Involvement of Astrocytes in the Formation, Maintenance, and Function of the Blood–Brain Barrier
Abstract
1. Introduction
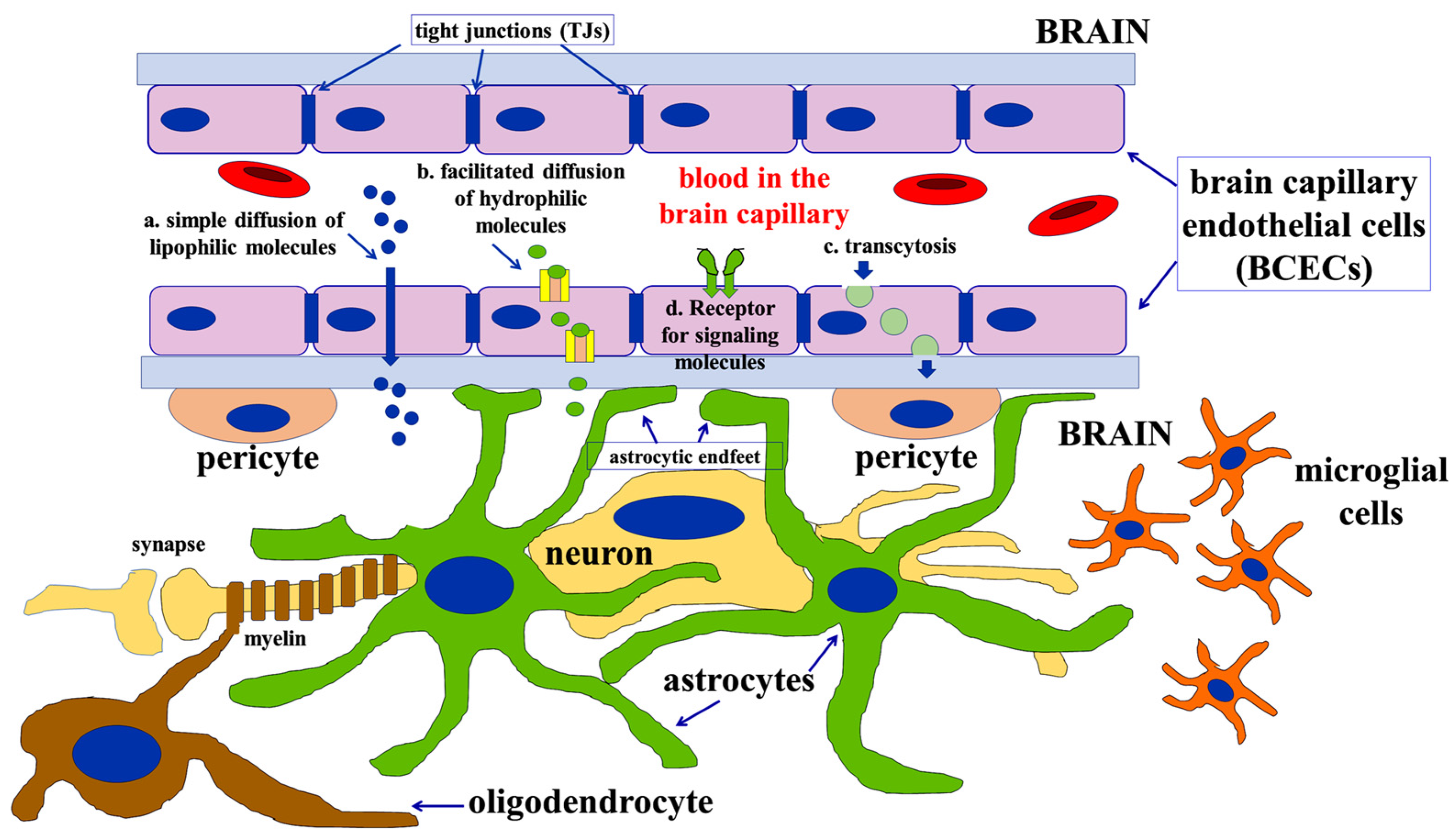
2. Developmental Appearance and Maintenance of the Barrier’s Function
2.1. The General Metabolic Role of Brain Astrocytes
2.2. Astrocytes and BBB Formation and Maintenance
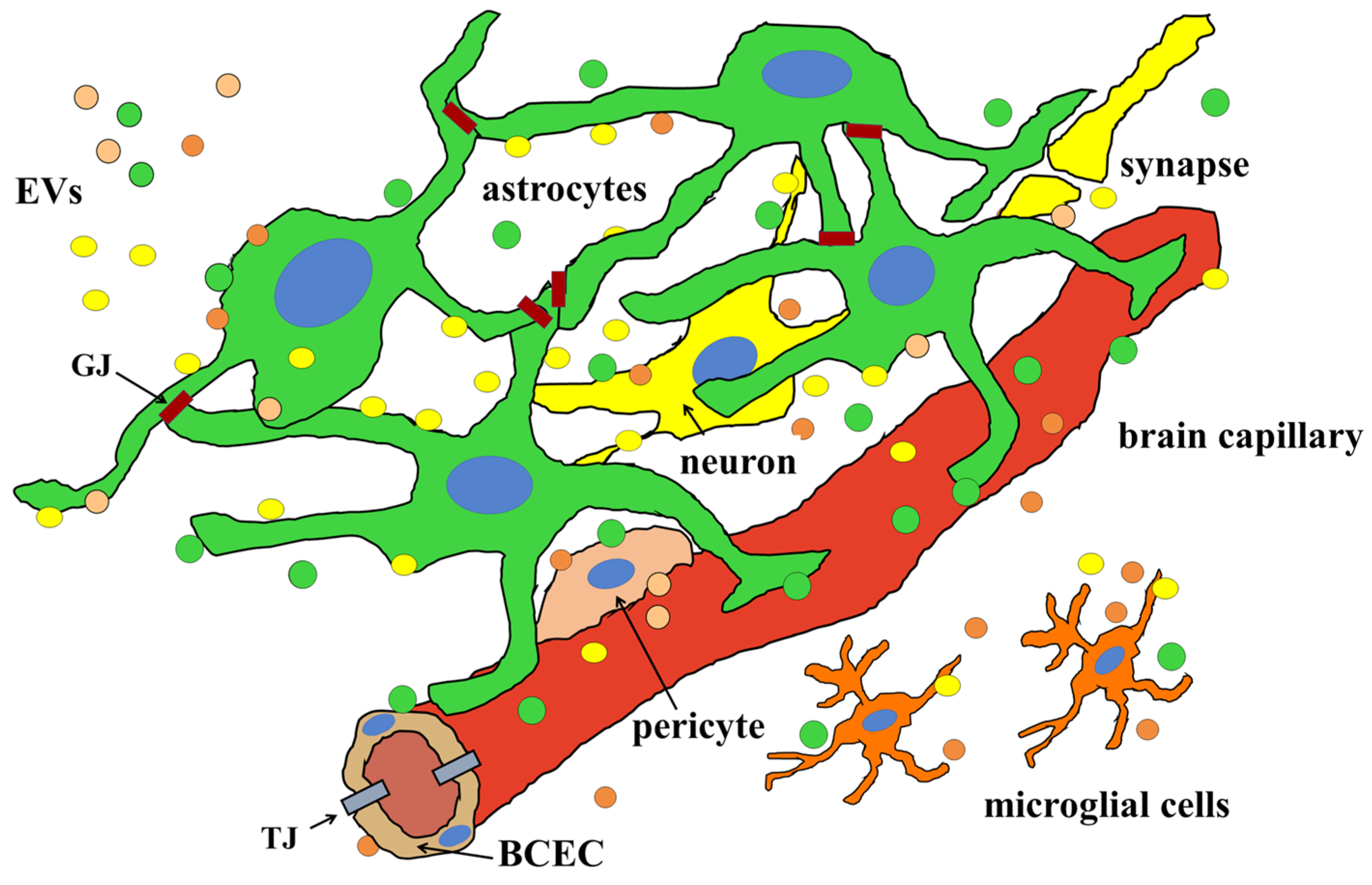
2.3. The Role of Extracellular Vesicles (EVs) in the Formation and Maintenance of the BBB
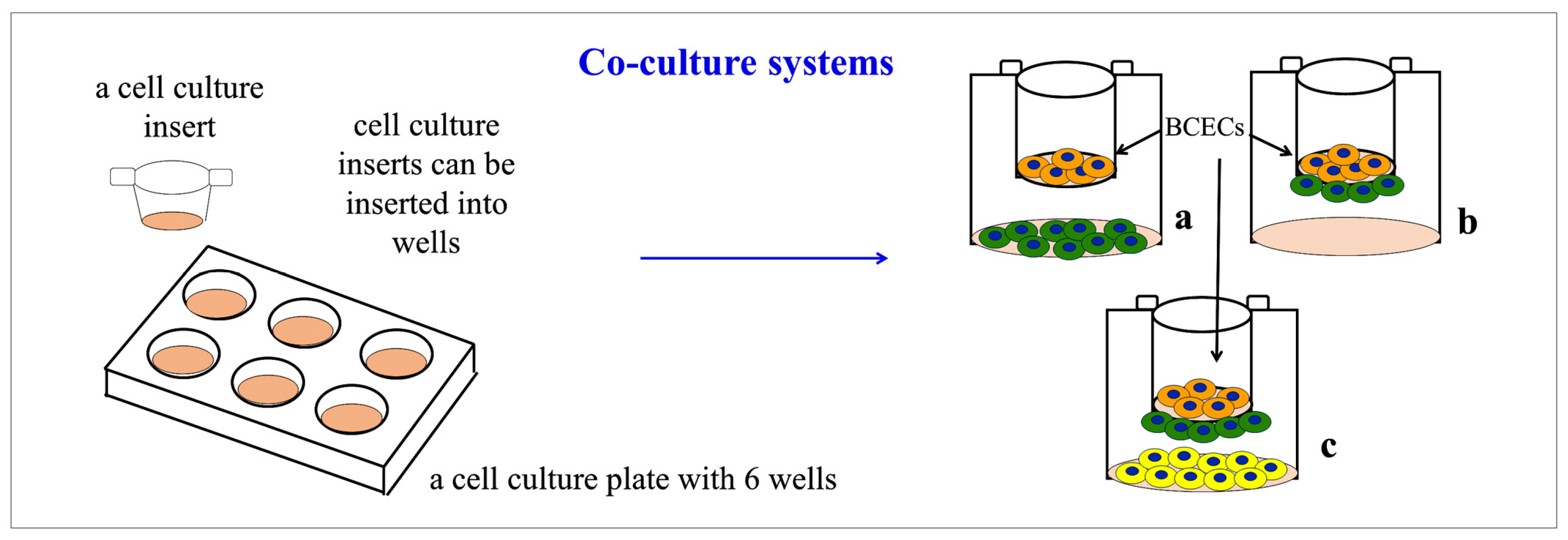
2.4. In Vitro Models Used to Study the Formation of the BBB
2.4.1. Co-Culture Models for Studying BBB Formation and Maintenance In Vitro
2.4.2. In Vitro Models Aimed at Studying Modifications of the BBB’s Function under Pathological Conditions as well as the Ability of Drugs to cross a Functional BBB
3. Pathological Alterations of the BBB
3.1. Alzheimer’s Disease and BBB Alteration: A Focus on Astrocytes
3.2. Multiple Sclerosis: Astrocytes of the BBB
3.3. Astrocyte and BBB Alteration under Other Pathological Conditions
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Serlin, Y.; Shelef, I.; Knyazer, B.; Friedman, A. Anatomy and physiology of the blood-brain barrier. Semin. Cell Dev. Biol. 2015, 38, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Harder, D.R.; Zhang, C.; Gebremedhin, D. Astrocytes function in matching blood flow to metabolic activity. News Physiol. Sci. 2002, 17, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Blanchette, M.; Daneman, R. Formation and maintenance of the BBB. Mech. Dev. 2015, 138, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Archie, S.R.; Al Shoyaib, A.; Cucullo, L. Blood-Brain Barrier Dysfunction in CNS Disorders and Putative Therapeutic Targets: An Overview. Pharmaceutics 2021, 13, 1779. [Google Scholar] [CrossRef]
- Cibelli, A.; Stout, R.; Timmermann, A.; de Menezes, L.; Guo, P.; Maass, K.; Seifert, G.; Steinhäuser, C.; Spray, D.C.; Scemes, E. Cx43 carboxyl terminal domain determines AQP4 and Cx30 endfoot organization and blood brain barrier permeability. Sci. Rep. 2021, 11, 24334. [Google Scholar] [CrossRef]
- Kugler, E.C.; Greenwood, J.; MacDonald, R.B. The “Neuro-Glial-Vascular” Unit: The Role of Glia in Neurovascular Unit Formation and Dysfunction. Front. Cell Dev. Biol. 2021, 9, 732820. [Google Scholar] [CrossRef]
- McConnell, H.L.; Mishra, A. Cells of the Blood-Brain Barrier: An Overview of the Neurovascular Unit in Health and Disease. Methods Mol. Biol. 2022, 2492, 3–24. [Google Scholar] [CrossRef]
- Naranjo, O.; Osborne, O.; Torices, S.; Toborek, M. In Vivo Targeting of the Neurovascular Unit: Challenges and Advancements. Cell Mol. Neurobiol. 2022, 42, 2131–2146. [Google Scholar] [CrossRef]
- Zidarič, T.; Gradišnik, L.; Velnar, T. Astrocytes and human artificial blood-brain barrier models. Bosn. J. Basic Med. Sci. 2022, 22, 651–672. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhen, Y.; Wang, X.; Wang, J.; Zhu, G. Neurovascular glial unit: A target of phytotherapy for cognitive impairments. Phytomedicine 2023, 119, 155009. [Google Scholar] [CrossRef]
- Cresto, N.; Janvier, A.; Marchi, N. From neurons to the neuro-glio-vascular unit: Seizures and brain homeostasis in networks. Rev. Neurol. 2023, 179, 308–315. [Google Scholar] [CrossRef]
- Gnanasekaran, R.; Aickareth, J.; Hawwar, M.; Sanchez, N.; Croft, J.; Zhang, J. CmPn/CmP Signaling Networks in the Maintenance of the Blood Vessel Barrier. J. Pers. Med. 2023, 13, 751. [Google Scholar] [CrossRef] [PubMed]
- Hourfar, H.; Aliakbari, F.; Aqdam, S.R.; Nayeri, Z.; Bardania, H.; Otzen, D.E.; Morshedi, D. The impact of α-synuclein aggregates on blood-brain barrier integrity in the presence of neurovascular unit cells. Int. J. Biol. Macromol. 2023, 229, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Jiang, H.; Hou, Y.; Zhang, Y.; Zhao, Q.; Zeng, Y.; Meng, X.; Wang, X. Barrier Functional Integrity Recording on bEnd.3 Vascular Endothelial Cells via Transendothelial Electrical Resistance Detection. J. Vis. Exp. 2023, 199, e65938. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Brain endothelial cell-cell junctions: How to "open" the blood brain barrier. Curr. Neuropharmacol. 2008, 6, 179–192. [Google Scholar] [CrossRef]
- Furuse, M.; Sasaki, H.; Fujimoto, K.; Tsukita, S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J. Cell Biol. 1998, 143, 391–401. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M. Occludin and claudins in tight-junction strands: Leading or supporting players? Trends Cell Biol. 1999, 9, 268–273. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Haas, M.J.; Chehade, J.M. Age-related changes in rat cerebral occludin and zonula occludens-1 (ZO-1). Mech. Ageing Dev. 2003, 124, 143–146. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Rogan, S.; Yu, A.; Vidal, L.S.; Holmes, J.; Anderson, J.M. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am. J. Physiol. Renal Physiol. 2006, 291, F1288–F1299. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Tanaka, H.; Tamura, A. The Claudins: From Tight Junctions to Biological Systems. Trends Biochem. Sci. 2019, 44, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Chiba, H.; Ichikawa-Tomikawa, N.; Imura, T.; Sugimoto, K. The region-selective regulation of endothelial claudin-5 expression and signaling in brain health and disorders. J. Cell Physiol. 2021, 236, 7134–7143. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Greene, C.; Munnich, A.; Campbell, M. The CLDN5 gene at the blood-brain barrier in health and disease. Fluids Barriers CNS 2023, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, L.A. The molecular structure of the tight junction. Adv. Drug Deliv. Rev. 2000, 41, 255–264. [Google Scholar] [CrossRef]
- Schiera, G.; Bono, E.; Raffa, M.P.; Gallo, A.; Pitarresi, G.L.; Di Liegro, I.; Savettieri, G. Synergistic effects of neurons and astrocytes on the differentiation of brain capillary endothelial cells in culture. J. Cell Mol. Med. 2003, 7, 165–170. [Google Scholar] [CrossRef]
- Schiera, G.; Sala, S.; Gallo, A.; Raffa, M.P.; Pitarresi, G.L.; Savettieri, G.; Di Liegro, I. Permeability properties of a three-cell type in vitro model of blood-brain barrier. J. Cell Mol. Med. 2005, 9, 373–379. [Google Scholar] [CrossRef]
- Bazzoni, G.; Martínez Estrada, O.; Dejana, E. Molecular structure and functional role of vascular tight junctions. Trends Cardiovasc. Med. 1999, 9, 147–152. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Engelhardt, B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012, 12, 623–635. [Google Scholar] [CrossRef]
- Kniesel, U.; Wolburg, H. Tight junctions of the blood-brain barrier. Cell Mol. Neurobiol. 2000, 20, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Risau, W.; Wolburg, H. Development of the blood-brain barrier. Trends Neurosci. 1990, 13, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Swanson, R.A. Physiologic coupling of glial glycogen metabolism to neuronal activity in brain. Can. J. Physiol. Pharmacol. 1992, 70 (Suppl. S1), S138–S144. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A.; Miller, J.J.; Payne, R.S.; Rigor, B.M. An increase in lactate output by brain tissue serves to meet the energy needs of glutamate-activated neurons. J. Neurosci. 1999, 19, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Pernet, A.; Hallett, W.A.; Bingham, E.; Marsden, P.K.; Amiel, S.A. Lactate: A preferred fuel for human brain metabolism in vivo. J. Cereb. Blood Flow Metab. 2003, 23, 658–664. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Sweet sixteen for ANLS. J. Cereb. Blood Flow Metab. 2012, 32, 1152–1166. [Google Scholar] [CrossRef]
- Dienel, G.A. The metabolic trinity, glucose-glycogen-lactate, links astrocytes and neurons in brain energetics, signaling, memory, and gene expression. Neurosci. Lett. 2015, 637, 18–25. [Google Scholar] [CrossRef]
- Proia, P.; Di Liegro, C.M.; Schiera, G.; Fricano, A.; Di Liegro, I. Lactate as a Metabolite and a Regulator in the Central Nervous System. Int. J. Mol. Sci. 2016, 17, 1450. [Google Scholar] [CrossRef]
- Barros, L.F.; Weber, B. CrossTalk proposal: An important astrocyte-to-neuron lactate shuttle couples neuronal activity to glucose utilisation in the brain. J. Physiol. 2018, 596, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Dembitskaya, Y.; Piette, C.; Perez, S.; Berry, H.; Magistretti, P.J.; Venance, L. Lactate supply overtakes glucose when neural computational and cognitive loads scale up. Proc. Natl. Acad. Sci. USA 2022, 119, e2212004119. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, M.S.; Frostig, R.D. Astrocyte-neuron lactate shuttle plays a pivotal role in sensory-based neuroprotection in a rat model of permanent middle cerebral artery occlusion. Sci. Rep. 2023, 13, 12799. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef]
- Adamsky, A.; Goshen, I. Astrocytes in Memory Function: Pioneering Findings and Future Directions. Neuroscience 2018, 370, 14–26. [Google Scholar] [CrossRef]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 2018, 174, 59–71.e14. [Google Scholar] [CrossRef]
- Harris, R.A.; Lone, A.; Lim, H.; Martinez, F.; Frame, A.K.; Scholl, T.J.; Cumming, R.C. Aerobic Glycolysis is required for spatial memory acquisition but not for memory retrieval in mice. eNeuro 2019, 6, ENEURO.0389-18.2019. [Google Scholar] [CrossRef]
- Schiera, G.; Di Liegro, C.M.; Di Liegro, I. Cell-to-Cell Communication in Learning and Memory: From Neuro- and Glio-Transmission to Information Exchange Mediated by Extracellular Vesicles. Int. J. Mol. Sci. 2019, 21, 266. [Google Scholar] [CrossRef]
- Akter, M.; Hasan, M.; Ramkrishnan, A.S.; Iqbal, Z.; Zheng, X.; Fu, Z.; Lei, Z.; Karim, A.; Li, Y. Astrocyte and L-lactate in the anterior cingulate cortex modulate schema memory and neuronal mitochondrial biogenesis. Elife 2023, 12, e85751. [Google Scholar] [CrossRef]
- Lauritzen, K.H.; Morland, C.; Puchades, M.; Holm-Hansen, S.; Hagelin, E.M.; Lauritzen, F.; Attramadal, H.; Storm-Mathisen, J.; Gjedde, A.; Bergersen, L.H. Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb. Cortex 2014, 24, 2784–2795. [Google Scholar] [CrossRef] [PubMed]
- Morland, C.; Lauritzen, K.H.; Puchades, M.; Holm-Hansen, S.; Andersson, K.; Gjedde, A.; Attramadal, H.; Storm-Mathisen, J.; Bergersen, L.H. The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: Expression and action in brain. J. Neurosci. Res. 2015, 93, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- de Castro Abrantes, H.; Briquet, M.; Schmuziger, C.; Restivo, L.; Puyal, J.; Rosenberg, N.; Rocher, A.B.; Offermanns, S.; Chatton, J.Y. The lactate receptor HCAR1 modulates neuronal network activity through the activation of Gα and Gβγ subunits. J. Neurosci. 2019, 39, 4422–4433. [Google Scholar] [CrossRef]
- Colucci, A.C.M.; Tassinari, I.D.; Loss, E.D.S.; de Fraga, L.S. History and Function of the Lactate Receptor GPR81/HCAR1 in the Brain: A Putative Therapeutic Target for the Treatment of Cerebral Ischemia. Neuroscience 2023, 526, 144–163. [Google Scholar] [CrossRef] [PubMed]
- Vela, D. Hepcidin, an emerging and important player in brain iron homeostasis. J. Transl. Med. 2018, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Yanase, K.; Uemura, N.; Chiba, Y.; Murakami, R.; Fujihara, R.; Matsumoto, K.; Shirakami, G.; Araki, N.; Ueno, M. Immunoreactivities for hepcidin, ferroportin, and hephaestin in astrocytes and choroid plexus epithelium of human brains. Neuropathology 2020, 40, 75–83. [Google Scholar] [CrossRef]
- Zhang, X.; Gou, Y.J.; Zhang, Y.; Li, J.; Han, K.; Xu, Y.; Li, H.; You, L.H.; Yu, P.; Chang, Y.Z.; et al. Hepcidin overexpression in astrocytes alters brain iron metabolism and protects against amyloid-β induced brain damage in mice. Cell Death Discov. 2020, 6, 113. [Google Scholar] [CrossRef]
- You, L.; Yu, P.P.; Dong, T.; Guo, W.; Chang, S.; Zheng, B.; Ci, Y.; Wang, F.; Yu, P.; Gao, G.; et al. Astrocyte-derived hepcidin controls iron traffic at the blood-brain-barrier via regulating ferroportin 1 of microvascular endothelial cells. Cell Death Dis. 2022, 13, 667. [Google Scholar] [CrossRef]
- Davaanyam, D.; Lee, H.; Seol, S.I.; Oh, S.A.; Kim, S.W.; Lee, J.K. HMGB1 induces hepcidin upregulation in astrocytes and causes an acute iron surge and subsequent ferroptosis in the postischemic brain. Exp. Mol. Med. 2023, 55, 2402–2416. [Google Scholar] [CrossRef]
- Gordleeva, S.Y.; Tsybina, Y.A.; Krivonosov, M.I.; Ivanchenko, M.V.; Zaikin, A.A.; Kazantsev, V.B.; Gorban, A.N. Modeling Working Memory in a Spiking Neuron Network Accompanied by Astrocytes. Front. Cell Neurosci. 2021, 15, 631485. [Google Scholar] [CrossRef]
- Linsambarth, S.; Carvajal, F.J.; Moraga-Amaro, R.; Mendez, L.; Tamburini, G.; Jimenez, I.; Verdugo, D.A.; Gómez, G.I.; Jury, N.; Martínez, P.; et al. Astroglial gliotransmitters released via Cx43 hemichannels regulate NMDAR-dependent transmission and short-term fear memory in the basolateral amygdala. FASEB J. 2022, 36, e22134. [Google Scholar] [CrossRef]
- Foubert, D.; Cookson, F.; Ruthazer, E.S. Capturing a rising star: The emerging role of astrocytes in neural circuit wiring and plasticity-lessons from the visual system. Neurophotonics 2023, 10, 044408. [Google Scholar] [CrossRef] [PubMed]
- Goenaga, J.; Araque, A.; Kofuji, P.; Herrera Moro Chao, D. Calcium signaling in astrocytes and gliotransmitter release. Front. Synaptic Neurosci. 2023, 15, 1138577. [Google Scholar] [CrossRef]
- Purushotham, S.S.; Buskila, Y. Astrocytic modulation of neuronal signalling. Front. Netw. Physiol. 2023, 3, 1205544. [Google Scholar] [CrossRef] [PubMed]
- Rasia-Filho, A.A.; Calcagnotto, M.E.; von Bohlen Und Halbach, O. Glial Cell Modulation of Dendritic Spine Structure and Synaptic Function. Adv. Neurobiol. 2023, 34, 255–310. [Google Scholar] [CrossRef] [PubMed]
- Rennels, M.L.; Gregory, T.F.; Blaumanis, O.R.; Fujimoto, K.; Grady, P.A. Evidence for a “paravascular” fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985, 326, 47–63. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Zeppenfeld, D.M.; Venkataraman, A.; Plog, B.A.; Liao, Y.; Deane, R.; Nedergaard, M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 2013, 33, 18190–18199. [Google Scholar] [CrossRef]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef]
- Maugeri, R.; Schiera, G.; Di Liegro, C.M.; Fricano, A.; Iacopino, D.G.; Di Liegro, I. Aquaporins and Brain Tumors. Int. J. Mol. Sci. 2016, 17, 1029. [Google Scholar] [CrossRef]
- de Leon, M.J.; Li, Y.; Okamura, N.; Tsui, W.H.; Saint-Louis, L.A.; Glodzik, L.; Osorio, R.S.; Fortea, J.; Butler, T.; Pirraglia, E.; et al. Cerebrospinal fluid clearance in Alzheimer disease measured with dynamic PET. J. Nucl. Med. 2017, 58, 1471–1476. [Google Scholar] [CrossRef]
- Lv, T.; Zhao, B.; Hu, Q.; Zhang, X. The Glymphatic System: A Novel Therapeutic Target for Stroke Treatment. Front. Aging Neurosci. 2021, 13, 689098. [Google Scholar] [CrossRef] [PubMed]
- Generoso, J.S.; Thorsdottir, S.; Collodel, A.; Dominguini, D.; Santo, R.R.E.; Petronilho, F.; Barichello, T.; Iovino, F. Dysfunctional Glymphatic System with Disrupted Aquaporin 4 Expression Pattern on Astrocytes Causes Bacterial Product Accumulation in the CSF during Pneumococcal Meningitis. mBio 2022, 13, e0188622. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Castro, B.; Robel, S.; Mishra, A. Astrocyte Endfeet in Brain Function and Pathology: Open Questions. Annu. Rev. Neurosci. 2023, 46, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Bloch, O.; Manley, G.T. The Role of aquaporin-4 in cerebral water transport and edema. Neurosurg. Focus 2007, 22, E3. [Google Scholar] [CrossRef]
- Valente, O.; Messina, R.; Ingravallo, G.; Bellitti, E.; Zimatore, D.S.; de Gennaro, L.; Abbrescia, P.; Pati, R.; Palazzo, C.; Nicchia, G.P.; et al. Alteration of the translational readthrough isoform AQP4ex induces redistribution and downregulation of AQP4 in human glioblastoma. Cell Mol. Life Sci. 2022, 79, 140. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.M.; Kitchen, P.; Halsey, A.; Wang, M.X.; Törnroth-Horsefield, S.; Conner, A.C.; Badaut, J.; Iliff, J.J.; Bill, R.M. Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain 2022, 145, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.Y.; Gao, M.C.; Bai, S.W.; Xie, L.; Song, Y.Y.; Ding, J.; Wu, Y.F.; Xue, C.R.; Hao, Y.; Zhang, Y.; et al. Enlarged perivascular spaces, neuroinflammation and neurological dysfunction in NMOSD patients. Front. Immunol. 2022, 13, 966781. [Google Scholar] [CrossRef]
- Mader, S.; Brimberg, L.; Vo, A.; Strohl, J.J.; Crawford, J.M.; Bonnin, A.; Carrión, J.; Campbell, D.; Huerta, T.S.; La Bella, A.; et al. In utero exposure to maternal anti-aquaporin-4 antibodies alters brain vasculature and neural dynamics in male mouse offspring. Sci. Transl. Med. 2022, 14, eabe9726. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Shen, Y.; Zhu, W.; Wang, Y.; Wang, J.; Zhang, N.; Li, C.; Xie, L.; Wu, Q. Moxibustion improves hypothalamus Aqp4 polarization in APP/PS1 mice: Evidence from spatial transcriptomics. Front. Aging Neurosci. 2023, 15, 1069155. [Google Scholar] [CrossRef]
- Mueller, S.M.; White, K.M.; Fass, S.B.; Chen, S.; Shi, Z.; Ge, X.; Engelbach, J.A.; Gaines, S.H.; Bice, A.R.; Vasek, M.J.; et al. Evaluation of gliovascular functions of Aqp4 readthrough isoforms. bioRxiv 2023. [Google Scholar] [CrossRef]
- Tanida, I.; Mizushima, N.; Kiyooka, M.; Ohsumi, M.; Ueno, T.; Ohsumi, Y.; Kominami, E. Apg7p/Cvt2p: A novel protein-activating enzyme essential for autophagy. Mol. Biol. Cell. 1999, 10, 1367–1379. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Ueno, T.; Iwata, J.; Murata, S.; Tanida, I.; Ezaki, J.; Mizushima, N.; Ohsumi, Y.; Uchiyama, Y.; et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 2005, 169, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, J.Y.; Li, Y.; Ban, M.; Sun, Q.; Wang, H.J.; Zhao, D.; Tong, P.G.; Wang, L.; Wang, K.J.; et al. Endothelial depletion of Atg7 triggers astrocyte-microvascular disassociation at blood-brain barrier. J. Cell Biol. 2023, 222, e202103098. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kelly, J.R.; Morales, J.E.; Sun, R.C.; De, A.; Burkin, D.J.; McCarty, J.H. The alpha7 integrin subunit in astrocytes promotes endothelial blood-brain barrier integrity. Development 2023, 150, dev201356. [Google Scholar] [CrossRef]
- Araya, R.; Kudo, M.; Kawano, M.; Ishii, K.; Hashikawa, T.; Iwasato, T.; Itohara, S.; Terasaki, T.; Oohira, A.; Mishina, Y.; et al. BMP signaling through BMPRIA in astrocytes is essential for proper cerebral angiogenesis and formation of the blood-brain-barrier. Mol. Cell Neurosci. 2008, 38, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Heithoff, B.P.; George, K.K.; Phares, A.N.; Zuidhoek, I.A.; Munoz-Ballester, C.; Robel, S. Astrocytes are necessary for blood-brain barrier maintenance in the adult mouse brain. Glia 2021, 69, 436–472. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Jo, J.; Wang, C.-Y.; Oh, H.; Tiffany J Choy, T.J.; Kim, K.; D’Alessandro, A.; Reshetnyak, Y.K.; Jung, S.Y.; Chen, Z.; et al. Astrocytic Slc4a4 regulates blood-brain barrier integrity in healthy and stroke brains via a NO-CCL2-CCR2 pathway. bioRxiv 2023. [Google Scholar] [CrossRef]
- Savettieri, G.; Di Liegro, I.; Catania, C.; Licata, L.; Pitarresi, G.L.; D’Agostino, S.; Schiera, G.; De Caro, V.; Giandalia, G.; Giannola, L.I.; et al. Neurons and ECM regulate occludin localization in brain endothelial cells. Neuroreport 2000, 11, 1081–1084. [Google Scholar] [CrossRef]
- Cestelli, A.; Catania, C.; D’Agostino, S.; Di Liegro, I.; Licata, L.; Schiera, G.; Pitarresi, G.L.; Savettieri, G.; De Caro, V.; Giandalia, G.; et al. Functional feature of a novel model of blood brain barrier: Studies on permeation of test compounds. J. Control. Release 2001, 76, 139–147. [Google Scholar] [CrossRef]
- Schiera, G.; Proia, P.; Alberti, C.; Mineo, M.; Savettieri, G.; Di Liegro, I. Neurons produce FGF2 and VEGF and secrete them at least in part by shedding extracellular vesicles. J. Cell Mol. Med. 2007, 11, 1384–1394. [Google Scholar] [CrossRef]
- Proia, P.; Schiera, G.; Mineo, M.; Ingrassia, A.M.; Santoro, G.; Savettieri, G.; Di Liegro, I. Astrocytes shed extracellular vesicles that contain fibroblast growth factor-2 and vascular endothelial growth factor. Int. J. Mol. Med. 2008, 21, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Tabata, H. Crosstalk between Blood Vessels and Glia during the Central Nervous System Development. Life 2022, 12, 1761. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, L.; Huo, D.; Zhi, S.; Yang, R.; Yang, B.; Xu, B.; Zhang, T.; Dai, M.; Tan, C.; et al. Astrocyte-Derived TGFβ1 Facilitates Blood-Brain Barrier Function via Non-Canonical Hedgehog Signaling in Brain Microvascular Endothelial Cells. Brain Sci. 2021, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Guérit, S.; Fidan, E.; Macas, J.; Czupalla, C.J.; Figueiredo, R.; Vijikumar, A.; Yalcin, B.H.; Thom, S.; Winter, P.; Gerhardt, H.; et al. Astrocyte-derived Wnt growth factors are required for endothelial blood-brain barrier maintenance. Prog. Neurobiol. 2021, 199, 101937. [Google Scholar] [CrossRef]
- Han, D.; Li, F.; Zhang, H.; Ji, C.; Shu, Q.; Wang, C.; Ni, H.; Zhu, Y.; Wang, S. Mesencephalic astrocyte-derived neurotrophic factor restores blood-brain barrier integrity of aged mice after ischaemic stroke/reperfusion through anti-inflammation via TLR4/MyD88/NF-κB pathway. J. Drug Target. 2022, 30, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Savidge, T.C.; Newman, P.; Pothoulakis, C.; Ruhl, A.; Neunlist, M.; Bourreille, A.; Hurst, R.; Sofroniew, M.V. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 2007, 132, 1344–1358. [Google Scholar] [CrossRef] [PubMed]
- Haseloff, R.F.; Blasig, I.E.; Bauer, H.C.; Bauer, H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol. Neurobiol. 2005, 25, 25–39. [Google Scholar] [CrossRef]
- Saunders, N.R.; Ek, C.J.; Habgood, M.D.; Dziegielewska, K.M. Barriers in the brain: A renaissance? Trends Neurosci. 2008, 31, 279–286. [Google Scholar] [CrossRef]
- Weidenfeller, C.; Svendsen, C.N.; Shusta, E.V. Differentiating embryonic neural progenitor cells induce blood-brain barrier properties. J. Neurochem. 2007, 101, 555–565. [Google Scholar] [CrossRef]
- Schiera, G.; Di Liegro, C.M.; Di Liegro, I. Extracellular Membrane Vesicles as Vehicles for Brain Cell-to-Cell Interaction in Physiological as well as Pathological Conditions. Biomed. Res. Int. 2015, 2015, 152926. [Google Scholar] [CrossRef]
- Mateescu, B.; Kowal, E.J.K.; van Balkom, B.W.M.; Bartel, S.; Bhattacharyya, S.N.; Buzás, E.I.; Buck, A.H.; de Candia, P.; Chow, F.W.N.; Das, S.; et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—An ISEV position paper. J. Extracell. Vesicles 2017, 6, 1286095. [Google Scholar] [CrossRef] [PubMed]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J. Microbe-Host Communication by Small RNAs in Extracellular Vesicles: Vehicles for Transkingdom RNA Transportation. Int. J. Mol. Sci. 2019, 20, 1487. [Google Scholar] [CrossRef]
- Court, F.A.; Hendriks, W.T.J.; MacGillavry, H.D.; Alvarez, J.; Van Minnen, J. Schwann cell to axon transfer of ribosomes: Toward a novel understanding of the role of glia in the nervous system. J. Neurosci. 2008, 28, 11024–11029. [Google Scholar] [CrossRef] [PubMed]
- Twiss, J.L.; Fainzilber, M. Ribosomes in axons–scrounging from the neighbors? Trends Cell Biol. 2009, 19, 236–243. [Google Scholar] [CrossRef]
- Sotelo, J.R.; Canclini, L.; Kun, A.; Sotelo-Silveira, J.R.; Calliari, A.; Cal, K.; Bresque, M.; Dipaolo, A.; Farias, J.; Mercer, J.A. Glia to axon RNA transfer. Dev. Neurobiol. 2014, 74, 292–302. [Google Scholar] [CrossRef]
- Karnati, H.K.; Garcia, J.H.; Tweedie, D.; Becker, R.E.; Kapogiannis, D.; Greig, N.H. Neuronal enriched extracellular vesicle proteins as biomarkers for traumatic brain injury. J. Neurotrauma 2019, 36, 975–987. [Google Scholar] [CrossRef]
- Ruan, Z.; Pathak, D.; Venkatesan, K.S.; Yoshii-Kitahara, A.; Muraoka, S.; Bhatt, N.; Takamatsu-Yukawa, K.; Hu, J.; Wang, Y.; Hersh, S.; et al. Alzheimer’s disease brain-derived extracellular vesicles spread tau pathology in interneurons. Brain 2021, 144, 288–309. [Google Scholar] [CrossRef]
- Gabrielli, M.; Prada, I.; Joshi, P.; Falcicchia, C.; D’Arrigo, G.; Rutigliano, G.; Battocchio, E.; Zenatelli, R.; Tozzi, F.; Radeghieri, A.; et al. Microglial large extracellular vesicles propagate early synaptic dysfunction in Alzheimer’s disease. Brain 2022, 145, 2849–2868. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, L.; Mao, Z.; Wang, Z.; Zhang, Z.; Li, M. Bidirectional Communication Between the Brain and Other Organs: The Role of Extracellular Vesicles. Cell Mol. Neurobiol. 2023, 43, 2675–2696. [Google Scholar] [CrossRef]
- Bakhti, M.; Winter, C.; Simons, M. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J. Biol. Chem. 2011, 286, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cesca, F.; Loers, G.; Schweizer, M.; Buck, F.; Benfenati, F.; Schachner, M.; Kleene, R. Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J. Neurosci. 2011, 31, 7275–7290. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ge, X.; Yu, J.; Han, Z.; Yin, Z.; Li, Y.; Chen, F.; Wang, H.; Zhang, J.; Lei, P. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 2018, 32, 512–528. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Li, Y.; Zhu, L.; Zhou, Z. Astrocyte-derived exosomes suppress autophagy and ameliorate neuronal damage in experimental ischemic stroke. Exp. Cell Res. 2019, 382, 111474. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, M.; Parolisi, R.; Scaroni, F.; Bonfanti, E.; Gualerzi, A.; Gabrielli, M.; Kerlero, D.R.N.; Uccelli, A.; Giussani, P.; Viani, P.; et al. Detrimental and protective action of microglial extracellular vesicles on myelin lesions: Astrocyte involvement in remyelination failure. Acta Neuropathol. 2019, 138, 987–1012. [Google Scholar] [CrossRef] [PubMed]
- Datta, C.A.; Dasgheyb, R.M.; DeVine, L.R.; Bi, H.; Cole, R.N.; Haughey, N.J. Stimulus-dependent modifications in astrocyte-derived extracellular vesicle cargo regulate neuronal excitability. Glia 2020, 68, 128–144. [Google Scholar] [CrossRef]
- Nogueras-Ortiz, C.J.; Mahairaki, V.; Delgado-Peraza, F.; Das, D.; Avgerinos, K.; Eren, E.; Hentschel, M.; Goetzl, E.J.; Mattson, M.P.; Kapogiannis, D. Astrocyte- and neuron-derived extracellular vesicles from Alzheimer’s disease patients effect complement-mediated neurotoxicity. Cells 2020, 9, 1618. [Google Scholar] [CrossRef]
- Li, Z.; Song, Y.; He, T.; Wen, R.; Li, Y.; Chen, T.; Huang, S.; Wang, Y.; Tang, Y.; Shen, F.; et al. M2 microglial small extracellular vesicles reduce glial scar formation via the miR-124/STAT3 pathway after ischemic stroke in mice. Theranostics 2021, 11, 1232–1248. [Google Scholar] [CrossRef]
- Durur, D.Y.; Tastan, B.; Ugur, T.K.; Olcum, M.; Uzuner, H.; Karakulah, G.; Yener, G.; Genc, S. Alteration of miRNAs in small neuron derived extracellular vesicles of Alzheimer’s disease patients and the effect of extracellular vesicles on microglial immune responses. J. Mol. Neurosci. 2022, 72, 1182–1194. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, H.; Zhang, M.; He, Y.; Li, X.; Xu, Y.; Liu, X. Hypoxia induced changes of exosome cargo and subsequent biological effects. Front. Immunol. 2022, 13, 824188. [Google Scholar] [CrossRef]
- Makrygianni, E.A.; Chrousos, G.P. Extracellular Vesicles and the Stress System. Neuroendocrinology 2023, 113, 120–167. [Google Scholar] [CrossRef]
- Chen, C.C.; Liu, L.; Ma, F.; Wong, C.W.; Guo, X.E.; Chacko, J.V.; Farhoodi, H.P.; Zhang, S.X.; Zimak, J.; Ségaliny, A.; et al. Elucidation of Exosome Migration across the Blood-Brain Barrier Model In Vitro. Cell Mol. Bioeng. 2016, 9, 509–529. [Google Scholar] [CrossRef] [PubMed]
- Morad, G.; Carman, C.V.; Hagedorn, E.J.; Perlin, J.R.; Zon, L.I.; Mustafaoglu, N.; Park, T.E.; Ingber, D.E.; Daisy, C.C.; Moses, M.A. Tumor-Derived Extracellular Vesicles Breach the Intact Blood-Brain Barrier via Transcytosis. ACS Nano 2019, 13, 13853–13865. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Guan, S.; Lu, P.; Li, Y.; Xu, H. Extracellular vesicles: Critical bilateral communicators in periphery-brain crosstalk in central nervous system disorders. Biomed. Pharmacother. 2023, 160, 114354. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; McKee, C.; Halassy, S.; Kojan, S.; Feinstein, D.L.; Chaudhry, G.R. Neural stem cells derived from primitive mesenchymal stem cells reversed disease symptoms and promoted neurogenesis in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Stem Cell Res. Ther. 2021, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fan, M.; Xu, J.X.; Yang, L.J.; Qi, C.C.; Xia, Q.R.; Ge, J.F. Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J. Neuroinflamm. 2022, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Yari, H.; Mikhailova, M.V.; Mardasi, M.; Jafarzadehgharehziaaddin, M.; Shahrokh, S.; Thangavelu, L.; Ahmadi, H.; Shomali, N.; Yaghoubi, Y.; Zamani, M.; et al. Emerging role of mesenchymal stromal cells (MSCs)-derived exosome in neurodegeneration-associated conditions: A groundbreaking cell-free approach. Stem Cell Res. Ther. 2022, 13, 423. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Zhang, Z.; Zhang, X.; Zhu, Y.; Zhang, C.; Bi, Y. Extracellular vesicles mediate the communication of adipose tissue with brain and promote cognitive impairment associated with insulin resistance. Cell Metab. 2022, 34, 1264–1279.e8. [Google Scholar] [CrossRef]
- Morris, G.P.; Clark, I.A.; Zinn, R.; Vissel, B. Microglia: A new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiol. Learn. Mem. 2013, 105, 40–53. [Google Scholar] [CrossRef]
- Luarte, A.; Henzi, R.; Fernández, A.; Gaete, D.; Cisternas, P.; Pizarro, M.; Batiz, L.F.; Villalobos, I.; Masalleras, M.; Vergara, R.; et al. Astrocyte-Derived Small Extracellular Vesicles Regulate Dendritic Complexity through miR-26a-5p Activity. Cells 2020, 9, 930. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Bernuz, M.; Puerta, R.; Esteban de Antonio, E.; Sánchez-López, E.; Souto, E.B.; Camins, A.; Martí, M.; Pividori, M.I.; et al. Extracellular vesicles, the emerging mirrors of brain physiopathology. Int. J. Biol. Sci. 2023, 19, 721–743. [Google Scholar] [CrossRef] [PubMed]
- Busatto, S.; Morad, G.; Guo, P.; Moses, M.A. The role of extracellular vesicles in the physiological and pathological regulation of the blood-brain barrier. FASEB Bioadv. 2021, 3, 665–675. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.; Burch, A.; Dave, K.M.; Sreeram, A.; Reynolds, M.J.; Dobbins, D.X.; Kamte, Y.S.; Zhao, W.; Sabatelle, C.; Joy, G.M.; et al. Microvesicles transfer mitochondria and increase mitochondrial function in brain endothelial cells. J. Control. Release 2021, 338, 505–526. [Google Scholar] [CrossRef]
- Ramos-Zaldívar, H.M.; Polakovicova, I.; Salas-Huenuleo, E.; Corvalán, A.H.; Kogan, M.J.; Yefi, C.P.; Andia, M.E. Extracellular vesicles through the blood-brain barrier: A review. Fluids Barriers CNS 2022, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Zhang, Y.; Paudel, K.R.; Kachelmeier, A.; Hansbro, P.M.; Shi, X. The Emerging Role of Pericyte-Derived Extracellular Vesicles in Vascular and Neurological Health. Cells 2022, 11, 3108. [Google Scholar] [CrossRef]
- López-Cepeda, L.; Castro, J.D.; Aristizábal-Pachón, A.F.; González-Giraldo, Y.; Pinzón, A.; Puentes-Rozo, P.J.; González, J. Modulation of Small RNA Signatures by Astrocytes on Early Neurodegeneration Stages; Implications for Biomarker Discovery. Life 2022, 12, 1720. [Google Scholar] [CrossRef] [PubMed]
- Fevrier, B.; Vilette, D.; Archer, F.; Loew, D.; Faigle, W.; Vidal, M.; Laude, H.; Raposo, G. Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. USA 2004, 101, 9683–9688. [Google Scholar] [CrossRef]
- Rajendran, L.; Honsho, M.; Zahn, T.R.; Keller, P.; Geiger, K.D.; Verkade, P.; Simons, K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc. Natl. Acad. Sci. USA 2006, 103, 11172–11177. [Google Scholar] [CrossRef]
- Emmanouilidou, E.; Melachroinou, K.; Roumeliotis, T.; Garbis, S.D.; Ntzouni, M.; Margaritis, L.H.; Stefanis, L.; Vekrellis, K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 2010, 30, 6838–6851. [Google Scholar] [CrossRef]
- Shi, M.; Liu, C.; Cook, T.J.; Bullock, K.M.; Zhao, Y.; Ginghina, C.; Li, Y.; Aro, P.; Dator, R.; He, C.; et al. Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014, 128, 639–650. [Google Scholar] [CrossRef]
- DeLeo, A.M.; Ikezu, T. Extracellular Vesicle Biology in Alzheimer’s Disease and Related Tauopathy. J. Neuroimmune Pharmacol. 2018, 13, 292–308. [Google Scholar] [CrossRef]
- Kaur, S.; Verma, H.; Dhiman, M.; Tell, G.; Gigli, G.L.; Janes, F.; Mantha, A.K. Brain Exosomes: Friend or Foe in Alzheimer’s Disease? Mol. Neurobiol. 2021, 58, 6610–6624. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, R.; Li, Y.; Makarava, N.; Pandit, N.P.; Molesworth, K.; Birukov, K.G.; Baskakov, I.V. Reactive astrocytes associated with prion disease impair the blood brain barrier. Neurobio. Dis. 2023, 185, 106264. [Google Scholar] [CrossRef] [PubMed]
- Soukup, J.; Moško, T.; Kereïche, S.; Holada, K. Large extracellular vesicles transfer more prions and infect cell culture better than small extracellular vesicles. Biochem. Biophys. Res. Commun. 2023, 687, 149208. [Google Scholar] [CrossRef]
- Stewart, P.A.; Wiley, M.J. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: A study using quail–chick transplantation chimeras. Dev. Biol. 1981, 84, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Joó, F. The cerebral microvessels in culture, an update. J. Neurochem. 1992, 58, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, T.; Tsuji, A. Drug delivery to the brain utilizing blood–brain barrier transport systems. J. Control. Release 1994, 29, 163–169. [Google Scholar] [CrossRef]
- Wade, L.A.; Katzman, R. Synthetic amino acids and the nature of L-DOPA transport at the blood-brain barrier. J. Neurochem. 1975, 25, 837–842. [Google Scholar] [CrossRef]
- Pardridge, W.M. CNS drug design based on principles of blood-brain barrier transport. J. Neurochem. 1998, 70, 1781–1792. [Google Scholar] [CrossRef]
- Dehouck, M.P.; Méresse, S.; Dehouck, B.; Fruchart, J.C.; Cecchelli, R. In vitro reconstituted blood–brain barrier. J. Control. Release 1992, 21, 81–92. [Google Scholar] [CrossRef]
- Gomes, P.; Soares-da-Silva, P. Interaction between L-DOPA and 3-O-methyl-L-DOPA for transport in immortalised rat capillary cerebral endothelial cells. Neuropharmacology 1999, 38, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Barberio, C.; Withers, A.; Mishra, Y.; Couraud, P.O.; Romero, I.A.; Weksler, B.; Owens, R.M. A human-derived neurovascular unit in vitro model to study the effects of cellular cross-talk and soluble factors on barrier integrity. Front. Cell Neurosci. 2022, 16, 1065193. [Google Scholar] [CrossRef] [PubMed]
- Ledwig, V.; Reichl, S. Isolation and Cultivation of Porcine Endothelial Cells, Pericytes and Astrocytes to Develop an In Vitro Blood-Brain Barrier Model for Drug Permeation Testing. Pharmaceutics 2023, 15, 1688. [Google Scholar] [CrossRef] [PubMed]
- Cucullo, L.; McAllister, M.S.; Kight, K.; Krizanac-Bengez, L.; Marroni, M.; Mayberg, M.R.; Stanness, K.A.; Janigro, D. A new dynamic in vitro model for the multidimensional study of astrocyte-endothelial cell interactions at the blood-brain barrier. Brain Res. 2002, 951, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Nakagawa, S.; Morofuji, Y.; Tóth, A.E.; Vastag, M.; Aruga, J.; Niwa, M.; Deli, M.A. Characterization of a Primate Blood-Brain Barrier Co-Culture Model Prepared from Primary Brain Endothelial Cells, Pericytes and Astrocytes. Pharmaceutics 2021, 13, 1484. [Google Scholar] [CrossRef]
- Sharma, S.; Zhang, Y.; Akter, K.A.; Nozohouri, S.; Archie, S.R.; Patel, D.; Villalba, H.; Abbruscato, T. Permeability of Metformin across an In Vitro Blood-Brain Barrier Model during Normoxia and Oxygen-Glucose Deprivation Conditions: Role of Organic Cation Transporters (Octs). Pharmaceutics 2023, 15, 1357. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Humle, N.; Hede, E.; Moos, T.; Burkhart, A.; Thomsen, L.B. The blood-brain barrier studied in vitro across species. PLoS ONE 2021, 16, e0236770. [Google Scholar] [CrossRef]
- Gonzales-Aloy, E.; Ahmed-Cox, A.; Tsoli, M.; Ziegler, D.S.; Kavallaris, M. From cells to organoids: The evolution of blood-brain barrier technology for modelling drug delivery in brain cancer. Adv. Drug Deliv. Rev. 2023, 196, 114777. [Google Scholar] [CrossRef]
- Park, J.S.; Choe, K.; Khan, A.; Jo, M.H.; Park, H.Y.; Kang, M.H.; Park, T.J.; Kim, M.O. Establishing Co-Culture Blood-Brain Barrier Models for Different Neurodegeneration Conditions to Understand Its Effect on BBB Integrity. Int. J. Mol. Sci. 2023, 24, 5283. [Google Scholar] [CrossRef]
- de Rus Jacquet, A.; Alpaugh, M.; Denis, H.L.; Tancredi, J.L.; Boutin, M.; Decaestecker, J.; Beauparlant, C.; Herrmann, L.; Saint-Pierre, M.; Parent, M.; et al. The contribution of inflammatory astrocytes to BBB impairments in a brain-chip model of Parkinson’s disease. Nat. Commun. 2023, 14, 3651. [Google Scholar] [CrossRef]
- Stanton, A.E.; Bubnys, A.; Agbas, E.; James, B.; Park, D.S.; Jiang, A.; Pinals, R.L.; Truong, N.; Loon, A.; Staab, C.; et al. Engineered 3D Immuno-Glial-Neurovascular Human Brain Model. bioRxiv 2023. [Google Scholar] [CrossRef]
- Bolden, C.T.; Skibber, M.A.; Olson, S.D.; Zamorano Rojas, M.; Milewicz, S.; Gill, B.S.; Cox, C.S., Jr. Validation and characterization of a novel blood-brain barrier platform for investigating traumatic brain injury. Sci. Rep. 2023, 13, 16150. [Google Scholar] [CrossRef] [PubMed]
- Potjewyd, G.; Kellett, K.A.B.; Hooper, N.M. 3D hydrogel models of the neurovascular unit to investigate blood-brain barrier dysfunction. Neuronal Signal. 2021, 5, NS20210027. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, H.; Shi, Y.; Xiao, Y.; Yin, Y.; Jiang, B.; Ren, H.; Chen, W.; Xue, Q.; Xu, X. Reconstituting neurovascular unit with primary neural stem cells and brain microvascular endothelial cells in three-dimensional matrix. Brain. Pathol. 2021, 31, e12940. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, Y.; Zhang, Z.; Liu, Q.; Wang, M.; Li, W.; Yang, G.Y. Engineering Neurovascular Unit and Blood-Brain Barrier for Ischemic Stroke Modeling. Adv. Healthc. Mater. 2023, 12, e2202638. [Google Scholar] [CrossRef] [PubMed]
- Wevers, N.R.; Nair, A.L.; Fowke, T.M.; Pontier, M.; Kasi, D.G.; Spijkers, X.; Hallard, C.; Rabussier, G.; van Vught, R.; Vulto, P.; et al. Modeling ischemic stroke in a triculture neurovascular unit on-a-chip. Fluids Barriers CNS 2021, 18, 59. [Google Scholar] [CrossRef]
- Spitzer, D.; Guérit, S.; Puetz, T.; Khel, M.I.; Armbrust, M.; Dunst, M.; Macas, J.; Zinke, J.; Devraj, G.; Jia, X.; et al. Profiling the neurovascular unit unveils detrimental effects of osteopontin on the blood-brain barrier in acute ischemic stroke. Acta Neuropathol. 2022, 144, 305–337. [Google Scholar] [CrossRef]
- Stafford, P.; Mitra, S.; Debot, M.; Lutz, P.; Stem, A.; Hadley, J.; Hom, P.; Schaid, T.R.; Cohen, M.J. Astrocytes and pericytes attenuate severely injured patient plasma mediated expression of tight junction proteins in endothelial cells. PLoS ONE 2022, 17, e0270817. [Google Scholar] [CrossRef]
- Floryanzia, S.D.; Nance, E. Applications and Considerations for Microfluidic Systems to Model the Blood-Brain Barrier. ACS Appl. Bio. Mater. 2023, 6, 3617–3632. [Google Scholar] [CrossRef]
- Galpayage Dona, K.N.U.; Ramirez, S.H.; Andrews, A.M. A Next-Generation 3D Tissue-Engineered Model of the Human Brain Microvasculature to Study the Blood-Brain Barrier. Bioengineering 2023, 10, 817. [Google Scholar] [CrossRef]
- Nakayama-Kitamura, K.; Shigemoto-Mogami, Y.; Toyoda, H.; Mihara, I.; Moriguchi, H.; Naraoka, H.; Furihata, T.; Ishida, S.; Sato, K. Usefulness of a humanized tricellular static transwell blood-brain barrier model as a microphysiological system for drug development applications—A case study based on the benchmark evaluations of blood-brain barrier microphysiological system. Regen Ther. 2023, 22, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Palma-Florez, S.; López-Canosa, A.; Moralez-Zavala, F.; Castaño, O.; Kogan, M.J.; Samitier, J.; Lagunas, A.; Mir, M. BBB-on-a-chip with integrated micro-TEER for permeability evaluation of multi-functionalized gold nanorods against Alzheimer’s disease. J. Nanobiotechnol. 2023, 21, 115. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Cucullo, L. Evaluation of Barrier Integrity Using a Two-Layered Microfluidic Device Mimicking the Blood-Brain Barrier. Methods Mol. Biol. 2024, 2711, 77–88. [Google Scholar] [CrossRef]
- Xiao, T.; Pan, M.; Wang, Y.; Huang, Y.; Tsunoda, M.; Zhang, Y.; Wang, R.; Hu, W.; Yang, H.; Li, L.S.; et al. In vitro bloodbrain barrier permeability study of four main active ingredients from Alpiniae oxyphyllae fructus. J. Pharm. Biomed. Anal. 2023, 235, 115637. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Pekrun, K.; Khan, T.A.; Zhang, F.; Paşca, S.P.; Kay, M.A. Selection of rAAV vectors that cross the human blood-brain barrier and target the central nervous system using a transwell model. Mol. Ther. Methods Clin. Dev. 2022, 27, 73–88. [Google Scholar] [CrossRef]
- Lauranzano, E.; Rasile, M.; Matteoli, M. Integrating Primary Astrocytes in a Microfluidic Model of the Blood-Brain Barrier. Methods Mol. Biol. 2022, 2492, 225–240. [Google Scholar] [CrossRef]
- Matsuki, H.; Mandai, S.; Shiwaku, H.; Koide, T.; Takahashi, N.; Yanagi, T.; Inaba, S.; Ida, S.; Fujiki, T.; Mori, Y.; et al. Chronic kidney disease causes blood-brain barrier breakdown via urea-activated matrix metalloproteinase-2 and insolubility of tau protein. Aging 2023, 15, 10972–10995. [Google Scholar] [CrossRef]
- Claeys, W.; Van Hoecke, L.; Lefere, S.; Geerts, A.; Verhelst, X.; Van Vlierberghe, H.; Degroote, H.; Devisscher, L.; Vandenbroucke, R.E.; Van Steenkiste, C. The neurogliovascular unit in hepatic encephalopathy. JHEP Rep. 2021, 3, 100352. [Google Scholar] [CrossRef]
- Garvin, J.; Semenikhina, M.; Liu, Q.; Rarick, K.; Isaeva, E.; Levchenko, V.; Staruschenko, A.; Palygin, O.; Harder, D.; Cohen, S. Astrocytic responses to high glucose impair barrier formation in cerebral microvessel endothelial cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 322, R571–R580. [Google Scholar] [CrossRef]
- Sun, N.; Hu, H.; Wang, F.; Li, L.; Zhu, W.; Shen, Y.; Xiu, J.; Xu, Q. Antibiotic-induced microbiome depletion in adult mice disrupts blood-brain barrier and facilitates brain infiltration of monocytes after bone-marrow transplantation. Brain Behav. Immun. 2021, 92, 102–114. [Google Scholar] [CrossRef]
- Ju, J.; Su, Y.; Zhou, Y.; Wei, H.; Xu, Q. The SARS-CoV-2 envelope protein disrupts barrier function in an in vitro human blood-brain barrier model. Front. Cell Neurosci. 2022, 16, 897564. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Parra, H.; Reyes-Hernández, O.D.; Figueroa-González, G.; González-Del Carmen, M.; González-Torres, M.; Peña-Corona, S.I.; Florán, B.; Cortés, H.; Leyva-Gómez, G. Alteration of the blood-brain barrier by COVID-19 and its implication in the permeation of drugs into the brain. Front. Cell Neurosci. 2023, 17, 1125109. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, J.; Ji, W.; Xu, L.; Xie, Y.; He, S.; Lai, C.; Hou, K.; Li, Z.; Chen, G.; et al. High-titer AAV disrupts cerebrovascular integrity and induces lymphocyte infiltration in adult mouse brain. Mol. Ther. Methods Clin. Dev. 2023, 31, 101102. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Aenlle, K.K.; Cohen, J.; Mathew, A.; Isler, D.; Pangeni, R.P.; Nathanson, L.; Theoharides, T.C.; Klimas, N.G. COVID-19 and Long COVID: Disruption of the Neurovascular Unit, Blood-Brain Barrier, and Tight Junctions. Neuroscientist 2023, 11, 10738584231194927. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Pant, P.; Bhagat, R.; Seth, P. Zika virus E protein modulates functions of human brain microvascular endothelial cells and astrocytes: Implications on blood-brain barrier properties. Front. Cell Neurosci. 2023, 17, 1173120. [Google Scholar] [CrossRef] [PubMed]
- Segura-Collar, B.; Mata-Martínez, P.; Hernández-Laín, A.; Sánchez-Gómez, P.; Gargini, R. Blood-Brain Barrier Disruption: A Common Driver of Central Nervous System Diseases. Neuroscientist 2022, 28, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Burn, L.; Gutowski, N.; Whatmore, J.; Giamas, G.; Pranjol, M.Z.I. The role of astrocytes in brain metastasis at the interface of circulating tumour cells and the blood brain barrier. Front. Biosci. 2021, 26, 590–601. [Google Scholar] [CrossRef]
- Wang, P.; Wu, Y.; Chen, W.; Zhang, M.; Qin, J. Malignant Melanoma-Derived Exosomes Induce Endothelial Damage and Glial Activation on a Human BBB Chip Model. Biosensors 2022, 12, 89. [Google Scholar] [CrossRef]
- Li, X.; Li, L.; Zhou, K.; Zhang, H.; Maalim, A.A.; Chen, X.; He, X.; Ding, X.; Xu, C.; Wang, Y. Glioma Shapes Blood-Brain Barrier Integrity and Remodels the Tumor Microenvironment: Links with Clinical Features and Prognosis. J. Clin. Med. 2022, 11, 5863. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Li, C.; Li, X.; Chu, Y.; Guo, Q.; Zhang, Y.; Xia, W.; Liu, P.; Chen, H.; et al. Microenvironment-tailored micelles restrain carcinoma-astrocyte crosstalk for brain metastasis. J. Control. Release 2022, 349, 520–532. [Google Scholar] [CrossRef]
- Michinaga, S.; Koyama, Y. Pathophysiological Responses and Roles of Astrocytes in Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 6418. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Song, L.; Ding, Z.; Wang, Q.; Yan, Y.; Huang, J.; Ma, C. The Important Double-Edged Role of Astrocytes in Neurovascular Unit after Ischemic Stroke. Front. Aging Neurosci. 2022, 14, 833431. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wang, J.; Chen, S.; Liu, G.; Wu, C.; Lv, Q.; He, X.; Bai, X.; Huang, W.; Liao, H. Astrocytic p75NTR expression provoked by ischemic stroke exacerbates the blood-brain barrier disruption. Glia 2022, 70, 892–912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, X.; Zhang, Y.; Zeng, F.; Yan, S.; Chen, Y.; Li, Z.; Zhou, D.; Liu, L. The role of circadian clock in astrocytes: From cellular functions to ischemic stroke therapeutic targets. Front. Neurosci. 2022, 16, 1013027. [Google Scholar] [CrossRef] [PubMed]
- Michinaga, S.; Hishinuma, S.; Koyama, Y. Roles of Astrocytic Endothelin ETB Receptor in Traumatic Brain Injury. Cells 2023, 12, 719. [Google Scholar] [CrossRef] [PubMed]
- Reiss, Y.; Bauer, S.; David, B.; Devraj, K.; Fidan, E.; Hattingen, E.; Liebner, S.; Melzer, N.; Meuth, S.G.; Rosenow, F.; et al. The neurovasculature as a target in temporal lobe epilepsy. Brain Pathol. 2023, 33, e13147. [Google Scholar] [CrossRef]
- Sun, J.; Wu, J.; Hua, F.; Chen, Y.; Zhan, F.; Xu, G. Sleep Deprivation Induces Cognitive Impairment by Increasing Blood-Brain Barrier Permeability via CD44. Front. Neurol. 2020, 11, 563916. [Google Scholar] [CrossRef]
- Kyrtata, N.; Emsley, H.C.A.; Sparasci, O.; Parkes, L.M.; Dickie, B.R. A Systematic Review of Glucose Transport Alterations in Alzheimer’s Disease. Front. Neurosci. 2021, 15, 626636. [Google Scholar] [CrossRef]
- Canepa, E.; Fossati, S. Impact of Tau on Neurovascular Pathology in Alzheimer’s Disease. Front. Neurol. 2021, 11, 573324. [Google Scholar] [CrossRef]
- Zenaro, E.; Piacentino, G.; Constantin, G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 2017, 107, 41–56. [Google Scholar] [CrossRef]
- Soto-Rojas, L.O.; Campa-Córdoba, B.B.; Harrington, C.R.; Salas-Casas, A.; Hernandes-Alejandro, M.; Villanueva-Fierro, I.; Bravo-Muñoz, M.; Garcés-Ramírez, L.; De La Cruz-López, F.; Ontiveros-Torres, M.Á.; et al. Insoluble Vascular Amyloid Deposits Trigger Disruption of the Neurovascular Unit in Alzheimer’s Disease Brains. Int. J. Mol. Sci. 2021, 22, 3654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; O’Callaghan, P.; Li, H.; Tan, Y.; Zhang, G.; Barash, U.; Wang, X.; Lannfelt, L.; Vlodavsky, I.; Lindahl, U.; et al. Heparanase overexpression impedes perivascular clearance of amyloid-β from murine brain: Relevance to Alzheimer’s disease. Acta Neuropathol. Commun. 2021, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Yue, Q.; Zhou, X.; Zhang, Z.; Hoi, M.P.M. Murine Beta-Amyloid (1-42) Oligomers Disrupt Endothelial Barrier Integrity and VEGFR Signaling via Activating Astrocytes to Release Deleterious Soluble Factors. Int. J. Mol. Sci. 2022, 23, 1878. [Google Scholar] [CrossRef]
- Nakamura, T.; Hashita, T.; Chen, Y.; Gao, Y.; Sun, Y.; Islam, S.; Sato, H.; Shibuya, Y.; Zou, K.; Matsunaga, T.; et al. Aβ42 treatment of the brain side reduced the level of flotillin from endothelial cells on the blood side via FGF-2 signaling in a blood-brain barrier model. Mol. Brain. 2023, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.M.; Özkan, E.; Gürsoy-Özdemir, Y. The role of extracellular matrix alterations in mediating astrocyte damage and pericyte dysfunction in Alzheimer’s disease: A comprehensive review. Eur. J. Neurosci. 2022, 56, 5453–5475. [Google Scholar] [CrossRef]
- Yue, Q.; Hoi, M.P.M. Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer’s disease. Neural Regen Res. 2023, 18, 1890–1902. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, W.; Tan, Z.; Zhang, L.; Dong, Z.; Cui, W.; Zhao, K.; Wang, H.; Jing, H.; Cao, R. A role of low-density lipoprotein receptor-related protein 4 (LRP4) in astrocytic Aβ clearance. J Neurosci. 2020, 40, 5347–5361. [Google Scholar] [CrossRef]
- Duong, M.T.; Nasrallah, I.M.; Wolk, D.A.; Chang, C.C.Y.; Chang, T.-Y. Cholesterol, Atherosclerosis, and APOE in Vascular Contributions to Cognitive Impairment and Dementia (VCID): Potential Mechanisms and Therapy. Front. Aging Neurosci. 2021, 13, 647990. [Google Scholar] [CrossRef]
- Jackson, R.J.; Meltzer, J.C.; Nguyen, H.; Commins, C.; Bennett, R.E.; Hudry, E.; Hyman, B.T. APOE4 derived from astrocytes leads to blood-brain barrier impairment. Brain 2022, 145, 3582–3593. [Google Scholar] [CrossRef]
- Zenaro, E.; Pietronigro, E.; Della Bianca, V.; Piacentino, G.; Marongiu, L.; Budui, S.; Turano, E.; Rossi, B.; Angiari, S.; Dusi, S.; et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 2015, 21, 880–886. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Merlo, S.; Fagone, E.; Fruciano, M.; Sano, Y.; Kanda, T.; Sortino, M.A. Reciprocal Interplay Between Astrocytes and CD4+ Cells Affects Blood-Brain Barrier and Neuronal Function in Response to β Amyloid. Front. Mol. Neurosci. 2020, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Troili, F.; Cipollini, V.; Moci, M.; Morena, E.; Palotai, M.; Rinaldi, V.; Romano, C.; Ristori, G.; Giubilei, F.; Salvetti, M.; et al. Perivascular Unit: This Must Be the Place. The Anatomical Crossroad between the Immune, Vascular and Nervous System. Front. Neuroanat. 2020, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Toga, A.W.; Zlokovic, B.V. Blood-Brain Barrier Permeability and Gadolinium: Benefits and Potential Pitfalls in Research. JAMA Neurol. 2016, 73, 13–14. [Google Scholar] [CrossRef]
- Sanmarco, L.M.; Polonio, C.M.; Wheeler, M.A.; Quintana, F.J. Functional immune cell-astrocyte interactions. J. Exp. Med. 2021, 218, e20202715. [Google Scholar] [CrossRef]
- Kunkl, M.; Amormino, C.; Tedeschi, V.; Fiorillo, M.T.; Tuosto, L. Astrocytes and Inflammatory T Helper Cells: A Dangerous Liaison in Multiple Sclerosis. Front. Immunol. 2022, 13, 824411. [Google Scholar] [CrossRef]
- Cashion, J.M.; Young, K.M.; Sutherland, B.A. How does neurovascular unit dysfunction contribute to multiple sclerosis? Neurobiol. Dis. 2023, 178, 106028. [Google Scholar] [CrossRef] [PubMed]
- Argaw, A.T.; Asp, L.; Zhang, J.; Navrazhina, K.; Pham, T.; Mariani, J.N.; Mahase, S.; Dutta, D.J.; Seto, J.; Kramer, E.G.; et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Investig. 2012, 122, 2454–2468. [Google Scholar] [CrossRef]
- Chapouly, C.; Tadesse Argaw, A.; Horng, S.; Castro, K.; Zhang, J.; Asp, L.; Loo, H.; Laitman, B.M.; Mariani, J.N.; Straus Farber, R.; et al. Astrocytic TYMP and VEGFA Drive Blood-Brain Barrier Opening in Inflammatory Central Nervous System Lesions. Brain 2015, 138, 1548–1567. [Google Scholar] [CrossRef]
- Absinta, M.; Maric, D.; Gharagozloo, M.; Garton, T.; Smith, M.D.; Jin, J.; Fitzgerald, K.C.; Song, A.; Liu, P.; Lin, J.P.; et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature 2021, 597, 709–714. [Google Scholar] [CrossRef]
- Moll, N.M.; Rietsch, A.M.; Thomas, S.; Ransohoff, A.J.; Lee, J.C.; Fox, R.; Chang, A.; Ransohoff, R.M.; Fisher, E. Multiple sclerosis normal-appearing white matter: Pathology-imaging correlations. Ann. Neurol. 2011, 70, 764–773. [Google Scholar] [CrossRef]
- Dolcetti, E.; Bruno, A.; Guadalupi, L.; Rizzo, F.R.; Musella, A.; Gentile, A.; De Vito, F.; Caioli, S.; Bullitta, S.; Fresegna, D.; et al. Emerging Role of Extracellular Vesicles in the Pathophysiology of Multiple Sclerosis. Int. J. Mol. Sci. 2020, 21, 7336. [Google Scholar] [CrossRef] [PubMed]
- Maciak, K.; Dziedzic, A.; Miller, E.; Saluk-Bijak, J. miR-155 as an Important Regulator of Multiple Sclerosis Pathogenesis. A Review. Int. J. Mol. Sci. 2021, 22, 4332. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ding, J.; Wang, X.; Gu, C.; He, Y.; Li, Y.; Fan, H.; Xie, Q.; Qi, X.; Wang, Z.; et al. Transfer of neuron-derived α-synuclein to astrocytes induces neuroinflammation and blood-brain barrier damage after methamphetamine exposure: Involving the regulation of nuclear receptor-associated protein 1. Brain Behav. Immun. 2022, 106, 247–261. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, M.; Park, W.; Lim, J.S.; Lee, E.; Cha, H.; Ahn, J.S.; Kim, J.H.; Hong, S.H.; Park, J.E.; et al. Upregulation of AQP4 Improves Blood-Brain Barrier Integrity and Perihematomal Edema Following Intracerebral Hemorrhage. Neurotherapeutics 2021, 18, 2692–2706. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.; Wrzos, C.; Haberl, M.; Weil, M.T.; Gao, M.; Möbius, W.; Odoardi, F.; Thal, D.R.; Chang, M.; Opdenakker, G.; et al. Blood-brain barrier resealing in neuromyelitis optica occurs independently of astrocyte regeneration. Clin. Investig. 2021, 131, e141694. [Google Scholar] [CrossRef]
- Mills, W.A., 3rd; Woo, A.M.; Jiang, S.; Martin, J.; Surendran, D.; Bergstresser, M.; Kimbrough, I.F.; Eyo, U.B.; Sofroniew, M.V.; Sontheimer, H. Astrocyte plasticity in mice ensures continued endfoot coverage of cerebral blood vessels following injury and declines with age. Nat. Commun. 2022, 13, 1794. [Google Scholar] [CrossRef]
- Preininger, M.K.; Kaufer, D. Blood-Brain Barrier Dysfunction and Astrocyte Senescence as Reciprocal Drivers of Neuropathology in Aging. Int. J. Mol. Sci. 2022, 23, 6217. [Google Scholar] [CrossRef]
- Lin, S.; Zhou, F.Q.; Cheng, J.B.; Sun, X.D.; He, G.Q. Editorial: The role of astrocyte in vascular aging. Front. Aging Neurosci. 2022, 14, 961288. [Google Scholar] [CrossRef]
- Knopp, R.C.; Erickson, M.A.; Rhea, E.M.; Reed, M.J.; Banks, W.A. Cellular senescence and the blood-brain barrier: Implications for aging and age-related diseases. Exp. Biol. Med. 2023, 248, 399–411. [Google Scholar] [CrossRef]
- Siqueira, M.; Araujo, A.P.B.; Gomes, F.C.A.; Stipursky, J. Ethanol Gestational Exposure Impairs Vascular Development and Endothelial Potential to Control BBB-Associated Astrocyte Function in the Developing Cerebral Cortex. Mol. Neurobiol. 2021, 58, 1755–1768. [Google Scholar] [CrossRef]
- Archie, S.R.; Sifat, A.E.; Zhang, Y.; Villalba, H.; Sharma, S.; Nozohouri, S.; Abbruscato, T.J. Maternal e-cigarette use can disrupt postnatal blood-brain barrier (BBB) integrity and deteriorates motor, learning and memory function: Influence of sex and age. Fluids Barriers CNS 2023, 20, 17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiera, G.; Di Liegro, C.M.; Schirò, G.; Sorbello, G.; Di Liegro, I. Involvement of Astrocytes in the Formation, Maintenance, and Function of the Blood–Brain Barrier. Cells 2024, 13, 150. https://doi.org/10.3390/cells13020150
Schiera G, Di Liegro CM, Schirò G, Sorbello G, Di Liegro I. Involvement of Astrocytes in the Formation, Maintenance, and Function of the Blood–Brain Barrier. Cells. 2024; 13(2):150. https://doi.org/10.3390/cells13020150
Chicago/Turabian StyleSchiera, Gabriella, Carlo Maria Di Liegro, Giuseppe Schirò, Gabriele Sorbello, and Italia Di Liegro. 2024. "Involvement of Astrocytes in the Formation, Maintenance, and Function of the Blood–Brain Barrier" Cells 13, no. 2: 150. https://doi.org/10.3390/cells13020150
APA StyleSchiera, G., Di Liegro, C. M., Schirò, G., Sorbello, G., & Di Liegro, I. (2024). Involvement of Astrocytes in the Formation, Maintenance, and Function of the Blood–Brain Barrier. Cells, 13(2), 150. https://doi.org/10.3390/cells13020150






