New Perspectives in Radiological and Radiopharmaceutical Hybrid Imaging in Progressive Supranuclear Palsy: A Systematic Review
Abstract
:1. Introduction
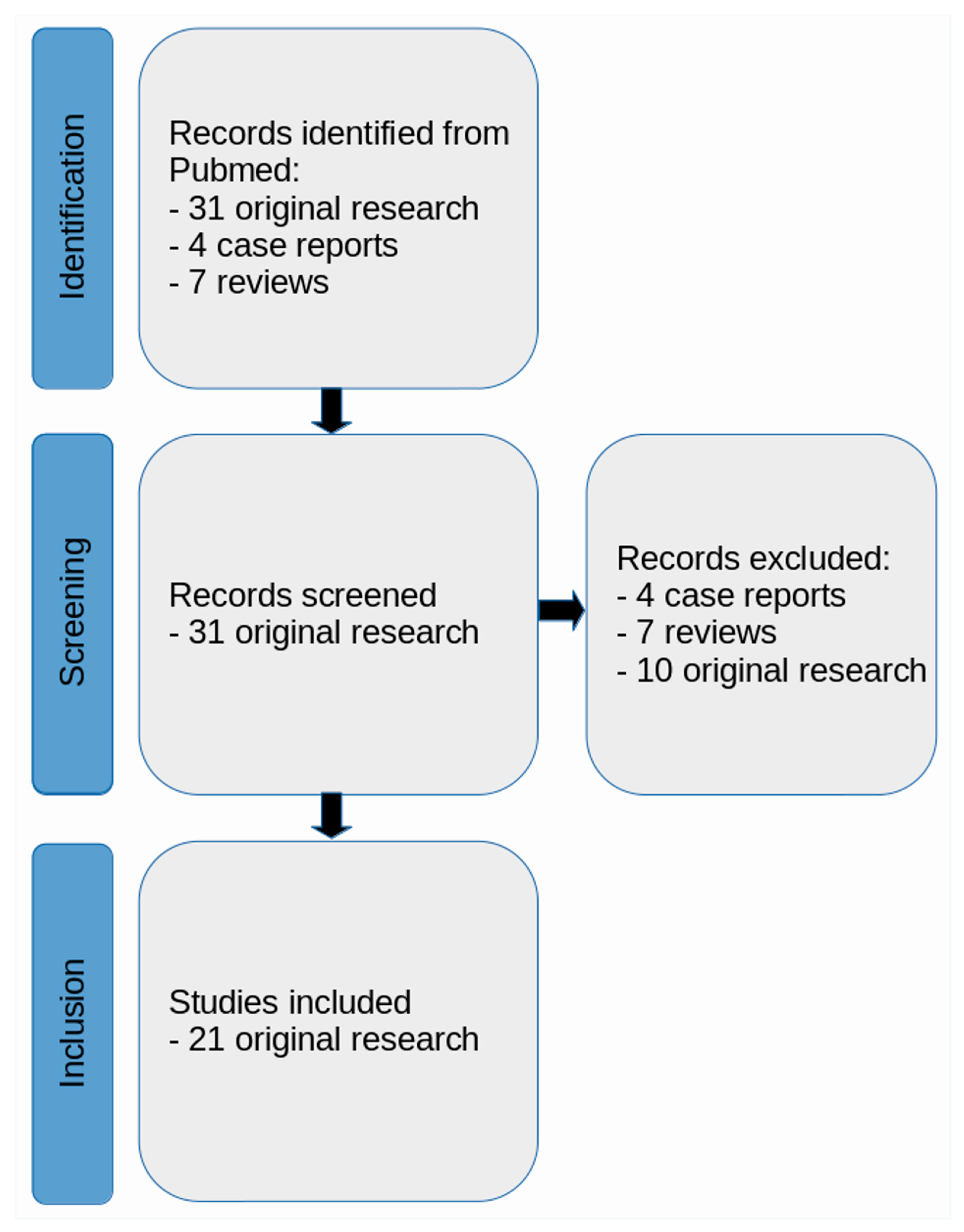
2. Methods
2.1. Literature Search Strategy
2.2. Identified Articles
3. Results
3.1. Methodological Overview for PET
3.1.1. First-Generation Tau Tracers
3.1.2. Second-Generation Tau Tracers
3.1.3. Recent Development of Tracers
3.2. General Characteristics of Identified Literature
3.2.1. Findings from PET/CT with MRI Co-Registration
[18F]AV-1451
[11C]UCB-J
[11C]PK11195 and [18F]AV-1451
[18F]RO948
[18F]-PM-PBB3
3.2.2. Findings from PET/MRI
[18F]PI-2620
[11C]UCB-J
3.2.3. Findings from PET with Multi-Modal MRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Boxer, A.L.; Yu, J.-T.; Golbe, L.I.; Litvan, I.; Lang, A.E.; Höglinger, G.U. Advances in progressive supranuclear palsy: New diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol. 2017, 16, 552–563. [Google Scholar] [CrossRef]
- VandeVrede, L.; Ljubenkov, P.A.; Rojas, J.C.; Welch, A.E.; Boxer, A.L. Four-Repeat Tauopathies: Current Management and Future Treatments. Neurother. J. Am. Soc. Exp. NeuroTher. 2020, 17, 1563–1581. [Google Scholar] [CrossRef]
- Kovacs, G.G.; Lukic, M.J.; Irwin, D.J.; Arzberger, T.; Respondek, G.; Lee, E.B.; Coughlin, D.; Giese, A.; Grossman, M.; Kurz, C.; et al. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. 2020, 140, 99–119. [Google Scholar] [CrossRef]
- Stamelou, M.; Respondek, G.; Giagkou, N.; Whitwell, J.L.; Kovacs, G.G.; Höglinger, G.U. Evolving concepts in progressive supranuclear palsy and other 4-repeat tauopathies. Nat. Rev. Neurol. 2021, 17, 601–620. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. Off. J. Mov. Disord. Soc. 2017, 32, 853–864. [Google Scholar] [CrossRef]
- Chung, D.C.; Roemer, S.; Petrucelli, L.; Dickson, D.W. Cellular and pathological heterogeneity of primary tauopathies. Mol. Neurodegener. 2021, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Svenningsson, P. Corticobasal degeneration: Advances in clinicopathology and biomarkers. Curr. Opin. Neurol. 2019, 32, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G. Tauopathies. In Handbook of Clinical Neurology; North-Holland Publishing Company: Amsterdam, The Netherlands, 2017; Volume 145, pp. 355–368. [Google Scholar] [CrossRef]
- Greene, P. Progressive Supranuclear Palsy, Corticobasal Degeneration, and Multiple System Atrophy. Continuum 2019, 25, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.C.; Ye, X.; Mellor, J.A.; Golden, K.J.; Zamudio, J.; Chiodo, L.A.; Bao, Y.; Xie, T. Disease course and treatment patterns in progressive supranuclear palsy: A real-world study. J. Neurol. Sci. 2021, 421, 117293. [Google Scholar] [CrossRef]
- Greten, S.; Wegner, F.; Jensen, I.; Krey, L.; Rogozinski, S.; Fehring, M.; Heine, J.; Doll-Lee, J.; Pötter-Nerger, M.; Zeitzschel, M.; et al. The comorbidity and co-medication profile of patients with progressive supranuclear palsy. J. Neurol. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Barer, Y.; Cohen, R.; Grabarnik-John, M.; Ye, X.; Zamudio, J.; Gurevich, T.; Chodick, G. Progressive supranuclear palsy’s economical burden: The use and costs of healthcare resources in a large health provider in Israel. J. Neurol. 2023, 270, 3770–3778. [Google Scholar] [CrossRef] [PubMed]
- Barer, Y.; Chodick, G.; Cohen, R.; Grabarnik-John, M.; Ye, X.; Zamudio, J.; Gurevich, T. Epidemiology of Progressive Supranuclear Palsy: Real World Data from the Second Largest Health Plan in Israel. Brain Sci. 2022, 12, 1126. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.T.; Casteau, S.; Archibald, N. Spatial attention and spatial short term memory in PSP and Parkinson’s disease. Cortex A J. Devoted Study Nerv. Syst. Behav. 2021, 137, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, J.L.; Höglinger, G.U.; Antonini, A.; Bordelon, Y.; Boxer, A.L.; Colosimo, C.; van Eimeren, T.; Golbe, L.I.; Kassubek, J.; Kurz, C.; et al. Radiological biomarkers for diagnosis in PSP: Where are we and where do we need to be? Mov. Disord. Off. J. Mov. Disord. Soc. 2017, 32, 955–971. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, J.L.; Tosakulwong, N.; Clark, H.M.; Ali, F.; Botha, H.; Weigand, S.D.; Sintini, I.; Machulda, M.M.; Schwarz, C.G.; Reid, R.I.; et al. Diffusion tensor imaging analysis in three progressive supranuclear palsy variants. J. Neurol. 2021, 268, 3409–3420. [Google Scholar] [CrossRef]
- Saeed, U.; Lang, A.E.; Masellis, M. Neuroimaging Advances in Parkinson’s Disease and Atypical Parkinsonian Syndromes. Front. Neurol. 2020, 11, 572976. [Google Scholar] [CrossRef] [PubMed]
- Messerschmidt, K.; Barthel, H.; Brendel, M.; Scherlach, C.; Hoffmann, K.-T.; Rauchmann, B.-S.; Rullmann, M.; Marek, K.; Villemagne, V.L.; Rumpf, J.-J.; et al. 18F-PI-2620 Tau PET Improves the Imaging Diagnosis of Progressive Supranuclear Palsy. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2022, 63, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, I.; Gallivanone, F.; Gilardi, M.C. Quantitation and Data Analysis in Hybrid PET/MRI Systems. In PET-CT and PET-MRI in Neurology; Ciarmiello, A., Mansi, L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 23–30. [Google Scholar]
- Panic, N.; Leoncini, E.; de Belvis, G.; Ricciardi, W.; Boccia, S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE 2013, 8, e83138. [Google Scholar] [CrossRef]
- Ichise, M.; Meyer, J.H.; Yonekura, Y. An introduction to PET and SPECT neuroreceptor quantification models. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2001, 42, 755–763. [Google Scholar]
- Mena, A.M.; Strafella, A.P. Imaging pathological tau in atypical parkinsonisms: A review. Clin. Park. Relat. Disord. 2022, 7, 100155. [Google Scholar] [CrossRef]
- Crișan, G.; Moldovean-Cioroianu, N.S.; Timaru, D.-G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022, 23, 5023. [Google Scholar] [CrossRef]
- Hellwig, S.; Frings, L.; Amtage, F.; Buchert, R.; Spehl, T.S.; Rijntjes, M.; Tüscher, O.; Weiller, C.; Weber, W.A.; Vach, W.; et al. 18F-FDG PET Is an Early Predictor of Overall Survival in Suspected Atypical Parkinsonism. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2015, 56, 1541–1546. [Google Scholar] [CrossRef]
- Wu, P.; Zhao, Y.; Wu, J.; Brendel, M.; Lu, J.; Ge, J.; Bernhardt, A.; Li, L.; Alberts, I.; Katzdobler, S.; et al. Differential Diagnosis of Parkinsonism Based on Deep Metabolic Imaging Indices. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2022, 63, 1741–1747. [Google Scholar] [CrossRef]
- Brajkovic, L.; Kostic, V.; Sobic-Saranovic, D.; Stefanova, E.; Jecmenica-Lukic, M.; Jesic, A.; Stojiljkovic, M.; Odalovic, S.; Gallivanone, F.; Castiglioni, I.; et al. The utility of FDG-PET in the differential diagnosis of Parkinsonism. Neurol. Res. 2017, 39, 675–684. [Google Scholar] [CrossRef]
- Eidelberg, D.; Moeller, J.R.; Ishikawa, T.; Dhawan, V.; Spetsieris, P.; Chaly, T.; Belakhlef, A.; Mandel, F.; Przedborski, S.; Fahn, S. Early differential diagnosis of Parkinson’s disease with 18F-fluorodeoxyglucose and positron emission tomography. Neurology 1995, 45, 1995–2004. [Google Scholar] [CrossRef]
- Eckert, T.; Tang, C.; Ma, Y.; Brown, N.; Lin, T.; Frucht, S.; Feigin, A.; Eidelberg, D. Abnormal metabolic networks in atypical parkinsonism. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Pin, G.; Labouré, J.; Guedj, E.; Felician, O.; Grimaldi, S.; Azulay, J.P.; Ceccaldi, M.; Koric, L. Brain FDG-PET correlates of saccadic disorders in early PSP. J. Neurol. 2023, 270, 4841–4850. [Google Scholar] [CrossRef]
- Seiffert, A.P.; Gómez-Grande, A.; Alonso-Gómez, L.; Méndez-Guerrero, A.; Villarejo-Galende, A.; Gómez, E.J.; Sánchez-González, P. Differences in Striatal Metabolism in 18FFDG PET in Parkinson’s Disease and Atypical Parkinsonism. Diagnostics 2022, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Houssein, N.J.; Henriksen, A.C.; Hejl, A.-M.; Marner, L. Diagnostic accuracy of cerebral 18FFDG PET in atypical parkinsonism. EJNMMI Res. 2023, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Buchert, R.; Wegner, F.; Huppertz, H.-J.; Berding, G.; Brendel, M.; Apostolova, I.; Buhmann, C.; Dierks, A.; Katzdobler, S.; Klietz, M.; et al. Automatic covariance pattern analysis outperforms visual reading of 18 F-fluorodeoxyglucose-positron emission tomography (FDG-PET) in variant progressive supranuclear palsy. Mov. Disord. Off. J. Mov. Disord. Soc. 2023, 38, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Shoghi-Jadid, K.; Small, G.W.; Agdeppa, E.D.; Kepe, V.; Ercoli, L.M.; Siddarth, P.; Read, S.; Satyamurthy, N.; Petric, A.; Huang, S.-C.; et al. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2002, 10, 24–35. [Google Scholar] [CrossRef]
- Coakeley, S.; Strafella, A.P. Imaging tau pathology in Parkinsonisms. NPJ Park. Dis. 2017, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; Tolboom, N.; Foster-Dingley, J.C.; Adriaanse, S.F.; Boellaard, R.; Yaqub, M.; Windhorst, A.D.; Barkhof, F.; Lammertsma, A.A.; Scheltens, P.; et al. Longitudinal imaging of Alzheimer pathology using 11CPIB, 18FFDDNP and 18FFDG PET. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 990–1000. [Google Scholar] [CrossRef]
- Kepe, V.; Bordelon, Y.; Boxer, A.; Huang, S.-C.; Liu, J.; Thiede, F.C.; Mazziotta, J.C.; Mendez, M.F.; Donoghue, N.; Small, G.W.; et al. PET imaging of neuropathology in tauopathies: Progressive supranuclear palsy. J. Alzheimer’s Dis. JAD 2013, 36, 145–153. [Google Scholar] [CrossRef]
- Ricci, M.; Cimini, A.; Camedda, R.; Chiaravalloti, A.; Schillaci, O. Tau Biomarkers in Dementia: Positron Emission Tomography Radiopharmaceuticals in Tauopathy Assessment and Future Perspective. Int. J. Mol. Sci. 2021, 22, 13002. [Google Scholar] [CrossRef]
- Buongiorno, M.; Antonelli, F.; Compta, Y.; Fernandez, Y.; Pavia, J.; Lomeña, F.; Ríos, J.; Ramírez, I.; García, J.R.; Soler, M.; et al. Cross-Sectional and Longitudinal Cognitive Correlates of FDDNP PET and CSF Amyloid-β and Tau in Parkinson’s Disease1. J. Alzheimer’s Dis. JAD 2017, 55, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Jie, C.V.M.L.; Treyer, V.; Schibli, R.; Mu, L. Tauvid™: The First FDA-Approved PET Tracer for Imaging Tau Pathology in Alzheimer’s Disease. Pharmaceuticals 2021, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Alizadeh, H.; Marton, J.; Cumming, P. The Sensitivity of Tau Tracers for the Discrimination of Alzheimer’s Disease Patients and Healthy Controls by PET. Biomolecules 2023, 13, 290. [Google Scholar] [CrossRef] [PubMed]
- Marquié, M.; Normandin, M.D.; Meltzer, A.C.; Siao Tick Chong, M.; Andrea, N.V.; Antón-Fernández, A.; Klunk, W.E.; Mathis, C.A.; Ikonomovic, M.D.; Debnath, M.; et al. Pathological correlations of F-18-AV-1451 imaging in non-alzheimer tauopathies. Ann. Neurol. 2017, 81, 117–128. [Google Scholar] [CrossRef]
- Nicastro, N.; Rodriguez, P.V.; Malpetti, M.; Bevan-Jones, W.R.; Simon Jones, P.; Passamonti, L.; Aigbirhio, F.I.; O’Brien, J.T.; Rowe, J.B. 18F-AV1451 PET imaging and multimodal MRI changes in progressive supranuclear palsy. J. Neurol. 2020, 267, 341–349. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Lowe, V.J.; Tosakulwong, N.; Weigand, S.D.; Senjem, M.L.; Schwarz, C.G.; Spychalla, A.J.; Petersen, R.C.; Jack, C.R.; Josephs, K.A. 18 FAV-1451 tau positron emission tomography in progressive supranuclear palsy. Mov. Disord. Off. J. Mov. Disord. Soc. 2017, 32, 124–133. [Google Scholar] [CrossRef]
- Ghirelli, A.; Tosakulwong, N.; Weigand, S.D.; Clark, H.M.; Ali, F.; Botha, H.; Duffy, J.R.; Utianski, R.L.; Buciuc, M.; Murray, M.E.; et al. Sensitivity-Specificity of Tau and Amyloid β Positron Emission Tomography in Frontotemporal Lobar Degeneration. Ann. Neurol. 2020, 88, 1009–1022. [Google Scholar] [CrossRef]
- Murugan, N.A.; Chiotis, K.; Rodriguez-Vieitez, E.; Lemoine, L.; Ågren, H.; Nordberg, A. Cross-interaction of tau PET tracers with monoamine oxidase B: Evidence from in silico modelling and in vivo imaging. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Fodero-Tavoletti, M.T.; Okamura, N.; Furumoto, S.; Mulligan, R.S.; Connor, A.R.; McLean, C.A.; Cao, D.; Rigopoulos, A.; Cartwright, G.A.; O’Keefe, G.; et al. 18F-THK523: A novel in vivo tau imaging ligand for Alzheimer’s disease. Brain A J. Neurol. 2011, 134, 1089–1100. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Okamura, N. In vivo tau imaging: Obstacles and progress. Alzheimer’s Dement. 2014, 10, S254–S264. [Google Scholar] [CrossRef] [PubMed]
- Fodero-Tavoletti, M.T.; Furumoto, S.; Taylor, L.; McLean, C.A.; Mulligan, R.S.; Birchall, I.; Harada, R.; Masters, C.L.; Yanai, K.; Kudo, Y.; et al. Assessing THK523 selectivity for tau deposits in Alzheimer’s disease and non-Alzheimer’s disease tauopathies. Alzheimer’s Res. Ther. 2014, 6, 11. [Google Scholar] [CrossRef]
- Choi, Y.; Ha, S.; Lee, Y.-S.; Kim, Y.K.; Lee, D.S.; Kim, D.J. Development of tau PET Imaging Ligands and their Utility in Preclinical and Clinical Studies. Nucl. Med. Mol. Imaging 2017, 52, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Brendel, M.; Yousefi, B.H.; Blume, T.; Herz, M.; Focke, C.; Deussing, M.; Peters, F.; Lindner, S.; Ungern-Sternberg, B.v.; Drzezga, A.; et al. Comparison of 18F-T807 and 18F-THK5117 PET in a Mouse Model of Tau Pathology. Front. Aging Neurosci. 2018, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Chiotis, K.; Saint-Aubert, L.; Savitcheva, I.; Jelic, V.; Andersen, P.; Jonasson, M.; Eriksson, J.; Lubberink, M.; Almkvist, O.; Wall, A.; et al. Imaging in-vivo tau pathology in Alzheimer’s disease with THK5317 PET in a multimodal paradigm. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1686–1699. [Google Scholar] [CrossRef] [PubMed]
- Chiotis, K.; Saint-Aubert, L.; Rodriguez-Vieitez, E.; Leuzy, A.; Almkvist, O.; Savitcheva, I.; Jonasson, M.; Lubberink, M.; Wall, A.; Antoni, G.; et al. Longitudinal changes of tau PET imaging in relation to hypometabolism in prodromal and Alzheimer’s disease dementia. Mol. Psychiatry 2018, 23, 1666–1673. [Google Scholar] [CrossRef]
- Harada, R.; Ishiki, A.; Kai, H.; Sato, N.; Furukawa, K.; Furumoto, S.; Tago, T.; Tomita, N.; Watanuki, S.; Hiraoka, K.; et al. Correlations of 18F-THK5351 PET with Postmortem Burden of Tau and Astrogliosis in Alzheimer Disease. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2018, 59, 671–674. [Google Scholar] [CrossRef]
- Okamura, N.; Harada, R.; Ishiki, A.; Kikuchi, A.; Nakamura, T.; Kudo, Y. The development and validation of tau PET tracers: Current status and future directions. Clin. Transl. Imaging 2018, 6, 305–316. [Google Scholar] [CrossRef]
- Betthauser, T.J.; Lao, P.J.; Murali, D.; Barnhart, T.E.; Furumoto, S.; Okamura, N.; Stone, C.K.; Johnson, S.C.; Christian, B.T. In Vivo Comparison of Tau Radioligands 18F-THK-5351 and 18F-THK-5317. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2017, 58, 996–1002. [Google Scholar] [CrossRef]
- Ishiki, A.; Harada, R.; Kai, H.; Sato, N.; Totsune, T.; Tomita, N.; Watanuki, S.; Hiraoka, K.; Ishikawa, Y.; Funaki, Y.; et al. Neuroimaging-pathological correlations of 18FTHK5351 PET in progressive supranuclear palsy. Acta Neuropathol. Commun. 2018, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Sone, D.; Imabayashi, E.; Maikusa, N.; Okamura, N.; Furumoto, S.; Kudo, Y.; Ogawa, M.; Takano, H.; Yokoi, Y.; Sakata, M.; et al. Regional tau deposition and subregion atrophy of medial temporal structures in early Alzheimer’s disease: A combined positron emission tomography/magnetic resonance imaging study. Alzheimer’s Dement. 2017, 9, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Shimada, H.; Suhara, T.; Shinotoh, H.; Ji, B.; Maeda, J.; Zhang, M.-R.; Trojanowski, J.Q.; Lee, V.M.-Y.; Ono, M.; et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron 2013, 79, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Shimada, H.; Sahara, N.; Ono, M.; Koga, S.; Kitamura, S.; Niwa, F.; Hirano, S.; Kimura, Y.; Ichise, M.; et al. In vivo binding of a tau imaging probe, 11 CPBB3, in patients with progressive supranuclear palsy. Mov. Disord. Off. J. Mov. Disord. Soc. 2019, 34, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Schröter, N.; Blazhenets, G.; Frings, L.; Barkhausen, C.; Jost, W.H.; Weiller, C.; Rijntjes, M.; Meyer, P.T. Tau Imaging in the 4-Repeat-Tauopathies Progressive Supranuclear Palsy and Corticobasal Syndrome: A 11C-Pyridinyl-Butadienyl-Benzothiazole 3 PET Pilot Study. Clin. Nucl. Med. 2020, 45, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Uzuegbunam, B.C.; Librizzi, D.; Hooshyar Yousefi, B. PET Radiopharmaceuticals for Alzheimer’s Disease and Parkinson’s Disease Diagnosis, the Current and Future Landscape. Molecules 2020, 25, 977. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.-C.; Hsiao, I.-T.; Yang, Q.-F.; Yao, C.-H.; Tai, C.-Y.; Wu, M.-F.; Yen, T.-C.; Jang, M.-K.; Lin, K.-J. Characterization of 18F-PM-PBB3 (18F-APN-1607) Uptake in the rTg4510 Mouse Model of Tauopathy. Molecules 2020, 25, 1750. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Kitamura, S.; Ono, M.; Kimura, Y.; Ichise, M.; Takahata, K.; Moriguchi, S.; Kubota, M.; Ishii, T.; Takado, Y.; et al. [P3–378]: First-in-human pet study with 18 F-AM-PBB3 and 18 F-PM-PBB3. Alzheimer’s Dement. 2017, 13, P1104. [Google Scholar] [CrossRef]
- Li, L.; Liu, F.-T.; Li, M.; Lu, J.-Y.; Sun, Y.-M.; Liang, X.; Bao, W.; Chen, Q.-S.; Li, X.-Y.; Zhou, X.-Y.; et al. Clinical Utility of 18 F-APN-1607 Tau PET Imaging in Patients with Progressive Supranuclear Palsy. Mov. Disord. Off. J. Mov. Disord. Soc. 2021, 36, 2314–2323. [Google Scholar] [CrossRef]
- Mashima, K.; Konishi, M.; Tezuka, T.; Ito, D.; Mimura, M. A case of tauopathy with auditory agnosia and dysprosody diagnosed by 18FPM-PBB3 tau PET scan. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2021, 42, 3471–3474. [Google Scholar] [CrossRef]
- Zhou, X.-Y.; Lu, J.-Y.; Liu, F.-T.; Wu, P.; Zhao, J.; Ju, Z.-Z.; Tang, Y.-L.; Shi, Q.-Y.; Lin, H.-M.; Wu, J.-J.; et al. In Vivo 18 F-APN-1607 Tau Positron Emission Tomography Imaging in MAPT Mutations: Cross-Sectional and Longitudinal Findings. Mov. Disord. Off. J. Mov. Disord. Soc. 2022, 37, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Ishizuchi, K.; Takizawa, T.; Tezuka, T.; Takahata, K.; Seki, M.; Tabuchi, H.; Ueda, R.; Kubota, M.; Mimura, M.; Nakahara, J.; et al. A case of progressive supranuclear palsy with predominant cerebellar ataxia diagnosed by 18FPM-PBB3 tau PET. J. Neurol. Sci. 2021, 425, 117440. [Google Scholar] [CrossRef] [PubMed]
- Katzdobler, S.; Nitschmann, A.; Barthel, H.; Bischof, G.; Beyer, L.; Marek, K.; Song, M.; Wagemann, O.; Palleis, C.; Weidinger, E.; et al. Additive value of 18FPI-2620 perfusion imaging in progressive supranuclear palsy and corticobasal syndrome. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 423–434. [Google Scholar] [CrossRef]
- Kroth, H.; Oden, F.; Molette, J.; Schieferstein, H.; Capotosti, F.; Mueller, A.; Berndt, M.; Schmitt-Willich, H.; Darmency, V.; Gabellieri, E.; et al. Discovery and preclinical characterization of 18FPI-2620, a next-generation tau PET tracer for the assessment of tau pathology in Alzheimer’s disease and other tauopathies. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Rullmann, M.; Brendel, M.; Schroeter, M.L.; Saur, D.; Levin, J.; Perneczky, R.G.; Tiepolt, S.; Patt, M.; Mueller, A.; Villemagne, V.L.; et al. Multicenter 18F-PI-2620 PET for In Vivo Braak Staging of Tau Pathology in Alzheimer’s Disease. Biomolecules 2022, 12, 458. [Google Scholar] [CrossRef]
- Kroth, H.; Oden, F.; Molette, J.; Schieferstein, H.; Gabellieri, E.; Mueller, A.; Berndt, M.; Sreenivasachary, N.; Serra, A.M.; Capotosti, F.; et al. PI-2620 Lead Optimization Highlights the Importance of Off-Target Assays to Develop a PET Tracer for the Detection of Pathological Aggregated Tau in Alzheimer’s Disease and Other Tauopathies. J. Med. Chem. 2021, 64, 12808–12830. [Google Scholar] [CrossRef]
- Brendel, M.; Schönecker, S.; Höglinger, G.; Lindner, S.; Havla, J.; Blautzik, J.; Sauerbeck, J.; Rohrer, G.; Zach, C.; Vettermann, F.; et al. 18F-THK5351 PET Correlates with Topology and Symptom Severity in Progressive Supranuclear Palsy. Front. Aging Neurosci. 2017, 9, 440. [Google Scholar] [CrossRef]
- Schönecker, S.; Palleis, C.; Franzmeier, N.; Katzdobler, S.; Ferschmann, C.; Schuster, S.; Finze, A.; Scheifele, M.; Prix, C.; Fietzek, U.; et al. Symptomatology in 4-repeat tauopathies is associated with data-driven topology of 18F-PI-2620 tau-PET signal. NeuroImage Clin. 2023, 38, 103402. [Google Scholar] [CrossRef]
- Schonhaut, D.R.; McMillan, C.T.; Spina, S.; Dickerson, B.C.; Siderowf, A.; Devous, M.D.; Tsai, R.; Winer, J.; Russell, D.S.; Litvan, I.; et al. 18 F-flortaucipir tau positron emission tomography distinguishes established progressive supranuclear palsy from controls and Parkinson disease: A multicenter study. Ann. Neurol. 2017, 82, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Brendel, M.; Barthel, H.; van Eimeren, T.; Marek, K.; Beyer, L.; Song, M.; Palleis, C.; Gehmeyr, M.; Fietzek, U.; Respondek, G.; et al. Assessment of 18F-PI-2620 as a Biomarker in Progressive Supranuclear Palsy. JAMA Neurol. 2020, 77, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Walji, A.M.; Hostetler, E.D.; Selnick, H.; Zeng, Z.; Miller, P.; Bennacef, I.; Salinas, C.; Connolly, B.; Gantert, L.; Holahan, M.; et al. Discovery of 6-(Fluoro-(18)F)-3-(1H-pyrrolo2,3-cpyridin-1-yl)isoquinolin-5-amine ((18)F-MK-6240): A Positron Emission Tomography (PET) Imaging Agent for Quantification of Neurofibrillary Tangles (NFTs). J. Med. Chem. 2016, 59, 4778–4789. [Google Scholar] [CrossRef] [PubMed]
- Betthauser, T.J.; Cody, K.A.; Zammit, M.D.; Murali, D.; Converse, A.K.; Barnhart, T.E.; Stone, C.K.; Rowley, H.A.; Johnson, S.C.; Christian, B.T. In Vivo Characterization and Quantification of Neurofibrillary Tau PET Radioligand 18F-MK-6240 in Humans from Alzheimer Disease Dementia to Young Controls. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2019, 60, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, T.A.; Benedet, A.L.; Tudorascu, D.L.; Therriault, J.; Mathotaarachchi, S.; Savard, M.; Lussier, F.Z.; Tissot, C.; Chamoun, M.; Kang, M.S.; et al. Longitudinal 18F-MK-6240 tau tangles accumulation follows Braak stages. Brain A J. Neurol. 2021, 144, 3517–3528. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, T.A.; Shin, M.; Kang, M.S.; Chamoun, M.; Chartrand, D.; Mathotaarachchi, S.; Bennacef, I.; Therriault, J.; Ng, K.P.; Hopewell, R.; et al. In vivo quantification of neurofibrillary tangles with 18FMK-6240. Alzheimer’s Res. Ther. 2018, 10, 74. [Google Scholar] [CrossRef]
- Gogola, A.; Minhas, D.S.; Villemagne, V.L.; Cohen, A.D.; Mountz, J.M.; Pascoal, T.A.; Laymon, C.M.; Mason, N.S.; Ikonomovic, M.D.; Mathis, C.A.; et al. Direct Comparison of the Tau PET Tracers 18F-Flortaucipir and 18F-MK-6240 in Human Subjects. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2022, 63, 108–116. [Google Scholar] [CrossRef]
- Kim, H.; Choe, Y.S. High-yield synthesis of a tau PET radioligand and its nonradioactive ligand using an alternative protection and deprotection strategy. J. Label. Compd. Radiopharm. 2021, 64, 150–158. [Google Scholar] [CrossRef]
- Santillo, A.F.; Leuzy, A.; Honer, M.; Landqvist Waldö, M.; Tideman, P.; Harper, L.; Ohlsson, T.; Moes, S.; Giannini, L.; Jögi, J.; et al. 18FRO948 tau positron emission tomography in genetic and sporadic frontotemporal dementia syndromes. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1371–1383. [Google Scholar] [CrossRef]
- Leuzy, A.; Pascoal, T.A.; Strandberg, O.; Insel, P.; Smith, R.; Mattsson-Carlgren, N.; Benedet, A.L.; Cho, H.; Lyoo, C.H.; La Joie, R.; et al. A multicenter comparison of 18Fflortaucipir, 18FRO948, and 18FMK6240 tau PET tracers to detect a common target ROI for differential diagnosis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2295–2305. [Google Scholar] [CrossRef]
- Lindberg, A.; Knight, A.C.; Sohn, D.; Rakos, L.; Tong, J.; Radelet, A.; Mason, N.S.; Stehouwer, J.S.; Lopresti, B.J.; Klunk, W.E.; et al. Radiosynthesis, In Vitro and In Vivo Evaluation of 18FCBD-2115 as a First-in-Class Radiotracer for Imaging 4R-Tauopathies. ACS Chem. Neurosci. 2021, 12, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.D.; Matuskey, D.; Finnema, S.J. Positron emission tomography imaging of the γ-aminobutyric acid system. Neurosci. Lett. 2019, 691, 35–43. [Google Scholar] [CrossRef]
- Bigio, E.H.; Vono, M.B.; Satumtira, S.; Adamson, J.; Sontag, E.; Hynan, L.S.; White, C.L.; Baker, M.; Hutton, M. Cortical synapse loss in progressive supranuclear palsy. J. Neuropathol. Exp. Neurol. 2001, 60, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.; Jones, P.S.; Savulich, G.; Wiggins, J.K.; Hong, Y.T.; Fryer, T.D.; Manavaki, R.; Sephton, S.M.; Boros, I.; Malpetti, M.; et al. Synaptic Loss in Primary Tauopathies Revealed by 11 CUCB-J Positron Emission Tomography. Mov. Disord. Off. J. Mov. Disord. Soc. 2020, 35, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.; Jones, P.S.; Savulich, G.; Naessens, M.; Malpetti, M.; Whiteside, D.J.; Street, D.; Swann, P.; Hong, Y.T.; Fryer, T.D.; et al. Longitudinal Synaptic Loss in Primary Tauopathies: An In Vivo 11 CUCB-J Positron Emission Tomography Study. Mov. Disord. Off. J. Mov. Disord. Soc. 2023, 38, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.; Malpetti, M.; Rittman, T.; Mak, E.E.; Passamonti, L.; Kaalund, S.S.; Hezemans, F.H.; Jones, P.S.; Savulich, G.; Hong, Y.T.; et al. Molecular pathology and synaptic loss in primary tauopathies: An 18F-AV-1451 and 11C-UCB-J PET study. Brain A J. Neurol. 2022, 145, 340–348. [Google Scholar] [CrossRef]
- Chauveau, F.; Becker, G.; Boutin, H. Have (R)-11CPK11195 challengers fulfilled the promise? A scoping review of clinical TSPO PET studies. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Ji, B.; Kong, Y.; Qin, L.; Ren, W.; Guan, Y.; Ni, R. PET Imaging of Neuroinflammation in Alzheimer’s Disease. Front. Immunol. 2021, 12, 739130. [Google Scholar] [CrossRef] [PubMed]
- van Camp, N.; Lavisse, S.; Roost, P.; Gubinelli, F.; Hillmer, A.; Boutin, H. TSPO imaging in animal models of brain diseases. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 77–109. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, K.; Shao, T.; Hou, L.; Zhang, S.; Ye, W.; Josephson, L.; Meyer, J.H.; Zhang, M.-R.; Vasdev, N.; et al. Recent developments on PET radiotracers for TSPO and their applications in neuroimaging. Acta Pharm. Sin. B 2021, 11, 373–393. [Google Scholar] [CrossRef]
- Aghakhanyan, G.; Rullmann, M.; Rumpf, J.; Schroeter, M.L.; Scherlach, C.; Patt, M.; Brendel, M.; Koglin, N.; Stephens, A.W.; Classen, J.; et al. Interplay of tau and functional network connectivity in progressive supranuclear palsy: A 18FPI-2620 PET/MRI study. Eur. J. Nucl. Med. Mol. Imaging 2022, 50, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Coakeley, S.; Cho, S.S.; Koshimori, Y.; Rusjan, P.; Ghadery, C.; Kim, J.; Lang, A.E.; Houle, S.; Strafella, A.P. 18FAV-1451 binding to neuromelanin in the substantia nigra in PD and PSP. Brain Struct. Funct. 2018, 223, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Coakeley, S.; Cho, S.S.; Koshimori, Y.; Rusjan, P.; Harris, M.; Ghadery, C.; Kim, J.; Lang, A.E.; Wilson, A.; Houle, S.; et al. Positron emission tomography imaging of tau pathology in progressive supranuclear palsy. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2017, 37, 3150–3160. [Google Scholar] [CrossRef] [PubMed]
- Cope, T.E.; Rittman, T.; Borchert, R.J.; Jones, P.S.; Vatansever, D.; Allinson, K.; Passamonti, L.; Vazquez Rodriguez, P.; Bevan-Jones, W.R.; O’Brien, J.T.; et al. Tau burden and the functional connectome in Alzheimer’s disease and progressive supranuclear palsy. Brain A J. Neurol. 2018, 141, 550–567. [Google Scholar] [CrossRef]
- Mak, E.; Holland, N.; Jones, P.S.; Savulich, G.; Low, A.; Malpetti, M.; Kaalund, S.S.; Passamonti, L.; Rittman, T.; Romero-Garcia, R.; et al. In vivo coupling of dendritic complexity with presynaptic density in primary tauopathies. Neurobiol. Aging 2021, 101, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Malpetti, M.; Passamonti, L.; Jones, P.S.; Street, D.; Rittman, T.; Fryer, T.D.; Hong, Y.T.; Vàsquez Rodriguez, P.; Bevan-Jones, W.R.; Aigbirhio, F.I.; et al. Neuroinflammation predicts disease progression in progressive supranuclear palsy. J. Neurol. Neurosurg. Psychiatry 2021, 92, 769–775. [Google Scholar] [CrossRef]
- Malpetti, M.; Passamonti, L.; Rittman, T.; Jones, P.S.; Vázquez Rodríguez, P.; Bevan-Jones, W.R.; Hong, Y.T.; Fryer, T.D.; Aigbirhio, F.I.; O’Brien, J.T.; et al. Neuroinflammation and Tau Colocalize in vivo in Progressive Supranuclear Palsy. Ann. Neurol. 2020, 88, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Takado, Y.; Tagai, K.; Kubota, M.; Sano, Y.; Takahata, K.; Ono, M.; Seki, C.; Matsumoto, H.; Endo, H.; et al. Two pathways differentially linking tau depositions, oxidative stress, and neuronal loss to apathetic phenotypes in progressive supranuclear palsy. J. Neurol. Sci. 2023, 444, 120514. [Google Scholar] [CrossRef]
- Oliveira Hauer, K.; Pawlik, D.; Leuzy, A.; Janelidze, S.; Hall, S.; Hansson, O.; Smith, R. Performance of 18FRO948 PET, MRI and CSF neurofilament light in the differential diagnosis of progressive supranuclear palsy. Park. Relat. Disord. 2023, 106, 105226. [Google Scholar] [CrossRef]
- Passamonti, L.; Rodríguez, P.V.; Hong, Y.T.; Allinson, K.S.J.; Bevan-Jones, W.R.; Williamson, D.; Jones, P.S.; Arnold, R.; Borchert, R.J.; Surendranathan, A.; et al. 11CPK11195 binding in Alzheimer disease and progressive supranuclear palsy. Neurology 2018, 90, e1989–e1996. [Google Scholar] [CrossRef]
- Passamonti, L.; Vázquez Rodríguez, P.; Hong, Y.T.; Allinson, K.S.J.; Williamson, D.; Borchert, R.J.; Sami, S.; Cope, T.E.; Bevan-Jones, W.R.; Jones, P.S.; et al. 18F-AV-1451 positron emission tomography in Alzheimer’s disease and progressive supranuclear palsy. Brain A J. Neurol. 2017, 140, 781–791. [Google Scholar] [CrossRef]
- Seckin, Z.I.; Duffy, J.R.; Strand, E.A.; Clark, H.M.; Utianski, R.L.; Machulda, M.M.; Botha, H.; Ali, F.; Thu Pham, N.T.; Lowe, V.J.; et al. The evolution of parkinsonism in primary progressive apraxia of speech: A 6-year longitudinal study. Park. Relat. Disord. 2020, 81, 34–40. [Google Scholar] [CrossRef]
- Sintini, I.; Schwarz, C.G.; Senjem, M.L.; Reid, R.I.; Botha, H.; Ali, F.; Ahlskog, J.E.; Jack, C.R.; Lowe, V.J.; Josephs, K.A.; et al. Multimodal neuroimaging relationships in progressive supranuclear palsy. Park. Relat. Disord. 2019, 66, 56–61. [Google Scholar] [CrossRef]
- Tagai, K.; Ikoma, Y.; Endo, H.; Debnath, O.B.; Seki, C.; Matsuoka, K.; Matsumoto, H.; Oya, M.; Hirata, K.; Shinotoh, H.; et al. An optimized reference tissue method for quantification of tau protein depositions in diverse neurodegenerative disorders by PET with 18F-PM-PBB3 (18F-APN-1607). NeuroImage 2022, 264, 119763. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Ahlskog, J.E.; Tosakulwong, N.; Senjem, M.L.; Spychalla, A.J.; Petersen, R.C.; Jack, C.R.; Lowe, V.J.; Josephs, K.A. Pittsburgh Compound B and AV-1451 positron emission tomography assessment of molecular pathologies of Alzheimer’s disease in progressive supranuclear palsy. Park. Relat. Disord. 2018, 48, 3–9. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Tosakulwong, N.; Botha, H.; Ali, F.; Clark, H.M.; Duffy, J.R.; Utianski, R.L.; Stevens, C.A.; Weigand, S.D.; Schwarz, C.G.; et al. Brain volume and flortaucipir analysis of progressive supranuclear palsy clinical variants. NeuroImage Clin. 2020, 25, 102152. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Tosakulwong, N.; Schwarz, C.G.; Botha, H.; Senjem, M.L.; Spychalla, A.J.; Ahlskog, J.E.; Knopman, D.S.; Petersen, R.C.; Jack, C.R.; et al. MRI Outperforms 18FAV-1451 PET as a Longitudinal Biomarker in Progressive Supranuclear Palsy. Mov. Disord. Off. J. Mov. Disord. Soc. 2019, 34, 105–113. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, B.; Gao, S.; Li, X. Clinical, MRI and 18F-FDG-PET/CT analysis of progressive supranuclear palsy. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas 2020, 80, 318–323. [Google Scholar] [CrossRef]
- Assaf, Y.; Pasternak, O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: A review. J. Mol. Neurosci. 2008, 34, 51–61. [Google Scholar] [CrossRef]
- Basser, P.J.; Mattiello, J.; LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994, 66, 259–267. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Kassubek, J.; Csoti, I.; Ehret, R.; Herbst, H.; Wellach, I.; Winkler, J.; Jost, W.H. Differentiation of atypical Parkinson syndromes. J. Neural Transm. 2017, 124, 997–1004. [Google Scholar] [CrossRef]
- Kassubek, J. MRI-based neuroimaging: Atypical parkinsonisms and other movement disorders. Curr. Opin. Neurol. 2018, 31, 425–430. [Google Scholar] [CrossRef]
- Tournier, J.-D.; Mori, S.; Leemans, A. Diffusion tensor imaging and beyond. Magn. Reson. Med. 2011, 65, 1532–1556. [Google Scholar] [CrossRef]
- Abhinav, K.; Yeh, F.-C.; Pathak, S.; Suski, V.; Lacomis, D.; Friedlander, R.M.; Fernandez-Miranda, J.C. Advanced diffusion MRI fiber tracking in neurosurgical and neurodegenerative disorders and neuroanatomical studies: A review. Biochim. Biophys. Acta 2014, 1842, 2286–2297. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Heras, E.; Grussu, F.; Prados, F.; Solana, E.; Llufriu, S. Diffusion-Weighted Imaging: Recent Advances and Applications. Semin. Ultrasound CT MR 2021, 42, 490–506. [Google Scholar] [CrossRef] [PubMed]
- Winston, G.P. The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant. Imaging Med. Surg. 2012, 2, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Metzler-Baddeley, C.; O’Sullivan, M.J.; Bells, S.; Pasternak, O.; Jones, D.K. How and how not to correct for CSF-contamination in diffusion MRI. NeuroImage 2012, 59, 1394–1403. [Google Scholar] [CrossRef]
- Planetta, P.J.; Ofori, E.; Pasternak, O.; Burciu, R.G.; Shukla, P.; DeSimone, J.C.; Okun, M.S.; McFarland, N.R.; Vaillancourt, D.E. Free-water imaging in Parkinson’s disease and atypical parkinsonism. Brain A J. Neurol. 2016, 139, 495–508. [Google Scholar] [CrossRef]
- Tondo, G.; Esposito, M.; Dervenoulas, G.; Wilson, H.; Politis, M.; Pagano, G. Hybrid PET-MRI Applications in Movement Disorders. Int. Rev. Neurobiol. 2019, 144, 211–257. [Google Scholar] [CrossRef] [PubMed]
- Afaq, A.; Faul, D.; Chebrolu, V.V.; Wan, S.; Hope, T.A.; Haibach, P.V.; Bomanji, J. Pitfalls on PET/MRI. Semin. Nucl. Med. 2021, 51, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Catana, C. Motion correction options in PET/MRI. Semin. Nucl. Med. 2015, 45, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Catana, C. Attenuation correction for human PET/MRI studies. Phys. Med. Biol. 2020, 65, 23TR02. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jamadar, S.D.; Li, S.; Sforazzini, F.; Baran, J.; Ferris, N.; Shah, N.J.; Egan, G.F. From simultaneous to synergistic MR-PET brain imaging: A review of hybrid MR-PET imaging methodologies. Hum. Brain Mapp. 2018, 39, 5126–5144. [Google Scholar] [CrossRef] [PubMed]
- Aiello, M.; Cavaliere, C.; Marchitelli, R.; d’Albore, A.; De Vita, E.; Salvatore, M. Hybrid PET/MRI Methodology. Int. Rev. Neurobiol. 2018, 141, 97–128. [Google Scholar] [CrossRef] [PubMed]
- Sari, H.; Reaungamornrat, J.; Catalano, O.A.; Vera-Olmos, J.; Izquierdo-Garcia, D.; Morales, M.A.; Torrado-Carvajal, A.; Ng, T.S.C.; Malpica, N.; Kamen, A.; et al. Evaluation of Deep Learning-Based Approaches to Segment Bowel Air Pockets and Generate Pelvic Attenuation Maps from CAIPIRINHA-Accelerated Dixon MR Images. J. Nucl. Med. 2022, 63, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhu, X. MRI-Driven PET Image Optimization for Neurological Applications. Front. Neurosci. 2019, 13, 782. [Google Scholar] [CrossRef]
- Jung, J.H.; Choi, Y.; Im, K.C. PET/MRI: Technical Challenges and Recent Advances. Nucl. Med. Mol. Imaging 2016, 50, 3–12. [Google Scholar] [CrossRef]
- Greve, T.; Sollmann, N.; Hock, A.; Hey, S.; Gnanaprakasam, V.; Nijenhuis, M.; Zimmer, C.; Kirschke, J.S. Highly accelerated time-of-flight magnetic resonance angiography using spiral imaging improves conspicuity of intracranial arterial branches while reducing scan time. Eur. Radiol. 2020, 30, 855–865. [Google Scholar] [CrossRef]
- Greve, T.; Sollmann, N.; Hock, A.; Zimmer, C.; Kirschke, J.S. Novel Ultrafast Spiral Head MR Angiography Compared to Standard MR and CT Angiography. J. Neuroimaging 2021, 31, 45–56. [Google Scholar] [CrossRef]
- Monch, S.; Sollmann, N.; Hock, A.; Zimmer, C.; Kirschke, J.S.; Hedderich, D.M. Magnetic Resonance Imaging of the Brain Using Compressed Sensing—Quality Assessment in Daily Clinical Routine. Clin. Neuroradiol. 2020, 30, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Radmanesh, A.; Muckley, M.J.; Murrell, T.; Lindsey, E.; Sriram, A.; Knoll, F.; Sodickson, D.K.; Lui, Y.W. Exploring the Acceleration Limits of Deep Learning Variational Network-based Two-dimensional Brain MRI. Radiol. Artif. Intell. 2022, 4, e210313. [Google Scholar] [CrossRef] [PubMed]
- Bilgic, B.; Gagoski, B.A.; Cauley, S.F.; Fan, A.P.; Polimeni, J.R.; Grant, P.E.; Wald, L.L.; Setsompop, K. Wave-CAIPI for highly accelerated 3D imaging. Magn. Reson. Med. 2015, 73, 2152–2162. [Google Scholar] [CrossRef] [PubMed]
- Franzmeier, N.; Brendel, M.; Beyer, L.; Slemann, L.; Kovacs, G.G.; Arzberger, T.; Kurz, C.; Respondek, G.; Lukic, M.J.; Biel, D.; et al. Tau deposition patterns are associated with functional connectivity in primary tauopathies. Nat. Commun. 2022, 13, 1362. [Google Scholar] [CrossRef]
- Quattrone, A.; Nicoletti, G.; Messina, D.; Fera, F.; Condino, F.; Pugliese, P.; Lanza, P.; Barone, P.; Morgante, L.; Zappia, M.; et al. MR imaging index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple system atrophy. Radiology 2008, 246, 214–221. [Google Scholar] [CrossRef]
- Quattrone, A.; Morelli, M.; Nigro, S.; Quattrone, A.; Vescio, B.; Arabia, G.; Nicoletti, G.; Nistico, R.; Salsone, M.; Novellino, F.; et al. A new MR imaging index for differentiation of progressive supranuclear palsy-parkinsonism from Parkinson’s disease. Park. Relat. Disord. 2018, 54, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Nigro, S.; Antonini, A.; Vaillancourt, D.E.; Seppi, K.; Ceravolo, R.; Strafella, A.P.; Augimeri, A.; Quattrone, A.; Morelli, M.; Weis, L.; et al. Automated MRI Classification in Progressive Supranuclear Palsy: A Large International Cohort Study. Mov. Disord. 2020, 35, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Quattrone, A.; Bianco, M.G.; Antonini, A.; Vaillancourt, D.E.; Seppi, K.; Ceravolo, R.; Strafella, A.P.; Tedeschi, G.; Tessitore, A.; Cilia, R.; et al. Development and Validation of Automated Magnetic Resonance Parkinsonism Index 2.0 to Distinguish Progressive Supranuclear Palsy-Parkinsonism From Parkinson’s Disease. Mov. Disord. 2022, 37, 1272–1281. [Google Scholar] [CrossRef]
- Coughlin, D.G.; Litvan, I. Progressive supranuclear palsy: Advances in diagnosis and management. Park. Relat. Disord. 2020, 73, 105–116. [Google Scholar] [CrossRef]
- Ali, F.; Josephs, K. The diagnosis of progressive supranuclear palsy: Current opinions and challenges. Expert Rev. Neurother. 2018, 18, 603–616. [Google Scholar] [CrossRef]
- Bluett, B.; Pantelyat, A.Y.; Litvan, I.; Ali, F.; Apetauerova, D.; Bega, D.; Bloom, L.; Bower, J.; Boxer, A.L.; Dale, M.L.; et al. Best Practices in the Clinical Management of Progressive Supranuclear Palsy and Corticobasal Syndrome: A Consensus Statement of the CurePSP Centers of Care. Front. Neurol. 2021, 12, 694872. [Google Scholar] [CrossRef]
- Currie, G.M.; Kamvosoulis, P.; Bushong, S. PET/MRI, Part 2: Technologic Principles. J. Nucl. Med. Technol. 2021, 49, 217–225. [Google Scholar] [CrossRef]
- Erlandsson, K.; Buvat, I.; Pretorius, P.H.; Thomas, B.A.; Hutton, B.F. A review of partial volume correction techniques for emission tomography and their applications in neurology, cardiology and oncology. Phys. Med. Biol. 2012, 57, R119–R159. [Google Scholar] [CrossRef]
- Alavi, A.; Werner, T.J.; Høilund-Carlsen, P.F.; Zaidi, H. Correction for Partial Volume Effect Is a Must, Not a Luxury, to Fully Exploit the Potential of Quantitative PET Imaging in Clinical Oncology. Mol. Imaging Biol. 2018, 20, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Marquis, H.; Willowson, K.P.; Bailey, D.L. Partial volume effect in SPECT & PET imaging and impact on radionuclide dosimetry estimates. Asia Ocean. J. Nucl. Med. Biol. 2023, 11, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Gallivanone, F.; Stefano, A.; Canevari, C.; Messa, C.; Gilardi, M.C.; Castiglioni, I. PVE correction in PET from PVE affected images. In Proceedings of the 2009 IEEE Nuclear Science Symposium Conference Record (NSS/MIC), Orlando, FL, USA, 24 October–1 November 2009; pp. 3146–3150. [Google Scholar]
- Erlandsson, K.; Dickson, J.; Arridge, S.; Atkinson, D.; Ourselin, S.; Hutton, B.F. MR Imaging-Guided Partial Volume Correction of PET Data in PET/MR Imaging. PET Clin. 2016, 11, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Steinke, F.; Scheel, V.; Charpiat, G.; Farquhar, J.; Aschoff, P.; Brady, M.; Schölkopf, B.; Pichler, B.J. MRI-based attenuation correction for PET/MRI: A novel approach combining pattern recognition and atlas registration. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2008, 49, 1875–1883. [Google Scholar] [CrossRef]
- Lu, Y.; Toyonaga, T.; Naganawa, M.; Gallezot, J.-D.; Chen, M.-K.; Mecca, A.P.; van Dyck, C.H.; Carson, R.E. Partial volume correction analysis for 11C-UCB-J PET studies of Alzheimer’s disease. NeuroImage 2021, 238, 118248. [Google Scholar] [CrossRef]
- Rousset, O.G.; Ma, Y.; Evans, A.C. Correction for partial volume effects in PET: Principle and validation. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1998, 39, 904–911. [Google Scholar]
- Ali, F.; Martin, P.R.; Botha, H.; Ahlskog, J.E.; Bower, J.H.; Masumoto, J.Y.; Maraganore, D.; Hassan, A.; Eggers, S.; Boeve, B.F.; et al. Sensitivity and Specificity of Diagnostic Criteria for Progressive Supranuclear Palsy. Mov. Disord. Off. J. Mov. Disord. Soc. 2019, 34, 1144–1153. [Google Scholar] [CrossRef]
- Owolabi, L. Progressive supranuclear palsy misdiagnosed as Parkinson’s disease: A case report and review of literature. Ann. Med. Health Sci. Res. 2013, 3, S44–S47. [Google Scholar] [CrossRef]
- Johnson, K.B.; Wei, W.Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision Medicine, AI, and the Future of Personalized Health Care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kurz, C.; Ebersbach, G.; Respondek, G.; Giese, A.; Arzberger, T.; Hoglinger, G.U. An autopsy-confirmed case of progressive supranuclear palsy with predominant postural instability. Acta Neuropathol. Commun. 2016, 4, 120. [Google Scholar] [CrossRef] [PubMed]
- Soleimani-Meigooni, D.N.; Iaccarino, L.; La Joie, R.; Baker, S.; Bourakova, V.; Boxer, A.L.; Edwards, L.; Eser, R.; Gorno-Tempini, M.-L.; Jagust, W.J.; et al. 18F-flortaucipir PET to autopsy comparisons in Alzheimer’s disease and other neurodegenerative diseases. Brain A J. Neurol. 2020, 143, 3477–3494. [Google Scholar] [CrossRef] [PubMed]

| Author, Year and Study Number (SN) | Study Cohort | Radio- Pharmaceutical | Image Acquisition | Main Findings |
|---|---|---|---|---|
| Coakeley 2017 [96] (1) |
| [18F]AV-1451 (also known as [18F]-T807) |
|
|
| Coakeley 2018 [95] (2) |
| [18F]AV-1451 ([18F]-T807) |
|
|
| Passamonti 2017 [104] (3) |
| 18F-AV-1451 & 11C-PiB radiotracer |
|
|
| Schonhaut 2017 [74] (4) |
| 18F-flortaucipir (formerly 18F-AV1451 or 18F-T807) |
|
|
| Cope 2018 [97] (5) |
| 18F-AV-1451 and 11C-PiB |
|
|
| Passamonti 2018 [103] (6) |
| [11C]PK11195 and 11C-PiB |
|
|
| Whitwell 2018 [108] (7) |
18 f)
| [18F]AV-1451 PET |
|
|
| Sintini 2019 [106] (8) |
19 f)
| Flortaucipir PET scans-[18F]AV-1451 PET |
|
|
| Whitwell 2019 [110] (9) |
| [18F]AV-1451 tau-PET |
|
|
| Holland 2020 [87] (10) |
| [11C]UCB-J and 11C-PiB |
|
|
| Malpetti 2020 [100] (11) |
| [11C]PK11195 and [18F]AV-1451 BPND |
|
|
| Nicastro 2020 [42] (12) |
| 18F AV1451 |
|
|
| Seckin 2020 [105] (13) |
| [18F]fluorodeoxyglucose |
|
|
| Whitwell 2020 [109] (14) |
53 f)
| [18F]flortaucipir ([18F]AV-1451) |
|
|
| Zhao 2020 [111] (15) |
| 18F-FDG-PET/CT |
|
|
| Mak 2021 [98] (16) |
| [11C]UCB-J PET. |
|
|
| Malpetti 2021 [99] (17) |
| [11C]PK11195 and [18F]AV-1451 (18F-flortaucipir) |
|
|
| Tagai 2022 [107] (18) |
| 18F-PM-PBB3 |
|
|
| Aghakhanyan 2022 [94] (19) |
| [18F]PI-2620 |
|
|
| Matsuoka 2023 [101] (20) |
| 18F-PM-PBB3 |
|
|
| Oliveira Hauer 2023 [102] (21) |
| [18F]RO948 |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strobel, J.; Müller, H.-P.; Ludolph, A.C.; Beer, A.J.; Sollmann, N.; Kassubek, J. New Perspectives in Radiological and Radiopharmaceutical Hybrid Imaging in Progressive Supranuclear Palsy: A Systematic Review. Cells 2023, 12, 2776. https://doi.org/10.3390/cells12242776
Strobel J, Müller H-P, Ludolph AC, Beer AJ, Sollmann N, Kassubek J. New Perspectives in Radiological and Radiopharmaceutical Hybrid Imaging in Progressive Supranuclear Palsy: A Systematic Review. Cells. 2023; 12(24):2776. https://doi.org/10.3390/cells12242776
Chicago/Turabian StyleStrobel, Joachim, Hans-Peter Müller, Albert C. Ludolph, Ambros J. Beer, Nico Sollmann, and Jan Kassubek. 2023. "New Perspectives in Radiological and Radiopharmaceutical Hybrid Imaging in Progressive Supranuclear Palsy: A Systematic Review" Cells 12, no. 24: 2776. https://doi.org/10.3390/cells12242776






