Modulating Growth Factor Receptor Signaling to Promote Corneal Epithelial Homeostasis
Abstract
1. Introduction
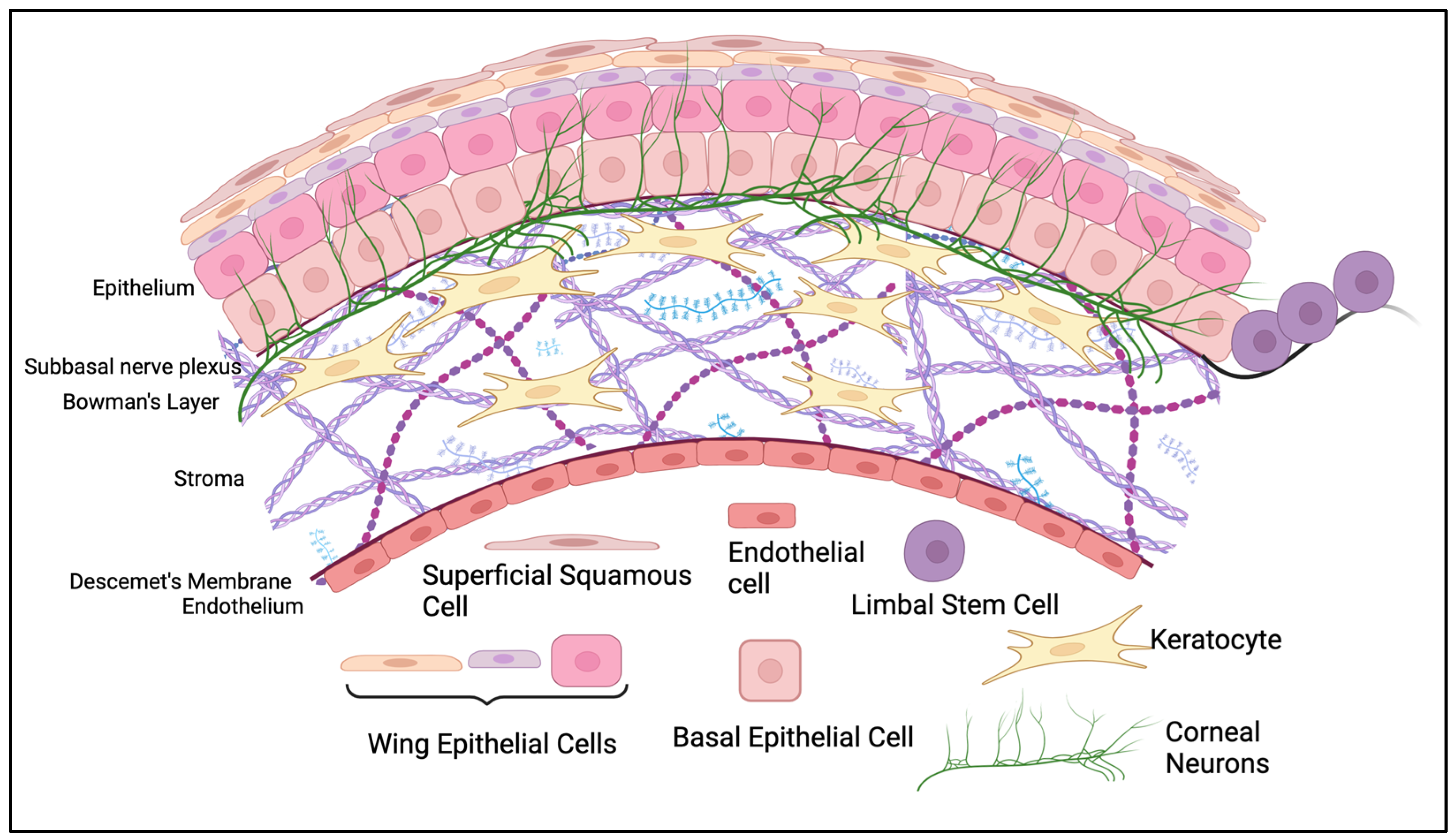
2. Corneal Epithelium
2.1. Anatomical Structure
2.2. Tear Fluid
2.3. Corneal Nerves
3. Growth Factors and Their Receptors
3.1. Receptor Expression
3.2. Growth Factor Expression

3.3. Receptor Mechanism of Action
| Receptor | Factor | Effects on Corneal Wound Healing | Regulation by CBL |
|---|---|---|---|
| c-Met | Hepatocyte Growth Factor (HGF) |
| |
| EGFR | Epidermal Growth Factor (EGF) | ||
| Heparin binding-EGF (HB-EGF) | |||
| Betacellulin (BTC) | |||
| Transforming Growth Factor-α (TGF-α) | |||
| KGFR | Keratinocyte Growth Factor (KGF) |
|
|
| FGFR | Fibroblast Growth Factor (FGF) |
| |
| IGF-1R; IGF-2R | Insulin Growth Factor (IGF) Insulin | ||
| PDGF-αR & PDGF-βR | Platelet Derived Growth Factor (PDGF) | ||
| VEGFR 2 | Vascular Endothelial Growth Factor (VEGF) |
| |
| TrkA | Nerve Growth Factor (NGF) | ||
| TrkB | Brain derived neurotrophic factor (BDNF) |
| |
| TGF-βR | Transforming Growth Factor-β (TGF-β1,2,3) |
|
|
| RET/GFR-α | Glial cell line- derived neurotrophic factor (GDNF) |
Alternative Signaling Mechanisms
3.4. Growth Factor Alterations in Disease States
4. Growth Factor-Mediated Corneal Epithelial Restoration
4.1. The Role of Growth Factors for Corneal Epithelial Homeostasis and Restoration
4.2. Opportunities for the Use of Growth Factors
4.2.1. Current FDA-Approved Growth Factor Therapies: Amniotic Membrane
4.2.2. Current FDA-Approved Growth Factor Therapies: Recombinant Growth Factors
5. Emerging Opportunities
5.1. EGF: The Catalyst of Corneal Homeostasis Studies
5.2. CBL-Mediated Desensitization of RTKs

5.3. HGF: The Multi-Faceted Growth Factor
5.3.1. Inhibition of Fibrosis

5.3.2. Corneal Neovascularization
5.3.3. Neuro-Regeneration
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scott, P. Scott’s Anatomy of the Eye and Orbit; Ridgevue Publishing: Atascadero, CA, USA, 2019. [Google Scholar]
- Müller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M.T. Corneal nerves: Structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Rao, S.K.; Srinivas, S.P.; Tong, L.; Young, A.L. Evaluation of ocular surface and tear function—A review of current approaches for dry eye. Indian J. Ophthalmol. 2022, 70, 1883–1891. [Google Scholar] [CrossRef]
- Klenkler, B.; Sheardown, H.; Jones, L. Growth factors in the tear film: Role in tissue maintenance, wound healing, and ocular pathology. Ocul. Surf. 2007, 5, 228–239. [Google Scholar] [CrossRef]
- Bonanno, J.A. Molecular mechanisms underlying the corneal endothelial pump. Exp. Eye Res. 2012, 95, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Thoft, R.A.; Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1442–1443. [Google Scholar]
- Hanna, C.; Bicknell, D.S.; O’Brien, J.E. Cell Turnover in the Adult Human Eye. Arch. Ophthalmol. 1961, 65, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A. Renewal of the rabbit corneal epithelium as investigated by autoradiography after intravitreal injection of 3H-thymidine. Cornea 2000, 19, 378–383. [Google Scholar] [CrossRef]
- Thoft, R.A.; Wiley, L.A.; Sundarraj, N. The multipotential cells of the limbus. Eye 1989, 3, 109–113. [Google Scholar] [CrossRef]
- Ludwig, P.E.; Lopez, M.J.; Sevensma, K.E. Anatomy, Head and Neck, Eye Cornea. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yazdani, M. Tear film lipid layer and corneal oxygenation: A new function? Eye 2023, 37, 3534–3541. [Google Scholar] [CrossRef]
- Beuerman, R.W.; Pedroza, L. Ultrastructure of the human cornea. Microsc. Res. Tech. 1996, 33, 320–335. [Google Scholar] [CrossRef]
- Driver, P.J.; Lemp, M.A. Meibomian gland dysfunction. Surv. Ophthalmol. 1996, 40, 343–367. [Google Scholar] [CrossRef] [PubMed]
- Sack, R.A.; Conradi, L.; Krumholz, D.; Beaton, A.; Sathe, S.; Morris, C. Membrane array characterization of 80 chemokines, cytokines, and growth factors in open- and closed-eye tears: Angiogenin and other defense system constituents. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, J.M. Tears in health and disease. Eye 2003, 17, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Rolando, M.; Zierhut, M. The ocular surface and tear film and their dysfunction in dry eye disease. Surv. Ophthalmol. 2001, 45 (Suppl. S2), S203–S210. [Google Scholar] [CrossRef] [PubMed]
- Rózsa, A.; Beuerman, R.W. Density and Organization of Free Nerve Endings in the Corneal Epithelium of the Rabbit. Pain 1982, 14, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Marfurt, C.F.; Kingsley, R.E.; Echtenkamp, S.E. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Investig. Ophthalmol. Vis. Sci. 1989, 30, 461–472. [Google Scholar]
- Marfurt, C.F.; Jones, M.A.; Thrasher, K. Parasympathetic Innervation of the Rat Cornea. Exp. Eye Res. 1998, 66, 437–448. [Google Scholar] [CrossRef]
- Marfurt, C.F. Sympathetic innervation of the rat cornea as demonstrated by the retrograde and anterograde transport of horseradish peroxidase–wheat germ agglutinin. J. Comp. Neurol. 1988, 268, 147–160. [Google Scholar] [CrossRef]
- Marfurt, C.F. Corneal Nerves: Anatomy. In Encyclopedia of the Eye; Dartt, D.A., Ed.; Academic Press: Oxford, UK, 2010; pp. 485–492. [Google Scholar]
- Dartt, D.A. Neural regulation of lacrimal gland secretory processes: Relevance in dry eye diseases. Prog. Retin. Eye Res. 2009, 28, 155–177. [Google Scholar] [CrossRef]
- Labetoulle, M.; Baudouin, C.; Calonge, M.; Merayo-Lloves, J.; Boboridis, K.G.; Akova, Y.A.; Aragona, P.; Geerling, G.; Messmer, E.M.; Benitez-Del-Castillo, J. Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmol. 2019, 97, 137–145. [Google Scholar] [CrossRef]
- Stepp, M.A.; Tadvalkar, G.; Hakh, R.; Pal-Ghosh, S. Corneal epithelial cells function as surrogate Schwann cells for their sensory nerves. Glia 2017, 65, 851–863. [Google Scholar] [CrossRef]
- Bargagna-Mohan, P.; Schultz, G.; Rheaume, B.; Trakhtenberg, E.F.; Robson, P.; Pal-Ghosh, S.; Stepp, M.A.; Given, K.S.; Macklin, W.B.; Mohan, R. Corneal nonmyelinating Schwann cells illuminated by single-cell transcriptomics and visualized by protein biomarkers. J. Neurosci. Res. 2021, 99, 731–749. [Google Scholar] [CrossRef] [PubMed]
- Mirmoeini, K.; Tajdaran, K.; Zhang, J.; Gordon, T.; Ali, A.; Kaplan, D.R.; Feinberg, K.; Borschel, G.H. Schwann Cells Are Key Regulators of Corneal Epithelial Renewal. Investig. Ophthalmol. Vis. Sci. 2023, 64, 7. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, K.; Tajdaran, K.; Mirmoeini, K.; Daeschler, S.C.; Henriquez, M.A.; Stevens, K.E.; Mulenga, C.M.; Hussain, A.; Hamrah, P.; Ali, A.; et al. The Role of Sensory Innervation in Homeostatic and Injury-Induced Corneal Epithelial Renewal. Int. J. Mol. Sci. 2023, 24, 12615. [Google Scholar] [CrossRef] [PubMed]
- Kowtharapu, B.S.; Stahnke, T.; Wree, A.; Guthoff, R.F.; Stachs, O. Corneal epithelial and neuronal interactions: Role in wound healing. Exp. Eye Res. 2014, 125, 53–61. [Google Scholar] [CrossRef]
- Yu, F.S.; Yin, J.; Xu, K.; Huang, J. Growth factors and corneal epithelial wound healing. Brain Res. Bull. 2010, 81, 229–235. [Google Scholar] [CrossRef]
- Bukowiecki, A.; Hos, D.; Cursiefen, C.; Eming, S.A. Wound-Healing Studies in Cornea and Skin: Parallels, Differences and Opportunities. Int. J. Mol. Sci. 2017, 18, 1257. [Google Scholar] [CrossRef]
- Imanishi, J.; Kamiyama, K.; Iguchi, I.; Kita, M.; Sotozono, C.; Kinoshita, S. Growth factors: Importance in wound healing and maintenance of transparency of the cornea. Prog. Retin. Eye Res. 2000, 19, 113–129. [Google Scholar] [CrossRef]
- Tervo, T.; Vesaluoma, M.; Bennett, G.L.; Schwall, R.; Helena, M.; Liang, Q.; Wilson, S.E. Tear hepatocyte growth factor (HGF) availability increases markedly after excimer laser surface ablation. Exp. Eye Res. 1997, 64, 501–504. [Google Scholar] [CrossRef]
- Wilson, S.E.; Liang, Q.; Kim, W.J. Lacrimal gland HGF, KGF, and EGF mRNA levels increase after corneal epithelial wounding. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2185–2190. [Google Scholar]
- Wilson, S.E.; Lloyd, S.A. Epidermal growth factor and its receptor, basic fibroblast growth factor, transforming growth factor beta-1, and interleukin-1 alpha messenger RNA production in human corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2747–2756. [Google Scholar]
- Shaheen, B.S.; Bakir, M.; Jain, S. Corneal nerves in health and disease. Surv. Ophthalmol. 2014, 59, 263–285. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, L.; Massaro-Giordano, G.; Nubile, M.; Sacchetti, M. Understanding the Pathogenesis of Neurotrophic Keratitis: The Role of Corneal Nerves. J. Cell. Physiol. 2017, 232, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Huang, Y.H.; Cunningham, C.M.; Han, K.Y.; Chang, M.; Seiki, M.; Zhou, Z.; Azar, D.T. Matrix metalloproteinase 14 modulates signal transduction and angiogenesis in the cornea. Surv. Ophthalmol. 2016, 61, 478–497. [Google Scholar] [CrossRef] [PubMed]
- Zbodakova, O.; Chalupsky, K.; Sarnova, L.; Kasparek, P.; Jirouskova, M.; Gregor, M.; Sedlacek, R. ADAM10 and ADAM17 regulate EGFR, c-Met and TNF RI signalling in liver regeneration and fibrosis. Sci. Rep. 2021, 11, 11414. [Google Scholar] [CrossRef]
- Yin, J.; Fu-Shin, X.Y. ERK1/2 mediate wounding-and G-protein-coupled receptor ligands-induced EGFR activation via regulating ADAM17 and HB-EGF shedding. Investig. Ophthalmol. Vis. Sci. 2009, 50, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, B.; Tuft, S.J.; Khaw, P.T. Matrix metalloproteinase distribution during early corneal wound healing. Eye 2005, 19, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.-P.; Ding, Y.; Ling, J.; Dong, Z.; Yu, F.-S.X. Wound-Induced HB-EGF Ectodomain Shedding and EGFR Activation in Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2004, 45, 813–820. [Google Scholar] [CrossRef]
- Breyer, J.A.; Cohen, S. The epidermal growth factor precursor isolated from murine kidney membranes. Chemical characterization and biological properties. J. Biol. Chem. 1990, 265, 16564–16570. [Google Scholar] [CrossRef]
- Gutmann, T.; Kim, K.H.; Grzybek, M.; Walz, T.; Coskun, Ü. Visualization of ligand-induced transmembrane signaling in the full-length human insulin receptor. J. Cell Biol. 2018, 217, 1643–1649. [Google Scholar] [CrossRef]
- Omoto, M.; Suri, K.; Amouzegar, A.; Li, M.; Katikireddy, K.R.; Mittal, S.K.; Chauhan, S.K. Hepatocyte Growth Factor Suppresses Inflammation and Promotes Epithelium Repair in Corneal Injury. Mol. Ther. 2017, 25, 1881–1888. [Google Scholar] [CrossRef]
- Tarvestad-Laise, K.; Ceresa, B.P. Knockout of c-Cbl/Cbl-b slows c-Met trafficking resulting in enhanced signaling in corneal epithelial cells. J. Biol. Chem. 2023, 299, 105233. [Google Scholar] [CrossRef]
- Takahashi, M.; Ota, S.; Shimada, T.; Hamada, E.; Kawabe, T.; Okudaira, T.; Matsumura, M.; Kaneko, N.; Terano, A.; Nakamura, T.; et al. Hepatocyte growth factor is the most potent endogenous stimulant of rabbit gastric epithelial cell proliferation and migration in primary culture. J. Clin. Investig. 1995, 95, 1994–2003. [Google Scholar] [CrossRef]
- Shukla, M.N.; Rose, J.L.; Ray, R.; Lathrop, K.L.; Ray, A.; Ray, P. Hepatocyte growth factor inhibits epithelial to myofibroblast transition in lung cells via Smad7. Am. J. Respir. Cell Mol. Biol. 2009, 40, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Eagleson, K.L.; Wu, H.H.; Levitt, P. Hepatocyte Growth Factor Modulates MET Receptor Tyrosine Kinase and beta-Catenin Functional Interactions to Enhance Synapse Formation. eNeuro 2016, 3, e0074-16. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Yamanaka, H.; Fukuoka, T.; Dai, Y.; Obata, K.; Mashimo, T.; Noguchi, K. Expression of HGF and cMet in the peripheral nervous system of adult rats following sciatic nerve injury. Clin. Neurosci. Neuropathol. 2001, 12, 1403–1407. [Google Scholar] [CrossRef]
- Lee, N.; Kim, S.; Lee, J. Impairment of peripheral nerve regeneration by insufficient activation of the HGF/c-Met/c-Jun pathway in aged mice. Heliyon 2022, 8, e11411. [Google Scholar] [CrossRef]
- Hamanoue, M.; Takemoto, N.; Matsumoto, K.; Nakamura, T.; Nakajima, K.; Kohsaka, S. Neurotrophic effect of hepatocyte growth factor on central nervous system neurons in vitro. J. Neurosci. Res. 1996, 43, 554–564. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, H.; Zheng, Y.; Gu, Q.; Ma, J.; Xu, X. A novel antiangiogenic peptide derived from hepatocyte growth factor inhibits neovascularization in vitro and in vivo. Mol. Vis. 2010, 16, 1982–1995. [Google Scholar]
- Xin, X.; Yang, S.; Ingle, G.; Zlot, C.; Rangell, L.; Kowalski, J.; Schwall, R.; Ferrara, N.; Gerritsen, M.E. Hepatocyte Growth Factor Enhances Vascular Endothelial Growth Factor-Induced Angiogenesis in Vitro and in Vivo. Am. J. Pathol. 2001, 158, 1111–1120. [Google Scholar] [CrossRef]
- Ucuzian, A.A.; Gassman, A.A.; East, A.T.; Greisler, H.P. Molecular mediators of angiogenesis. J. Burn Care Res. 2010, 31, 158–175. [Google Scholar] [CrossRef]
- Sun, Y.; Su, L.; Wang, Z.; Xu, Y.; Xu, X. H-RN, a peptide derived from hepatocyte growth factor, inhibits corneal neovascularization by inducing endothelial apoptosis and arresting the cell cycle. BMC Cell Biol. 2013, 14, 8. [Google Scholar] [CrossRef]
- Peschard, P.; Fournier, T.M.; Lamorte, L.; Naujokas, M.A.; Band, H.; Langdon, W.Y.; Park, M. Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol. Cell 2001, 8, 995–1004. [Google Scholar] [CrossRef]
- Peschard, P.; Ishiyama, N.; Lin, T.; Lipkowitz, S.; Park, M. A conserved DpYR motif in the juxtamembrane domain of the Met receptor family forms an atypical c-Cbl/Cbl-b tyrosine kinase binding domain binding site required for suppression of oncogenic activation. J. Biol. Chem. 2004, 279, 29565–29571. [Google Scholar] [CrossRef] [PubMed]
- Rush, J.S.; Boeving, M.A.; Berry, W.L.; Ceresa, B.P. Antagonizing c-Cbl enhances EGFR-dependent corneal epithelial homeostasis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4691–4699. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daniele, S.; Frati, L.; Fiore, C.; Santoni, G. The effect of the epidermal growth factor (EGF) on the corneal epithelium in humans. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 1979, 210, 159–165. [Google Scholar] [CrossRef]
- Watanabe, H.; Ohashi, Y.; Kinoshita, S.; Manabe, R.; Ohshiden, K. Distribution of epidermal growth factor in rat ocular and periocular tissues. Graefe’s Arch. Clin. Exp. Ophthalmol. 1993, 231, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E.; He, Y.G.; Lloyd, S.A. EGF, EGF receptor, basic FGF, TGF beta-1, and IL-1 alpha mRNA in human corneal epithelial cells and stromal fibroblasts. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1756–1765. [Google Scholar]
- Boisjoly, H.M.; Laplante, C.; Bernatchez, S.F.; Salesse, C.; Giasson, M.; Joly, M.-C. Effects of EGF, IL-1 and their combination on in vitro corneal epithelial wound closure and cell chemotaxis. Exp. Eye Res. 1993, 57, 293–300. [Google Scholar] [CrossRef]
- Savage, C.R.; Cohen, S. Proliferation of corneal epithelium induced by epidermal growth factor. Exp. Eye Res. 1973, 15, 361–366. [Google Scholar] [CrossRef]
- Tao, W.; Liou, G.I.; Wu, X.; Abney, T.O.; Reinach, P.S. ETB and epidermal growth factor receptor stimulation of wound closure in bovine corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2614–2622. [Google Scholar]
- Crotchett, B.L.M.; Ceresa, B.P. Knockout of c-Cbl slows EGFR endocytic trafficking and enhances EGFR signaling despite incompletely blocking receptor ubiquitylation. Pharmacol. Res. Perspect. 2021, 9, e00756. [Google Scholar] [CrossRef]
- Pinilla-Macua, I.; Sorkin, A. Cbl and Cbl-b independently regulate EGFR through distinct receptor interaction modes. Mol. Biol. Cell 2023, 34, mbc E23-02-0058. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Langdon, W.Y.; Zhang, J. Negative regulation of receptor tyrosine kinases by ubiquitination: Key roles of the Cbl family of E3 ubiquitin ligases. Front. Endocrinol. 2022, 13, 971162. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.L.; Phelps, E.D.; Doll, M.A.; Schaal, S.; Ceresa, B.P. The role of endogenous epidermal growth factor receptor ligands in mediating corneal epithelial homeostasis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2870–2880. [Google Scholar] [CrossRef]
- Tolino, M.A.; Block, E.R.; Klarlund, J.K. Brief treatment with heparin-binding EGF-like growth factor, but not with EGF, is sufficient to accelerate epithelial wound healing. Biochim. Biophys. Acta BBA Gen. Subj. 2011, 1810, 875–878. [Google Scholar] [CrossRef]
- Jeong, W.-Y.; Yoo, H.-Y.; Kim, C.-W. β-cellulin promotes the proliferation of corneal epithelial stem cells through the phosphorylation of erk1/2. Biochem. Biophys. Res. Commun. 2018, 496, 359–366. [Google Scholar] [CrossRef]
- McClintock, J.L.; Ceresa, B.P. Transforming growth factor-{alpha} enhances corneal epithelial cell migration by promoting EGFR recycling. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3455–3461. [Google Scholar] [CrossRef]
- Sotozono, C.; Inatomi, T.; Nakamura, M.; Kinoshita, S. Keratinocyte growth factor accelerates corneal epithelial wound healing in vivo. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1524–1529. [Google Scholar]
- Sotozono, C.; Kinoshita, S.; Kita, M.; Imanishi, J. Paracrine role of keratinocyte growth factor in rabbit corneal epithelial cell growth. Exp. Eye Res. 1994, 59, 385–392. [Google Scholar] [CrossRef]
- Chandrasekher, G.; Kakazu, A.H.; Bazan, H.E. HGF- and KGF-induced activation of PI-3K/p70 s6 kinase pathway in corneal epithelial cells: Its relevance in wound healing. Exp. Eye Res. 2001, 73, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Belleudi, F.; Leone, L.; Nobili, V.; Raffa, S.; Francescangeli, F.; Maggio, M.; Morrone, S.; Marchese, C.; Torrisi, M.R. Keratinocyte Growth Factor Receptor Ligands Target the Receptor to Different Intracellular Pathways. Traffic 2007, 8, 1854–1872. [Google Scholar] [CrossRef] [PubMed]
- Rieck, P.; Assouline, M.; Savoldelli, M.; Hartmann, C.; Jacob, C.; Pouliquen, Y.; Courtois, Y. Recombinant human basic fibroblast growth factor (Rh-bFGF) in three different wound models in rabbits: Corneal wound healing effect and pharmacology. Exp. Eye Res. 1992, 54, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Barry-Lane, P.A.; Cavanagh, H.D.; Petroll, W.M. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea 1996, 15, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Huang, J.; Petroll, W.M.; Cavanagh, H.D. TGFβ induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFβ, PDGF and integrin signaling. Exp. Eye Res. 2002, 75, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E.; Liu, J.J.; Mohan, R.R. Stromal-epithelial interactions in the cornea. Prog. Retin. Eye Res. 1999, 18, 293–309. [Google Scholar] [CrossRef]
- Shakiba, Y.; Mansouri, K.; Arshadi, D.; Rezaei, N. Corneal neovascularization: Molecular events and therapeutic options. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 221–231. [Google Scholar] [CrossRef]
- Wong, A.; Lamothe, B.; Lee, A.; Schlessinger, J.; Lax, I. FRS2α attenuates FGF receptor signaling by Grb2-mediated recruitment of the ubiquitin ligase Cbl. Proc. Natl. Acad. Sci. USA 2002, 99, 6684–6689. [Google Scholar] [CrossRef]
- Stuard, W.L.; Titone, R.; Robertson, D.M. The IGF/Insulin-IGFBP Axis in Corneal Development, Wound Healing, and Disease. Front. Endocrinol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Trosan, P.; Svobodova, E.; Chudickova, M.; Krulova, M.; Zajicova, A.; Holan, V. The key role of insulin-like growth factor I in limbal stem cell differentiation and the corneal wound-healing process. Stem Cells Dev. 2012, 21, 3341–3350. [Google Scholar] [CrossRef]
- Nakamura, M.; Nagano, T.; Chikama, T.-i.; Nishida, T. Up-Regulation of Phosphorylation of Focal Adhesion Kinase and Paxillin by Combination of Substance P and IGF-1 in SV-40 Transformed Human Corneal Epithelial Cells. Biochem. Biophys. Res. Commun. 1998, 242, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Shanley, L.J.; McCaig, C.D.; Forrester, J.V.; Zhao, M. Insulin, not leptin, promotes in vitro cell migration to heal monolayer wounds in human corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Lee, K.-S.; Joo, C.-K. Transactivation of EGFR mediates insulin-stimulated ERK1/2 activation and enhanced cell migration in human corneal epithelial cells. Mol. Vis. 2006, 12, 1403–1410. [Google Scholar] [PubMed]
- Sehat, B.; Andersson, S.; Girnita, L.; Larsson, O. Identification of c-Cbl as a new ligase for insulin-like growth factor-I receptor with distinct roles from Mdm2 in receptor ubiquitination and endocytosis. Cancer Res. 2008, 68, 5669–5677. [Google Scholar] [CrossRef]
- Li, H.; Xu, L.; Li, C.; Zhao, L.; Ma, Y.; Zheng, H.; Li, Z.; Zhang, Y.; Wang, R.; Liu, Y. Ubiquitin ligase Cbl-b represses IGF-I-induced epithelial mesenchymal transition via ZEB2 and microRNA-200c regulation in gastric cancer cells. Mol. Cancer 2014, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, K.; Iguchi, I.; Wang, X.; Imanishi, J. Effects of PDGF on the migration of rabbit corneal fibroblasts and epithelial cells. Cornea 1998, 17, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Rorsman, C.; Tsioumpekou, M.; Heldin, C.-H.; Lennartsson, J. The ubiquitin ligases c-Cbl and Cbl-b negatively regulate platelet-derived growth factor (PDGF) BB-induced chemotaxis by affecting PDGF receptor β (PDGFRβ) internalization and signaling. J. Biol. Chem. 2016, 291, 11608–11618. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Fukuoka, S.; Karagianni, N.; Guaiquil, V.H.; Rosenblatt, M.I. Vascular endothelial growth factor promotes anatomical and functional recovery of injured peripheral nerves in the avascular cornea. FASEB J. 2013, 27, 2756–2767. [Google Scholar] [CrossRef]
- Li, Z.; Burns, A.R.; Han, L.; Rumbaut, R.E.; Smith, C.W. IL-17 and VEGF Are Necessary for Efficient Corneal Nerve Regeneration. Am. J. Pathol. 2011, 178, 1106–1116. [Google Scholar] [CrossRef]
- Singh, A.J.; Meyer, R.D.; Navruzbekov, G.; Shelke, R.; Duan, L.; Band, H.; Leeman, S.E.; Rahimi, N. A critical role for the E3-ligase activity of c-Cbl in VEGFR-2-mediated PLCγ1 activation and angiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 5413–5418. [Google Scholar] [CrossRef]
- Blanco-Mezquita, T.; Martinez-Garcia, C.; Proença, R.; Zieske, J.D.; Bonini, S.; Lambiase, A.; Merayo-Lloves, J. Nerve growth factor promotes corneal epithelial migration by enhancing expression of matrix metalloprotease-9. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3880–3890. [Google Scholar] [CrossRef]
- Fixman, E.D.; Fournier, T.M.; Kamikura, D.M.; Naujokas, M.A.; Park, M. Pathways Downstream of Shc and Grb2 Are Required for Cell Transformation by the Tpr-Met Oncoprotein. J. Biol. Chem. 1996, 271, 13116–13122. [Google Scholar] [CrossRef] [PubMed]
- Pedrotti, E.; Bonacci, E.; Chierego, C.; De Gregorio, A.; Cozzini, T.; Brighenti, T.; Caldarella, G.; Pastore, G.; Fasolo, A.; Marchini, G. Eight months follow-up of corneal nerves and sensitivity after treatment with cenegermin for neurotrophic keratopathy. Orphanet J. Rare Dis. 2022, 17, 63. [Google Scholar] [CrossRef]
- Takahashi, Y.; Shimokawa, N.; Esmaeili-Mahani, S.; Morita, A.; Masuda, H.; Iwasaki, T.; Tamura, J.; Haglund, K.; Koibuchi, N. Ligand-induced downregulation of TrkA is partly regulated through ubiquitination by Cbl. FEBS Lett. 2011, 585, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-K.; Li, Y.-Z.; Ge, A.-N.; Zhu, Y.-B.; Wu, S.-J.; Bai, X.; Bai, H.-H.; Liu, Y.-N. Cbl-b modulated TrkA ubiquitination and function in the dorsal root ganglion of mice. Eur. J. Pharmacol. 2022, 921, 174876. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Kruse, F.E.; Völcker, H.E. Neurotrophic Factors in the Human Cornea. Investig. Ophthalmol. Vis. Sci. 2000, 41, 692–702. [Google Scholar]
- McCarty, J.H.; Feinstein, S.C. The TrkB receptor tyrosine kinase regulates cellular proliferation via signal transduction pathways involving SHC, PLCgamma, and CBL. J. Recept. Signal Transduct. Res. 1999, 19, 953–974. [Google Scholar] [CrossRef] [PubMed]
- Pandya, C.; Kutiyanawalla, A.; Turecki, G.; Pillai, A. Glucocorticoid regulates TrkB protein levels via c-Cbl dependent ubiquitination: A decrease in c-Cbl mRNA in the prefrontal cortex of suicide subjects. Psychoneuroendocrinology 2014, 45, 108–118. [Google Scholar] [CrossRef]
- Pancholi, S.; Tullo, A.; Khaliq, A.; Foreman, D.; Boulton, M. The effects of growth factors and conditioned media on the proliferation of human corneal epithelial cells and keratocytes. Graefe’s Arch. Clin. Exp. Ophthalmol. 1998, 236, 1–8. [Google Scholar] [CrossRef]
- Haber, M.; Cao, Z.; Panjwani, N.; Bedenice, D.; Li, W.W.; Provost, P.J. Effects of growth factors (EGF, PDGF-BB and TGF-β1) on cultured equine epithelial cells and keratocytes: Implications for wound healing. Vet. Ophthalmol. 2003, 6, 211–217. [Google Scholar] [CrossRef]
- Mishima, H.; Nakamura, M.; Murakami, J.; Nishida, T.; Otori, T. Transforming growth factor-β modulates effects of epidermal growth factor on corneal epithelial cells. Curr. Eye Res. 1992, 11, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Honma, Y.; Nishida, K.; Sotozono, C.; Kinoshita, S. Effect of transforming growth factor-β1 and-β2 onin vitroRabbit corneal epithelial cell proliferation promoted by epidermal growth factor, keratinocyte growth factor, or hepatocyte growth factor. Exp. Eye Res. 1997, 65, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Ho-Chang, J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp. Eye Res. 2003, 77, 581–592. [Google Scholar] [CrossRef]
- MøSller-Pedersen, T.; Cavanagh, H.D.; Petroll, W.M.; Jester, J.V. Neutralizing antibody to TGFβ modulates stromal fibrosis but not regression of photoablative effect following PRK. Curr. Eye Res. 1998, 17, 736–747. [Google Scholar] [CrossRef]
- Karamichos, D.; Hutcheon, A.; Zieske, J. Reversal of fibrosis by TGF-β3 in a 3D in vitro model. Exp. Eye Res. 2014, 124, 31–36. [Google Scholar] [CrossRef]
- Zuo, W.; Huang, F.; Chiang, Y.J.; Li, M.; Du, J.; Ding, Y.; Zhang, T.; Lee, H.W.; Jeong, L.S.; Chen, Y.; et al. c-Cbl-Mediated Neddylation Antagonizes Ubiquitination and Degradation of the TGF-β Type II Receptor. Mol. Cell 2013, 49, 499–510. [Google Scholar] [CrossRef]
- Wohlfert, E.A.; Gorelik, L.; Mittler, R.; Flavell, R.A.; Clark, R.B. Cutting edge: Deficiency in the E3 ubiquitin ligase Cbl-b results in a multifunctional defect in T cell TGF-β sensitivity in vitro and in vivo. J. Immunol. 2006, 176, 1316–1320. [Google Scholar] [CrossRef]
- Di, G.; Qi, X.; Zhao, X.; Zhang, S.; Danielson, P.; Zhou, Q. Corneal Epithelium-Derived Neurotrophic Factors Promote Nerve Regeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4695–4702. [Google Scholar] [CrossRef]
- You, L.; Ebner, S.; Kruse, F.E. Glial Cell–Derived Neurotrophic Factor (GDNF)–Induced Migration and Signal Transduction in Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2496–2504. [Google Scholar]
- Bian, F.; Qi, H.; Ma, P.; Zhang, L.; Yoon, K.-C.; Pflugfelder, S.C.; Li, D.-Q. An Immunoprotective Privilege of Corneal Epithelial Stem Cells Against Th17 Inflammatory Stress by Producing Glial Cell-Derived Neurotrophic Factor. Stem Cells 2010, 28, 2172–2181. [Google Scholar] [CrossRef]
- Hyndman, B.D.; Crupi, M.J.F.; Peng, S.; Bone, L.N.; Rekab, A.N.; Lian, E.Y.; Wagner, S.M.; Antonescu, C.N.; Mulligan, L.M. Differential recruitment of E3 ubiquitin ligase complexes regulates RET isoform internalization. J. Cell Sci. 2017, 130, 3282–3296. [Google Scholar] [CrossRef] [PubMed]
- Kales, S.C.; Nau, M.M.; Merchant, A.S.; Lipkowitz, S. Enigma prevents Cbl-c-mediated ubiquitination and degradation of RETMEN2A. PLoS ONE 2014, 9, e87116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, H.; Zhang, F.; Pan, Z.; Capó-Aponte, J.; Reinach, P.S. Dependence of EGF-Induced Increases in Corneal Epithelial Proliferation and Migration on GSK-3 Inactivation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4828–4835. [Google Scholar] [CrossRef][Green Version]
- Reinach, P.S.; Mergler, S.; Okada, Y.; Saika, S. Ocular transient receptor potential channel function in health and disease. BMC Ophthalmol. 2015, 15, 153. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Capó-Aponte, J.E.; Zhang, F.; Pan, Z.; Reinach, P.S. Epidermal growth factor receptor transactivation by the cannabinoid receptor (CB1) and transient receptor potential vanilloid 1 (TRPV1) induces differential responses in corneal epithelial cells. Exp. Eye Res. 2010, 91, 462–471. [Google Scholar] [CrossRef]
- Okada, Y.; Sumioka, T.; Reinach, P.S.; Miyajima, M.; Saika, S. Roles of Epithelial and Mesenchymal TRP Channels in Mediating Inflammatory Fibrosis. Front. Immunol. 2021, 12, 731674. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, Y.; Li, X.; Shen, Y.; Yin, M.; Guo, Y.; Diao, L.; Liu, Y.; Yue, D. Critical role of TRPC6 channels in the development of human renal cell carcinoma. Mol. Biol. Rep. 2013, 40, 5115–5122. [Google Scholar] [CrossRef]
- Waning, J.; Vriens, J.; Owsianik, G.; Stüwe, L.; Mally, S.; Fabian, A.; Frippiat, C.; Nilius, B.; Schwab, A. A novel function of capsaicin-sensitive TRPV1 channels: Involvement in cell migration. Cell Calcium 2007, 42, 17–25. [Google Scholar] [CrossRef]
- Pflugfelder, S.C. Tear dysfunction and the cornea: LXVIII Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 2011, 152, 900–909.e901. [Google Scholar] [CrossRef]
- Willcox, M.D.P.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef]
- Lam, H.; Bleiden, L.; de Paiva, C.S.; Farley, W.; Stern, M.E.; Pflugfelder, S.C. Tear cytokine profiles in dysfunctional tear syndrome. Am. J. Ophthalmol. 2009, 147, 198–205.e191. [Google Scholar] [CrossRef]
- García-López, C.; Rodríguez-Calvo-de-Mora, M.; Borroni, D.; Sánchez-González, J.M.; Romano, V.; Rocha-de-Lossada, C. The role of matrix metalloproteinases in infectious corneal ulcers. Surv. Ophthalmol. 2023, 68, 929–939. [Google Scholar] [CrossRef]
- Choi, M.; Park, Y.M.; Ko, B.Y. Comparative Evaluation of Matrix Metalloproteinase-9 Immunoassay and Tear Osmolarity Measurement for Diagnosing Severity of Dry Eye Disease. Korean J. Ophthalmol. 2023, 37, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Block, E.R.; Klarlund, J.K. Wounding sheets of epithelial cells activates the epidermal growth factor receptor through distinct short- and long-range mechanisms. Mol. Biol. Cell 2008, 19, 4909–4917. [Google Scholar] [CrossRef] [PubMed]
- Stepp, M.A.; Pal-Ghosh, S.; Tadvalkar, G.; Williams, A.; Pflugfelder, S.C.; de Paiva, C.S. Reduced intraepithelial corneal nerve density and sensitivity accompany desiccating stress and aging in C57BL/6 mice. Exp. Eye Res. 2018, 169, 91–98. [Google Scholar] [CrossRef]
- Sacchetti, M.; Lambiase, A. Neurotrophic factors and corneal nerve regeneration. Neural Regen. Res. 2017, 12, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, M.; Lambiase, A. Diagnosis and management of neurotrophic keratitis. Clin. Ophthalmol. 2014, 8, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Micera, A.; Sacchetti, M.; Cortes, M.; Mantelli, F.; Bonini, S. Alterations of Tear Neuromediators in Dry Eye Disease. Arch. Ophthalmol. 2011, 129, 981–986. [Google Scholar] [CrossRef]
- van Setten, G.B.; Tervo, T.; Viinikka, L.; Perheentupa, J.; Tarkkanen, A. Epidermal growth factor in human tear fluid: A minireview. Int. Ophthalmol. 1991, 15, 359–362. [Google Scholar] [CrossRef]
- Saghizadeh, M.; Chwa, M.; Aoki, A.; Lin, B.; Pirouzmanesh, A.; Brown, D.J.; Ljubimov, A.V.; Kenney, M.C. Altered Expression of Growth Factors and Cytokines in Keratoconus, Bullous Keratopathy and Diabetic Human Corneas. Exp. Eye Res. 2001, 73, 179–189. [Google Scholar] [CrossRef]
- Saghizadeh, M.; Kramerov, A.A.; Tajbakhsh, J.; Aoki, A.M.; Wang, C.; Chai, N.N.; Ljubimova, J.Y.; Sasaki, T.; Sosne, G.; Carlson, M.R.; et al. Proteinase and growth factor alterations revealed by gene microarray analysis of human diabetic corneas. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3604–3615. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.; Monroy, D.C.; Nava, A.; Pflugfelder, S.C. Inflammatory cytokines in the tears of patients with ocular rosacea. Ophthalmology 1997, 104, 1868–1874. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Willcox, M. Cytokine and lipid inflammatory mediator profile of human tears during contact lens associated inflammatory diseases. Exp. Eye Res. 1998, 67, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; McClellan, S.A.; Barrett, R.; Foldenauer, M.; Hazlett, L.D. HGF signaling impacts severity of Pseudomonas aeruginosa keratitis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2180–2190. [Google Scholar] [CrossRef][Green Version]
- Jiang, X.; McClellan, S.A.; Barrett, R.P.; Berger, E.A.; Zhang, Y.; Hazlett, L.D. VIP and growth factors in the infected cornea. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6154–6161. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.; Verma, A.; Singh, V.K.; Jaffet, J.; Chaurasia, S.; Sahel, D.K.; Ramappa, M.; Singh, V. New Frontier in the Management of Corneal Dystrophies: Basics, Development, and Challenges in Corneal Gene Therapy and Gene Editing. Asia Pac. J. Ophthalmol. 2022, 11, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Burdon, K.P.; Macgregor, S.; Bykhovskaya, Y.; Javadiyan, S.; Li, X.; Laurie, K.J.; Muszynska, D.; Lindsay, R.; Lechner, J.; Haritunians, T.; et al. Association of Polymorphisms in the Hepatocyte Growth Factor Gene Promoter with Keratoconus. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8514–8519. [Google Scholar] [CrossRef]
- Lucas, S.E.M.; Zhou, T.; Blackburn, N.B.; Mills, R.A.; Ellis, J.; Leo, P.; Souzeau, E.; Ridge, B.; Charlesworth, J.C.; Lindsay, R.; et al. Rare, potentially pathogenic variants in 21 keratoconus candidate genes are not enriched in cases in a large Australian cohort of European descent. PLoS ONE 2018, 13, e0199178. [Google Scholar] [CrossRef]
- Alswailmi, F.K. Role of HGF polymorphisms in the development of keratoconus in South Asian population. Electron. J. Gen. Med. 2023, 20, em439. [Google Scholar] [CrossRef]
- Cohen, S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J. Biol. Chem. 1962, 237, 1555–1562. [Google Scholar] [CrossRef]
- Zieske, J.D.; Wasson, M. Regional variation in distribution of EGF receptor in developing and adult corneal epithelium. J. Cell Sci. 1993, 106 Pt 1, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Dabin, I.; Courtois, Y. Acidic fibroblast growth factor overexpression in corneal epithelial wound healing. Growth Factors 1991, 5, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Stepp, M.A.; Zieske, J.D.; Trinkaus-Randall, V.; Kyne, B.M.; Pal-Ghosh, S.; Tadvalkar, G.; Pajoohesh-Ganji, A. Wounding the cornea to learn how it heals. Exp. Eye Res. 2014, 121, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Friend, J.; Ishii, Y.; Thoft, R.A. Corneal epithelial changes in diabetic rats. Ophthalmic Res. 1982, 14, 269–278. [Google Scholar] [CrossRef]
- Kaji, Y. Prevention of diabetic keratopathy. Br. J. Ophthalmol. 2005, 89, 254–255. [Google Scholar] [CrossRef]
- Ljubimov, A.V. Diabetic complications in the cornea. Vis. Res. 2017, 139, 138–152. [Google Scholar] [CrossRef]
- Liu, Y.C.; Francis, J.H.; Abramson, D.H.; FACS. Ocular Side Effects of Systemically Administered Chemotherapy. 2023. Available online: https://www.uptodate.com/contents/ocular-side-effects-of-systemically-administered-chemotherapy/print (accessed on 20 October 2023).
- Foerster, C.G.; Cursiefen, C.; Kruse, F.E. Persisting corneal erosion under cetuximab (Erbitux) treatment (epidermal growth factor receptor antibody). Cornea 2008, 27, 612–614. [Google Scholar] [CrossRef]
- Wood, G.E.; Hockings, H.; Hilton, D.M.; Kermorgant, S. The role of MET in chemotherapy resistance. Oncogene 2021, 40, 1927–1941. [Google Scholar] [CrossRef]
- Murphy, S.V.; Skardal, A.; Nelson Jr, R.A.; Sunnon, K.; Reid, T.; Clouse, C.; Kock, N.D.; Jackson, J.; Soker, S.; Atala, A. Amnion membrane hydrogel and amnion membrane powder accelerate wound healing in a full thickness porcine skin wound model. Stem Cells Transl. Med. 2020, 9, 80–92. [Google Scholar] [CrossRef]
- Puyana, S.; Ruiz, S.; Elkbuli, A.; Bernal, E.; McKenney, M.; Lim, R.; Askari, M.; Mir, H. Using Dehydrated Amniotic Membrane Skin Substitute in Facial Burns: Is There a Outcome Difference Between Adult and Pediatric Patients? J. Craniofacial Surg. 2020, 31, e145–e147. [Google Scholar] [CrossRef]
- Farhadihosseinabadi, B.; Farahani, M.; Tayebi, T.; Jafari, A.; Biniazan, F.; Modaresifar, K.; Moravvej, H.; Bahrami, S.; Redl, H.; Tayebi, L. Amniotic membrane and its epithelial and mesenchymal stem cells as an appropriate source for skin tissue engineering and regenerative medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Bajaj, A.; Gundappa, M. Human Amnion Membrane: Potential Applications in Oral and Periodontal Field. J. Int. Soc. Prev. Community Dent. 2017, 7, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Dadkhah Tehrani, F.; Firouzeh, A.; Shabani, I.; Shabani, A. A Review on Modifications of Amniotic Membrane for Biomedical Applications. Front. Bioeng. Biotechnol. 2021, 8, 606982. [Google Scholar] [CrossRef] [PubMed]
- Hortensius, R.A.; Ebens, J.H.; Dewey, M.J.; Harley, B.A.C. Incorporation of the Amniotic Membrane as an Immunomodulatory Design Element in Collagen Scaffolds for Tendon Repair. ACS Biomater. Sci. Eng. 2018, 4, 4367–4377. [Google Scholar] [CrossRef]
- He, H.; Li, W.; Chen, S.-Y.; Zhang, S.; Chen, Y.-T.; Hayashida, Y.; Zhu, Y.-T.; Tseng, S.C. Suppression of activation and induction of apoptosis in RAW264. 7 cells by amniotic membrane extract. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4468–4475. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Saller, J.; Cuttica, D.; Neufeld, S. Review of Use of Amniotic Membrane Allograft in Total Ankle Replacements. Foot Ankle Orthop. 2019, 4, 2473011419S2473000222. [Google Scholar] [CrossRef]
- Tandel, P.R.; Surati, D.; Patel, S.R. Study of human amniotic membrane as a biological dressing in burn wounds. IJSS J. Surg. 2018, 4, 46–52. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/sea-189839 (accessed on 20 October 2023).
- Koob, T.J.; Lim, J.J.; Zabek, N.; Massee, M. Cytokines in single layer amnion allografts compared to multilayer amnion/chorion allografts for wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1133–1140. [Google Scholar] [CrossRef]
- Koizumi, N.; Inatomi, T.; Sotozono, C.; Fullwood, N.J.; Quantock, A.J.; Kinoshita, S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr. Eye Res. 2000, 20, 173–177. [Google Scholar] [CrossRef]
- De Rötth, A. Plastic repair of conjunctival defects with fetal membranes. Arch. Ophthalmol. 1940, 23, 522–525. [Google Scholar] [CrossRef]
- Silveira, B.C.; Ribeiro, A.P.; Pizzinatto, F.D.; Lobo, P.M.; Miranda, H.R.; de Assis Pereira, N. Effects of commercial amniotic membrane extract on the re-epithelialization time and the early expression of matrix metalloproteinase-9 in cats with experimentally induced corneal ulcers. Vet. Ophthalmol. 2023, 26, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Tseng, S.C.G. Amniotic Membrane Transplantation for Persistent Epithelial Defects With Ulceration. Am. J. Ophthalmol. 1997, 123, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.H.; Riaz, K.M.; Karamichos, D. Treatment of Non-Infectious Corneal Injury: Review of Diagnostic Agents, Therapeutic Medications, and Future Targets. Drugs 2022, 82, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Murri, M.S.; Moshirfar, M.; Birdsong, O.C.; Ronquillo, Y.C.; Ding, Y.; Hoopes, P.C. Amniotic membrane extract and eye drops: A review of literature and clinical application. Clin. Ophthalmol. 2018, 12, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Walkden, A. Amniotic Membrane Transplantation in Ophthalmology: An Updated Perspective. Clin. Ophthalmol. 2020, 14, 2057–2072. [Google Scholar] [CrossRef]
- Soykan, M.N.; Altug, B.; Bas, H.; Ghorbanpoor, H.; Avci, H.; Eroglu, S.; Butun Sengel, S.; Eker Sariboyaci, A.; Gunes Bagis, S.; Uysal, O.; et al. Developing a Novel Platelet-Rich Plasma-Laden Bioadhesive Hydrogel Contact Lens for the Treatment of Ocular Surface Chemical Injuries. Macromol. Biosci. 2023, e2300204. [Google Scholar] [CrossRef] [PubMed]
- Jumelle, C.; Sani, E.S.; Taketani, Y.; Yung, A.; Gantin, F.; Chauhan, S.K.; Annabi, N.; Dana, R. Growth factor-eluting hydrogels for management of corneal defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111790. [Google Scholar] [CrossRef] [PubMed]
- Pal-Ghosh, S.; Pajoohesh-Ganji, A.; Tadvalkar, G.; Kyne, B.M.; Guo, X.; Zieske, J.D.; Stepp, M.A. Topical Mitomycin-C enhances subbasal nerve regeneration and reduces erosion frequency in the debridement wounded mouse cornea. Exp. Eye Res. 2016, 146, 361–369. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Massaro-Giordano, M.; Perez, V.L.; Hamrah, P.; Deng, S.X.; Espandar, L.; Foster, C.S.; Affeldt, J.; Seedor, J.A.; Afshari, N.A.; et al. Topical Recombinant Human Nerve Growth Factor (Cenegermin) for Neurotrophic Keratopathy: A Multicenter Randomized Vehicle-Controlled Pivotal Trial. Ophthalmology 2020, 127, 14–26. [Google Scholar] [CrossRef]
- Zhang, X.; Muddana, S.; Kumar, S.R.; Burton, J.N.; Labroo, P.; Shea, J.; Stocking, P.; Siegl, C.; Archer, B.; Agarwal, J.; et al. Topical Pergolide Enhance Corneal Nerve Regrowth Following Induced Corneal Abrasion. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4. [Google Scholar] [CrossRef]
- Nishida, T.; Chikama, T.-i.; Morishige, N.; Yanai, R.; Yamada, N.; Saito, J. Persistent Epithelial Defects Due to Neurotrophic Keratopathy Treated with a Substance P-Derived Peptide and Insulin-Like Growth Factor 1. Jpn. J. Ophthalmol. 2007, 51, 442–447. [Google Scholar] [CrossRef]
- Bruscolini, A.; Marenco, M.; Albanese, G.M.; Lambiase, A.; Sacchetti, M. Long-term clinical efficacy of topical treatment with recombinant human nerve growth factor in neurotrophic keratopathy: A novel cure for a rare degenerative corneal disease? Orphanet J. Rare Dis. 2022, 17, 57. [Google Scholar] [CrossRef]
- Deeks, E.D.; Lamb, Y.N. Cenegermin: A Review in Neurotrophic Keratitis. Drugs 2020, 80, 489–494. [Google Scholar] [CrossRef]
- Aloe, L. Rita Levi-Montalcini: The discovery of nerve growth factor and modern neurobiology. Trends Cell Biol. 2004, 14, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Fleeman, N.; Mahon, J.; Nevitt, S.; Duarte, R.; Boland, A.; Kotas, E.; Dundar, Y.; McEntee, J.; Ahmad, S. Cenegermin for Treating Neurotrophic Keratitis: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. Pharmacoecon. Open 2019, 3, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.S.; Patel, A.R. Cenegermin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Sheha, H.; Tighe, S.; Hashem, O.; Hayashida, Y. Update On Cenegermin Eye Drops In The Treatment Of Neurotrophic Keratitis. Clin. Ophthalmol. 2019, 13, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chang, C.; Levian, B.; Woodley, D.T.; Li, W. Why Are There So Few FDA-Approved Therapeutics for Wound Healing? Int. J. Mol. Sci. 2023, 24, 15109. [Google Scholar] [CrossRef]
- Mohan, V.K. Recombinant human epidermal growth factor (REGEN-D™ 150): Effect on healing of diabetic foot ulcers. Diabetes Res. Clin. Pract. 2007, 78, 405–411. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Fernández-Montequín, J.; Valdés-Pérez, C.; Savigne-Gutiérrez, W.; Mendoza-Marí, Y.; García-Ojalvo, A.; Falcón-Cama, V.; García del Barco-Herrera, D.; Fernández-Mayola, M.; Pérez-Saad, H.; et al. Diabetic Foot Ulcers and Epidermal Growth Factor: Revisiting the Local Delivery Route for a Successful Outcome. BioMed Res. Int. 2017, 2017, 2923759. [Google Scholar] [CrossRef]
- Tuyet, H.L.; Nguyen Quynh, T.T.; Vo Hoang Minh, H.; Thi Bich, D.N.; Do Dinh, T.; Le Tan, D.; Van, H.L.; Le Huy, T.; Doan Huu, H.; Tran Trong, T.N. The efficacy and safety of epidermal growth factor in treatment of diabetic foot ulcers: The preliminary results. Int. Wound J. 2009, 6, 159–166. [Google Scholar] [CrossRef]
- Zieske, J.D.; Takahashi, H.; Hutcheon, A.E.K.; Dalbone, A.C. Activation of Epidermal Growth Factor Receptor during Corneal Epithelial Migration. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1346–1355. [Google Scholar]
- Scardovi, C.; De Felice, G.; Gazzaniga, A. Epidermal growth factor in the topical treatment of traumatic corneal ulcers. Ophthalmologica 1993, 206, 119–124. [Google Scholar] [CrossRef]
- Pastor, J.C.; Calonge, M. Epidermal Growth Factor and Corneal Wound Healing: A Multicenter Study. Cornea 1992, 11, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.; Dean, W.H.; Price, N.; Gomaa, A.; Ayre, G.; Guglani, S.; Sallam, A. Perforating corneal ulceration in a patient with lung metastatic adenocarcinoma treated with gefitinib: A case report. Case Rep. Ophthalmol. Med. 2012, 2012, 379132. [Google Scholar] [CrossRef]
- Dellaert, M.; Casey, T.; Wiffen, S.; Gordon, J.; Johnson, P.; Geerards, A.; Rijneveld, W.; Remeijer, L.; Mulder, P.; Beekhuis, W. Influence of topical human epidermal growth factor on postkeratoplasty re-epithelialisation. Br. J. Ophthalmol. 1997, 81, 391–395. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kandarakis, A.S.; Page, C.; Kaufman, H.E. The effect of epidermal growth factor on epithelial healing after penetrating keratoplasty in human eyes. Am. J. Ophthalmol. 1984, 98, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.; Davidson, J.M.; Kamerath, C.D.; Wolt, T.B.; Woodward, S.C. Sustained release of epidermal growth factor accelerates wound repair. Proc. Natl. Acad. Sci. USA 1985, 82, 7340–7344. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R.; Anderson, F.L.; Port, J.D.; Skerl, L.; Hershberger, R.E.; Larrabee, P.; O’Connell, J.B.; Renlund, D.G.; Volkman, K.; Murray, J.; et al. Differences in beta-adrenergic neuroeffector mechanisms in ischemic versus idiopathic dilated cardiomyopathy. Circulation 1991, 84, 1024–1039. [Google Scholar] [CrossRef]
- Bristow, M.R.; Ginsburg, R.; Minobe, W.; Cubicciotti, R.S.; Sageman, W.S.; Lurie, K.; Billingham, M.E.; Harrison, D.C.; Stinson, E.B. Decreased Catecholamine Sensitivity and β-Adrenergic-Receptor Density in Failing Human Hearts. N. Engl. J. Med. 1982, 307, 205–211. [Google Scholar] [CrossRef]
- Ledda, F.; Paratcha, G. Negative Regulation of Receptor Tyrosine Kinase (RTK) Signaling: A Developing Field. Biomark. Insights 2007, 2, 45–58. [Google Scholar] [CrossRef]
- Neben, C.L.; Lo, M.; Jura, N.; Klein, O.D. Feedback regulation of RTK signaling in development. Dev. Biol. 2019, 447, 71–89. [Google Scholar] [CrossRef]
- Goh, L.K.; Sorkin, A. Endocytosis of receptor tyrosine kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a017459. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, G.; Tsygankov, A.Y. The Cbl family proteins: Ring leaders in regulation of cell signaling. J. Cell. Physiol. 2006, 209, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Peschard, P.; Park, M. Escape from Cbl-mediated downregulation. Cancer Cell 2003, 3, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Viticchie, G.; Muller, P.A.J. c-Met and Other Cell Surface Molecules: Interaction, Activation and Functional Consequences. Biomedicines 2015, 3, 46–70. [Google Scholar] [CrossRef]
- Weathington, N.M.; Mallampalli, R.K. Emerging therapies targeting the ubiquitin proteasome system in cancer. J. Clin. Investig. 2014, 124, 6–12. [Google Scholar] [CrossRef]
- Chang, C.H.; Bijian, K.; Qiu, D.; Su, J.; Saad, A.; Dahabieh, M.S.; Miller, W.H., Jr.; Alaoui-Jamali, M.A. Endosomal sorting and c-Cbl targeting of paxillin to autophagosomes regulate cell-matrix adhesion turnover in human breast cancer cells. Oncotarget 2017, 8, 31199–31214. [Google Scholar] [CrossRef]
- Li, N.; Lorinczi, M.; Ireton, K.; Elferink, L.A. Specific Grb2-mediated Interactions Regulate Clathrin-dependent Endocytosis of the cMet-tyrosine Kinase. J. Biol. Chem. 2007, 282, 16764–16775. [Google Scholar] [CrossRef]
- van der Bliek, A.M.; Redelmeier, T.E.; Damke, H.; Tisdale, E.J.; Meyerowitz, E.M.; Schmid, S.L. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol. 1993, 122, 553–563. [Google Scholar] [CrossRef]
- van der Bliek, A.M.; Meyerowitz, E.M. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature 1991, 351, 411–414. [Google Scholar] [CrossRef]
- Herskovits, J.S.; Burgess, C.C.; Obar, R.A.; Vallee, R.B. Effects of mutant rat dynamin on endocytosis. J. Cell Biol. 1993, 122, 565–578. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.C.; Murillo, S.; Saikia, P.; Wilson, S.E. The Efficacy of Topical HGF on Corneal Fibrosis and Epithelial Healing after Scar-Producing PRK Injury in Rabbits. Transl. Vis. Sci. Technol. 2020, 9, 29. [Google Scholar] [CrossRef]
- Funakoshi, H.; Nakamura, T. Hepatocyte growth factor (HGF): Neurotrophic functions and therapeutic implications for neuronal injury/diseases. Curr. Signal Transduct. Ther. 2011, 6, 156–167. [Google Scholar] [CrossRef]
- Nishida, K.; Sotozono, C.; Adachi, W.; Yamamoto, S.; Yokoi, N.; Kinoshita, S. Transforming growth factor-beta 1, -beta 2 and -beta 3 mRNA expression in human cornea. Curr. Eye Res. 1995, 14, 235–241. [Google Scholar] [CrossRef]
- Stramer, B.M.; Zieske, J.D.; Jung, J.C.; Austin, J.S.; Fini, M.E. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: Implications for surgical outcomes. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4237–4246. [Google Scholar] [CrossRef]
- Masur, S.K.; Dewal, H.S.; Dinh, T.T.; Erenburg, I.; Petridou, S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc. Natl. Acad. Sci. USA 1996, 93, 4219–4223. [Google Scholar] [CrossRef]
- Nakamura, K.; Kurosaka, D.; Yoshino, M.; Oshima, T.; Kurosaka, H. Injured corneal epithelial cells promote myodifferentiation of corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2603–2608. [Google Scholar]
- Kaur, H.; Chaurasia, S.S.; de Medeiros, F.W.; Agrawal, V.; Salomao, M.Q.; Singh, N.; Ambati, B.K.; Wilson, S.E. Corneal stroma PDGF blockade and myofibroblast development. Exp. Eye Res. 2009, 88, 960–965. [Google Scholar] [CrossRef]
- Kaur, H.; Chaurasia, S.S.; Agrawal, V.; Suto, C.; Wilson, S.E. Corneal myofibroblast viability: Opposing effects of IL-1 and TGF β1. Exp. Eye Res. 2009, 89, 152–158. [Google Scholar] [CrossRef]
- Singh, V.; Barbosa, F.L.; Torricelli, A.A.; Santhiago, M.R.; Wilson, S.E. Transforming growth factor β and platelet-derived growth factor modulation of myofibroblast development from corneal fibroblasts in vitro. Exp. Eye Res. 2014, 120, 152–160. [Google Scholar] [CrossRef]
- Wilson, S.E. Corneal wound healing. Exp. Eye Res. 2020, 197, 108089. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Moller-Pedersen, T.; Huang, J.; Sax, C.M.; Kays, W.T.; Cavangh, H.D.; Petroll, W.M.; Piatigorsky, J. The cellular basis of corneal transparency: Evidence for ‘corneal crystallins’. J. Cell Sci. 1999, 112 Pt 5, 613–622. [Google Scholar] [CrossRef]
- Mizuno, S.; Matsumoto, K.; Kurosawa, T.; Mizuno-Horikawa, Y.; Nakamura, T. Reciprocal balance of hepatocyte growth factor and transforming growth factor-beta 1 in renal fibrosis in mice. Kidney Int. 2000, 57, 937–948. [Google Scholar] [CrossRef]
- Nakamura, T.; Mizuno, S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 588–610. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Okada, H.; Kobayashi, T.; Watanabe, Y.; Kanno, Y.; Kopp, J.B.; Nishida, T.; Takigawa, M.; Ueno, M.; Nakamura, T.; et al. Hepatocyte growth factor counteracts transforming growth factor-beta1, through attenuation of connective tissue growth factor induction, and prevents renal fibrogenesis in 5/6 nephrectomized mice. FASEB J. 2003, 17, 268–270. [Google Scholar] [CrossRef]
- Iekushi, K.; Taniyama, Y.; Azuma, J.; Sanada, F.; Kusunoki, H.; Yokoi, T.; Koibuchi, N.; Okayama, K.; Rakugi, H.; Morishita, R. Hepatocyte growth factor attenuates renal fibrosis through TGF-β1 suppression by apoptosis of myofibroblasts. J. Hypertens. 2010, 28, 2454–2461. [Google Scholar] [CrossRef]
- Ishizaki, M.; Zhu, G.; Haseba, T.; Shafer, S.S.; Kao, W.W. Expression of collagen I, smooth muscle alpha-actin, and vimentin during the healing of alkali-burned and lacerated corneas. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3320–3328. [Google Scholar]
- Kurosaka, H.; Kurosaka, D.; Kato, K.; Mashima, Y.; Tanaka, Y. Transforming growth factor-beta 1 promotes contraction of collagen gel by bovine corneal fibroblasts through differentiation of myofibroblasts. Investig. Ophthalmol. Vis. Sci. 1998, 39, 699–704. [Google Scholar]
- Nagaraj, N.S.; Datta, P.K. Targeting the transforming growth factor-beta signaling pathway in human cancer. Expert Opin. Investig. Drugs 2010, 19, 77–91. [Google Scholar] [CrossRef]
- Kamimoto, M.; Mizuno, S.; Matsumoto, K.; Nakamura, T. Hepatocyte growth factor prevents multiple organ injuries in endotoxemic mice through a heme oxygenase-1-dependent mechanism. Biochem. Biophys. Res. Commun. 2009, 380, 333–337. [Google Scholar] [CrossRef]
- Gupta, S.; Sinha, N.R.; Martin, L.M.; Keele, L.M.; Sinha, P.R.; Rodier, J.T.; Landreneau, J.R.; Hesemann, N.P.; Mohan, R.R. Long-Term Safety and Tolerability of BMP7 and HGF Gene Overexpression in Rabbit Cornea. Transl. Vis. Sci. Technol. 2021, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Fink, M.K.; Ghosh, A.; Tripathi, R.; Sinha, P.R.; Sharma, A.; Hesemann, N.P.; Chaurasia, S.S.; Giuliano, E.A.; Mohan, R.R. Novel Combination BMP7 and HGF Gene Therapy Instigates Selective Myofibroblast Apoptosis and Reduces Corneal Haze In Vivo. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1045–1057. [Google Scholar] [CrossRef]
- Uchikawa, E.; Chen, Z.; Xiao, G.Y.; Zhang, X.; Bai, X.C. Structural basis of the activation of c-MET receptor. Nat. Commun. 2021, 12, 4074. [Google Scholar] [CrossRef] [PubMed]
- Paumelle, R.; Tulashe, D.; Kherrouche, Z.; Plaza, S.; Leroy, C.; Reveneau, S.; Vandenbunder, B.; Fafeur, V. Hepatocyte growth factor/scatter factor activates the ETS1 transcription factor by a RAS-RAF-MEK-ERK signaling pathway. Oncogene 2002, 21, 2309–2319. [Google Scholar] [CrossRef]
- Kakazu, A.; Chandrasekher, G.; Bazan, H.E. HGF protects corneal epithelial cells from apoptosis by the PI-3K/Akt-1/Bad- but not the ERK1/2-mediated signaling pathway. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3485–3492. [Google Scholar] [CrossRef]
- Rodrigues, G.A.; Park, M.; Schlessinger, J. Activation of the JNK pathway is essential for transformation by the Met oncogene. EMBO J. 1997, 16, 2634–2645. [Google Scholar] [CrossRef]
- Garcia-Guzman, M.; Dolfi, F.; Zeh, K.; Vuori, K. Met-induced JNK activation is mediated by the adapter protein Crk and correlates with the Gab1—Crk signaling complex formation. Oncogene 1999, 18, 7775–7786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-W.; Wang, L.-M.; Jove, R.; Vande Woude, G.F. Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene 2002, 21, 217–226. [Google Scholar] [CrossRef]
- Syed, Z.A.; Yin, W.; Hughes, K.; Gill, J.N.; Shi, R.; Clifford, J.L. HGF/c-met/Stat3 signaling during skin tumor cell invasion: Indications for a positive feedback loop. BMC Cancer 2011, 11, 180. [Google Scholar] [CrossRef]
- Biswas, S.; Shafiquzzaman, M.; Yu, G.; Li, P.; Yu, Q.; Zhao, P.; Li, B.; Li, J. Notch1 signaling in keratocytes maintains corneal transparency by suppressing VEGF expression. Stem Cell Rep. 2022, 17, 1442–1457. [Google Scholar] [CrossRef]
- Sakamoto, T.; Ueno, H.; Sonoda, K.; Hisatomi, T.; Shimizu, K.; Ohashi, H.; Inomata, H. Blockade of TGF-β by in vivo gene transfer of a soluble TGF-β type II receptor in the muscle inhibits corneal opacification, edema and angiogenesis. Gene Ther. 2000, 7, 1915–1924. [Google Scholar] [CrossRef][Green Version]
- Colombo, E.S.; Menicucci, G.; McGuire, P.G.; Das, A. Hepatocyte growth factor/scatter factor promotes retinal angiogenesis through increased urokinase expression. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1793–1800. [Google Scholar] [CrossRef]
- Boulton, M. A role for hepatocyte growth factor in diabetic retinopathy? BMJ Publishing Group Ltd.: Hoboken, NJ, USA, 1999; Volume 83, pp. 763–764. [Google Scholar]
- Ramos-Lopez, M.; Hirose, T.; Lashkari, K. Hepatocyte growth factor receptor is selectively activated in the vascular compartment of stage 5 retinopathy of prematurity. Investig. Ophthalmol. Vis. Sci. 2003, 44, 566. [Google Scholar]
- He, M.; Han, T.; Wang, Y.; Wu, Y.H.; Qin, W.S.; Du, L.Z.; Zhao, C.Q. Effects of HGF and KGF gene silencing on vascular endothelial growth factor and its receptors in rat ultraviolet radiation-induced corneal neovascularization. Int. J. Mol. Med. 2019, 43, 1888–1899. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Lee, S.H.; Lee, J.; Lee, M.Y.; Lim, J.; Kim, S.; Kim, S. Hepatocyte growth factor is necessary for efficient outgrowth of injured peripheral axons in in vitro culture system and in vivo nerve crush mouse model. Biochem. Biophys. Rep. 2021, 26, 100973. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Kagoshima, M.; Wanaka, A.; Tohyama, M.; Matsumoto, K.; Nakamura, T. Localization and functional coupling of HGF and c-Met/HGF receptor in rat brain: Implication as neurotrophic factor. Brain Res. Mol. Brain Res. 1995, 32, 197–210. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarvestad-Laise, K.E.; Ceresa, B.P. Modulating Growth Factor Receptor Signaling to Promote Corneal Epithelial Homeostasis. Cells 2023, 12, 2730. https://doi.org/10.3390/cells12232730
Tarvestad-Laise KE, Ceresa BP. Modulating Growth Factor Receptor Signaling to Promote Corneal Epithelial Homeostasis. Cells. 2023; 12(23):2730. https://doi.org/10.3390/cells12232730
Chicago/Turabian StyleTarvestad-Laise, Kate E., and Brian P. Ceresa. 2023. "Modulating Growth Factor Receptor Signaling to Promote Corneal Epithelial Homeostasis" Cells 12, no. 23: 2730. https://doi.org/10.3390/cells12232730
APA StyleTarvestad-Laise, K. E., & Ceresa, B. P. (2023). Modulating Growth Factor Receptor Signaling to Promote Corneal Epithelial Homeostasis. Cells, 12(23), 2730. https://doi.org/10.3390/cells12232730







