NLRP3-Induced NETosis: A Potential Therapeutic Target for Ischemic Thrombotic Diseases?
Abstract
:1. Introduction
2. NETosis: Bridging Immune Defense to Thrombotic Triggers
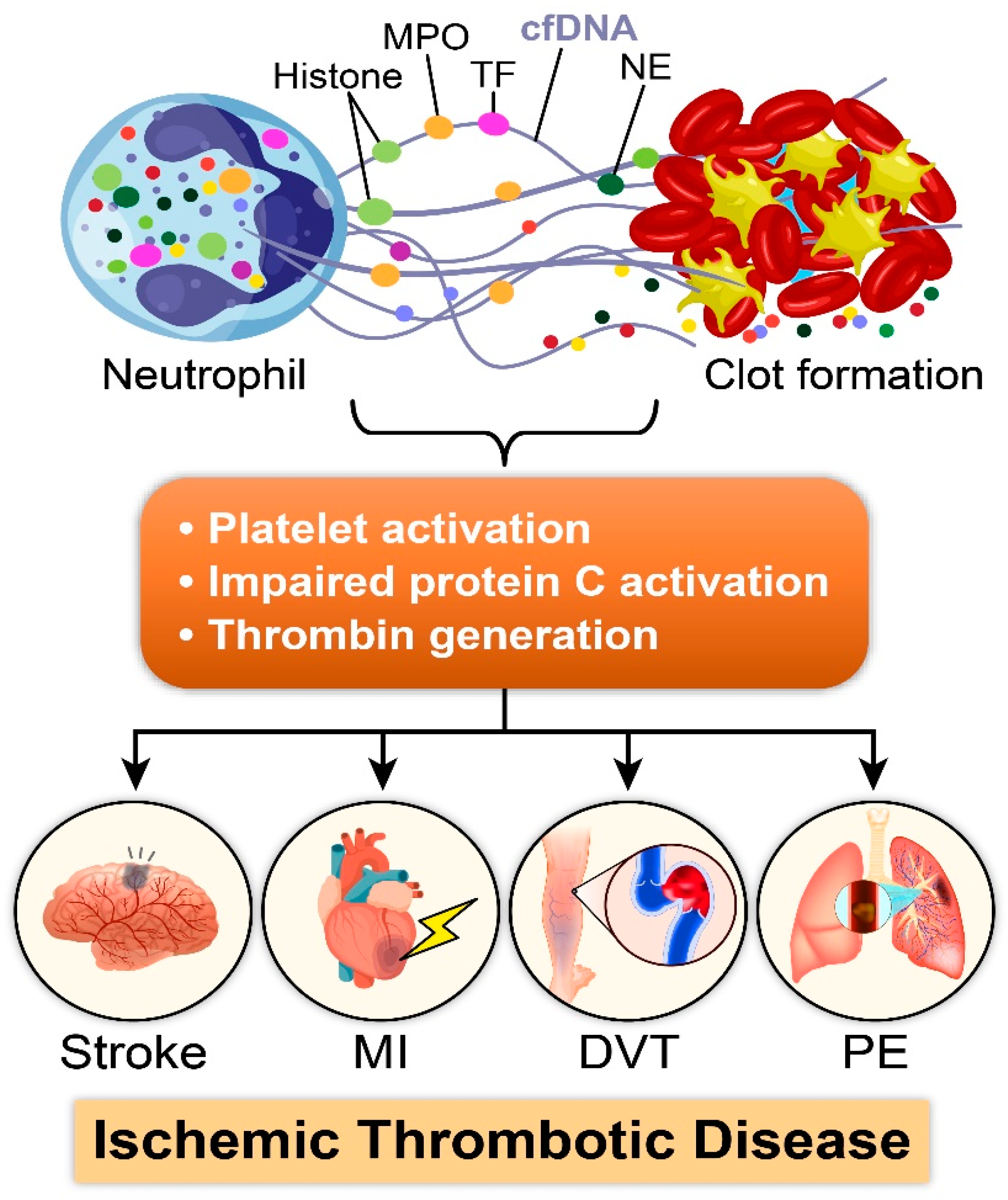
2.1. Role of NETs in Thrombosis: A Multifaceted Mechanism
2.2. Prothrombotic Effects of Individual Components of NETs
2.3. Platelet-Induced NETosis
3. NETosis in Disease Condition
3.1. NETosis in Acute Ischemic Conditions
3.2. NETosis in Cancer-Associated Thrombosis
3.3. NETosis and Age in the Context of COVID-19
4. NETosis in Non-ischemic Conditions
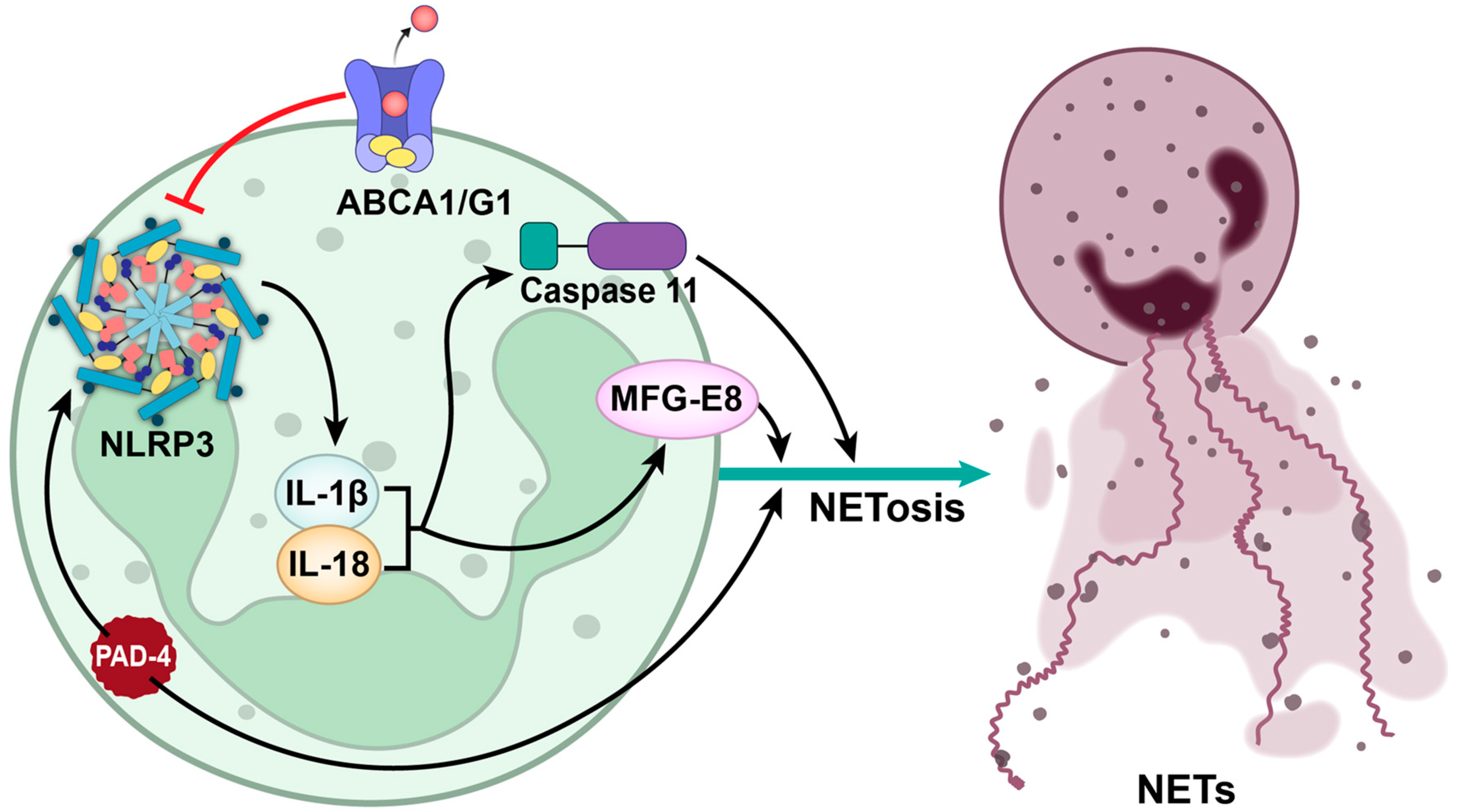
5. NLRP3 Inflammasome: A Key Mediator of NETosis-Driven Thrombosis
Inhibiting NLRP3: A Promising Therapeutic Approach for Thrombotic Disorders
| Compound | Model | Results | IC50 | Reference |
|---|---|---|---|---|
| Glyburide | Bone marrow-derived macrophages treated with LPS and ATP, and blood-derived monocytes treated with LPS and ATP | ↓ secretion of mature IL-1β | 12 μM (monocytes) | [154,163] |
| 2-aminoethoxy diphenylborinate (2APB) | Peritoneal macrophages treated with LPS and ATP | ↓ secretion of mature IL-1β | 67 μM | [162] |
| EC144 | Bone marrow-derived macrophages treated with nigericin and LPS | ↓ formation of ASC specks | 99 nM | [164] |
| MCC950 | Bone marrow-derived macrophages stimulated with LPS and ATP | ↓ secretion of mature IL-1β | 7.5 nM | [165] |
| Compound 44 (ester-substitute of MCC 950) | Peripheral blood mononuclear cells stimulated with LPS and ATP | ↓ secretion of mature IL-1β | 36 nM | [156] |
| Compound 45 (ester-substitute of MCC 950) | Peripheral blood mononuclear cells stimulated with LPS and ATP | ↓ secretion of mature IL-1β | 30 nM | [156] |
| AMS-17 | N9 microglial cells stimulated with LPS | ↓ secretion of mature IL-1β | 2.8 µM of AMS-17 reduced the IL-1β protein level by 30% | [157] |
| JC124 | J774A.1 macrophage cells stimulated with LPS and ATP | ↓ secretion of mature IL-1β | 3.25 µM | [158] |
| Compound 5 | THP-1 macrophage cells stimulated with LPS and ATP | ↓ pyroptosis | 10 µM of compound reduced pyroptosis by 100% | [159] |
| INF58 | THP-1 macrophage cells stimulated with LPS and ATP | NLRP3 ATPase activity | 74 μM | [160] |
| NDT-30805g | Peripheral blood mononuclear cells stimulated with LPS and ATP | ↓ secretion of mature IL-1β | 13 nM | [156] |
| NIC7w | THP-1 macrophage cells stimulated with LPS and nigericin | ↓ secretion of mature IL-1β | 9 µM | [161] |
| NBC6 | Mouse peritoneal macrophages stimulated with LPS and ATP | ↓ secretion of mature IL-1β | 574 nM | [162] |
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Wilkerson, W.R.; Sane, D.C. Aging and thrombosis. Semin. Thromb. Hemost. 2002, 28, 555–568. [Google Scholar] [CrossRef]
- Nurmohamed, M.T.; Büller, H.R.; ten Cate, J.W. Physiological changes due to age. Implications for the prevention and treatment of thrombosis in older patients. Drugs Aging 1994, 5, 20–33. [Google Scholar] [CrossRef]
- Engbers, M.J.; van Hylckama Vlieg, A.; Rosendaal, F.R. Venous thrombosis in the elderly: Incidence, risk factors and risk groups. J. Thromb. Haemost. 2010, 8, 2105–2112. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Scharrig, E.; Carestia, A.; Ferrer, M.F.; Cedola, M.; Pretre, G.; Drut, R.; Picardeau, M.; Schattner, M.; Gomez, R.M. Neutrophil Extracellular Traps are Involved in the Innate Immune Response to Infection with Leptospira. PLoS Negl. Trop. Dis. 2015, 9, e0003927. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Hull, R.D.; Kayali, F.; Ghali, W.A.; Alshab, A.K.; Olson, R.E. Venous thromboembolism according to age: The impact of an aging population. Arch. Intern. Med. 2004, 164, 2260–2265. [Google Scholar] [CrossRef] [PubMed]
- Anderson, F.A., Jr.; Spencer, F.A. Risk factors for venous thromboembolism. Circulation 2003, 107, I9–I16. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Torrero, J.F.; Bounameaux, H.; Pedrajas, J.M.; Lorenzo, A.; Rubio, S.; Kearon, C.; Hernández, L.; Monreal, M. Effects of age on the risk of dying from pulmonary embolism or bleeding during treatment of deep vein thrombosis. J. Vasc. Surg. 2011, 54, 26s–32s. [Google Scholar] [CrossRef] [PubMed]
- Gregson, J.; Kaptoge, S.; Bolton, T.; Pennells, L.; Willeit, P.; Burgess, S.; Bell, S.; Sweeting, M.; Rimm, E.B.; Kabrhel, C.; et al. Cardiovascular Risk Factors Associated With Venous Thromboembolism. JAMA Cardiol. 2019, 4, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Brill, A.; Fuchs, T.A.; Savchenko, A.S.; Thomas, G.M.; Martinod, K.; De Meyer, S.F.; Bhandari, A.A.; Wagner, D.D. Neutrophil extracellular traps promote deep vein thrombosis in mice. J. Thromb. Haemost. 2012, 10, 136–144. [Google Scholar] [CrossRef]
- von Kockritz-Blickwede, M.; Goldmann, O.; Thulin, P.; Heinemann, K.; Norrby-Teglund, A.; Rohde, M.; Medina, E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 2008, 111, 3070–3080. [Google Scholar] [CrossRef]
- Yousefi, S.; Gold, J.A.; Andina, N.; Lee, J.J.; Kelly, A.M.; Kozlowski, E.; Schmid, I.; Straumann, A.; Reichenbach, J.; Gleich, G.J.; et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008, 14, 949–953. [Google Scholar] [CrossRef]
- Kumar, R.; Sonkar, V.K.; Swamy, J.; Ahmed, A.; Sharathkumar, A.A.; Pierce, G.L.; Dayal, S. DNase 1 Protects From Increased Thrombin Generation and Venous Thrombosis During Aging: Cross-Sectional Study in Mice and Humans. J. Am. Heart Assoc. 2022, 11, e021188. [Google Scholar] [CrossRef]
- Dayal, S.; Wilson, K.M.; Motto, D.G.; Miller, F.J., Jr.; Chauhan, A.K.; Lentz, S.R. Hydrogen peroxide promotes aging-related platelet hyperactivation and thrombosis. Circulation 2013, 127, 1308–1316. [Google Scholar] [CrossRef]
- Sonkar, V.K.; Eustes, A.S.; Ahmed, A.; Jensen, M.; Solanki, M.V.; Swamy, J.; Kumar, R.; Fidler, T.P.; Houtman, J.C.D.; Allen, B.G.; et al. Endogenous SOD2 (Superoxide Dismutase) Regulates Platelet-Dependent Thrombin Generation and Thrombosis During Aging. Arter. Thromb. Vasc. Biol. 2023, 43, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, J.; Jiang, Y. Association between hypertension and deep vein thrombosis after orthopedic surgery: A meta-analysis. Eur. J. Med. Res. 2016, 21, 13. [Google Scholar] [CrossRef]
- Kuller, L.H.; Velentgas, P.; Barzilay, J.; Beauchamp, N.J.; O’Leary, D.H.; Savage, P.J. Diabetes mellitus: Subclinical cardiovascular disease and risk of incident cardiovascular disease and all-cause mortality. Arter. Thromb. Vasc. Biol. 2000, 20, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Bennett, M. Aging and atherosclerosis: Mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ. Res. 2012, 111, 245–259. [Google Scholar] [CrossRef]
- Darvall, K.A.; Sam, R.C.; Silverman, S.H.; Bradbury, A.W.; Adam, D.J. Obesity and thrombosis. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 223–233. [Google Scholar] [CrossRef] [PubMed]
- LaVasseur, C.; Neukam, S.; Kartika, T.; Samuelson Bannow, B.; Shatzel, J.; DeLoughery, T.G. Hormonal therapies and venous thrombosis: Considerations for prevention and management. Res. Pr. Thromb. Haemost. 2022, 6, e12763. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Mäkikallio, T.H.; Seidu, S.; de Araújo, C.G.S.; Dey, R.S.; Blom, A.W.; Laukkanen, J.A. Physical activity and risk of venous thromboembolism: Systematic review and meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2020, 35, 431–442. [Google Scholar] [CrossRef]
- North, B.J.; Sinclair, D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Njeim, R.; Azar, W.S.; Fares, A.H.; Azar, S.T.; Kfoury Kassouf, H.; Eid, A.A. NETosis contributes to the pathogenesis of diabetes and its complications. J. Mol. Endocrinol. 2020, 65, R65–R76. [Google Scholar] [CrossRef]
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 2015, 21, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Takei, H.; Araki, A.; Watanabe, H.; Ichinose, A.; Sendo, F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc. Biol. 1996, 59, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Stadler, S.; Correll, S.; Li, P.; Wang, D.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef]
- de Bont, C.M.; Boelens, W.C.; Pruijn, G.J.M. NETosis, complement, and coagulation: A triangular relationship. Cell. Mol. Immunol. 2019, 16, 19–27. [Google Scholar] [CrossRef]
- Martinod, K.; Demers, M.; Fuchs, T.A.; Wong, S.L.; Brill, A.; Gallant, M.; Hu, J.; Wang, Y.; Wagner, D.D. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 8674–8679. [Google Scholar] [CrossRef] [PubMed]
- Martinod, K.; Witsch, T.; Farley, K.; Gallant, M.; Remold-O’Donnell, E.; Wagner, D.D. Neutrophil elastase-deficient mice form neutrophil extracellular traps in an experimental model of deep vein thrombosis. J. Thromb. Haemost. 2016, 14, 551–558. [Google Scholar] [CrossRef]
- Pertiwi, K.R.; de Boer, O.J.; Mackaaij, C.; Pabittei, D.R.; de Winter, R.J.; Li, X.; van der Wal, A.C. Extracellular traps derived from macrophages, mast cells, eosinophils and neutrophils are generated in a time-dependent manner during atherothrombosis. J. Pathol. 2019, 247, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Schorn, C.; Janko, C.; Latzko, M.; Chaurio, R.; Schett, G.; Herrmann, M. Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells. Front. Immunol. 2012, 3, 277. [Google Scholar] [CrossRef] [PubMed]
- Behzadifard, M.; Soleimani, M. NETosis and SARS-COV-2 infection related thrombosis: A narrative review. Thromb. J. 2022, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Ammollo, C.T.; Semeraro, F.; Xu, J.; Esmon, N.L.; Esmon, C.T. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J. Thromb. Haemost. 2011, 9, 1795–1803. [Google Scholar] [CrossRef]
- Urak, K.T.; Blanco, G.N.; Shubham, S.; Lin, L.H.; Dassie, J.P.; Thiel, W.H.; Chen, Y.; Sonkar, V.K.; Lei, B.; Murthy, S.; et al. RNA inhibitors of nuclear proteins responsible for multiple organ dysfunction syndrome. Nat. Commun. 2019, 10, 116. [Google Scholar] [CrossRef]
- Semeraro, F.; Ammollo, C.T.; Morrissey, J.H.; Dale, G.L.; Friese, P.; Esmon, N.L.; Esmon, C.T. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: Involvement of platelet TLR2 and TLR4. Blood 2011, 118, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.; Leslie, B.A.; Stafford, A.R.; Zhou, J.; Fredenburgh, J.C.; Weitz, J.I. Histidine-rich glycoprotein binds DNA and RNA and attenuates their capacity to activate the intrinsic coagulation pathway. Thromb. Haemost. 2016, 115, 89–98. [Google Scholar] [CrossRef]
- Bhagirath, V.C.; Dwivedi, D.J.; Liaw, P.C. Comparison of the Proinflammatory and Procoagulant Properties of Nuclear, Mitochondrial, and Bacterial DNA. Shock 2015, 44, 265–271. [Google Scholar] [CrossRef]
- Gould, T.J.; Vu, T.T.; Swystun, L.L.; Dwivedi, D.J.; Mai, S.H.; Weitz, J.I.; Liaw, P.C. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arter. Thromb. Vasc. Biol. 2014, 34, 1977–1984. [Google Scholar] [CrossRef]
- Longstaff, C.; Varju, I.; Sotonyi, P.; Szabo, L.; Krumrey, M.; Hoell, A.; Bota, A.; Varga, Z.; Komorowicz, E.; Kolev, K. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J. Biol. Chem. 2013, 288, 6946–6956. [Google Scholar] [CrossRef]
- Varjú, I.; Longstaff, C.; Szabó, L.; Farkas, Á.Z.; Varga-Szabó, V.J.; Tanka-Salamon, A.; Machovich, R.; Kolev, K. DNA, histones and neutrophil extracellular traps exert anti-fibrinolytic effects in a plasma environment. Thromb. Haemost. 2015, 113, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Ducroux, C.; Di Meglio, L.; Loyau, S.; Delbosc, S.; Boisseau, W.; Deschildre, C.; Ben Maacha, M.; Blanc, R.; Redjem, H.; Ciccio, G.; et al. Thrombus Neutrophil Extracellular Traps Content Impair tPA-Induced Thrombolysis in Acute Ischemic Stroke. Stroke 2018, 49, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Komissarov, A.A.; Florova, G.; Idell, S. Effects of extracellular DNA on plasminogen activation and fibrinolysis. J. Biol. Chem. 2011, 286, 41949–41962. [Google Scholar] [CrossRef] [PubMed]
- Noubouossie, D.F.; Whelihan, M.F.; Yu, Y.B.; Sparkenbaugh, E.; Pawlinski, R.; Monroe, D.M.; Key, N.S. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood 2017, 129, 1021–1029. [Google Scholar] [CrossRef]
- Stakos, D.A.; Kambas, K.; Konstantinidis, T.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Tsironidou, V.; Giatromanolaki, A.; Skendros, P.; Konstantinides, S.; et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur. Heart J. 2015, 36, 1405–1414. [Google Scholar] [CrossRef]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Invest. 2020, 130, 6151–6157. [Google Scholar] [CrossRef]
- Etulain, J.; Martinod, K.; Wong, S.L.; Cifuni, S.M.; Schattner, M.; Wagner, D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood 2015, 126, 242–246. [Google Scholar] [CrossRef]
- Constantinescu-Bercu, A.; Grassi, L.; Frontini, M.; Salles, C., II; Woollard, K.; Crawley, J.T. Activated alpha(IIb)beta(3) on platelets mediates flow-dependent NETosis via SLC44A2. Elife 2020, 9, e53353. [Google Scholar] [CrossRef]
- Apipongrat, D.; Numbenjapon, T.; Prayoonwiwat, W.; Arnutti, P.; Nathalang, O. Association between SLC44A2 rs2288904 polymorphism and risk of recurrent venous thromboembolism among Thai patients. Thromb. Res. 2019, 174, 163–165. [Google Scholar] [CrossRef]
- Germain, M.; Chasman, D.I.; de Haan, H.; Tang, W.; Lindström, S.; Weng, L.C.; de Andrade, M.; de Visser, M.C.; Wiggins, K.L.; Suchon, P.; et al. Meta-analysis of 65,734 individuals identifies TSPAN15 and SLC44A2 as two susceptibility loci for venous thromboembolism. Am. J. Hum. Genet. 2015, 96, 532–542. [Google Scholar] [CrossRef]
- Hinds, D.A.; Buil, A.; Ziemek, D.; Martinez-Perez, A.; Malik, R.; Folkersen, L.; Germain, M.; Malarstig, A.; Brown, A.; Soria, J.M.; et al. Genome-wide association analysis of self-reported events in 6135 individuals and 252 827 controls identifies 8 loci associated with thrombosis. Hum Mol Genet 2016, 25, 1867–1874. [Google Scholar] [CrossRef]
- Caudrillier, A.; Kessenbrock, K.; Gilliss, B.M.; Nguyen, J.X.; Marques, M.B.; Monestier, M.; Toy, P.; Werb, Z.; Looney, M.R. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Invest. 2012, 122, 2661–2671. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, M.; Bona, E.; Novello, G.; Migliario, M.; Renò, F. Aging hampers neutrophil extracellular traps (NETs) efficacy. Aging Clin. Exp. Res. 2022, 34, 2345–2353. [Google Scholar] [CrossRef]
- Martinod, K.; Witsch, T.; Erpenbeck, L.; Savchenko, A.; Hayashi, H.; Cherpokova, D.; Gallant, M.; Mauler, M.; Cifuni, S.M.; Wagner, D.D. Peptidylarginine deiminase 4 promotes age-related organ fibrosis. J. Exp. Med. 2017, 214, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, W.; Kolaczkowska, E. Age is the work of art? Impact of neutrophil and organism age on neutrophil extracellular trap formation. Cell Tissue Res. 2018, 371, 473–488. [Google Scholar] [CrossRef]
- Matsuda, Y.; Itabashi, M.; Tachibana, Y.; Sugihara, T.; Sakashita, Y.; Matsubara, T.; Murayama, S.; Yumura, W.; Shimizu, A.; Takei, T.; et al. Citrullinated histone H3 expression in anti-neutrophil cytoplasmic antibody-associated vasculitis in older Japanese autopsy patients. Geriatr. Gerontol. Int. 2019, 19, 259–264. [Google Scholar] [CrossRef]
- Matsuda, Y.; Hamayasu, H.; Seki, A.; Nonaka, K.; Wang, T.; Matsumoto, T.; Hamano, Y.; Sumikura, H.; Kumasaka, T.; Murayama, S.; et al. Presence of Citrullinated Histone H3-Positive Neutrophils in Microscopic Polyangiitis from the Early Phase: An Autopsy Proven Case. Pathol. Int. 2016, 66, 466–471. [Google Scholar] [CrossRef]
- Shah, M.; He, Z.; Rauf, A.; Beikoghli Kalkhoran, S.; Heiestad, C.M.; Stenslokken, K.O.; Parish, C.R.; Soehnlein, O.; Arjun, S.; Davidson, S.M.; et al. Extracellular histones are a target in myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 2022, 118, 1115–1125. [Google Scholar] [CrossRef]
- De Meyer, S.F.; Suidan, G.L.; Fuchs, T.A.; Monestier, M.; Wagner, D.D. Extracellular chromatin is an important mediator of ischemic stroke in mice. Arter. Thromb. Vasc. Biol. 2012, 32, 1884–1891. [Google Scholar] [CrossRef]
- Valles, J.; Lago, A.; Santos, M.T.; Latorre, A.M.; Tembl, J.I.; Salom, J.B.; Nieves, C.; Moscardo, A. Neutrophil extracellular traps are increased in patients with acute ischemic stroke: Prognostic significance. Thromb. Haemost. 2017, 117, 1919–1929. [Google Scholar] [CrossRef]
- Demyanets, S.; Stojkovic, S.; Mauracher, L.M.; Kopp, C.W.; Wojta, J.; Thaler, J.; Panzer, S.; Gremmel, T. Surrogate Markers of Neutrophil Extracellular Trap Formation are Associated with Ischemic Outcomes and Platelet Activation after Peripheral Angioplasty and Stenting. J. Clin. Med. 2020, 9, 304. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Brill, A.; Wagner, D.D. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arter. Thromb. Vasc. Biol. 2012, 32, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Borissoff, J.I.; Joosen, I.A.; Versteylen, M.O.; Brill, A.; Fuchs, T.A.; Savchenko, A.S.; Gallant, M.; Martinod, K.; Ten Cate, H.; Hofstra, L.; et al. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arter. Thromb. Vasc. Biol. 2013, 33, 2032–2040. [Google Scholar] [CrossRef]
- Franck, G.; Mawson, T.L.; Folco, E.J.; Molinaro, R.; Ruvkun, V.; Engelbertsen, D.; Liu, X.; Tesmenitsky, Y.; Shvartz, E.; Sukhova, G.K.; et al. Roles of PAD4 and NETosis in Experimental Atherosclerosis and Arterial Injury: Implications for Superficial Erosion. Circ. Res. 2018, 123, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Liu, H.; Long, Y.; Wan, W.; Li, Q.; Zhu, W.; Wu, Y. The association between circulating neutrophil extracellular trap related biomarkers and retinal vein occlusion incidence: A case-control pilot study. Exp. Eye Res. 2021, 210, 108702. [Google Scholar] [CrossRef] [PubMed]
- Fukui, Y.; Kawashima, M.; Kawaguchi, K.; Takeuchi, M.; Hirata, M.; Kataoka, T.R.; Sakurai, T.; Kataoka, M.; Kanao, S.; Nakamoto, Y.; et al. Granulocyte-colony-stimulating factor-producing metaplastic carcinoma of the breast with significant elevation of serum interleukin-17 and vascular endothelial growth factor levels. Int. Cancer Conf. J. 2018, 7, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, F.; Xu, Z.; Chen, C.; Wu, X.; Li, G.; Li, J. Expression of granulocyte colony stimulating factor receptor in human colorectal cancer. Postgrad. Med. J. 2005, 81, 333–337. [Google Scholar] [CrossRef]
- Uematsu, T.; Tsuchie, K.; Ukai, K.; Kimoto, E.; Funakawa, T.; Mizuno, R. Granulocyte-colony stimulating factor produced by pancreatic carcinoma. Int. J. Pancreatol. 1996, 19, 135–139. [Google Scholar] [CrossRef]
- Kowanetz, M.; Wu, X.; Lee, J.; Tan, M.; Hagenbeek, T.; Qu, X.; Yu, L.; Ross, J.; Korsisaari, N.; Cao, T.; et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 21248–21255. [Google Scholar] [CrossRef]
- Jiang, X.; Lopez, A.; Holyoake, T.; Eaves, A.; Eaves, C. Autocrine production and action of IL-3 and granulocyte colony-stimulating factor in chronic myeloid leukemia. Proc. Natl. Acad. Sci. USA 1999, 96, 12804–12809. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Krause, D.S.; Schatzberg, D.; Martinod, K.; Voorhees, J.R.; Fuchs, T.A.; Scadden, D.T.; Wagner, D.D. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13076–13081. [Google Scholar] [CrossRef] [PubMed]
- Mauracher, L.M.; Posch, F.; Martinod, K.; Grilz, E.; Daullary, T.; Hell, L.; Brostjan, C.; Zielinski, C.; Ay, C.; Wagner, D.D.; et al. Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J. Thromb. Haemost. 2018, 16, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Guy, A.; Favre, S.; Labrouche-Colomer, S.; Deloison, L.; Gourdou-Latyszenok, V.; Renault, M.A.; Riviere, E.; James, C. High circulating levels of MPO-DNA are associated with thrombosis in patients with MPN. Leukemia 2019, 33, 2544–2548. [Google Scholar] [CrossRef] [PubMed]
- Thalin, C.; Demers, M.; Blomgren, B.; Wong, S.L.; von Arbin, M.; von Heijne, A.; Laska, A.C.; Wallen, H.; Wagner, D.D.; Aspberg, S. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb. Res. 2016, 139, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Oklu, R.; Sheth, R.A.; Wong, K.H.K.; Jahromi, A.H.; Albadawi, H. Neutrophil extracellular traps are increased in cancer patients but does not associate with venous thrombosis. Cardiovasc. Diagn. Ther. 2017, 7, S140–S149. [Google Scholar] [CrossRef]
- Kumar, R.; Katare, P.B.; Lentz, S.R.; Modi, A.J.; Sharathkumar, A.A.; Dayal, S. Thrombotic potential during pediatric acute lymphoblastic leukemia induction: Role of cell-free DNA. Res. Pr. Thromb. Haemost. 2021, 5, e12557. [Google Scholar] [CrossRef] [PubMed]
- Hisada, Y.; Grover, S.P.; Maqsood, A.; Houston, R.; Ay, C.; Noubouossie, D.F.; Cooley, B.C.; Wallen, H.; Key, N.S.; Thalin, C.; et al. Neutrophils and neutrophil extracellular traps enhance venous thrombosis in mice bearing human pancreatic tumors. Haematologica 2019, 105, 218–225. [Google Scholar] [CrossRef]
- Leal, A.C.; Mizurini, D.M.; Gomes, T.; Rochael, N.C.; Saraiva, E.M.; Dias, M.S.; Werneck, C.C.; Sielski, M.S.; Vicente, C.P.; Monteiro, R.Q. Tumor-Derived Exosomes Induce the Formation of Neutrophil Extracellular Traps: Implications For The Establishment of Cancer-Associated Thrombosis. Sci. Rep. 2017, 7, 6438. [Google Scholar] [CrossRef]
- Varady, C.B.S.; Oliveira, A.C.; Monteiro, R.Q.; Gomes, T. Recombinant human DNase I for the treatment of cancer-associated thrombosis: A pre-clinical study. Thromb. Res. 2021, 203, 131–137. [Google Scholar] [CrossRef]
- Gomes, T.; Varady, C.B.S.; Lourenco, A.L.; Mizurini, D.M.; Rondon, A.M.R.; Leal, A.C.; Goncalves, B.S.; Bou-Habib, D.C.; Medei, E.; Monteiro, R.Q. IL-1beta Blockade Attenuates Thrombosis in a Neutrophil Extracellular Trap-Dependent Breast Cancer Model. Front. Immunol. 2019, 10, 2088. [Google Scholar] [CrossRef] [PubMed]
- Wolach, O.; Sellar, R.S.; Martinod, K.; Cherpokova, D.; McConkey, M.; Chappell, R.J.; Silver, A.J.; Adams, D.; Castellano, C.A.; Schneider, R.K.; et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci. Transl. Med. 2018, 10, eaan8292. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Estes, S.K.; Ali, R.A.; Gandhi, A.A.; Yalavarthi, S.; Shi, H.; Sule, G.; Gockman, K.; Madison, J.A.; Zuo, M.; et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci. Transl. Med. 2020, 12, eabd3876. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Zuo, M.; Yalavarthi, S.; Gockman, K.; Madison, J.A.; Shi, H.; Woodard, W.; Lezak, S.P.; Lugogo, N.L.; Knight, J.S.; et al. Neutrophil extracellular traps and thrombosis in COVID-19. J. Thromb. Thrombolysis 2021, 51, 446–453. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Ng, H.; Havervall, S.; Rosell, A.; Aguilera, K.; Parv, K.; von Meijenfeldt, F.A.; Lisman, T.; Mackman, N.; Thalin, C.; Phillipson, M. Circulating Markers of Neutrophil Extracellular Traps Are of Prognostic Value in Patients With COVID-19. Arter. Thromb. Vasc. Biol. 2021, 41, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Janiuk, K.; Jabłońska, E.; Garley, M. Significance of NETs Formation in COVID-19. Cells 2021, 10, 151. [Google Scholar] [CrossRef]
- Yaqinuddin, A.; Kvietys, P.; Kashir, J. COVID-19: Role of neutrophil extracellular traps in acute lung injury. Respir. Investig. 2020, 58, 419–420. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, X.; Liu, X. NETosis and Neutrophil Extracellular Traps in COVID-19: Immunothrombosis and Beyond. Front. Immunol. 2022, 13, 838011. [Google Scholar] [CrossRef] [PubMed]
- Hanna, E.B.; Rossen, J.; Eustes, A.S.; Dayal, S. Heavy lone coronary artery thrombosis treated by stent retriever, in the setting of COVID-19 infection. Catheter. Cardiovasc. Interv. 2022, 99, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Schurink, B.; Roos, E.; Radonic, T.; Barbe, E.; Bouman, C.S.C.; de Boer, H.H.; de Bree, G.J.; Bulle, E.B.; Aronica, E.M.; Florquin, S.; et al. Viral presence and immunopathology in patients with lethal COVID-19: A prospective autopsy cohort study. Lancet Microbe 2020, 1, e290–e299. [Google Scholar] [CrossRef]
- Morgan, W.H.; Hazelton, M.L.; Yu, D.Y. Retinal venous pulsation: Expanding our understanding and use of this enigmatic phenomenon. Prog. Retin. Eye Res. 2016, 55, 82–107. [Google Scholar] [CrossRef] [PubMed]
- Freund, K.B.; Sarraf, D.; Leong, B.C.S.; Garrity, S.T.; Vupparaboina, K.K.; Dansingani, K.K. Association of Optical Coherence Tomography Angiography of Collaterals in Retinal Vein Occlusion With Major Venous Outflow Through the Deep Vascular Complex. JAMA Ophthalmol. 2018, 136, 1262–1270. [Google Scholar] [CrossRef]
- Pietronigro, E.C.; Della Bianca, V.; Zenaro, E.; Constantin, G. NETosis in Alzheimer’s Disease. Front. Immunol. 2017, 8, 211. [Google Scholar] [CrossRef]
- Byun, D.J.; Lee, J.; Yu, J.W.; Hyun, Y.M. NLRP3 Exacerbate NETosis-Associated Neuroinflammation in an LPS-Induced Inflamed Brain. Immune Netw. 2023, 23, e27. [Google Scholar] [CrossRef]
- Grabcanovic-Musija, F.; Obermayer, A.; Stoiber, W.; Krautgartner, W.D.; Steinbacher, P.; Winterberg, N.; Bathke, A.C.; Klappacher, M.; Studnicka, M. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir. Res. 2015, 16, 59. [Google Scholar] [CrossRef]
- Lee, K.H.; Kronbichler, A.; Park, D.D.; Park, Y.; Moon, H.; Kim, H.; Choi, J.H.; Choi, Y.; Shim, S.; Lyu, I.S.; et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun. Rev. 2017, 16, 1160–1173. [Google Scholar] [CrossRef]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013, 5, 178ra140. [Google Scholar] [CrossRef]
- Song, W.; Ye, J.; Pan, N.; Tan, C.; Herrmann, M. Neutrophil Extracellular Traps Tied to Rheumatoid Arthritis: Points to Ponder. Front. Immunol. 2020, 11, 578129. [Google Scholar] [CrossRef] [PubMed]
- Corsiero, E.; Pratesi, F.; Prediletto, E.; Bombardieri, M.; Migliorini, P. NETosis as Source of Autoantigens in Rheumatoid Arthritis. Front. Immunol. 2016, 7, 485. [Google Scholar] [CrossRef]
- Salazar-Gonzalez, H.; Zepeda-Hernandez, A.; Melo, Z.; Saavedra-Mayorga, D.E.; Echavarria, R. Neutrophil Extracellular Traps in the Establishment and Progression of Renal Diseases. Medicina 2019, 55, 431. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Yang, S.; Li, T.; Gao, R.; Hu, J.; Luo, T.; Qing, H.; Zhen, Q.; Hu, R.; Li, X.; et al. Role of neutrophil extracellular traps in chronic kidney injury induced by bisphenol-A. J. Endocrinol. 2019, 241, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Moutsopoulos, N.M.; Hajishengallis, E.; Chavakis, T. Immune and regulatory functions of neutrophils in inflammatory bone loss. Semin. Immunol. 2016, 28, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- de Zoete, M.R.; Flavell, R.A. Interactions between Nod-Like Receptors and Intestinal Bacteria. Front. Immunol. 2013, 4, 462. [Google Scholar] [CrossRef]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.; Durco, F.; Miller-Ocuin, J.L.; Takedai, T.; Shankar, S.; Liang, X.; Liu, X.; Cui, X.; Sachdev, U.; Rath, D.; et al. The NLRP3 inflammasome and bruton’s tyrosine kinase in platelets co-regulate platelet activation, aggregation, and in vitro thrombus formation. Biochem. Biophys. Res. Commun. 2017, 483, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Allen, I.C.; Scull, M.A.; Moore, C.B.; Holl, E.K.; McElvania-TeKippe, E.; Taxman, D.J.; Guthrie, E.H.; Pickles, R.J.; Ting, J.P. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 2009, 30, 556–565. [Google Scholar] [CrossRef]
- Gross, O.; Poeck, H.; Bscheider, M.; Dostert, C.; Hannesschläger, N.; Endres, S.; Hartmann, G.; Tardivel, A.; Schweighoffer, E.; Tybulewicz, V.; et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 2009, 459, 433–436. [Google Scholar] [CrossRef]
- Kanneganti, T.D.; Body-Malapel, M.; Amer, A.; Park, J.H.; Whitfield, J.; Franchi, L.; Taraporewala, Z.F.; Miller, D.; Patton, J.T.; Inohara, N.; et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 2006, 281, 36560–36568. [Google Scholar] [CrossRef]
- Menu, P.; Vince, J.E. The NLRP3 inflammasome in health and disease: The good, the bad and the ugly. Clin. Exp. Immunol. 2011, 166, 1–15. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Carmona-Rivera, C.; Smith, C.K.; Kaplan, M.J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 2013, 190, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Caution, K.; Young, N.; Robledo-Avila, F.; Krause, K.; Abu Khweek, A.; Hamilton, K.; Badr, A.; Vaidya, A.; Daily, K.; Gosu, H.; et al. Caspase-11 Mediates Neutrophil Chemotaxis and Extracellular Trap Formation During Acute Gouty Arthritis Through Alteration of Cofilin Phosphorylation. Front. Immunol. 2019, 10, 2519. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Jiao, J.; Liu, J.; Huang, M.; Hu, Y.; Ran, W.; Yan, L.; Xiong, Y.; Li, M.; Quan, Z.; et al. MFG-E8 accelerates wound healing in diabetes by regulating “NLRP3 inflammasome-neutrophil extracellular traps” axis. Cell Death Discov. 2020, 6, 84. [Google Scholar] [CrossRef]
- Yalcinkaya, M.; Fotakis, P.; Liu, W.; Endo-Umeda, K.; Dou, H.; Abramowicz, S.; Xiao, T.; Libby, P.; Wang, N.; Tall, A.R.; et al. Cholesterol accumulation in macrophages drives NETosis in atherosclerotic plaques via IL-1β secretion. Cardiovasc. Res. 2023, 119, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Münzer, P.; Negro, R.; Fukui, S.; di Meglio, L.; Aymonnier, K.; Chu, L.; Cherpokova, D.; Gutch, S.; Sorvillo, N.; Shi, L.; et al. NLRP3 Inflammasome Assembly in Neutrophils Is Supported by PAD4 and Promotes NETosis Under Sterile Conditions. Front. Immunol. 2021, 12, 683803. [Google Scholar] [CrossRef]
- Yang, S.; Feng, Y.; Chen, L.; Wang, Z.; Chen, J.; Ni, Q.; Guo, X.; Zhang, L.; Xue, G. Disulfiram accelerates diabetic foot ulcer healing by blocking NET formation via suppressing the NLRP3/Caspase-1/GSDMD pathway. Transl. Res. 2023, 254, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Aymonnier, K.; Ng, J.; Fredenburgh, L.E.; Zambrano-Vera, K.; Münzer, P.; Gutch, S.; Fukui, S.; Desjardins, M.; Subramaniam, M.; Baron, R.M.; et al. Inflammasome activation in neutrophils of patients with severe COVID-19. Blood Adv. 2022, 6, 2001–2013. [Google Scholar] [CrossRef]
- Harper, B.E.; Wills, R.; Pierangeli, S.S. Pathophysiological mechanisms in antiphospholipid syndrome. Int. J. Clin. Rheumtol 2011, 6, 157–171. [Google Scholar] [CrossRef]
- Wilson, W.A.; Gharavi, A.E.; Koike, T.; Lockshin, M.D.; Branch, D.W.; Piette, J.C.; Brey, R.; Derksen, R.; Harris, E.N.; Hughes, G.R.; et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: Report of an international workshop. Arthritis Rheum. 1999, 42, 1309–1311. [Google Scholar] [CrossRef]
- Martirosyan, A.; Petrek, M.; Navratilova, Z.; Blbulyan, A.; Boyajyan, A.; Manukyan, G. Differential regulation of proinflammatory mediators following LPS- and ATP-induced activation of monocytes from patients with antiphospholipid syndrome. Biomed. Res. Int. 2015, 2015, 292851. [Google Scholar] [CrossRef]
- Müller-Calleja, N.; Köhler, A.; Siebald, B.; Canisius, A.; Orning, C.; Radsak, M.; Stein, P.; Mönnikes, R.; Lackner, K.J. Cofactor-independent antiphospholipid antibodies activate the NLRP3-inflammasome via endosomal NADPH-oxidase: Implications for the antiphospholipid syndrome. Thromb. Haemost. 2015, 113, 1071–1083. [Google Scholar] [CrossRef]
- He, G.; Tan, W.; Wang, B.; Chen, J.; Li, G.; Zhu, S.; Xie, J.; Xu, B. Increased M1 Macrophages Infiltration Is Associated with Thrombogenesis in Rheumatic Mitral Stenosis Patients with Atrial Fibrillation. PLoS ONE 2016, 11, e0149910. [Google Scholar] [CrossRef]
- Vogel, S.; Kamimura, S.; Arora, T.; Smith, M.L.; Almeida, L.E.F.; Combs, C.A.; Thein, S.L.; Quezado, Z.M.N. NLRP3 inflammasome and bruton tyrosine kinase inhibition interferes with upregulated platelet aggregation and in vitro thrombus formation in sickle cell mice. Biochem. Biophys. Res. Commun. 2021, 555, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Usui-Kawanishi, F.; Komada, T.; Karasawa, T.; Kamata, R.; Yamada, N.; Kimura, H.; Dezaki, K.; Ohmori, T.; Takahashi, M. ASC regulates platelet activation and contributes to thrombus formation independent of NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2020, 531, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Holy, E.W.; Akhmedov, A.; Bonetti, N.R.; Nietlispach, F.; Matter, C.M.; Mach, F.; Montecucco, F.; Beer, J.H.; Paneni, F.; et al. Interleukin-1β Mediates Arterial Thrombus Formation via NET-Associated Tissue Factor. J. Clin. Med. 2019, 8, 2072. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Wu, X.; Luo, Q.; Wei, G.; Xu, M.; Wu, Y.; Liu, Y.; Li, X.; Zi, J.; Ju, W.; et al. NLRP3 regulates platelet integrin αIIbβ3 outside-in signaling, hemostasis and arterial thrombosis. Haematologica 2018, 103, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Chi, L.; Zhao, R.; Tourdot, B.E.; Yalavarthi, S.; Jacobs, B.N.; Banka, A.; Liao, H.; Koonse, S.; Anyanwu, A.C.; et al. Ectonucleotidase tri(di)phosphohydrolase-1 (ENTPD-1) disrupts inflammasome/interleukin 1β-driven venous thrombosis. J. Clin. Invest. 2019, 129, 2872–2877. [Google Scholar] [CrossRef]
- Hyman, M.C.; Petrovic-Djergovic, D.; Visovatti, S.H.; Liao, H.; Yanamadala, S.; Bouïs, D.; Su, E.J.; Lawrence, D.A.; Broekman, M.J.; Marcus, A.J.; et al. Self-regulation of inflammatory cell trafficking in mice by the leukocyte surface apyrase CD39. J. Clin. Invest. 2009, 119, 1136–1149. [Google Scholar] [CrossRef]
- Kanthi, Y.; Hyman, M.C.; Liao, H.; Baek, A.E.; Visovatti, S.H.; Sutton, N.R.; Goonewardena, S.N.; Neral, M.K.; Jo, H.; Pinsky, D.J. Flow-dependent expression of ectonucleotide tri(di)phosphohydrolase-1 and suppression of atherosclerosis. J. Clin. Invest. 2015, 125, 3027–3036. [Google Scholar] [CrossRef]
- Pinsky, D.J.; Broekman, M.J.; Peschon, J.J.; Stocking, K.L.; Fujita, T.; Ramasamy, R.; Connolly, E.S., Jr.; Huang, J.; Kiss, S.; Zhang, Y.; et al. Elucidation of the thromboregulatory role of CD39/ectoapyrase in the ischemic brain. J. Clin. Invest. 2002, 109, 1031–1040. [Google Scholar] [CrossRef]
- Gupta, N.; Sahu, A.; Prabhakar, A.; Chatterjee, T.; Tyagi, T.; Kumari, B.; Khan, N.; Nair, V.; Bajaj, N.; Sharma, M.; et al. Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc. Natl. Acad. Sci. USA 2017, 114, 4763–4768. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; He, T.; Liao, P.; Zhuang, K.; Yan, X.; Liu, F. 17-Allylamino-Demethoxygeldanamycin Ameliorate Microthrombosis Via HSP90/RIP3/NLRP3 Pathway After Subarachnoid Hemorrhage in Rats. Acta Neurochir. Suppl. 2020, 127, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.N.; Wang, X.; Wang, J.; Yang, Z.; Li, S.; Yang, J.; Liu, L.; Lei, X.; Shao, F. Chemical probing reveals insights into the signaling mechanism of inflammasome activation. Cell Res. 2010, 20, 1289–1305. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. J. Biomed. Biotechnol. 2012, 2012, 247597. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.Q.; Zhang, C.C.; Sun, X.L.; Cheng, X.X.; Wang, J.B.; Zhang, Y.D.; Xu, J.; Zou, H.Q. Antimalarial drug artemisinin extenuates amyloidogenesis and neuroinflammation in APPswe/PS1dE9 transgenic mice via inhibition of nuclear factor-κB and NLRP3 inflammasome activation. CNS Neurosci. Ther. 2013, 19, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Juliana, C.; Fernandes-Alnemri, T.; Wu, J.; Datta, P.; Solorzano, L.; Yu, J.W.; Meng, R.; Quong, A.A.; Latz, E.; Scott, C.P.; et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 2010, 285, 9792–9802. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.Y.; Zhang, D.F.; Zhong, X.; Du, K.; Qian, P.; Yao, W.F.; Gao, H.; Wei, M.J. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci. Ther. 2019, 25, 575–590. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, B.; Wei, F.; Ding, M.; Luo, Z.; Han, X.; Tan, X. Gegen Qinlian pills alleviate carrageenan-induced thrombosis in mice model by regulating the HMGB1/NF-κB/NLRP3 signaling. Phytomedicine 2022, 100, 154083. [Google Scholar] [CrossRef]
- Fei, J.; Qin, X.; Ma, H.; Zhang, X.; Wang, H.; Han, J.; Yu, C.; Jiang, J. Resveratrol Ameliorates Deep Vein Thrombosis-Induced Inflammatory Response Through Inhibiting HIF-1α/NLRP3 Pathway. Inflammation 2022, 45, 2268–2279. [Google Scholar] [CrossRef]
- Everett, B.M.; MacFadyen, J.G.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Inhibition of Interleukin-1β and Reduction in Atherothrombotic Cardiovascular Events in the CANTOS Trial. J. Am. Coll. Cardiol. 2020, 76, 1660–1670. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Thuren, T.; Libby, P. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1β inhibition with canakinumab: Further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur. Heart J. 2020, 41, 2153–2163. [Google Scholar] [CrossRef]
- Nordeng, J.; Schandiz, H.; Solheim, S.; Åkra, S.; Hoffman, P.; Roald, B.; Bendz, B.; Arnesen, H.; Helseth, R.; Seljeflot, I. The Inflammasome Signaling Pathway Is Actively Regulated and Related to Myocardial Damage in Coronary Thrombi from Patients with STEMI. Mediat. Inflamm. 2021, 2021, 5525917. [Google Scholar] [CrossRef] [PubMed]
- Perregaux, D.G.; McNiff, P.; Laliberte, R.; Hawryluk, N.; Peurano, H.; Stam, E.; Eggler, J.; Griffiths, R.; Dombroski, M.A.; Gabel, C.A. Identification and characterization of a novel class of interleukin-1 post-translational processing inhibitors. J. Pharmacol. Exp. Ther. 2001, 299, 187–197. [Google Scholar] [PubMed]
- Coll, R.C.; Robertson, A.A.; Chae, J.J.; Higgins, S.C.; Muñoz-Planillo, R.; Inserra, M.C.; Vetter, I.; Dungan, L.S.; Monks, B.G.; Stutz, A.; et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015, 21, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.; Boutard, N.; Brzozka, K.; Bugaj, M.; Chmielewski, S.; Cierpich, A.; Doedens, J.R.; Fabritius, C.R.Y.; Gabel, C.A.; Galezowski, M.; et al. Discovery of a series of ester-substituted NLRP3 inflammasome inhibitors. Bioorg Med. Chem. Lett. 2020, 30, 127560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sajith, A.M.; Xu, X.; Jiang, J.; Phillip Bowen, J.; Kulkarni, A.; Hao, J. Targeting NLRP3 signaling by a novel-designed sulfonylurea compound for inhibition of microglial inflammation. Bioorg Med. Chem. 2022, 58, 116645. [Google Scholar] [CrossRef]
- Fulp, J.; He, L.; Toldo, S.; Jiang, Y.; Boice, A.; Guo, C.; Li, X.; Rolfe, A.; Sun, D.; Abbate, A.; et al. Structural Insights of Benzenesulfonamide Analogues as NLRP3 Inflammasome Inhibitors: Design, Synthesis, and Biological Characterization. J. Med. Chem. 2018, 61, 5412–5423. [Google Scholar] [CrossRef]
- Cocco, M.; Garella, D.; Di Stilo, A.; Borretto, E.; Stevanato, L.; Giorgis, M.; Marini, E.; Fantozzi, R.; Miglio, G.; Bertinaria, M. Electrophilic warhead-based design of compounds preventing NLRP3 inflammasome-dependent pyroptosis. J. Med. Chem. 2014, 57, 10366–10382. [Google Scholar] [CrossRef]
- Cocco, M.; Miglio, G.; Giorgis, M.; Garella, D.; Marini, E.; Costale, A.; Regazzoni, L.; Vistoli, G.; Orioli, M.; Massulaha-Ahmed, R.; et al. Design, Synthesis, and Evaluation of Acrylamide Derivatives as Direct NLRP3 Inflammasome Inhibitors. ChemMedChem 2016, 11, 1790–1803. [Google Scholar] [CrossRef]
- Haseeb, M.; Javaid, N.; Yasmeen, F.; Jeong, U.; Han, J.H.; Yoon, J.; Seo, J.Y.; Heo, J.K.; Shin, H.C.; Kim, M.S.; et al. Novel Small-Molecule Inhibitor of NLRP3 Inflammasome Reverses Cognitive Impairment in an Alzheimer’s Disease Model. ACS Chem. Neurosci. 2022, 13, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.G.; Rivers-Auty, J.; Daniels, M.J.D.; White, C.S.; Schwalbe, C.H.; Schilling, T.; Hammadi, H.; Jaiyong, P.; Spencer, N.G.; England, H.; et al. Boron-Based Inhibitors of the NLRP3 Inflammasome. Cell Chem. Biol. 2017, 24, 1321–1335.e1325. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Mueller, J.L.; Vitari, A.C.; Misaghi, S.; Fedorova, A.; Deshayes, K.; Lee, W.P.; Hoffman, H.M.; Dixit, V.M. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J. Cell Biol. 2009, 187, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Nizami, S.; Arunasalam, K.; Green, J.; Cook, J.; Lawrence, C.B.; Zarganes-Tzitzikas, T.; Davis, J.B.; Di Daniel, E.; Brough, D. Inhibition of the NLRP3 inflammasome by HSP90 inhibitors. Immunology 2021, 162, 84–91. [Google Scholar] [CrossRef]
- Dempsey, C.; Rubio Araiz, A.; Bryson, K.J.; Finucane, O.; Larkin, C.; Mills, E.L.; Robertson, A.A.B.; Cooper, M.A.; O’Neill, L.A.J.; Lynch, M.A. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice. Brain Behav. Immun. 2017, 61, 306–316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Patil, G.; Dayal, S. NLRP3-Induced NETosis: A Potential Therapeutic Target for Ischemic Thrombotic Diseases? Cells 2023, 12, 2709. https://doi.org/10.3390/cells12232709
Kumar R, Patil G, Dayal S. NLRP3-Induced NETosis: A Potential Therapeutic Target for Ischemic Thrombotic Diseases? Cells. 2023; 12(23):2709. https://doi.org/10.3390/cells12232709
Chicago/Turabian StyleKumar, Rahul, Gokul Patil, and Sanjana Dayal. 2023. "NLRP3-Induced NETosis: A Potential Therapeutic Target for Ischemic Thrombotic Diseases?" Cells 12, no. 23: 2709. https://doi.org/10.3390/cells12232709
APA StyleKumar, R., Patil, G., & Dayal, S. (2023). NLRP3-Induced NETosis: A Potential Therapeutic Target for Ischemic Thrombotic Diseases? Cells, 12(23), 2709. https://doi.org/10.3390/cells12232709






