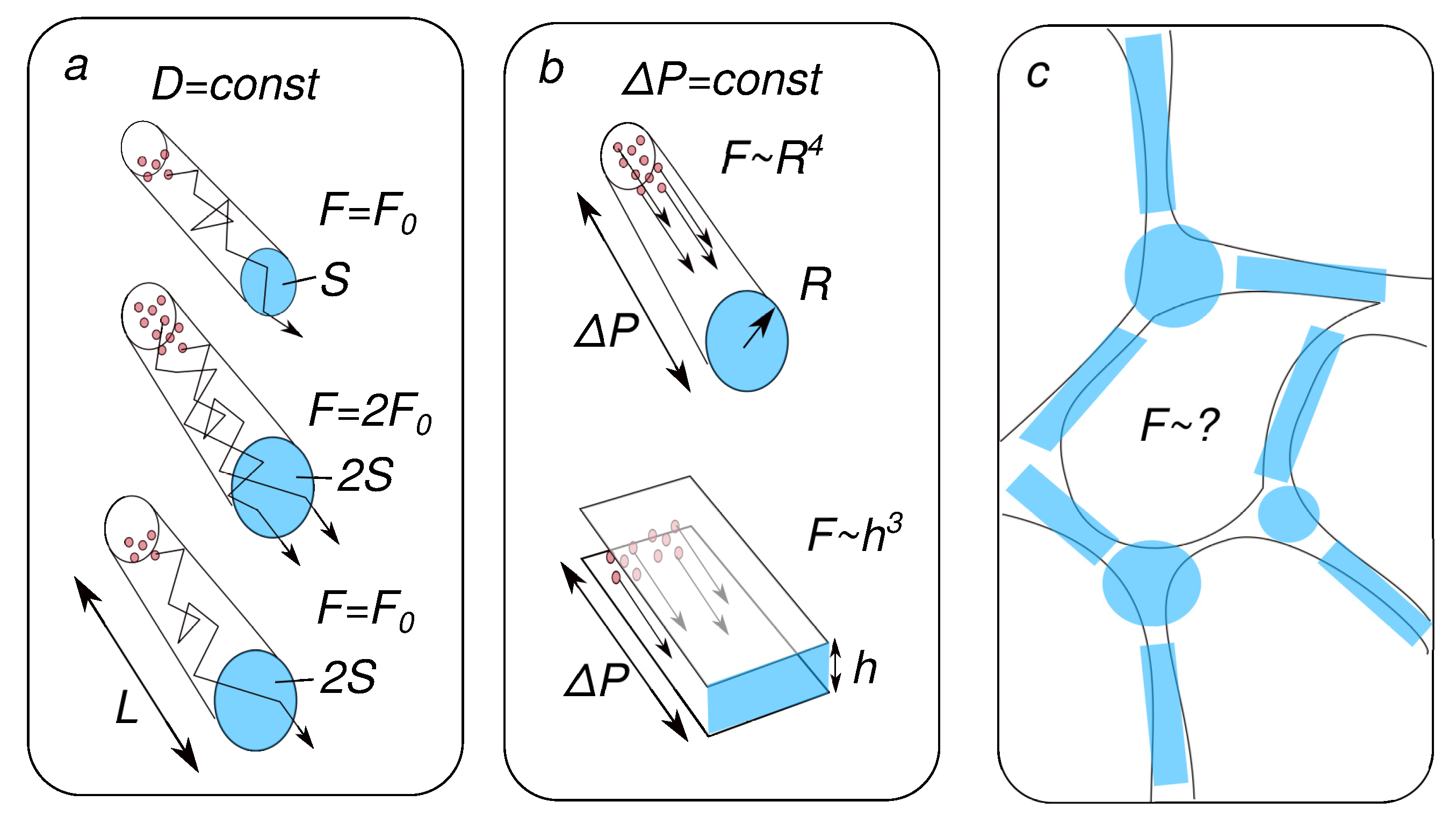
6. Physical Implications for Metabolite Clearance and Prospects for Future Research
7. Problems and Issues in Research of Gender Differences of Night Life of Astocytes
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A | amyloid-beta |
| AD | Alzheimer’s disease |
| AQP4 | aquaporin 4 |
| BWRS | brain waste removal system |
| CSF | cerebral spinal fluid |
| ECS | extracellular space |
| FITCD | fluorescein isothiocyanate-dextran |
| GANE | glutamate amplifies noradrenergic effects |
| ISF | interstitial fluid |
| IP3 | inositol-trisphosphate |
| LC | locus coeruleus |
| NA | noradrenaline |
| NMDA | N-methyl-D-aspartate |
| NGVU | neuro–glia–vascular unit |
| NKA | sodium–potassium pump, Na/K ATPase |
| NREM | non-rapid eye movement |
References
- Zielinski, M.R.; McKenna, J.T.; McCarley, R.W. Functions and mechanisms of sleep. AIMS Neurosci. 2016, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Carskadon, M.A.; Dement, W.C. Normal human sleep: An overview. In Principles and Practice of Sleep Medicine; Kryger, M., Roth, T., Dement, W., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2005; pp. 13–23. [Google Scholar]
- Bellesi, M.; de Vivo, L.; Chini, M.; Gilli, F.; Tononi, G.; Cirelli, C. Sleep loss promotes astrocytic phagocytosis and microglial activation in mouse cerebral cortex. J. Neurosci. 2017, 37, 5263–5273. [Google Scholar] [CrossRef] [PubMed]
- Deurveilher, S.; Golovin, T.; Hall, S.; Semba, K. Microglia dynamics in sleep/wake states and in response to sleep loss. Neurochem. Int. 2021, 143, 104944. [Google Scholar] [CrossRef] [PubMed]
- Ooms, S.; Overeem, S.; Besse, K.; Rikkert, M.O.; Verbeek, M.; Claassen, J.A. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: A randomized clinical trial. JAMA Neurol. 2014, 71, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Shokri-Kojori, E.; Wang, G.J.; Wiers, C.E.; Demiral, S.B.; Guo, M.; Kim, S.W.; Lindgren, E.; Ramirez, V.; Zehra, A.; Freeman, C.; et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. USA 2018, 115, 4483–4488. [Google Scholar] [CrossRef] [PubMed]
- Franks, N.P.; Wisden, W. The inescapable drive to sleep: Overlapping mechanisms of sleep and sedation. Science 2021, 374, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Sabia, S.; Fayosse, A.; Dumurgier, J.; van Hees, V.T.; Paquet, C.; Sommerlad, A.; Kivimäki, M.; Dugravot, A.; Singh-Manoux, A. Association of sleep duration in middle and old age with incidence of dementia. Nat. Commun. 2021, 12, 2289. [Google Scholar] [CrossRef] [PubMed]
- Hauglund, N.L.; Pavan, C.; Nedergaard, M. Cleaning the sleeping brain–the potential restorative function of the glymphatic system. Curr. Opin. Physiol. 2020, 15, 1–6. [Google Scholar] [CrossRef]
- Simon, K.C.; Nadel, L.; Payne, J.D. The functions of sleep: A cognitive neuroscience perspective. Proc. Natl. Acad. Sci. USA 2022, 119, e2201795119. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Fultz, N.E.; Bonmassar, G.; Setsompop, K.; Stickgold, R.A.; Rosen, B.R.; Polimeni, J.R.; Lewis, L.D. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 2019, 366, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Reddy, O.C.; van der Werf, Y.D. The sleeping brain: Harnessing the power of the glymphatic system through lifestyle choices. Brain Sci. 2020, 10, 868. [Google Scholar] [CrossRef]
- Silva, I.; Silva, J.; Ferreira, R.; Trigo, D. Glymphatic system, AQP4, and their implications in Alzheimer’s disease. Neurol. Res. Pract. 2021, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Gao, P.; Zhu, T.; Yin, H.; Jin, S. Glymphatic system dysfunction: A novel mediator of sleep disorders and headaches. Front. Neurol. 2022, 13, 885020. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Qiu, Y.; Yu, X.; Yang, L. Glymphatic dysfunction: A bridge between sleep disturbance and mood disorders. Front. Psychiatry 2021, 12, 658340. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Penzel, T.; Blokhina, I.; Khorovodov, A.; Fedosov, I.; Yu, T.; Karandin, G.; Evsukova, A.; Elovenko, D.; Adushkina, V.; et al. Night photostimulation of clearance of beta-amyloid from mouse brain: New strategies in preventing Alzheimer’s disease. Cells 2021, 10, 3289. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M.; Goldman, S.A. Glymphatic failure as a final common pathway to dementia. Science 2020, 370, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.Z.; St Louis, E.K.; Knopman, D.S.; Boeve, B.F.; Lowe, V.J.; Roberts, R.O.; Mielke, M.M.; Przybelski, S.A.; Machulda, M.M.; Petersen, R.C.; et al. Association of excessive daytime sleepiness with longitudinal β-amyloid accumulation in elderly persons without dementia. JAMA Neurol. 2018, 75, 672–680. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Postnov, D.; Penzel, T.; Kurths, J. Sleep as a novel biomarker and a promising therapeutic target for cerebral small vessel disease: A review focusing on Alzheimer’s disease and the blood-brain barrier. Int. J. Mol. Sci. 2020, 21, 6293. [Google Scholar] [CrossRef]
- Lucey, B.P.; McCullough, A.; Landsness, E.C.; Toedebusch, C.D.; McLeland, J.S.; Zaza, A.M.; Fagan, A.M.; McCue, L.; Xiong, C.; Morris, J.C.; et al. Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Sci. Transl. Med. 2019, 11, eaau6550. [Google Scholar] [CrossRef]
- Lutsey, P.L.; Misialek, J.R.; Mosley, T.H.; Gottesman, R.F.; Punjabi, N.M.; Shahar, E.; MacLehose, R.; Ogilvie, R.P.; Knopman, D.; Alonso, A. Sleep characteristics and risk of dementia and Alzheimer’s disease: The atherosclerosis risk in communities study. Alzheimer’s Dement. 2018, 14, 157–166. [Google Scholar] [CrossRef]
- Minakawa, E.N.; Wada, K.; Nagai, Y. Sleep disturbance as a potential modifiable risk factor for Alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 803. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Zhou, J.; Ma, J.; Chang, J.; Qiu, Y.; Zhuang, Z.; Xiao, H.; Zeng, L. Sleep disturbance mediates the relationship between depressive symptoms and cognitive function in older adults with mild cognitive impairment. Geriatr. Nurs. 2021, 42, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Heizhati, M.; Wang, L.; Li, M.; Yang, Z.; Lin, M.; Abudereyimu, R.; Hong, J.; Yang, W.; Yao, L.; et al. Poor sleep quality is negatively associated with low cognitive performance in general population independent of self-reported sleep disordered breathing. BMC Public Health 2022, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Izci-Balserak, B.; Zhu, B.; Wang, H.; Bronas, U.G.; Gooneratne, N.S. Independent associations between sleep duration, gamma gap, and cognitive function among older adults: Results from the NHANES 2013–2014. Geriatr. Nurs. 2022, 44, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, H.; Heerdt, P.M.; Fontes, M.; Rothman, D.L.; Volkow, N.D. Glymphatic system function in relation to anesthesia and sleep states. Anesth. Analg. 2019, 128, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Hablitz, L.M.; Plá, V.; Giannetto, M.; Vinitsky, H.S.; Stæger, F.F.; Metcalfe, T.; Nguyen, R.; Benrais, A.; Nedergaard, M. Circadian control of brain glymphatic and lymphatic fluid flow. Nat. Commun. 2020, 11, 4411. [Google Scholar] [CrossRef] [PubMed]
- Ingiosi, A.M.; Frank, M.G. Goodnight, astrocyte: Waking up to astroglial mechanisms in sleep. FEBS J. 2022, 290, 2553–2564. [Google Scholar] [CrossRef]
- Greene, R.W.; Bjorness, T.E.; Suzuki, A. The adenosine-mediated, neuronal-glial, homeostatic sleep response. Curr. Opin. Neurobiol. 2017, 44, 236–242. [Google Scholar] [CrossRef]
- Halassa, M.M.; Florian, C.; Fellin, T.; Munoz, J.R.; Lee, S.Y.; Abel, T.; Haydon, P.G.; Frank, M.G. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 2009, 61, 213–219. [Google Scholar] [CrossRef]
- Fellin, T.; Halassa, M.M.; Terunuma, M.; Succol, F.; Takano, H.; Frank, M.; Moss, S.J.; Haydon, P.G. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 15037–15042. [Google Scholar] [CrossRef] [PubMed]
- Ingiosi, A.; Hayworth, C.; Harvey, D.; Singletary, K.; Rempe, M.; Wisor, J.; Frank, M. A Role for Astroglial Calcium in Mammalian Sleep and Sleep Regulation. Curr. Biol. CB 2020, 30, 4373–4383. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, T.V.; Collard, M.; Yokoyama, S.; Reitman, M.E.; Poskanzer, K.E. Cortical astrocytes independently regulate sleep depth and duration via separate GPCR pathways. Elife 2021, 10, e63329. [Google Scholar] [CrossRef] [PubMed]
- Bojarskaite, L.; Bjørnstad, D.M.; Pettersen, K.H.; Cunen, C.; Hermansen, G.H.; Åbjørsbråten, K.S.; Chambers, A.R.; Sprengel, R.; Vervaeke, K.; Tang, W.; et al. Astrocytic Ca2+ signaling is reduced during sleep and is involved in the regulation of slow wave sleep. Nat. Commun. 2020, 11, 3240. [Google Scholar] [CrossRef] [PubMed]
- Davla, S.; Artiushin, G.; Li, Y.; Chitsaz, D.; Li, S.; Sehgal, A.; van Meyel, D.J. AANAT1 functions in astrocytes to regulate sleep homeostasis. Elife 2020, 9, e53994. [Google Scholar] [CrossRef] [PubMed]
- Blum, I.D.; Keleş, M.F.; Baz, E.S.; Han, E.; Park, K.; Luu, S.; Issa, H.; Brown, M.; Ho, M.C.; Tabuchi, M.; et al. Astroglial calcium signaling encodes sleep need in Drosophila. Curr. Biol. 2021, 31, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Strecker, R.E.; Morairty, S.; Thakkar, M.M.; Porkka-Heiskanen, T.; Basheer, R.; Dauphin, L.J.; Rainnie, D.G.; Portas, C.M.; Greene, R.W.; McCarley, R.W. Adenosinergic modulation of basal forebrain and preoptic/anterior hypothalamic neuronal activity in the control of behavioral state. Behav. Brain Res. 2000, 115, 183–204. [Google Scholar] [CrossRef]
- Szymusiak, R.; Gvilia, I.; McGinty, D. Hypothalamic control of sleep. Sleep Med. 2007, 8, 291–301. [Google Scholar] [CrossRef]
- Krueger, J.M.; Rector, D.M.; Roy, S.; Van Dongen, H.P.; Belenky, G.; Panksepp, J. Sleep as a fundamental property of neuronal assemblies. Nat. Rev. Neurosci. 2008, 9, 910–919. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Rodríguez, J.J.; Parpura, V. Calcium signalling in astroglia. Mol. Cell. Endocrinol. 2012, 353, 45–56. [Google Scholar] [CrossRef]
- Volterra, A.; Liaudet, N.; Savtchouk, I. Astrocyte Ca2+ signalling: An unexpected complexity. Nat. Rev. Neurosci. 2014, 15, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Rusakov, D.A. Disentangling calcium-driven astrocyte physiology. Nat. Rev. Neurosci. 2015, 16, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Shigetomi, E.; Patel, S.; Khakh, B.S. Probing the complexities of astrocyte calcium signaling. Trends Cell Biol. 2016, 26, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, W.; Gu, S.; Qi, D.; Smith, N.A.; Peng, W.; Dong, W.; Yuan, J.; Zhao, B.; Mao, Y.; et al. Distinct astrocytic modulatory roles in sensory transmission during sleep, wakefulness, and arousal states in freely moving mice. Nat. Commun. 2023, 14, 2186. [Google Scholar] [CrossRef] [PubMed]
- Acosta, C.; Anderson, H.D.; Anderson, C.M. Astrocyte dysfunction in Alzheimer disease. J. Neurosci. Res. 2017, 95, 2430–2447. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.F.; Herholz, K.; Rosa-Neto, P.; Pellerin, L.; Nordberg, A.; Zimmer, E.R. Astrocyte biomarkers in Alzheimer’s disease. Trends Mol. Med. 2019, 25, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Thorne, R.G.; Nicholson, C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc. Natl. Acad. Sci. USA 2006, 103, 5567–5572. [Google Scholar] [CrossRef]
- Mattson, M.P.; Chan, S.L. Neuronal and glial calcium signaling in Alzheimer’s disease. Cell Calcium 2003, 34, 385–397. [Google Scholar] [CrossRef]
- Tarantini, S.; Tran, C.H.T.; Gordon, G.R.; Ungvari, Z.; Csiszar, A. Impaired neurovascular coupling in aging and Alzheimer’s disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp. Gerontol. 2017, 94, 52–58. [Google Scholar] [CrossRef]
- Santello, M.; Toni, N.; Volterra, A. Astrocyte function from information processing to cognition and cognitive impairment. Nat. Neurosci. 2019, 22, 154–166. [Google Scholar] [CrossRef]
- Dallérac, G.; Rouach, N. Astrocytes as new targets to improve cognitive functions. Prog. Neurobiol. 2016, 144, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Pascual, O.; Casper, K.B.; Kubera, C.; Zhang, J.; Revilla-Sanchez, R.; Sul, J.Y.; Takano, H.; Moss, S.J.; McCarthy, K.; Haydon, P.G. Astrocytic purinergic signaling coordinates synaptic networks. Science 2005, 310, 113–116. [Google Scholar] [CrossRef]
- Fellin, T. Communication between neurons and astrocytes: Relevance to the modulation of synaptic and network activity. J. Neurochem. 2009, 108, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Fellin, T.; Ellenbogen, J.M.; De Pittà, M.; Ben-Jacob, E.; Halassa, M.M. Astrocyte regulation of sleep circuits: Experimental and modeling perspectives. Front. Comput. Neurosci. 2012, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Nadjar, A.; Blutstein, T.; Aubert, A.; Laye, S.; Haydon, P.G. Astrocyte-derived adenosine modulates increased sleep pressure during inflammatory response. Glia 2013, 61, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Hines, D.; Schmitt, L.; Hines, R.; Moss, S.; Haydon, P. Antidepressant effects of sleep deprivation require astrocyte-dependent adenosine mediated signaling. Transl. Psychiatry 2013, 3, e212. [Google Scholar] [CrossRef] [PubMed]
- Haydon, P.G. Astrocytes and the modulation of sleep. Curr. Opin. Neurobiol. 2017, 44, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, I.S.; Jeong, J.Y.; Jang, I.S.; Lee, M.G.; Suk, K. Astrocytes in the ventrolateral preoptic area promote sleep. J. Neurosci. 2020, 40, 8994–9011. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.S.; Kim, J.H.; Jeong, J.Y.; Lee, M.G.; Suk, K.; Jang, I.S. Astrocyte-derived adenosine excites sleep-promoting neurons in the ventrolateral preoptic nucleus: Astrocyte-neuron interactions in the regulation of sleep. Glia 2022, 70, 1864–1885. [Google Scholar] [CrossRef]
- Pelluru, D.; Konadhode, R.R.; Bhat, N.R.; Shiromani, P.J. Optogenetic stimulation of astrocytes in the posterior hypothalamus increases sleep at night in C57 BL/6J mice. Eur. J. Neurosci. 2016, 43, 1298–1306. [Google Scholar] [CrossRef]
- Peng, W.; Liu, X.; Ma, G.; Wu, Z.; Wang, Z.; Fei, X.; Qin, M.; Wang, L.; Li, Y.; Zhang, S.; et al. Adenosine-independent regulation of the sleep–wake cycle by astrocyte activity. Cell Discov. 2023, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G. Astroglial regulation of sleep homeostasis. Curr. Opin. Neurobiol. 2013, 23, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Franken, P.; Lopez-Molina, L.; Marcacci, L.; Schibler, U.; Tafti, M. The transcription factor DBP affects circadian sleep consolidation and rhythmic EEG activity. J. Neurosci. 2000, 20, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; McCarthy, K.D. Astrocyte calcium signaling: From observations to functions and the challenges therein. Cold Spring Harb. Perspect. Biol. 2015, 7, a020404. [Google Scholar] [CrossRef] [PubMed]
- Bazargani, N.; Attwell, D. Astrocyte calcium signaling: The third wave. Nat. Neurosci. 2016, 19, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, M.; Patton, A.P.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Astrocytes control circadian timekeeping in the suprachiasmatic nucleus via glutamatergic signaling. Neuron 2017, 93, 1420–1435. [Google Scholar] [CrossRef] [PubMed]
- Astiz, M.; Delgado-García, L.M.; López-Mascaraque, L. Astrocytes as essential time-keepers of the central pacemaker. Glia 2022, 70, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.H.; Brancaccio, M.; Gonzalez-Aponte, M.F.; Herzog, E.D. Circadian rhythms and astrocytes: The good, the bad, and the ugly. Annu. Rev. Neurosci. 2023, 46, 123–143. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Toescu, E. Neuronal-glial networks as substrate for CNS integration. J. Cell. Mol. Med. 2006, 10, 869–879. [Google Scholar] [CrossRef]
- Iadecola, C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef]
- Schaeffer, S.; Iadecola, C. Revisiting the neurovascular unit. Nat. Neurosci. 2021, 24, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Farr, H.; David, T. Models of neurovascular coupling via potassium and EET signalling. J. Theor. Biol. 2011, 286, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Dormanns, K.; Brown, R.; David, T. The role of nitric oxide in neurovascular coupling. J. Theor. Biol. 2016, 394, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kenny, A.; Plank, M.J.; David, T. The role of astrocytic calcium and TRPV4 channels in neurovascular coupling. J. Comput. Neurosci. 2018, 44, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Mathias, E.J.; Kenny, A.; Plank, M.J.; David, T. Integrated models of neurovascular coupling and BOLD signals: Responses for varying neural activations. NeuroImage 2018, 174, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Santello, M.; Calì, C.; Bezzi, P. Gliotransmission and the tripartite synapse. In Synaptic Plasticity: Dynamics, Development and Disease; Springer: Berlin/Heidelberg, Germany, 2012; pp. 307–331. [Google Scholar]
- Volterra, A.; Magistretti, P.; Haydon, P. The Tripartite Synapse: Glia in Synaptic Transmission; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Rose, C.R.; Chatton, J.Y. Astrocyte sodium signaling and neuro-metabolic coupling in the brain. Neuroscience 2016, 323, 121–134. [Google Scholar] [CrossRef]
- Araque, A.; Carmignoto, G.; Haydon, P.G.; Oliet, S.H.; Robitaille, R.; Volterra, A. Gliotransmitters travel in time and space. Neuron 2014, 81, 728–739. [Google Scholar] [CrossRef]
- Nadkarni, S.; Jung, P. Dressed neurons: Modeling neural-glial interactions. Phys. Biol. 2004, 1, 35–41. [Google Scholar] [CrossRef]
- Nadkarni, S.; Jung, P. Modeling synaptic transmission of the tripartite synapse. Phys. Biol. 2007, 4, 1. [Google Scholar] [CrossRef]
- Ding, F.; O’Donnell, J.; Xu, Q.; Kang, N.; Goldman, N.; Nedergaard, M. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science 2016, 352, 550–555. [Google Scholar] [CrossRef]
- O’Donnell, J.; Ding, F.; Nedergaard, M. Distinct functional states of astrocytes during sleep and wakefulness: Is norepinephrine the master regulator? Curr. Sleep Med. Rep. 2015, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kugler, E.C.; Greenwood, J.; MacDonald, R.B. The “neuro-glial-vascular” unit: The role of glia in neurovascular unit formation and dysfunction. Front. Cell Dev. Biol. 2021, 9, 732820. [Google Scholar] [CrossRef] [PubMed]
- Semyanov, A.; Verkhratsky, A. Astrocytic processes: From tripartite synapses to the active milieu. Trends Neurosci. 2021, 44, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef] [PubMed]
- Hama, K.; Arii, T.; Katayama, E.; Marton, M.; Ellisman, M.H. Tri-dimensional morphometric analysis of astrocytic processes with high voltage electron microscopy of thick Golgi preparations. J. Neurocytol. 2004, 33, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Zisis, E.; Keller, D.; Kanari, L.; Arnaudon, A.; Gevaert, M.; Delemontex, T.; Coste, B.; Foni, A.; Abdellah, M.; Calì, C.; et al. Digital reconstruction of the neuro-glia-vascular architecture. Cereb. Cortex 2021, 31, 5686–5703. [Google Scholar] [CrossRef]
- Saper, C.B.; Chou, T.C.; Scammell, T.E. The sleep switch: Hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001, 24, 726–731. [Google Scholar] [CrossRef]
- Webb, W.B. Biological Rhythms, Sleep, and Performance; John Wiley & Sons: Hoboken, NJ, USA, 1982. [Google Scholar]
- Krueger, J.M.; Nguyen, J.T.; Dykstra-Aiello, C.J.; Taishi, P. Local sleep. Sleep Med. Rev. 2019, 43, 14–21. [Google Scholar] [CrossRef]
- Nobili, L.; De Gennaro, L.; Proserpio, P.; Moroni, F.; Sarasso, S.; Pigorini, A.; De Carli, F.; Ferrara, M. Local aspects of sleep: Observations from intracerebral recordings in humans. Prog. Brain Res. 2012, 199, 219–232. [Google Scholar] [CrossRef]
- Siclari, F.; Tononi, G. Local aspects of sleep and wakefulness. Curr. Opin. Neurobiol. 2017, 44, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Pigarev, I.N.; Nothdurft, H.C.; Kastner, S. Evidence for asynchronous development of sleep in cortical areas. Neuroreport 1997, 8, 2557–2560. [Google Scholar] [CrossRef] [PubMed]
- Landolt, H.P.; Holst, S.C. Ionic control of sleep and wakefulness. Science 2016, 352, 517–518. [Google Scholar] [CrossRef] [PubMed]
- Charles, A. Intercellular calcium waves in glia. Glia 1998, 24, 39–49. [Google Scholar] [CrossRef]
- Scemes, E.; Giaume, C. Astrocyte calcium waves: What they are and what they do. Glia 2006, 54, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, T.M.; Kuhn, B.; Göbel, W.; Huang, W.; Nakai, J.; Helmchen, F.; Flint, J.; Wang, S.S. Radially expanding transglial calcium waves in the intact cerebellum. Proc. Natl. Acad. Sci. USA 2009, 106, 3496–3501. [Google Scholar] [CrossRef] [PubMed]
- Brazhe, A.; Mathiesen, C.; Lauritzen, M. Multiscale vision model highlights spontaneous glial calcium waves recorded by 2-photon imaging in brain tissue. Neuroimage 2013, 68, 192–202. [Google Scholar] [CrossRef]
- Chvátal, A.; Ande˘rová, M.; Hock, M.; Prajerová, I.; Nepras˘ová, H.; Chvátal, V.; Kirchhoff, F.; Syková, E. Three-dimensional confocal morphometry reveals structural changes in astrocyte morphology in situ. J. Neurosci. Res. 2007, 85, 260–271. [Google Scholar] [CrossRef]
- Risher, W.C.; Andrew, R.D.; Kirov, S.A. Real-time passive volume responses of astrocytes to acute osmotic and ischemic stress in cortical slices and in vivo revealed by two-photon microscopy. Glia 2009, 57, 207–221. [Google Scholar] [CrossRef]
- Dibaj, P.; Kaiser, M.; Hirrlinger, J.; Kirchhoff, F.; Neusch, C. Kir4. 1 channels regulate swelling of astroglial processes in experimental spinal cord edema. J. Neurochem. 2007, 103, 2620–2628. [Google Scholar] [CrossRef]
- Florence, C.M.; Baillie, L.D.; Mulligan, S.J. Dynamic volume changes in astrocytes are an intrinsic phenomenon mediated by bicarbonate ion flux. PLoS ONE 2012, 7, e51124. [Google Scholar] [CrossRef] [PubMed]
- Walch, E.; Murphy, T.R.; Cuvelier, N.; Aldoghmi, M.; Morozova, C.; Donohue, J.; Young, G.; Samant, A.; Garcia, S.; Alvarez, C.; et al. Astrocyte-selective volume increase in elevated extracellular potassium conditions is mediated by the Na+/K+ ATPase and occurs independently of aquaporin 4. ASN Neuro 2020, 12, 1759091420967152. [Google Scholar] [CrossRef] [PubMed]
- Risher, W.C.; Croom, D.; Kirov, S.A. Persistent astroglial swelling accompanies rapid reversible dendritic injury during stroke-induced spreading depolarizations. Glia 2012, 60, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Sword, J.; Masuda, T.; Croom, D.; Kirov, S.A. Evolution of neuronal and astroglial disruption in the peri-contusional cortex of mice revealed by in vivo two-photon imaging. Brain 2013, 136, 1446–1461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Gordon, G.R.; Feighan, D.; MacVicar, B.A. Transient swelling, acidification, and mitochondrial depolarization occurs in neurons but not astrocytes during spreading depression. Cereb. Cortex 2010, 20, 2614–2624. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G.; Schilling, K.; Steinhäuser, C. Astrocyte dysfunction in neurological disorders: A molecular perspective. Nat. Rev. Neurosci. 2006, 7, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Walch, E.; Fiacco, T.A. Honey, I shrunk the extracellular space: Measurements and mechanisms of astrocyte swelling. Glia 2022, 70, 2013–2031. [Google Scholar] [CrossRef] [PubMed]
- Walz, W.; Shargool, M.; Hertz, L. Barium-induced inhibition of K+ transport mechanisms in cortical astrocytes—Its possible contribution to the large Ba2+-evoked extracellular K+ signal in brain. Neuroscience 1984, 13, 945–949. [Google Scholar] [CrossRef]
- Newman, E.A. Regional Specialization of the Membrane of Retinal Glial Cells and Its Importance to K+ Spatial Buffering a. Ann. N. Y. Acad. Sci. 1986, 481, 273–286. [Google Scholar] [CrossRef]
- Kofuji, P.; Ceelen, P.; Zahs, K.R.; Surbeck, L.W.; Lester, H.A.; Newman, E.A. Genetic inactivation of an inwardly rectifying potassium channel (Kir4. 1 subunit) in mice: Phenotypic impact in retina. J. Neurosci. 2000, 20, 5733–5740. [Google Scholar] [CrossRef]
- Ballanyi, K.; Grafe, P.; Ten Bruggencate, G. Ion activities and potassium uptake mechanisms of glial cells in guinea-pig olfactory cortex slices. J. Physiol. 1987, 382, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Springer, C.S., Jr.; Plenz, D.; Basser, P.J. Fast, Na+/K+ pump driven, steady-state transcytolemmal water exchange in neuronal tissue: A study of rat brain cortical cultures. Magn. Reson. Med. 2018, 79, 3207–3217. [Google Scholar] [CrossRef] [PubMed]
- Springer, C.S., Jr. Using 1H2O MR to measure and map sodium pump activity in vivo. J. Magn. Reson. 2018, 291, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Walz, W.; Hertz, L. Intracellular ion changes of astrocytes in response to extracellular potassium. J. Neurosci. Res. 1983, 10, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.R.; Stoica, A.; MacAulay, N. Managing brain extracellular K+ during neuronal activity: The physiological role of the Na+/K+-ATPase subunit isoforms. Front. Physiol. 2016, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.R.; Ransom, B.R. Intracellular sodium homeostasis in rat hippocampal astrocytes. J. Physiol. 1996, 491, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, T.; Packey, D.; Trachtenberg, M.; Haber, B. K+-induced ion and water movements in the frog spinal cord and filum terminale. Exp. Neurol. 1981, 71, 356–369. [Google Scholar] [CrossRef]
- Chen, H.; Sun, D. The role of Na–K–Cl co-transporter in cerebral ischemia. Neurol. Res. 2005, 27, 280–286. [Google Scholar] [CrossRef]
- Kimelberg, H.K.; MacVicar, B.A.; Sontheimer, H. Anion channels in astrocytes: Biophysics, pharmacology, and function. Glia 2006, 54, 747–757. [Google Scholar] [CrossRef]
- Wilson, C.S.; Mongin, A.A. The signaling role for chloride in the bidirectional communication between neurons and astrocytes. Neurosci. Lett. 2019, 689, 33–44. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Untiet, V.; Rose, C.R. Ionic signalling in astroglia beyond calcium. J. Physiol. 2020, 598, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Lascola, C.D.; Nelson, D.J.; Kraig, R.P. Cytoskeletal actin gates a Cl- channel in neocortical astrocytes. J. Neurosci. 1998, 18, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Kimelberg, H.K. Review: Cell Volume in the CNS: Regulation and Implications for Nervous System Function and Pathology. Neuroscience 2000, 6, 14–25. [Google Scholar] [CrossRef]
- Inoue, H.; Mori, S.I.; Morishima, S.; Okada, Y. Volume-sensitive chloride channels in mouse cortical neurons: Characterization and role in volume regulation. Eur. J. Neurosci. 2005, 21, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef] [PubMed]
- Walz, W. Chloride/anion channels in glial cell membranes. Glia 2002, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Østby, I.; Øyehaug, L.; Einevoll, G.T.; Nagelhus, E.A.; Plahte, E.; Zeuthen, T.; Lloyd, C.M.; Ottersen, O.P.; Omholt, S.W. Astrocytic mechanisms explaining neural-activity-induced shrinkage of extraneuronal space. PLoS Comput. Biol. 2009, 5, e1000272. [Google Scholar]
- Walz, W. Accumulation of Intracellular Bicarbonate Accounts for the Missing Anion during Potassium-Evoked Swelling of Cortical Type-1-Like Astrocytes a. Ann. N. Y. Acad. Sci. 1991, 633, 589–591. [Google Scholar] [CrossRef]
- Walz, W.; Hinks, E.C. Carrier-mediated KCl accumulation accompanied by water movements is involved in the control of physiological K+ levels by astrocytes. Brain Res. 1985, 343, 44–51. [Google Scholar] [CrossRef]
- Hübel, N.; Ullah, G. Anions govern cell volume: A case study of relative astrocytic and neuronal swelling in spreading depolarization. PLoS ONE 2016, 11, e0147060. [Google Scholar] [CrossRef]
- Kettenmann, H.; Schachner, M. Pharmacological properties of gamma-aminobutyric acid-, glutamate-, and aspartate-induced depolarizations in cultured astrocytes. J. Neurosci. 1985, 5, 3295–3301. [Google Scholar] [CrossRef] [PubMed]
- Bekar, L.K.; Walz, W. Intracellular chloride modulates A-type potassium currents in astrocytes. Glia 2002, 39, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Untiet, V.; Beinlich, F.R.; Kusk, P.; Kang, N.; Ladrón-de Guevara, A.; Song, W.; Kjaerby, C.; Andersen, M.; Hauglund, N.; Bojarowska, Z.; et al. Astrocytic chloride is brain state dependent and modulates inhibitory neurotransmission in mice. Nat. Commun. 2023, 14, 1871. [Google Scholar] [CrossRef] [PubMed]
- Cederroth, C.R.; Albrecht, U.; Bass, J.; Brown, S.A.; Dyhrfjeld-Johnsen, J.; Gachon, F.; Green, C.B.; Hastings, M.H.; Helfrich-Förster, C.; Hogenesch, J.B.; et al. Medicine in the fourth dimension. Cell Metab. 2019, 30, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Hablitz, L.M.; Vinitsky, H.S.; Sun, Q.; Stæger, F.F.; Sigurdsson, B.; Mortensen, K.N.; Lilius, T.O.; Nedergaard, M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 2019, 5, eaav5447. [Google Scholar] [CrossRef] [PubMed]
- Pla, V.; Bork, P.; Harnpramukkul, A.; Olveda, G.; Ladron-de Guevara, A.; Giannetto, M.J.; Hussain, R.; Wang, W.; Kelley, D.H.; Hablitz, L.M.; et al. A real-time in vivo clearance assay for quantification of glymphatic efflux. Cell Rep. 2022, 40. [Google Scholar] [CrossRef]
- Hladky, S.B.; Barrand, M.A. The glymphatic hypothesis: The theory and the evidence. Fluids Barriers CNS 2022, 19, 1–33. [Google Scholar] [CrossRef]
- Smith, A.J.; Jin, B.J.; Verkman, A.S. Muddying the water in brain edema? Trends Neurosci. 2015, 38, 331. [Google Scholar] [CrossRef]
- Dupont, G.; Schmidt, C.; Yilmaz, E.; Oskouian, R.J.; Macchi, V.; De Caro, R.; Tubbs, R.S. Our current understanding of the lymphatics of the brain and spinal cord. Clin. Anat. 2019, 32, 117–121. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Postnov, D.; Kurths, J. Blood-brain barrier, lymphatic clearance, and recovery: Ariadne’s thread in labyrinths of hypotheses. Int. J. Mol. Sci. 2018, 19, 3818. [Google Scholar] [CrossRef]
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The role of brain barriers in fluid movement in the CNS: Is there a “glymphatic” system? Acta Neuropathol. 2018, 135, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.R.; Davila, D.; Cuvelier, N.; Young, L.R.; Lauderdale, K.; Binder, D.K.; Fiacco, T.A. Hippocampal and cortical pyramidal neurons swell in parallel with astrocytes during acute hypoosmolar stress. Front. Cell. Neurosci. 2017, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Haines, T.H. Water transport across biological membranes. FEBS Lett. 1994, 346, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Hannesschlaeger, C.; Horner, A.; Pohl, P. Intrinsic membrane permeability to small molecules. Chem. Rev. 2019, 119, 5922–5953. [Google Scholar] [CrossRef] [PubMed]
- Przybyło, M.; Drabik, D.; Doskocz, J.; Iglič, A.; Langner, M. The effect of the osmotically active compound concentration difference on the passive water and proton fluxes across a lipid bilayer. Int. J. Mol. Sci. 2021, 22, 11099. [Google Scholar] [CrossRef] [PubMed]
- Lauderdale, K.; Murphy, T.; Tung, T.; Davila, D.; Binder, D.K.; Fiacco, T.A. Osmotic edema rapidly increases neuronal excitability through activation of NMDA receptor-dependent slow inward currents in juvenile and adult hippocampus. ASN Neuro 2015, 7, 1759091415605115. [Google Scholar] [CrossRef] [PubMed]
- Samuels, E.R.; Szabadi, E. Functional neuroanatomy of the noradrenergic locus coeruleus: Its roles in the regulation of arousal and autonomic function part I: Principles of functional organisation. Curr. Neuropharmacol. 2008, 6, 235–253. [Google Scholar] [CrossRef]
- Schwarz, L.A.; Luo, L. Organization of the locus coeruleus-norepinephrine system. Curr. Biol. 2015, 25, R1051–R1056. [Google Scholar] [CrossRef]
- Mouton, P.R.; Pakkenberg, B.; Gundersen, H.J.G.; Price, D.L. Absolute number and size of pigmented locus coeruleus neurons in young and aged individuals. J. Chem. Neuroanat. 1994, 7, 185–190. [Google Scholar] [CrossRef]
- Cohen, Z.; Molinatti, G.; Hamel, E. Astroglial and vascular interactions of noradrenaline terminals in the rat cerebral cortex. J. Cereb. Blood Flow Metab. 1997, 17, 894–904. [Google Scholar] [CrossRef]
- Descarries, L.; Watkins, K.C.; Lapierre, Y. Noradrenergic axon terminals in the cerebral cortex of rat. III. Topometric ultrastructural analysis. Brain Res. 1977, 133, 197–222. [Google Scholar] [CrossRef] [PubMed]
- Schummers, J.; Yu, H.; Sur, M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 2008, 320, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Thrane, A.S.; Rangroo Thrane, V.; Zeppenfeld, D.; Lou, N.; Xu, Q.; Nagelhus, E.A.; Nedergaard, M. General anesthesia selectively disrupts astrocyte calcium signaling in the awake mouse cortex. Proc. Natl. Acad. Sci. USA 2012, 109, 18974–18979. [Google Scholar] [CrossRef] [PubMed]
- Berridge, C.W.; Waterhouse, B.D. The locus coeruleus–noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003, 42, 33–84. [Google Scholar] [CrossRef] [PubMed]
- Eldar, E.; Cohen, J.D.; Niv, Y. The effects of neural gain on attention and learning. Nat. Neurosci. 2013, 16, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Paukert, M.; Agarwal, A.; Cha, J.; Doze, V.A.; Kang, J.U.; Bergles, D.E. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 2014, 82, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Ingiosi, A.M.; Frank, M.G. Noradrenergic Signaling in Astrocytes Influences Mammalian Sleep Homeostasis. Clocks Sleep 2022, 4, 332–345. [Google Scholar] [CrossRef]
- Polack, P.O.; Friedman, J.; Golshani, P. Cellular mechanisms of brain state–dependent gain modulation in visual cortex. Nat. Neurosci. 2013, 16, 1331–1339. [Google Scholar] [CrossRef]
- Mather, M.; Clewett, D.; Sakaki, M.; Harley, C.W. Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behav. Brain Sci. 2016, 39, e200. [Google Scholar] [CrossRef]
- Verisokin, A.; Verveyko, D.; Kirsanov, A.; Brazhe, A.; Postnov, D. Computational Model of Noradrenaline Modulation of Astrocyte Responses to Synaptic Activity. Mathematics 2023, 11, 628. [Google Scholar] [CrossRef]
- Bar El, Y.; Kanner, S.; Barzilai, A.; Hanein, Y. Activity changes in neuron-astrocyte networks in culture under the effect of norepinephrine. PLoS ONE 2018, 13, e0203761. [Google Scholar] [CrossRef]
- Reitman, M.E.; Tse, V.; Mi, X.; Willoughby, D.D.; Peinado, A.; Aivazidis, A.; Myagmar, B.E.; Simpson, P.C.; Bayraktar, O.A.; Yu, G.; et al. Norepinephrine links astrocytic activity to regulation of cortical state. Nat. Neurosci. 2023, 26, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Lohela, T.J.; Lilius, T.O.; Nedergaard, M. The glymphatic system: Implications for drugs for central nervous system diseases. Nat. Rev. Drug Discov. 2022, 21, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Lewis, L.D.; Hirschler, L.; Rivera, L.R.; Naganawa, S.; Levendovszky, S.R.; Ringstad, G.; Klarica, M.; Wardlaw, J.; Iadecola, C.; et al. Current Understanding of the Anatomy, Physiology, and Magnetic Resonance Imaging of Neurofluids: Update From the 2022 “ISMRM Imaging Neurofluids Study group” Workshop in Rome. J. Magn. Reson. Imaging 2023, 2023, 28759. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, C.; Syková, E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998, 21, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, C. Diffusion and related transport mechanisms in brain tissue. Rep. Prog. Phys. 2001, 64, 815. [Google Scholar] [CrossRef]
- Hrabětová, S.; Nicholson, C. Contribution of dead-space microdomains to tortuosity of brain extracellular space. Neurochem. Int. 2004, 45, 467–477. [Google Scholar] [CrossRef]
- Syková, E.; Nicholson, C. Diffusion in brain extracellular space. Physiol. Rev. 2008, 88, 1277–1340. [Google Scholar] [CrossRef]
- Nicholson, C.; Hrabětová, S. Brain extracellular space: The final frontier of neuroscience. Biophys. J. 2017, 113, 2133–2142. [Google Scholar] [CrossRef]
- Postnikov, E.B.; Lavrova, A.I.; Postnov, D.E. Transport in the brain extracellular space: Diffusion, but which kind? Int. J. Mol. Sci. 2022, 23, 12401. [Google Scholar] [CrossRef]
- Metzler, R. Superstatistics and non-Gaussian diffusion. Eur. Phys. J. Spec. Top. 2020, 229, 711–728. [Google Scholar] [CrossRef]
- Sutera, S.P.; Skalak, R. The history of Poiseuille’s law. Annu. Rev. Fluid Mech. 1993, 25, 1–20. [Google Scholar] [CrossRef]
- Pfitzner, J. Poiseuille and his law. Anaesthesia 1976, 31, 273–275. [Google Scholar] [CrossRef]
- Batchelor, C.K.; Batchelor, G.K. An Introduction to Fluid Dynamics; Cambridge University Press: Cambridge, UK, 1967. [Google Scholar]
- Clennell, M.B. Tortuosity: A guide through the maze. Geol. Soc. Lond. Spec. Publ. 1997, 122, 299–344. [Google Scholar] [CrossRef]
- Mortensen, N.A.; Okkels, F.; Bruus, H. Reexamination of Hagen-Poiseuille flow: Shape dependence of the hydraulic resistance in microchannels. Phys. Rev. E 2005, 71, 057301. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Perfect, E.; Cheng, C.L.; Hu, X. Generalized modeling of spontaneous imbibition based on Hagen–Poiseuille flow in tortuous capillaries with variably shaped apertures. Langmuir 2014, 30, 5142–5151. [Google Scholar] [CrossRef]
- Zampogna, G.A.; Bottaro, A. Fluid flow over and through a regular bundle of rigid fibres. J. Fluid Mech. 2016, 792, 5–35. [Google Scholar] [CrossRef]
- Vanina, A.S.; Sychev, A.V.; Lavrova, A.I.; Gavrilov, P.V.; Andropova, P.L.; Grekhnyova, E.V.; Kudryavtseva, T.N.; Postnikov, E.B. Computed Tomography-Assisted Study of the Liquid Contrast Agents Spread in a Hydrogel Phantom of the Brain Tissue. Fluids 2023, 8, 167. [Google Scholar] [CrossRef]
- Arizono, M.; Inavalli, V.K.; Bancelin, S.; Fernández-Monreal, M.; Nägerl, U.V. Super-resolution shadow imaging reveals local remodeling of astrocytic microstructures and brain extracellular space after osmotic challenge. Glia 2021, 69, 1605–1613. [Google Scholar] [CrossRef]
- Zhu, D.; Montagne, A.; Zhao, Z. Alzheimer’s pathogenic mechanisms and underlying sex difference. Cell. Mol. Life Sci. 2021, 78, 4907–4920. [Google Scholar] [CrossRef]
- Mielke, M.M. Sex and gender differences in Alzheimer’s disease dementia. Psychiatr. Times 2018, 35, 14. [Google Scholar]
- Giannetto, M.; Xia, M.; Stæger, F.F.; Metcalfe, T.; Vinitsky, H.S.; Dang, J.A.; Xavier, A.L.; Kress, B.T.; Nedergaard, M.; Hablitz, L.M. Biological sex does not predict glymphatic influx in healthy young, middle aged or old mice. Sci. Rep. 2020, 10, 16073. [Google Scholar] [CrossRef]
- Han, F.; Liu, X.; Yang, Y.; Liu, X. Sex-specific age-related changes in glymphatic function assessed by resting-state functional magnetic resonance imaging. bioRxiv, 2023; in press. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postnov, D.; Semyachkina-Glushkovskaya, O.; Litvinenko, E.; Kurths, J.; Penzel, T. Mechanisms of Activation of Brain’s Drainage during Sleep: The Nightlife of Astrocytes. Cells 2023, 12, 2667. https://doi.org/10.3390/cells12222667
Postnov D, Semyachkina-Glushkovskaya O, Litvinenko E, Kurths J, Penzel T. Mechanisms of Activation of Brain’s Drainage during Sleep: The Nightlife of Astrocytes. Cells. 2023; 12(22):2667. https://doi.org/10.3390/cells12222667
Chicago/Turabian StylePostnov, Dmitry, Oxana Semyachkina-Glushkovskaya, Elena Litvinenko, Jürgen Kurths, and Thomas Penzel. 2023. "Mechanisms of Activation of Brain’s Drainage during Sleep: The Nightlife of Astrocytes" Cells 12, no. 22: 2667. https://doi.org/10.3390/cells12222667
APA StylePostnov, D., Semyachkina-Glushkovskaya, O., Litvinenko, E., Kurths, J., & Penzel, T. (2023). Mechanisms of Activation of Brain’s Drainage during Sleep: The Nightlife of Astrocytes. Cells, 12(22), 2667. https://doi.org/10.3390/cells12222667







