Chemistry of Hydrogen Sulfide—Pathological and Physiological Functions in Mammalian Cells
Abstract
:1. Introduction
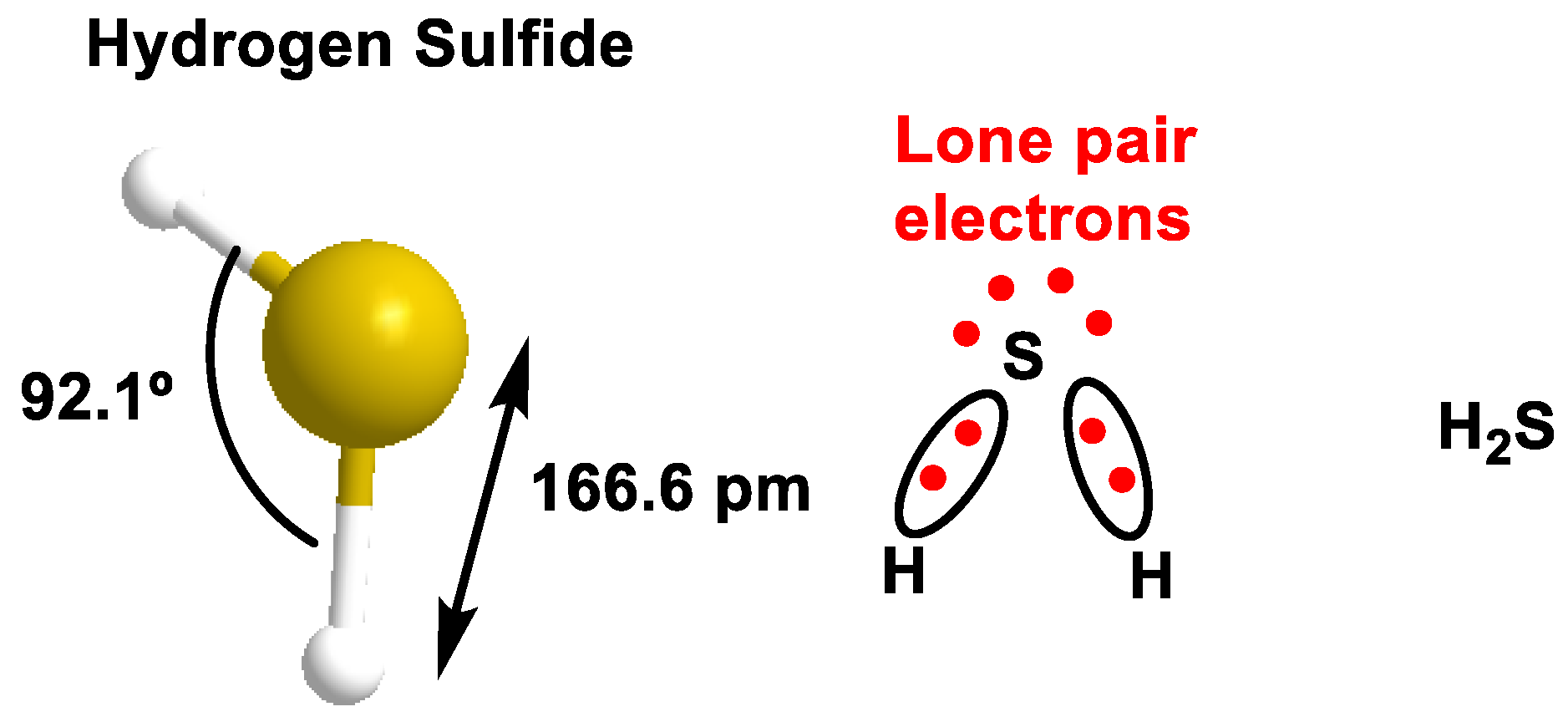
2. H2S Chemistry
3. Pathways of H2S Production
3.1. Environmental (Anthropogenic and Non-Anthropogenic) Sources
3.2. Endogenous Sources of H2S in Mammalian Cells
- (i)
- H2S is released immediately after its production by enzymes;
- (ii)
- H2S is stored and released in response to a physiological signal.
- Acidic conditions release H2S from acid-labile sulfur. Acid-labile sulfur is mainly sulfur atoms in iron–sulfur complexes, which play a key role in a wide range of redox reactions in respiratory chain enzymes in mitochondria. The critical pH below which H2S is released from labile sulfur to acid is 5.4 [36]. Because the mitochondrial pH is between 7 and 8, which is higher than the critical pH, acid-labile sulfur may not release H2S under physiological conditions.
- Another form of storage is called sulfane sulfur, which is localized in the cytoplasm, releasing H2S under reducing conditions [37]. This includes compounds such as polysulfides, thiosulfates, polyethionates, thiosulfonates, bisorganyl-polysulfanes or monoarylthiosulfonates and elemental sulfur. Sulfane sulfur compounds such as polysulfides release H2S under reducing conditions, suggesting that the cellular redox state is important in regulating their bioavailability [38]. The activity of reducing substances increases under alkaline conditions, and H2S can be released from sulfane sulfur when intracellular conditions become alkaline. In addition, free H2S can be incorporated into proteins as sulfane sulfur, where its divalent sulfur form binds only elemental sulfur, persulfides and polysulfides [39].
- (1)
- Hydrolysis of cysteine to form serine and H2S.
- (2)
- Condensation of cysteine and homocysteine to generate cystathionine and H2S.
- (3)
- Condensation of two cysteine molecules to form lanthionine and H2S [48].
3.3. Microbial Synthesis of H2S
3.4. Production in Laboratory or Industrially
4. H2S Consumption Routes
4.1. Mitochondrial Pathway of Sulfur Oxidation
4.2. Oxidation of Sulfides by Hemoproteins
5. H2S Reactivity
5.1. In Aqueous Solution
5.2. Reaction with Oxygen
5.3. Basic Chemical Reactivity of H2S
5.4. Reaction with Disulfide
6. Biological Functions of H2S
6.1. Cardiovascular System
6.2. Gastrointestinal System
6.3. Respiratory System
6.4. Nervous System
6.5. Endocrine System
6.6. Visual System
6.7. Age-Related Diseases
6.8. H2S in Cancer
6.9. H2S and Antimicrobial Resistance
7. Mechanisms of Action of H2S and Molecular Targets
7.1. Effect of H2S on Ion Channels
7.2. Direct Reaction of H2S with ROS and RNS
7.2.1. Non-Radical Species (Two-Electron Oxidation)
7.2.2. Radical Species (One-Electron Oxidation)
8. Persulfidation or S-Sulfhydration of Protein Thiols
9. Detection of H2S
9.1. Lead Acetate
9.2. Methylene Blue Method
9.3. Monobromobimane Derivatization
9.4. Methods Based on the Reducing Capacity of H2S
9.5. Methods Based on Nucleophilicity
9.6. Methods Based on the Ability to Bind Metal Cations
10. H2S Donors
10.1. Sulfur Salts
10.2. Natural Donors
10.3. Synthetic H2S Donors
10.4. H2S and •NO Hybrid Synthetic Donor
10.5. Mitochondrial H2S Donors
11. Hydrogen Sulfide as a Therapeutic Agent
11.1. Garlic Source of H2S Supplements
11.2. Sulfur Drugs and Their Therapeutic Potential
11.3. Balneotherapy and H2S
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef] [PubMed]
- Mathai, J.C.; Missner, A.; Kügler, P.; Saparov, S.M.; Zeidel, M.L.; Lee, J.K.; Pohl, P. No facilitator required for membrane transport of hydrogen sulfide. Proc. Natl. Acad. Sci. USA 2009, 106, 16633–16638. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Winyard, P.G. Hydrogen sulfide and inflammation: The good, the bad, the ugly and the promising. Expert. Rev. Clin. Pharmacol. 2011, 4, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Shibuya, N.; Kimura, Y. Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antioxid. Redox Signal. 2012, 17, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rose, P.; Moore, P.K. Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, M.-G.C. “GAS!”—The Story of the Special Brigade; Andrews UK Limited: Luton, UK, 2012. [Google Scholar]
- Yang, G.; Wang, R. H2S and Blood Vessels: An Overview. Handb. Exp. Pharmacol. 2015, 230, 85–110. [Google Scholar] [CrossRef]
- Rubio-Ruiz, M.E.; Pérez-Torres, I.; Soto, M.E.; Pastelín, G.; Guarner-Lans, V. Aging in blood vessels. Medicinal agents FOR systemic arterial hypertension in the elderly. Ageing Res. Rev. 2014, 18, 132–147. [Google Scholar] [CrossRef]
- Beauchamp, R.O., Jr.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A. A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol. 1984, 13, 25–97. [Google Scholar] [CrossRef]
- Reiffenstein, R.J.; Hulbert, W.C.; Roth, S.H. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Marshall, T.; Dorman, D.; Gardner, D.; Adeshina, F. Provisional advisory levels (PALs) for hydrogen sulfide (H2S). Inhal. Toxicol. 2009, 21, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef] [PubMed]
- Riahi, S.; Rowley, C.N. Why can hydrogen sulfide permeate cell membranes? J. Am. Chem. Soc. 2014, 136, 15111–15113. [Google Scholar] [CrossRef] [PubMed]
- Millero, F.J. The thermodynamics and kinetics of the hydrogen sulfide system in natural waters. Mar. Chem. 1986, 18, 121–147. [Google Scholar] [CrossRef]
- Fogg, P.G. Hydrogen Sulfide, Deuterium Sulfide & Hydrogen Selenide; Elsevier: Amsterdam, The Netherlands, 2013; Volume 32. [Google Scholar]
- Haouzi, P. Ventilatory and metabolic effects of exogenous hydrogen sulfide. Respir. Physiol. Neurobiol. 2012, 184, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lancaster, J.R. Chemical foundations of hydrogen sulfide biology. Nitric Oxide 2013, 35, 21–34. [Google Scholar] [CrossRef]
- Kimura, H. Production and physiological effects of hydrogen sulfide. Antioxid. Redox Signal. 2014, 20, 783–793. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H.; Kashfi, K. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol. 2021, 38, 101772. [Google Scholar] [CrossRef]
- Hanna, D.; Kumar, R.; Banerjee, R. A Metabolic Paradigm for Hydrogen Sulfide Signaling via Electron Transport Chain Plasticity. Antioxid. Redox Signal. 2023, 38, 57–67. [Google Scholar] [CrossRef]
- Szabo, C. A timeline of hydrogen sulfide (H2S) research: From environmental toxin to biological mediator. Biochem. Pharmacol. 2018, 149, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen sulfide: Its production and functions. Exp. Physiol. 2011, 96, 833–835. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, R.; Matsuki, N.; Kimura, H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef]
- Murphy, B.; Bhattacharya, R.; Mukherjee, P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019, 33, 13098–13125. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H. H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Randi, E.B.; Casili, G.; Jacquemai, S.; Szabo, C. Selenium-Binding Protein 1 (SELENBP1) Supports Hydrogen Sulfide Biosynthesis and Adipogenesis. Antioxidants 2021, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Kimura, H. Production of hydrogen sulfide from d-cysteine and its therapeutic potential. Front. Endocrinol. 2013, 4, 87. [Google Scholar] [CrossRef]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen sulfide: Its production, release and functions. Amino Acids 2011, 41, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, Y.; Ishii, K.; Togawa, T.; Tanabe, S. Determination of bound sulfur in serum by gas dialysis/high-performance liquid chromatography. Anal. Biochem. 1993, 215, 73–81. [Google Scholar] [CrossRef]
- Toohey, J.I. Sulphane sulphur in biological systems: A possible regulatory role. Biochem. J. 1989, 264, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, M.; Hiraki, K.; Umemura, K.; Ogasawara, Y.; Ishii, K.; Kimura, H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid. Redox Signal. 2009, 11, 205–214. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Isoda, S.; Tanabe, S. Tissue and subcellular distribution of bound and acid-labile sulfur, and the enzymic capacity for sulfide production in the rat. Biol. Pharm. Bull. 1994, 17, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T. Assay methods and biological roles of labile sulfur in animal tissues. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 781, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Westley, A.M.; Westley, J. Biological sulfane sulfur. Anal. Biochem. 1991, 195, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Gasotransmitters in cancer: From pathophysiology to experimental therapy. Nat. Rev. Drug Discov. 2016, 15, 185–203. [Google Scholar] [CrossRef] [PubMed]
- Searcy, D.G.; Lee, S.H. Sulfur reduction by human erythrocytes. J. Exp. Zool. 1998, 282, 310–322. [Google Scholar] [CrossRef]
- Mantle, D.; Yang, G. Hydrogen sulfide and metal interaction: The pathophysiological implications. Mol. Cell Biochem. 2022, 477, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- Kabil, O.; Banerjee, R. Enzymology of H2S biogenesis, decay and signaling. Antioxid. Redox Signal. 2014, 20, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.; Janosik, M.; Kery, V.; Kraus, J.P.; Burkhard, P. Structure of human cystathionine beta-synthase: A unique pyridoxal 5′-phosphate-dependent heme protein. EMBO J. 2001, 20, 3910–3916. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H. Sulfur amino acid metabolism: Pathways for production and removal of homocysteine and cysteine. Annu. Rev. Nutr. 2004, 24, 539–577. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Włodek, L. Biosynthesis and biological properties of compounds containing highly reactive, reduced sulfane sulfur. Pol. J. Pharmacol. 2001, 53, 215–225. [Google Scholar] [PubMed]
- Zheng, M.; Zeng, Q.; Shi, X.Q.; Zhao, J.; Tang, C.S.; Sun, N.L.; Geng, B. Erythrocytic or serum hydrogen sulfide association with hypertension development in untreated essential hypertension. Chin. Med. J. 2011, 124, 3693–3701. [Google Scholar] [PubMed]
- Singh, S.; Banerjee, R. PLP-dependent H2S biogenesis. Biochim. Biophys. Acta 2011, 1814, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.D.; Wang, H.; Zhu, Y.Z. The Drug Developments of Hydrogen Sulfide on Cardiovascular Disease. Oxid. Med. Cell Longev. 2018, 2018, 4010395. [Google Scholar] [CrossRef]
- Zuhra, K.; Augsburger, F.; Majtan, T.; Szabo, C. Cystathionine-β-Synthase: Molecular Regulation and Pharmacological Inhibition. Biomolecules 2020, 10, 697. [Google Scholar] [CrossRef]
- Bearden, S.E.; Beard, R.S., Jr.; Pfau, J.C. Extracellular transsulfuration generates hydrogen sulfide from homocysteine and protects endothelium from redox stress. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1568–H1576. [Google Scholar] [CrossRef]
- Rao, S.P.; Dobariya, P.; Bellamkonda, H.; More, S.S. Role of 3-Mercaptopyruvate Sulfurtransferase (3-MST) in Physiology and Disease. Antioxidants 2023, 12, 603. [Google Scholar] [CrossRef]
- Yadav, P.K.; Yamada, K.; Chiku, T.; Koutmos, M.; Banerjee, R. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J. Biol. Chem. 2013, 288, 20002–20013. [Google Scholar] [CrossRef] [PubMed]
- Hipólito, A.; Nunes, S.C.; Vicente, J.B.; Serpa, J. Cysteine Aminotransferase (CAT): A Pivotal Sponsor in Metabolic Remodeling and an Ally of 3-Mercaptopyruvate Sulfurtransferase (MST) in Cancer. Molecules 2020, 25, 3984. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Metabolic turnover of hydrogen sulfide. Front. Physiol. 2012, 3, 101. [Google Scholar] [CrossRef] [PubMed]
- Magee, E.A.; Richardson, C.J.; Hughes, R.; Cummings, J.H. Contribution of dietary protein to sulfide production in the large intestine: An in vitro and a controlled feeding study in humans. Am. J. Clin. Nutr. 2000, 72, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Levitt, M.D.; Springfield, J.; Furne, J.; Koenig, T.; Suarez, F.L. Physiology of sulfide in the rat colon: Use of bismuth to assess colonic sulfide production. J. Appl. Physiol. 2002, 92, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.K.; Rotbart, A.; Ou, J.Z.; Kalantar-Zadeh, K.; Muir, J.G.; Gibson, P.R. Modulation of colonic hydrogen sulfide production by diet and mesalazine utilizing a novel gas-profiling technology. Gut Microbes 2018, 9, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Lin, H.C. Hydrogen Sulfide in Physiology and Diseases of the Digestive Tract. Microorganisms 2015, 3, 866–889. [Google Scholar] [CrossRef] [PubMed]
- Barton, L.L.; Fauque, G.D. Biochemistry, physiology and biotechnology of sulfate-reducing bacteria. Adv. Appl. Microbiol. 2009, 68, 41–98. [Google Scholar] [CrossRef]
- Carbonero, F.; Benefiel, A.C.; Alizadeh-Ghamsari, A.H.; Gaskins, H.R. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 2012, 3, 448. [Google Scholar] [CrossRef]
- Bryan, N.S.; Lefer, D.J. Update on Gaseous Signaling Molecules Nitric Oxide and Hydrogen Sulfide: Strategies to Capture their Functional Activity for Human Therapeutics. Mol. Pharmacol. 2019, 96, 109–114. [Google Scholar] [CrossRef]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1479–R1485. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.C. The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim. Biophys. Acta BBA Bioenerg. 1977, 460, 299–307. [Google Scholar] [CrossRef] [PubMed]
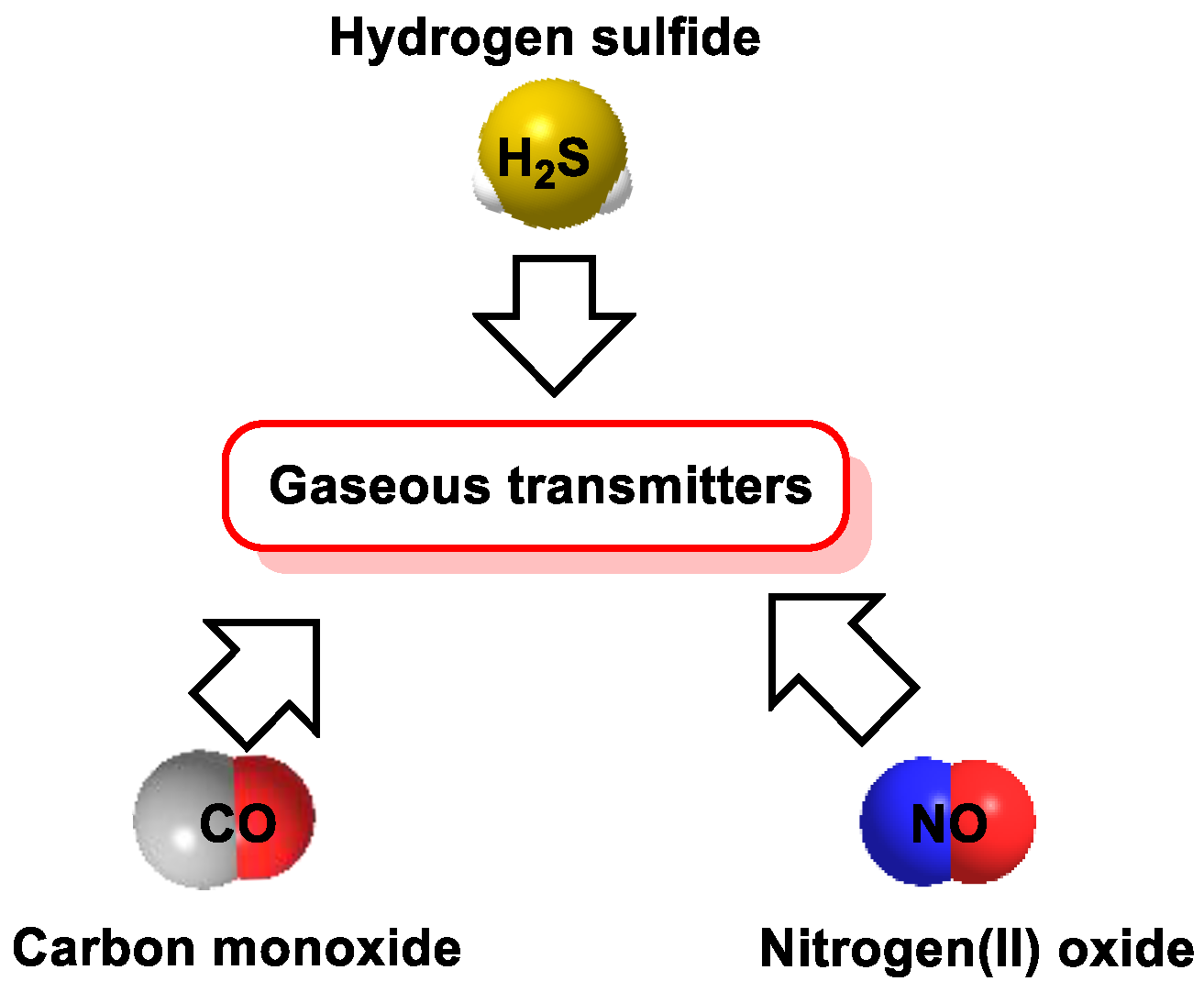
- Giuffrè, A.; Vicente, J.B. Hydrogen Sulfide Biochemistry and Interplay with Other Gaseous Mediators in Mammalian Physiology. Oxidative Med. Cell. Longev. 2018, 2018, 6290931. [Google Scholar] [CrossRef] [PubMed]
- Lagoutte, E.; Mimoun, S.; Andriamihaja, M.; Chaumontet, C.; Blachier, F.; Bouillaud, F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim. Biophys. Acta BBA Bioenerg. 2010, 1797, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Theissen, U.; Hoffmeister, M.; Grieshaber, M.; Martin, W. Single eubacterial origin of eukaryotic sulfide: Quinone oxidoreductase, a mitochondrial enzyme conserved from the early evolution of eukaryotes during anoxic and sulfidic times. Mol. Biol. Evol. 2003, 20, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.B.; Forte, E. Impact of Hydrogen Sulfide on Mitochondrial and Bacterial Bioenergetics. Int. J. Mol. Sci. 2021, 22, 2688. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; Arif, F.; Arora, K.; Patel, S.; DeLeon, E.R.; Sutton, T.R.; Feelisch, M.; Cortese-Krott, M.M.; Straub, K.D. Metabolism of hydrogen sulfide (H2S) and Production of Reactive Sulfur Species (RSS) by superoxide dismutase. Redox Biol. 2018, 15, 74–85. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; DeLeon, E.R.; Arif, M.; Arif, F.; Arora, N.; Straub, K.D. Catalase as a sulfide-sulfur oxido-reductase: An ancient (and modern?) regulator of reactive sulfur species (RSS). Redox Biol. 2017, 12, 325–339. [Google Scholar] [CrossRef]
- Searcy, D.G. HS-:O2 oxidoreductase activity of Cu, Zn superoxide dismutase. Arch. Biochem. Biophys. 1996, 334, 50–58. [Google Scholar] [CrossRef]
- Searcy, D.G.; Whitehead, J.P.; Maroney, M.J. Interaction of Cu, Zn superoxide dismutase with hydrogen sulfide. Arch. Biochem. Biophys. 1995, 318, 251–263. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Yadav, P.K.; Kurthen, A.; Banerjee, R. Sulfide oxidation by a noncanonical pathway in red blood cells generates thiosulfate and polysulfides. J. Biol. Chem. 2015, 290, 8310–8320. [Google Scholar] [CrossRef] [PubMed]
- Ríos-González, B.B.; Román-Morales, E.M.; Pietri, R.; López-Garriga, J. Hydrogen sulfide activation in hemeproteins: The sulfheme scenario. J. Inorg. Biochem. 2014, 133, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Pan, J.; Li, S.; Zhao, Y.; Wu, L.Y.; Berkman, C.E.; Whorton, A.R.; Xian, M. Capture and visualization of hydrogen sulfide by a fluorescent probe. Angew. Chem. Int. Ed. Engl. 2011, 50, 10327–10329. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Testai, L.; Breschi, M.C.; Blandizzi, C.; Virdis, A.; Taddei, S.; Calderone, V. Hydrogen sulphide: Novel opportunity for drug discovery. Med. Res. Rev. 2012, 32, 1093–1130. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Hartle, M.D.; Sommer, S.K.; Dietrich, S.R.; Pluth, M.D. Chemically reversible reactions of hydrogen sulfide with metal phthalocyanines. Inorg. Chem. 2014, 53, 7800–7802. [Google Scholar] [CrossRef]
- Aiuppa, A.; Federico, C.; Giudice, G.; Gurrieri, S.; Valenza, M. Hydrothermal buffering of the SO2/H2S ratio in volcanic gases: Evidence from La Fossa Crater fumarolic field, Vulcano Island. Geophys. Res. Lett. 2006, 33, 21. [Google Scholar] [CrossRef]
- Jiang, G.; Yang, Z.; Zhang, F.; Li, G.; Wei, Z.; Niu, B.; Zhao, M.; Zhang, Z.; Hao, Z. Thermal Decomposition of H2S at Low Temperature on Mo-Containing Catalysts Derived from MAlO (M = Mg, Co, and Ni) Layered Double Hydroxides. Ind. Eng. Chem. Res. 2023, 62, 7224–7234. [Google Scholar] [CrossRef]
- Vasas, A.; Dóka, É.; Fábián, I.; Nagy, P. Kinetic and thermodynamic studies on the disulfide-bond reducing potential of hydrogen sulfide. Nitric Oxide 2015, 46, 93–101. [Google Scholar] [CrossRef]
- Dilek, N.; Papapetropoulos, A.; Toliver-Kinsky, T.; Szabo, C. Hydrogen sulfide: An endogenous regulator of the immune system. Pharmacol. Res. 2020, 161, 105119. [Google Scholar] [CrossRef]
- Vandiver, M.; Snyder, S.H. Hydrogen sulfide: A gasotransmitter of clinical relevance. J. Mol. Med. 2012, 90, 255–263. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide 2013, 35, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Pan, L.; Ji, Y. H2S protects against diabetes-accelerated atherosclerosis by preventing the activation of NLRP3 inflammasome. J. Biomed. Res. 2019, 34, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Parsanathan, R.; Jain, S.K. Hydrogen sulfide regulates circadian-clock genes in C2C12 myotubes and the muscle of high-fat-diet-fed mice. Arch. Biochem. Biophys. 2019, 672, 108054. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Papapetropoulos, A. International Union of Basic and Clinical Pharmacology. CII: Pharmacological Modulation of H2S Levels: H2S Donors and H2S Biosynthesis Inhibitors. Pharmacol. Rev. 2017, 69, 497–564. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Nagai, Y.; Umemura, K.; Kimura, Y. Physiological roles of hydrogen sulfide: Synaptic modulation, neuroprotection, and smooth muscle relaxation. Antioxid. Redox Signal. 2005, 7, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Bilska-Wilkosz, A.; Kozdrowicki, M.; Górny, M. Reactive sulfur species and their significance in health and disease. Biosci. Rep. 2022, 42, BSR20221006. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Shackelford, R.E.; Shen, X.; Dominic, P.; Kevil, C.G. Sulfide regulation of cardiovascular function in health and disease. Nat. Rev. Cardiol. 2023, 20, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Rossoni, G.; Manfredi, B.; Tazzari, V.; Sparatore, A.; Trivulzio, S.; Del Soldato, P.; Berti, F. Activity of a new hydrogen sulfide-releasing aspirin (ACS14) on pathological cardiovascular alterations induced by glutathione depletion in rats. Eur. J. Pharmacol. 2010, 648, 139–145. [Google Scholar] [CrossRef]
- Li, Y.F.; Xiao, C.S.; Hui, R.T. Calcium sulfide (CaS), a donor of hydrogen sulfide (H2S): A new antihypertensive drug? Med. Hypotheses 2009, 73, 445–447. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, Z.; Luo, S.; Guo, W.; Zhu, Y.Z. The Cardioprotective Effects of Hydrogen Sulfide in Heart Diseases: From Molecular Mechanisms to Therapeutic Potential. Oxidative Med. Cell. Longev. 2015, 2015, 925167. [Google Scholar] [CrossRef] [PubMed]
- Tamizhselvi, R.; Koh, Y.H.; Sun, J.; Zhang, H.; Bhatia, M. Hydrogen sulfide induces ICAM-1 expression and neutrophil adhesion to caerulein-treated pancreatic acinar cells through NF-kappaB and Src-family kinases pathway. Exp. Cell Res. 2010, 316, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Morselli-Labate, A.M.; Fantini, L.; Pezzilli, R. Hydrogen sulfide, nitric oxide and a molecular mass 66 u substance in the exhaled breath of chronic pancreatitis patients. Pancreatology 2007, 7, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M. H2S and substance P in inflammation. Methods Enzymol. 2015, 555, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.R.; Levitt, M.D.; Farrugia, G.; Szurszewski, J.H. Endogenous production of H2S in the gastrointestinal tract: Still in search of a physiologic function. Antioxid. Redox Signal. 2010, 12, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Ganster, F.; Burban, M.; de la Bourdonnaye, M.; Fizanne, L.; Douay, O.; Loufrani, L.; Mercat, A.; Calès, P.; Radermacher, P.; Henrion, D.; et al. Effects of hydrogen sulfide on hemodynamics, inflammatory response and oxidative stress during resuscitated hemorrhagic shock in rats. Crit. Care 2010, 14, R165. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Vong, L.; McKnight, W.; Dicay, M.; Martin, G.R. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology 2009, 137, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Piña, A.E.; Tapia-Alvarez, G.R.; Navarrete, A. Inhibition of endogenous hydrogen sulfide synthesis by PAG protects against ethanol-induced gastric damage in the rat. Eur. J. Pharmacol. 2010, 630, 131–136. [Google Scholar] [CrossRef]
- Guo, W.; Cheng, Z.-y.; Zhu, Y.-z. Hydrogen sulfide and translational medicine. Acta Pharmacol. Sin. 2013, 34, 1284–1291. [Google Scholar] [CrossRef]
- Bhatia, M. H2S and Inflammation: An Overview. Handb. Exp. Pharmacol. 2015, 230, 165–180. [Google Scholar] [CrossRef]
- Flannigan, K.L.; Ferraz, J.G.; Wang, R.; Wallace, J.L. Enhanced synthesis and diminished degradation of hydrogen sulfide in experimental colitis: A site-specific, pro-resolution mechanism. PLoS ONE 2013, 8, e71962. [Google Scholar] [CrossRef] [PubMed]
- Chunyu, Z.; Junbao, D.; Dingfang, B.; Hui, Y.; Xiuying, T.; Chaoshu, T. The regulatory effect of hydrogen sulfide on hypoxic pulmonary hypertension in rats. Biochem. Biophys. Res. Commun. 2003, 302, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Khattak, S.; Zhang, Q.Q.; Sarfraz, M.; Muhammad, P.; Ngowi, E.E.; Khan, N.H.; Rauf, S.; Wang, Y.Z.; Qi, H.W.; Wang, D.; et al. The Role of Hydrogen Sulfide in Respiratory Diseases. Biomolecules 2021, 11, 682. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.H.; Iqbal, M.; Manhoosh, B.; Gholampoor, N.; Ma, D.; Marwah, M.; Sanchez-Aranguren, L. Hydrogen Sulphide-Based Therapeutics for Neurological Conditions: Perspectives and Challenges. Neurochem. Res. 2023, 48, 1981–1996. [Google Scholar] [CrossRef] [PubMed]
- Eto, K.; Asada, T.; Arima, K.; Makifuchi, T.; Kimura, H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002, 293, 1485–1488. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Ngowi, E.E.; Qian, L.; Li, T.; Qin, Y.Z.; Zhou, J.J.; Li, K.; Ji, X.Y.; Wu, D.D. Role of Hydrogen Sulfide in the Endocrine System. Front. Endocrinol. 2021, 12, 704620. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Gu, H.; Ni, X. Hydrogen sulfide in the endocrine and reproductive systems. Expert. Rev. Clin. Pharmacol. 2011, 4, 75–82. [Google Scholar] [CrossRef]
- Yu, M.; Sturgill-Short, G.; Ganapathy, P.; Tawfik, A.; Peachey, N.S.; Smith, S.B. Age-related changes in visual function in cystathionine-beta-synthase mutant mice, a model of hyperhomocysteinemia. Exp. Eye Res. 2012, 96, 124–131. [Google Scholar] [CrossRef]
- Ganapathy, P.S.; Moister, B.; Roon, P.; Mysona, B.A.; Duplantier, J.; Dun, Y.; Moister, T.K.; Farley, M.J.; Prasad, P.D.; Liu, K.; et al. Endogenous elevation of homocysteine induces retinal neuron death in the cystathionine-beta-synthase mutant mouse. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4460–4470. [Google Scholar] [CrossRef]
- Tawfik, A.; Markand, S.; Al-Shabrawey, M.; Mayo, J.N.; Reynolds, J.; Bearden, S.E.; Ganapathy, V.; Smith, S.B. Alterations of retinal vasculature in cystathionine-β-synthase heterozygous mice: A model of mild to moderate hyperhomocysteinemia. Am. J. Pathol. 2014, 184, 2573–2585. [Google Scholar] [CrossRef]
- Perridon, B.W.; Leuvenink, H.G.; Hillebrands, J.L.; van Goor, H.; Bos, E.M. The role of hydrogen sulfide in aging and age-related pathologies. Aging 2016, 8, 2264–2289. [Google Scholar] [CrossRef]
- Wilkie, S.E.; Borland, G.; Carter, R.N.; Morton, N.M.; Selman, C. Hydrogen sulfide in ageing, longevity and disease. Biochem. J. 2021, 478, 3485–3504. [Google Scholar] [CrossRef] [PubMed]
- Ngowi, E.E.; Afzal, A.; Sarfraz, M.; Khattak, S.; Zaman, S.U.; Khan, N.H.; Li, T.; Jiang, Q.Y.; Zhang, X.; Duan, S.F.; et al. Role of hydrogen sulfide donors in cancer development and progression. Int. J. Biol. Sci. 2021, 17, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.H.; Ngowi, E.E.; Li, Y.; Khattak, S.; Zhao, Y.; Shahid, M.; Zafar, U.; Waheed, I.; Khan, F.; Virk, R.; et al. Hydrogen sulfide donors and inhibitors in cancer research: A state-of-the-art review. Gene Protein Dis. 2022, 2, 164. [Google Scholar] [CrossRef]
- Fan, X.; Fei, W.; Zhang, M.; Yang, S.; Zhao, M.; Zheng, C. Nanotherapeutics for hydrogen sulfide-involved treatment: An emerging approach for cancer therapy. Nanotechnol. Rev. 2022, 11, 2320–2348. [Google Scholar] [CrossRef]
- Khattak, S.; Rauf, M.A.; Khan, N.H.; Zhang, Q.-Q.; Chen, H.-J.; Muhammad, P.; Ansari, M.A.; Alomary, M.N.; Jahangir, M.; Zhang, C.-Y. Hydrogen sulfide biology and its role in cancer. Molecules 2022, 27, 3389. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, M.R.; Szabo, C. Hydrogen Sulfide and Cancer. Handb. Exp. Pharmacol. 2015, 230, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, Z.H.; Ren, Z.; Qu, S.L.; Liu, M.H.; Liu, L.S.; Jiang, Z.S. Hydrogen sulfide, the next potent preventive and therapeutic agent in aging and age-associated diseases. Mol. Cell Biol. 2013, 33, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Han, S.; Li, Y.; Gao, H. Generation and Physiology of Hydrogen Sulfide and Reactive Sulfur Species in Bacteria. Antioxidants 2022, 11, 2487. [Google Scholar] [CrossRef]
- Shen, X.; Carlström, M.; Borniquel, S.; Jädert, C.; Kevil, C.G.; Lundberg, J.O. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic. Biol. Med. 2013, 60, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Walsh, B.J.; Costa, S.S.; Edmonds, K.A.; Trinidad, J.C.; Issoglio, F.M.; Brito, J.A.; Giedroc, D.P. Metabolic and Structural Insights into Hydrogen Sulfide Mis-Regulation in Enterococcus faecalis. Antioxidants 2022, 11, 1607. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Drlica, K. Reactive oxygen species and the bacterial response to lethal stress. Curr. Opin. Microbiol. 2014, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Seregina, T.A.; Lobanov, K.V.; Shakulov, R.S.; Mironov, A.S. Enhancement of the Bactericidal Effect of Antibiotics by Inhibition of Enzymes Involved in Production of Hydrogen Sulfide in Bacteria. Mol. Biol. 2022, 56, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, X.; Liu, W.; Zhang, Y.; Guan, H.; Wu, J.; Yu, S.; Xue, W. Revealing the antibacterial power of hydrogen-releasing PdH nanohydride against drug resistant Staphylococcus aureus: An in-depth mechanism study. J. Mater. Chem. B 2023, 11, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Shatalin, K.; Nuthanakanti, A.; Kaushik, A.; Shishov, D.; Peselis, A.; Shamovsky, I.; Pani, B.; Lechpammer, M.; Vasilyev, N.; Shatalina, E. Inhibitors of bacterial H2S biogenesis targeting antibiotic resistance and tolerance. Science 2021, 372, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Ong, K.X.; Surendran, S.T.; Sinha, A.; Lai, J.J.H.; Chen, J.; Liang, J.; Tay, L.K.S.; Cui, L.; Loo, H.L.; et al. Hydrogen sulfide sensitizes Acinetobacter baumannii to killing by antibiotics. Front. Microbiol. 2020, 11, 1875. [Google Scholar] [CrossRef] [PubMed]
- Pal, V.K.; Bandyopadhyay, P.; Singh, A. Hydrogen sulfide in physiology and pathogenesis of bacteria and viruses. IUBMB Life 2018, 70, 393–410. [Google Scholar] [CrossRef]
- Pietri, R.; Román-Morales, E.; López-Garriga, J. Hydrogen sulfide and hemeproteins: Knowledge and mysteries. Antioxid. Redox Signal. 2011, 15, 393–404. [Google Scholar] [CrossRef]
- Spassov, S.G.; Donus, R.; Ihle, P.M.; Engelstaedter, H.; Hoetzel, A.; Faller, S. Hydrogen Sulfide Prevents Formation of Reactive Oxygen Species through PI3K/Akt Signaling and Limits Ventilator-Induced Lung Injury. Oxidative Med. Cell. Longev. 2017, 2017, 3715037. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Streeter, E.; Hart, J.; Badoer, E. An investigation of the mechanisms of hydrogen sulfide-induced vasorelaxation in rat middle cerebral arteries. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.G.; Cao, Y.X.; Wang, W.W.; Ma, S.F.; Yao, T.; Zhu, Y.C. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc. Res. 2008, 79, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.T.; Neo, K.L.; Hu, L.F.; Yong, Q.C.; Bian, J.S. H2S preconditioning-induced PKC activation regulates intracellular calcium handling in rat cardiomyocytes. Am. J. Physiol. Cell Physiol. 2008, 294, C169–C177. [Google Scholar] [CrossRef]
- Tian, X.Y.; Wong, W.T.; Sayed, N.; Luo, J.; Tsang, S.Y.; Bian, Z.X.; Lu, Y.; Cheang, W.S.; Yao, X.; Chen, Z.Y.; et al. NaHS relaxes rat cerebral artery in vitro via inhibition of l-type voltage-sensitive Ca2+ channel. Pharmacol. Res. 2011, 65, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Shefa, U.; Kim, M.S.; Jeong, N.Y.; Jung, J. Antioxidant and Cell-Signaling Functions of Hydrogen Sulfide in the Central Nervous System. Oxidative Med. Cell. Longev. 2018, 2018, 1873962. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Wu, L.; Wang, R. Interaction of hydrogen sulfide with ion channels. Clin. Exp. Pharmacol. Physiol. 2010, 37, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.K.; Sikka, G.; Gazi, S.K.; Steppan, J.; Jung, S.M.; Bhunia, A.K.; Barodka, V.M.; Gazi, F.K.; Barrow, R.K.; Wang, R.; et al. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ. Res. 2011, 109, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Sivarajah, A.; McDonald, M.C.; Thiemermann, C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock 2006, 26, 154–161. [Google Scholar] [CrossRef]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H2S: A universal defense against antibiotics in bacteria. Science 2011, 334, 986–990. [Google Scholar] [CrossRef]
- Eghbal, M.A.; Pennefather, P.S.; O’Brien, P.J. H2S cytotoxicity mechanism involves reactive oxygen species formation and mitochondrial depolarisation. Toxicology 2004, 203, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Lalucat, J.; Bennasar, A.; Bosch, R.; García-Valdés, E.; Palleroni, N.J. Biology of Pseudomonas stutzeri. Microbiol. Mol. Biol. Rev. 2006, 70, 510–547. [Google Scholar] [CrossRef] [PubMed]
- Caro, A.A.; Thompson, S.; Tackett, J. Increased oxidative stress and cytotoxicity by hydrogen sulfide in HepG2 cells overexpressing cytochrome P450 2E1. Cell Biol. Toxicol. 2011, 27, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Mirzoyan, N.; Schreier, H.J. Effect of sulfide on growth of marine bacteria. Arch. Microbiol. 2014, 196, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Attene-Ramos, M.S.; Wagner, E.D.; Plewa, M.J.; Gaskins, H.R. Evidence that hydrogen sulfide is a genotoxic agent. Mol. Cancer Res. 2006, 4, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Cuevasanta, E.; Lange, M.; Bonanata, J.; Coitiño, E.L.; Ferrer-Sueta, G.; Filipovic, M.R.; Alvarez, B. Reaction of Hydrogen Sulfide with Disulfide and Sulfenic Acid to Form the Strongly Nucleophilic Persulfide. J. Biol. Chem. 2015, 290, 26866–26880. [Google Scholar] [CrossRef] [PubMed]
- Carballal, S.; Trujillo, M.; Cuevasanta, E.; Bartesaghi, S.; Möller, M.N.; Folkes, L.K.; García-Bereguiaín, M.A.; Gutiérrez-Merino, C.; Wardman, P.; Denicola, A.; et al. Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radic. Biol. Med. 2011, 50, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Cuevasanta, E.; Zeida, A.; Carballal, S.; Wedmann, R.; Morzan, U.N.; Trujillo, M.; Radi, R.; Estrin, D.A.; Filipovic, M.R.; Alvarez, B. Insights into the mechanism of the reaction between hydrogen sulfide and peroxynitrite. Free Radic. Biol. Med. 2015, 80, 93–100. [Google Scholar] [CrossRef]
- Nagy, P.; Winterbourn, C.C. Rapid reaction of hydrogen sulfide with the neutrophil oxidant hypochlorous acid to generate polysulfides. Chem. Res. Toxicol. 2010, 23, 1541–1543. [Google Scholar] [CrossRef]
- Benchoam, D.; Cuevasanta, E.; Möller, M.N.; Alvarez, B. Hydrogen Sulfide and Persulfides Oxidation by Biologically Relevant Oxidizing Species. Antioxidants 2019, 8, 48. [Google Scholar] [CrossRef]
- Hoffmann, M.R. Kinetics and mechanism of oxidation of hydrogen sulfide by hydrogen peroxide in acidic solution. Environ. Sci. Technol. 1977, 11, 61–66. [Google Scholar] [CrossRef]
- Rabai, G.; Orban, M.; Epstein, I.R. Systematic design of chemical oscillators. 77. A model for the pH-regulated oscillatory reaction between hydrogen peroxide and sulfide ion. J. Phys. Chem. 1992, 96, 5414–5419. [Google Scholar] [CrossRef]
- Mills, G.; Schmidt, K.H.; Matheson, M.S.; Meisel, D. Thermal and photochemical reactions of sulfhydryl radicals. Implications for colloid photocorrosion. J. Phys. Chem. 1987, 91, 1590–1596. [Google Scholar] [CrossRef]
- Creutz, C.; Sutin, N. Kinetics of the reactions of sodium dithionite with dioxygen and hydrogen peroxide. Inorg. Chem. 1974, 13, 2041–2043. [Google Scholar] [CrossRef]
- Giles, G.I.; Tasker, K.M.; Jacob, C. Hypothesis: The role of reactive sulfur species in oxidative stress. Free Radic. Biol. Med. 2001, 31, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Gruhlke, M.C.; Slusarenko, A.J. The biology of reactive sulfur species (RSS). Plant Physiol. Biochem. 2012, 59, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R.; Miljkovic, J.; Allgäuer, A.; Chaurio, R.; Shubina, T.; Herrmann, M.; Ivanovic-Burmazovic, I. Biochemical insight into physiological effects of H2S: Reaction with peroxynitrite and formation of a new nitric oxide donor, sulfinyl nitrite. Biochem. J. 2012, 441, 609–621. [Google Scholar] [CrossRef]
- Kang, M.; Hashimoto, A.; Gade, A.; Akbarali, H.I. Interaction between hydrogen sulfide-induced sulfhydration and tyrosine nitration in the KATP channel complex. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G532–G539. [Google Scholar] [CrossRef]
- Iciek, M.; Kowalczyk-Pachel, D.; Bilska-Wilkosz, A.; Kwiecień, I.; Górny, M.; Włodek, L. S-sulfhydration as a cellular redox regulation. Biosci. Rep. 2015, 36, e00304. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. H2S: A Novel Gasotransmitter that Signals by Sulfhydration. Trends Biochem. Sci. 2015, 40, 687–700. [Google Scholar] [CrossRef]
- Kabil, O.; Banerjee, R. Redox biochemistry of hydrogen sulfide. J. Biol. Chem. 2010, 285, 21903–21907. [Google Scholar] [CrossRef] [PubMed]
- Cuevasanta, E.; Reyes, A.M.; Zeida, A.; Mastrogiovanni, M.; De Armas, M.I.; Radi, R.; Alvarez, B.; Trujillo, M. Kinetics of formation and reactivity of the persulfide in the one-cysteine peroxiredoxin from Mycobacterium tuberculosis. J. Biol. Chem. 2019, 294, 13593–13605. [Google Scholar] [CrossRef] [PubMed]
- Cuevasanta, E.; Möller, M.N.; Alvarez, B. Biological chemistry of hydrogen sulfide and persulfides. Arch. Biochem. Biophys. 2017, 617, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Carroll, K.S. Cysteine-Mediated Redox Signaling: Chemistry, Biology, and Tools for Discovery. Chem. Rev. 2013, 113, 4633–4679. [Google Scholar] [CrossRef] [PubMed]
- Jencks, W.P.; Carriuolo, J. Reactivity of Nucleophilic Reagents toward Esters. J. Am. Chem. Soc. 1960, 82, 1778–1786. [Google Scholar] [CrossRef]
- Edwards, J.O.; Pearson, R.G. The Factors Determining Nucleophilic Reactivities. J. Am. Chem. Soc. 1962, 84, 16–24. [Google Scholar] [CrossRef]
- Toohey, J.I. Sulfur signaling: Is the agent sulfide or sulfane? Anal. Biochem. 2011, 413, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Akaike, T.; Sawa, T.; Kumagai, Y.; Wink, D.A.; Tantillo, D.J.; Hobbs, A.J.; Nagy, P.; Xian, M.; Lin, J.; et al. Redox chemistry and chemical biology of H2S, hydropersulfides, and derived species: Implications of their possible biological activity and utility. Free Radic. Biol. Med. 2014, 77, 82–94. [Google Scholar] [CrossRef]
- Filipovic, M.R. Persulfidation (S-sulfhydration) and H2S. Handb. Exp. Pharmacol. 2015, 230, 29–59. [Google Scholar] [CrossRef]
- Wood, J.L. Sulfane sulfur. Methods Enzymol. 1987, 143, 25–29. [Google Scholar] [CrossRef]
- Zuman, P.; Szafranski, W. Ultraviolet spectra of hydroxide, alkoxide, and hydrogen sulfide anions. Anal. Chem. 1976, 48, 2162–2163. [Google Scholar] [CrossRef]
- Warenycia, M.W.; Goodwin, L.R.; Benishin, C.G.; Reiffenstein, R.J.; Francom, D.M.; Taylor, J.D.; Dieken, F.P. Acute hydrogen sulfide poisoning: Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem. Pharmacol. 1989, 38, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Doeller, J.E.; Isbell, T.S.; Benavides, G.; Koenitzer, J.; Patel, H.; Patel, R.P.; Lancaster, J.R., Jr.; Darley-Usmar, V.M.; Kraus, D.W. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal. Biochem. 2005, 341, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim. Biophys. Acta BBA Bioenerg. 2009, 1787, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Dasgupta, P.K.; Li, J.; Tarver, G.A.; Zarus, G.M. Fluorometric field instrument for continuous measurement of atmospheric hydrogen sulfide. Anal. Chem. 2001, 73, 5716–5724. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jhee, K.H.; Kruger, W.D. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. J. Biol. Chem. 2004, 279, 52082–52086. [Google Scholar] [CrossRef]
- Siegel, L.M. A direct microdetermination for sulfide. Anal. Biochem. 1965, 11, 126–132. [Google Scholar] [CrossRef]
- Hine, C.; Mitchell, J.R. Endpoint or Kinetic Measurement of Hydrogen Sulfide Production Capacity in Tissue Extracts. Bio-Protocol 2017, 7, e2382. [Google Scholar] [CrossRef]
- Fogo, J.K.; Popowsky, M. Spectrophotometric Determination of Hydrogen Sulfide. Anal. Chem. 1949, 21, 732–734. [Google Scholar] [CrossRef]
- Wintner, E.A.; Deckwerth, T.L.; Langston, W.; Bengtsson, A.; Leviten, D.; Hill, P.; Insko, M.A.; Dumpit, R.; VandenEkart, E.; Toombs, C.F.; et al. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br. J. Pharmacol. 2010, 160, 941–957. [Google Scholar] [CrossRef]
- Pang, L.-J.; Wang, D.; Zhou, J.; Zhang, L.-H.; Ye, X.-S. Synthesis of neamine-derived pseudodisaccharides by stereo- and regio-selective functional group transformations. Org. Biomol. Chem. 2009, 7, 4252–4266. [Google Scholar] [CrossRef] [PubMed]
- Shabib, A.; Shoman, M.; Abdelhafez, E.M.N. Current Trends and Future Perspectives of Hydrogen Sulfide Donors. J. Adv. Biomed. Pharm. Sci. 2021, 4, 231–245. [Google Scholar] [CrossRef]
- Lippert, A.R.; New, E.J.; Chang, C.J. Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J. Am. Chem. Soc. 2011, 133, 10078–10080. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Baeza-Garza, C.D.; Logan, A.; Rosa, T.; Wedmann, R.; Prime, T.A.; Martin, J.L.; Saeb-Parsy, K.; Krieg, T.; Filipovic, M.R.; et al. Assessment of H2S in vivo using the newly developed mitochondria-targeted mass spectrometry probe MitoA. J. Biol. Chem. 2017, 292, 7761–7773. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, C.; Yang, Z.; Chen, J.; He, Y.; Jiao, Y.; He, W.; Qiu, L.; Cen, J.; Guo, Z. A ratiometric fluorescent probe for rapid detection of hydrogen sulfide in mitochondria. Angew. Chem. Int. Ed. Engl. 2013, 52, 1688–1691. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Q.; Liu, J.; Zhang, J.; Yang, T.; Guo, W. Fluorescent probe for biological gas SO2 derivatives bisulfite and sulfite. Chem. Commun. 2013, 49, 2637–2639. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2015, 44, 4596–4618. [Google Scholar] [CrossRef]
- Qian, Y.; Karpus, J.; Kabil, O.; Zhang, S.Y.; Zhu, H.L.; Banerjee, R.; Zhao, J.; He, C. Selective fluorescent probes for live-cell monitoring of sulphide. Nat. Commun. 2011, 2, 495. [Google Scholar] [CrossRef]
- Sasakura, K.; Hanaoka, K.; Shibuya, N.; Mikami, Y.; Kimura, Y.; Komatsu, T.; Ueno, T.; Terai, T.; Kimura, H.; Nagano, T. Development of a Highly Selective Fluorescence Probe for Hydrogen Sulfide. J. Am. Chem. Soc. 2011, 133, 18003–18005. [Google Scholar] [CrossRef]
- DeLeon, E.R.; Stoy, G.F.; Olson, K.R. Passive loss of hydrogen sulfide in biological experiments. Anal. Biochem. 2012, 421, 203–207. [Google Scholar] [CrossRef]
- Zhao, Y.; Biggs, T.D.; Xian, M. Hydrogen sulfide (H2S) releasing agents: Chemistry and biological applications. Chem. Commun. 2014, 50, 11788–11805. [Google Scholar] [CrossRef] [PubMed]
- Citi, V.; Martelli, A.; Gorica, E.; Brogi, S.; Testai, L.; Calderone, V. Role of hydrogen sulfide in endothelial dysfunction: Pathophysiology and therapeutic approaches. J. Adv. Res. 2021, 27, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, B.; Ji, K.; Pan, Z.; Chittavong, V.; Wang, B. Esterase-Sensitive Prodrugs with Tunable Release Rates and Direct Generation of Hydrogen Sulfide. Angew. Chem. Int. Ed. Engl. 2016, 55, 4514–4518. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Testai, L.; Citi, V.; Marino, A.; Pugliesi, I.; Barresi, E.; Nesi, G.; Rapposelli, S.; Taliani, S.; Da Settimo, F.; et al. Arylthioamides as H2S Donors: L-Cysteine-Activated Releasing Properties and Vascular Effects in Vitro and in Vivo. ACS Med. Chem. Lett. 2013, 4, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Bełtowski, J.; Jamroz-Wiśniewska, A. Hydrogen Sulfide in the Adipose Tissue-Physiology, Pathology and a Target for Pharmacotherapy. Molecules 2016, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bhushan, S.; Yang, C.; Otsuka, H.; Stein, J.D.; Pacheco, A.; Peng, B.; Devarie-Baez, N.O.; Aguilar, H.C.; Lefer, D.J.; et al. Controllable hydrogen sulfide donors and their activity against myocardial ischemia-reperfusion injury. ACS Chem. Biol. 2013, 8, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Steiger, A.K.; Pardue, S.; Kevil, C.G.; Pluth, M.D. Self-Immolative Thiocarbamates Provide Access to Triggered H2S Donors and Analyte Replacement Fluorescent Probes. J. Am. Chem. Soc. 2016, 138, 7256–7259. [Google Scholar] [CrossRef]
- Fox, B.; Schantz, J.T.; Haigh, R.; Wood, M.E.; Moore, P.K.; Viner, N.; Spencer, J.P.; Winyard, P.G.; Whiteman, M. Inducible hydrogen sulfide synthesis in chondrocytes and mesenchymal progenitor cells: Is H2S a novel cytoprotective mediator in the inflamed joint? J. Cell. Mol. Med. 2012, 16, 896–910. [Google Scholar] [CrossRef]
- Ali, R.; Pal, H.A.; Hameed, R.; Nazir, A.; Verma, S. Controlled release of hydrogen sulfide significantly reduces ROS stress and increases dopamine levels in transgenic C. elegans. Chem. Commun. 2019, 55, 10142–10145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, X.; Luo, W.; Zhu, J.; Zhao, J.; Wang, M.; Sang, L.; Chang, B.; Wang, B. Allicin in Digestive System Cancer: From Biological Effects to Clinical Treatment. Front. Pharmacol. 2022, 13, 903259. [Google Scholar] [CrossRef] [PubMed]
- Sarvizadeh, M.; Hasanpour, O.; Naderi Ghale-Noie, Z.; Mollazadeh, S.; Rezaei, M.; Pourghadamyari, H.; Masoud Khooy, M.; Aschner, M.; Khan, H.; Rezaei, N.; et al. Allicin and Digestive System Cancers: From Chemical Structure to Its Therapeutic Opportunities. Front. Oncol. 2021, 11, 650256. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Wasef, L.G.; Elewa, Y.H.A.; Al-Sagan, A.A.; Abd El-Hack, M.E.; Taha, A.E.; Abd-Elhakim, Y.M.; Prasad Devkota, H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Anwar, A.; Burkholz, T. Perspective on recent developments on sulfur-containing agents and hydrogen sulfide signaling. Planta Med. 2008, 74, 1580–1592. [Google Scholar] [CrossRef] [PubMed]
- Citi, V.; Martelli, A.; Testai, L.; Marino, A.; Breschi, M.C.; Calderone, V. Hydrogen sulfide releasing capacity of natural isothiocyanates: Is it a reliable explanation for the multiple biological effects of Brassicaceae? Planta Med. 2014, 80, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Magli, E.; Perissutti, E.; Santagada, V.; Caliendo, G.; Corvino, A.; Esposito, G.; Esposito, G.; Fiorino, F.; Migliaccio, M.; Scognamiglio, A.; et al. H2S Donors and Their Use in Medicinal Chemistry. Biomolecules 2021, 11, 1899. [Google Scholar] [CrossRef]
- Corvino, A.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Scognamiglio, A.; Caliendo, G.; Fiorino, F.; Severino, B. Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases. Antioxidants 2021, 10, 429. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, D.; Ma, F.; Yang, S.; Tan, B.; Xin, H.; Gu, X.; Chen, X.; Chen, S.; Mao, Y.; et al. Novel Angiogenic Activity and Molecular Mechanisms of ZYZ-803, a Slow-Releasing Hydrogen Sulfide-Nitric Oxide Hybrid Molecule. Antioxid. Redox Signal. 2016, 25, 498–514. [Google Scholar] [CrossRef]
- Xiong, Y.; Chang, L.L.; Tran, B.; Dai, T.; Zhong, R.; Mao, Y.C.; Zhu, Y.Z. ZYZ-803, a novel hydrogen sulfide-nitric oxide conjugated donor, promotes angiogenesis via cross-talk between STAT3 and CaMKII. Acta Pharmacol. Sin. 2020, 41, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Szczesny, B.; Módis, K.; Yanagi, K.; Coletta, C.; Le Trionnaire, S.; Perry, A.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide 2014, 41, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Gerő, D.; Torregrossa, R.; Perry, A.; Waters, A.; Le-Trionnaire, S.; Whatmore, J.L.; Wood, M.; Whiteman, M. The novel mitochondria-targeted hydrogen sulfide (H2S) donors AP123 and AP39 protect against hyperglycemic injury in microvascular endothelial cells in vitro. Pharmacol. Res. 2016, 113, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, J.L.; Burger, N.; Gawel, J.M.; Mulvey, J.F.; Norman, A.A.I.; Nishimura, T.; Tsujihata, Y.; Logan, A.; Sauchanka, O.; Caldwell, S.T.; et al. Rapid and selective generation of H2S within mitochondria protects against cardiac ischemia-reperfusion injury. Redox Biol. 2022, 55, 102429. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.H.; Wang, Q.; Pan, L.L.; Liu, X.H.; Xin, H.; Zhu, Y.Z. S-propargyl-cysteine, a novel hydrogen sulfide-modulated agent, attenuates lipopolysaccharide-induced spatial learning and memory impairment: Involvement of TNF signaling and NF-κB pathway in rats. Brain Behav. Immun. 2011, 25, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zaorska, E.; Tomasova, L.; Koszelewski, D.; Ostaszewski, R.; Ufnal, M. Hydrogen Sulfide in Pharmacotherapy, Beyond the Hydrogen Sulfide-Donors. Biomolecules 2020, 10, 323. [Google Scholar] [CrossRef] [PubMed]
- Sparatore, A.; Perrino, E.; Tazzari, V.; Giustarini, D.; Rossi, R.; Rossoni, G.; Erdmann, K.; Schröder, H.; Del Soldato, P. Pharmacological profile of a novel H2S-releasing aspirin. Free Radic. Biol. Med. 2009, 46, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Sulaieva, O.; Wallace, J.L. Gaseous mediator-based anti-inflammatory drugs. Curr. Opin. Pharmacol. 2015, 25, 1–6. [Google Scholar] [CrossRef]
- Lee, M.; Tazzari, V.; Giustarini, D.; Rossi, R.; Sparatore, A.; Del Soldato, P.; McGeer, E.; McGeer, P.L. Effects of hydrogen sulfide-releasing L-DOPA derivatives on glial activation: Potential for treating Parkinson disease. J. Biol. Chem. 2010, 285, 17318–17328. [Google Scholar] [CrossRef]
- Benedetti, S.; Canino, C.; Tonti, G.; Medda, V.; Calcaterra, P.; Nappi, G.; Salaffi, F.; Canestrari, F. Biomarkers of oxidation, inflammation and cartilage degradation in osteoarthritis patients undergoing sulfur-based spa therapies. Clin. Biochem. 2010, 43, 973–978. [Google Scholar] [CrossRef]
- Costantino, M.; Giuberti, G.; Caraglia, M.; Lombardi, A.; Misso, G.; Abbruzzese, A.; Ciani, F.; Lampa, E. Possible antioxidant role of SPA therapy with chlorine-sulphur-bicarbonate mineral water. Amino Acids 2009, 36, 161–165. [Google Scholar] [CrossRef]
- Benedetti, S.; Benvenuti, F.; Nappi, G.; Fortunati, N.A.; Marino, L.; Aureli, T.; De Luca, S.; Pagliarani, S.; Canestrari, F. Antioxidative effects of sulfurous mineral water: Protection against lipid and protein oxidation. Eur. J. Clin. Nutr. 2009, 63, 106–112. [Google Scholar] [CrossRef]
- Kabil, O.; Vitvitsky, V.; Xie, P.; Banerjee, R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid. Redox Signal. 2011, 15, 363–372. [Google Scholar] [CrossRef]




















































Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Sulfide—Pathological and Physiological Functions in Mammalian Cells. Cells 2023, 12, 2684. https://doi.org/10.3390/cells12232684
Andrés CMC, Pérez de la Lastra JM, Andrés Juan C, Plou FJ, Pérez-Lebeña E. Chemistry of Hydrogen Sulfide—Pathological and Physiological Functions in Mammalian Cells. Cells. 2023; 12(23):2684. https://doi.org/10.3390/cells12232684
Chicago/Turabian StyleAndrés, Celia María Curieses, José Manuel Pérez de la Lastra, Celia Andrés Juan, Francisco J. Plou, and Eduardo Pérez-Lebeña. 2023. "Chemistry of Hydrogen Sulfide—Pathological and Physiological Functions in Mammalian Cells" Cells 12, no. 23: 2684. https://doi.org/10.3390/cells12232684






