Scanning Probe Microscopy Techniques for Studying the Cell Glycocalyx
Abstract
:1. Introduction
2. Variations in the Structures and Functions of the Glycocalyx for Different Cell Phenotypes
3. Principles of Scanning Probe Microscopy Techniques
4. Sanning Probe Microscopy Imaging of the Glycocalyx
5. The Study of Glycocalyx Nanomechanics Using AFM Techniques
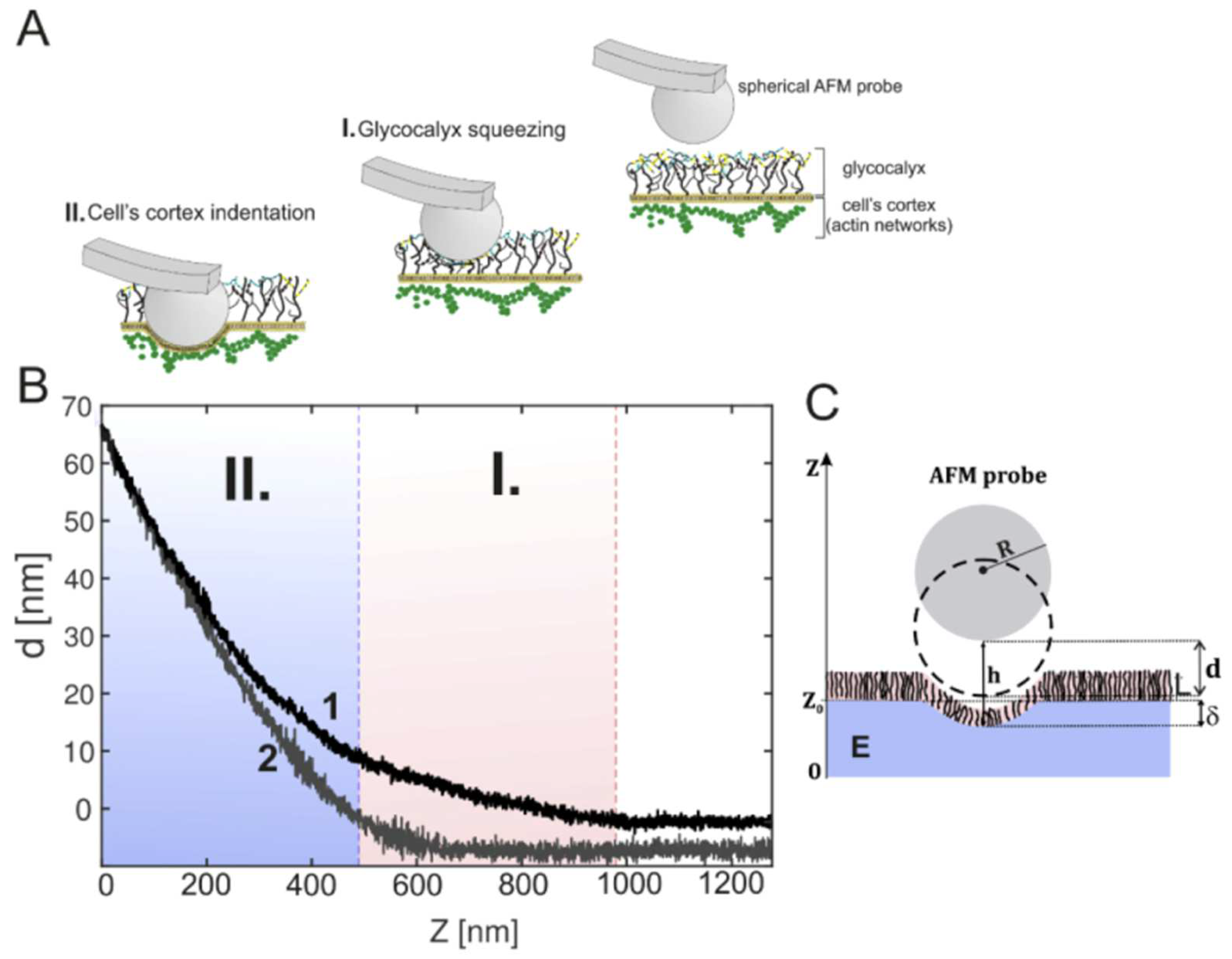
6. Examples of the Application of Nanomechanics to Studying the Glycocalyx in Pathophysiology
7. Other Scanning Probe Microscopy Methods for the Study of the Glycocalyx
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SPM | Scanning probe microscopy |
| AFM | Atomic force microscopy |
| AFS | Atomic force spectroscopy |
| GAG | Glycosaminoglycan |
| HUVEC | Human umbilical vein endothelial cells |
References
- Luft, J.H. Fine Structures of Capillary and Endocapillary Layer as Revealed by Ruthenium Red. Fed. Proc. 1966, 25, 1773–1783. [Google Scholar]
- Weinbaum, S.; Zhang, X.; Han, Y.; Vink, H.; Cowin, S.C. Mechanotransduction and Flow across the Endothelial Glycocalyx. Proc. Natl. Acad. Sci. USA 2003, 100, 7988–7995. [Google Scholar] [CrossRef] [PubMed]
- Weinbaum, S.; Tarbell, J.M.; Damiano, E.R. The Structure and Function of the Endothelial Glycocalyx Layer. Annu. Rev. Biomed. Eng. 2007, 9, 121–167. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Lipowsky, H.H. Composition of the Endothelial Glycocalyx and Its Relation to Its Thickness and Diffusion of Small Solutes. Microvasc. Res. 2010, 80, 394–401. [Google Scholar] [CrossRef]
- Squire, J.M.; Chew, M.; Nneji, G.; Neal, C.; Barry, J.; Michel, C. Quasi-Periodic Substructure in the Microvessel Endothelial Glycocalyx: A Possible Explanation for Molecular Filtering? J. Struct. Biol. 2001, 136, 239–255. [Google Scholar] [CrossRef]
- Nieuwdorp, M.; Meuwese, M.C.; Vink, H.; Hoekstra, J.B.; Kastelein, J.J.; Stroes, E.S. The Endothelial Glycocalyx: A Potential Barrier between Health and Vascular Disease. Curr. Opin. Lipidol. 2005, 16, 507–511. [Google Scholar] [CrossRef]
- Chevalier, L.; Selim, J.; Genty, D.; Baste, J.M.; Piton, N.; Boukhalfa, I.; Hamzaoui, M.; Pareige, P.; Richard, V. Electron Microscopy Approach for the Visualization of the Epithelial and Endothelial Glycocalyx. Morphologie 2017, 101, 55–63. [Google Scholar] [CrossRef]
- Chappell, D.; Hofmann-Kiefer, K.; Jacob, M.; Rehm, M.; Briegel, J.; Welsch, U.; Conzen, P.; Becker, B.F. TNF-α Induced Shedding of the Endothelial Glycocalyx Is Prevented by Hydrocortisone and Antithrombin. Basic Res. Cardiol. 2009, 104, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Mukai, S.; Takaki, T.; Nagumo, T.; Sano, M.; Kang, D.; Takimoto, M.; Honda, K. Three-Dimensional Electron Microscopy for Endothelial Glycocalyx Observation Using Alcian Blue with Silver Enhancement. Med. Mol. Morphol. 2021, 54, 95–107. [Google Scholar] [CrossRef]
- Ebong, E.E.; Macaluso, F.P.; Spray, D.C.; Tarbell, J.M. Imaging the Endothelial Glycocalyx In Vitro by Rapid Freezing/Freeze Substitution Transmission Electron Microscopy. Arter. Thromb. Vasc. Biol. 2011, 31, 1908–1915. [Google Scholar] [CrossRef]
- Salmon, A.H.; Satchell, S.C. Endothelial Glycocalyx Dysfunction in Disease: Albuminuria and Increased Microvascular Permeability: Glycocalyx and Microvascular Permeability. J. Pathol. 2012, 226, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Fu, B.M. Investigation of Endothelial Surface Glycocalyx Components and Ultrastructure by Single Molecule Localization Microscopy: Stochastic Optical Reconstruction Microscopy (STORM). Yale J. Biol. Med. 2018, 91, 257–266. [Google Scholar] [PubMed]
- Bae, Y.H.; Liu, S.; Byfield, F.J.; Janmey, P.A.; Assoian, R.K. Measuring the Stiffness of Ex Vivo Mouse Aortas Using Atomic Force Microscopy. J. Vis. Exp. 2016, 116, e54630. [Google Scholar] [CrossRef]
- Sun, M.; Graham, J.S.; Hegedüs, B.; Marga, F.; Zhang, Y.; Forgacs, G.; Grandbois, M. Multiple Membrane Tethers Probed by Atomic Force Microscopy. Biophys. J. 2005, 89, 4320–4329. [Google Scholar] [CrossRef]
- Yen, W.; Cai, B.; Yang, J.; Zhang, L.; Zeng, M.; Tarbell, J.M.; Fu, B.M. Endothelial Surface Glycocalyx Can Regulate Flow-Induced Nitric Oxide Production in Microvessels In Vivo. PLoS ONE 2015, 10, e0117133. [Google Scholar] [CrossRef]
- Van Den Berg, B.M.; Vink, H.; Spaan, J.A.E. The Endothelial Glycocalyx Protects Against Myocardial Edema. Circ. Res. 2003, 92, 592–594. [Google Scholar] [CrossRef]
- Kang, H.; Wu, Q.; Sun, A.; Liu, X.; Fan, Y.; Deng, X. Cancer Cell Glycocalyx and Its Significance in Cancer Progression. Int. J. Mol. Sci. 2018, 19, 2484. [Google Scholar] [CrossRef]
- Offeddu, G.S.; Hajal, C.; Foley, C.R.; Wan, Z.; Ibrahim, L.; Coughlin, M.F.; Kamm, R.D. The Cancer Glycocalyx Mediates Intravascular Adhesion and Extravasation during Metastatic Dissemination. Commun. Biol. 2021, 4, 255. [Google Scholar] [CrossRef]
- Kanyo, N.; Kovacs, K.D.; Saftics, A.; Szekacs, I.; Peter, B.; Santa-Maria, A.R.; Walter, F.R.; Dér, A.; Deli, M.A.; Horvath, R. Glycocalyx Regulates the Strength and Kinetics of Cancer Cell Adhesion Revealed by Biophysical Models Based on High Resolution Label-Free Optical Data. Sci. Rep. 2020, 10, 22422. [Google Scholar] [CrossRef]
- Curry, F.E.; Adamson, R.H. Endothelial Glycocalyx: Permeability Barrier and Mechanosensor. Ann. Biomed. Eng. 2012, 40, 828–839. [Google Scholar] [CrossRef]
- Villalba, N.; Baby, S.; Yuan, S.Y. The Endothelial Glycocalyx as a Double-Edged Sword in Microvascular Homeostasis and Pathogenesis. Front. Cell Dev. Biol. 2021, 9, 711003. [Google Scholar] [CrossRef] [PubMed]
- Gianesini, S.; Rimondi, E.; Raffetto, J.D.; Melloni, E.; Pellati, A.; Menegatti, E.; Avruscio, G.P.; Bassetto, F.; Costa, A.L.; Rockson, S. Human Collecting Lymphatic Glycocalyx Identification by Electron Microscopy and Immunohistochemistry. Sci. Rep. 2023, 13, 3022. [Google Scholar] [CrossRef] [PubMed]
- Cosgun, Z.C.; Fels, B.; Kusche-Vihrog, K. Nanomechanics of the Endothelial Glycocalyx: From Structure to Function. Am. J. Pathol. 2020, 190, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Culty, M. The Hyaluronate Receptor Is a Member of the CD44 (H-CAM) Family of Cell Surface Glycoproteins. J. Cell Biol. 1990, 111, 2765–2774, Erratum in J. Cell Biol. 1991, 112, 513. [Google Scholar] [CrossRef] [PubMed]
- Ochs, M.; Hegermann, J.; Lopez-Rodriguez, E.; Timm, S.; Nouailles, G.; Matuszak, J.; Simmons, S.; Witzenrath, M.; Kuebler, W.M. On Top of the Alveolar Epithelium: Surfactant and the Glycocalyx. Int. J. Mol. Sci. 2020, 21, 3075. [Google Scholar] [CrossRef] [PubMed]
- Walter, F.R.; Santa-Maria, A.R.; Mészáros, M.; Veszelka, S.; Dér, A.; Deli, M.A. Surface Charge, Glycocalyx, and Blood-Brain Barrier Function. Tissue Barriers 2021, 9, 1904773. [Google Scholar] [CrossRef]
- Tarbell, J.M.; Cancel, L.M. The Glycocalyx and Its Significance in Human Medicine. J. Intern. Med. 2016, 280, 97–113. [Google Scholar] [CrossRef]
- Hansson, G.C. Mucins and the Microbiome. Annu. Rev. Biochem. 2020, 89, 769–793. [Google Scholar] [CrossRef]
- Buffone, A.; Weaver, V.M. Don’t Sugarcoat It: How Glycocalyx Composition Influences Cancer Progression. J. Cell Biol. 2020, 219, e201910070. [Google Scholar] [CrossRef]
- Argüeso, P.; Woodward, A.M.; AbuSamra, D.B. The Epithelial Cell Glycocalyx in Ocular Surface Infection. Front. Immunol. 2021, 12, 729260. [Google Scholar] [CrossRef]
- Linnartz-Gerlach, B.; Mathews, M.; Neumann, H. Sensing the Neuronal Glycocalyx by Glial Sialic Acid Binding Immunoglobulin-like Lectins. Neuroscience 2014, 275, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Sonnino, S.; Chiricozzi, E.; Grassi, S.; Mauri, L.; Prioni, S.; Prinetti, A. Gangliosides in Membrane Organization. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2018; Volume 156, pp. 83–120. ISBN 978-0-12-812341-6. [Google Scholar]
- Rapoport, E.M.; Matveeva, V.K.; Vokhmyanina, O.A.; Belyanchikov, I.M.; Gabius, H.-J.; Bovin, N.V. Localization of Galectins within Glycocalyx. Biochemistry 2018, 83, 727–737. [Google Scholar] [CrossRef]
- Limozin, L.; Puech, P.-H. Membrane Organization and Physical Regulation of Lymphocyte Antigen Receptors: A Biophysicist’s Perspective. J. Membr. Biol. 2019, 252, 397–412. [Google Scholar] [CrossRef]
- Jackson, D.G. Leucocyte Trafficking via the Lymphatic Vasculature—Mechanisms and Consequences. Front. Immunol. 2019, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Fritsma, G.A. Platelet Structure and Function. Clin. Lab. Sci. 2015, 28, 125–131. [Google Scholar] [CrossRef]
- Binnig, G.; Rohrer, H. Scanning Tunneling Microscopy. Surf. Sci. 1983, 126, 236–244. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef]
- Baró, A.M.; Reifenberger, R.G. (Eds.) Atomic Force Microscopy in Liquid: Biological Applications, 1st ed.; Wiley-VCH: Weinheim, Germany; Hoboken, NJ, USA, 2012; ISBN 978-3-527-32758-4. [Google Scholar]
- Happel, P.; Thatenhorst, D.; Dietzel, I. Scanning Ion Conductance Microscopy for Studying Biological Samples. Sensors 2012, 12, 14983–15008. [Google Scholar] [CrossRef]
- Le Grimellec, C.; Lesniewska, E.; Cachia, C.; Schreiber, J.P.; De Fornel, F.; Goudonnet, J.P. Imaging of the Membrane Surface of MDCK Cells by Atomic Force Microscopy. Biophys. J. 1994, 67, 36–41. [Google Scholar] [CrossRef]
- Kalagara, T.; Moutsis, T.; Yang, Y.; Pappelbaum, K.I.; Farken, A.; Cladder-Micus, L.; Vidal-y-Sy, S.; John, A.; Bauer, A.T.; Moerschbacher, B.M.; et al. The Endothelial Glycocalyx Anchors von Willebrand Factor Fibers to the Vascular Endothelium. Blood Adv. 2018, 2, 2347–2357. [Google Scholar] [CrossRef]
- Chighizola, M.; Dini, T.; Marcotti, S.; D’Urso, M.; Piazzoni, C.; Borghi, F.; Previdi, A.; Ceriani, L.; Folliero, C.; Stramer, B.; et al. The Glycocalyx Affects the Mechanotransductive Perception of the Topographical Microenvironment. J. Nanobiotechnol. 2022, 20, 418. [Google Scholar] [CrossRef]
- Dufrêne, Y.F.; Martínez-Martín, D.; Medalsy, I.; Alsteens, D.; Müller, D.J. Multiparametric Imaging of Biological Systems by Force-Distance Curve–Based AFM. Nat. Methods 2013, 10, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Dokukin, M.E.; Sokolov, I. Nanoscale Compositional Mapping of Cells, Tissues, and Polymers with Ringing Mode of Atomic Force Microscopy. Sci. Rep. 2017, 7, 11828. [Google Scholar] [CrossRef] [PubMed]
- Marsh, G.; Waugh, R.E. Quantifying the Mechanical Properties of the Endothelial Glycocalyx with Atomic Force Microscopy. J. Vis. Exp. 2013, 72, 50163. [Google Scholar] [CrossRef]
- Wiesinger, A.; Peters, W.; Chappell, D.; Kentrup, D.; Reuter, S.; Pavenstädt, H.; Oberleithner, H.; Kümpers, P. Nanomechanics of the Endothelial Glycocalyx in Experimental Sepsis. PLoS ONE 2013, 8, e80905. [Google Scholar] [CrossRef] [PubMed]
- Oberleithner, H.; Peters, W.; Kusche-Vihrog, K.; Korte, S.; Schillers, H.; Kliche, K.; Oberleithner, K. Salt Overload Damages the Glycocalyx Sodium Barrier of Vascular Endothelium. Pflug. Arch.-Eur. J. Physiol. 2011, 462, 519–528. [Google Scholar] [CrossRef]
- Carl, P.; Schillers, H. Elasticity Measurement of Living Cells with an Atomic Force Microscope: Data Acquisition and Processing. Pflug. Arch.-Eur. J. Physiol. 2008, 457, 551–559. [Google Scholar] [CrossRef]
- Clifford, C.A.; Seah, M.P. Nanoindentation Measurement of Young’s Modulus for Compliant Layers on Stiffer Substrates Including the Effect of Poisson’s Ratios. Nanotechnology 2009, 20, 145708. [Google Scholar] [CrossRef]
- Sokolov, I.; Iyer, S.; Subba-Rao, V.; Gaikwad, R.M.; Woodworth, C.D. Detection of Surface Brush on Biological Cells in Vitro with Atomic Force Microscopy. Appl. Phys. Lett. 2007, 91, 023902. [Google Scholar] [CrossRef]
- De Gennes, P.G. Polymers at an Interface; a Simplified View. Adv. Colloid Interface Sci. 1987, 27, 189–209. [Google Scholar] [CrossRef]
- Iyer, S.; Gaikwad, R.M.; Subba-Rao, V.; Woodworth, C.D.; Sokolov, I. Atomic Force Microscopy Detects Differences in the Surface Brush of Normal and Cancerous Cells. Nat. Nanotechnol. 2009, 4, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, I.; Dokukin, M.E.; Guz, N.V. Method for Quantitative Measurements of the Elastic Modulus of Biological Cells in AFM Indentation Experiments. Methods 2013, 60, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Guz, N.; Dokukin, M.; Kalaparthi, V.; Sokolov, I. If Cell Mechanics Can Be Described by Elastic Modulus: Study of Different Models and Probes Used in Indentation Experiments. Biophys. J. 2014, 107, 564–575. [Google Scholar] [CrossRef]
- Dokukin, M.; Ablaeva, Y.; Kalaparthi, V.; Seluanov, A.; Gorbunova, V.; Sokolov, I. Pericellular Brush and Mechanics of Guinea Pig Fibroblast Cells Studied with AFM. Biophys. J. 2016, 111, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Giergiel, M.; Malek-Zietek, K.E.; Konior, J.; Targosz-Korecka, M. Endothelial Glycocalyx Detection and Characterization by Means of Atomic Force Spectroscopy: Comparison of Various Data Analysis Approaches. Micron 2021, 151, 103153. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, R.; Job, K.M.; Dull, R.O.; Hlady, V. Stiffness and Heterogeneity of the Pulmonary Endothelial Glycocalyx Measured by Atomic Force Microscopy. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2011, 301, L353–L360. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Wang, W. Spatio-Temporal Development of the Endothelial Glycocalyx Layer and Its Mechanical Property in Vitro. J. R. Soc. Interface 2012, 9, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Targosz-Korecka, M.; Malek-Zietek, K.E.; Brzezinka, G.D.; Jaglarz, M. Morphological and Nanomechanical Changes in Mechanosensitive Endothelial Cells Induced by Colloidal AFM Probes: Changes in Endothelial Cells Induced by AFM Probes. Scanning 2016, 38, 654–664. [Google Scholar] [CrossRef]
- Bartosch, A.M.W.; Mathews, R.; Tarbell, J.M. Endothelial Glycocalyx-Mediated Nitric Oxide Production in Response to Selective AFM Pulling. Biophys. J. 2017, 113, 101–108. [Google Scholar] [CrossRef]
- Targosz-Korecka, M.; Jaglarz, M.; Malek-Zietek, K.E.; Gregorius, A.; Zakrzewska, A.; Sitek, B.; Rajfur, Z.; Chlopicki, S.; Szymonski, M. AFM-Based Detection of Glycocalyx Degradation and Endothelial Stiffening in the Db/Db Mouse Model of Diabetes. Sci. Rep. 2017, 7, 15951. [Google Scholar] [CrossRef]
- Makarova, N.; Lekka, M.; Gnanachandran, K.; Sokolov, I. Mechanical Way To Study Molecular Structure of Pericellular Layer. ACS Appl. Mater. Interfaces 2023, 15, 35962–35972. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Zullo, J.A.; Liveris, D.; Dragovich, M.; Zhang, X.F.; Goligorsky, M.S. Therapeutic Restoration of Endothelial Glycocalyx in Sepsis. J. Pharmacol. Exp. Ther. 2017, 361, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Targosz-Korecka, M.; Malek-Zietek, K.E.; Kloska, D.; Rajfur, Z.; Stepien, E.Ł.; Grochot-Przeczek, A.; Szymonski, M. Metformin Attenuates Adhesion between Cancer and Endothelial Cells in Chronic Hyperglycemia by Recovery of the Endothelial Glycocalyx Barrier. Biochim. Biophys. Acta BBA-Gen. Subj. 2020, 1864, 129533. [Google Scholar] [CrossRef] [PubMed]
- Kolmogorov, V.S.; Erofeev, A.S.; Woodcock, E.; Efremov, Y.M.; Iakovlev, A.P.; Savin, N.A.; Alova, A.V.; Lavrushkina, S.V.; Kireev, I.I.; Prelovskaya, A.O.; et al. Mapping Mechanical Properties of Living Cells at Nanoscale Using Intrinsic Nanopipette–Sample Force Interactions. Nanoscale 2021, 13, 6558–6568. [Google Scholar] [CrossRef]
- Clarke, R.W.; Novak, P.; Zhukov, A.; Tyler, E.J.; Cano-Jaimez, M.; Drews, A.; Richards, O.; Volynski, K.; Bishop, C.; Klenerman, D. Low Stress Ion Conductance Microscopy of Sub-Cellular Stiffness. Soft Matter 2016, 12, 7953–7958. [Google Scholar] [CrossRef]
- Novak, P.; Li, C.; Shevchuk, A.I.; Stepanyan, R.; Caldwell, M.; Hughes, S.; Smart, T.G.; Gorelik, J.; Ostanin, V.P.; Lab, M.J.; et al. Nanoscale Live-Cell Imaging Using Hopping Probe Ion Conductance Microscopy. Nat. Methods 2009, 6, 279–281. [Google Scholar] [CrossRef]





| Cell Type | Associated Disease | Method/Model Used | Short Description | Reference |
|---|---|---|---|---|
| Rat fat pad endothelial cells (RFPECs) | Endothelial dysfunction | Atomic force spectroscopy | The authors declare glypican-1 and heparan sulfate as the primary components of the glycocalyx that are responsible for stress-associated NO production | [61] |
| Endothelial cells on ex vivo aorta samples | Endothelial dysfunction and diabetes | Nanomechanics, brush model | Strong degradation of glycocalyx in diabetic mice | [62] |
| Endothelial cells on ex vivo aorta samples/in vitro HUVEC, EA.hy 926, HPMEC, bEnd.3, and GM7373 endothelial cells | Endothelial dysfunction in sepsis | Nanomechanics, mechanical spring model | A decrease in glycocalyx thickness after intraperitoneal injection of lipopolysaccharides. An in vitro decrease in glycocalyx thickness after heparinase I, thrombin, LPS, or TNF-α treatment | [47] |
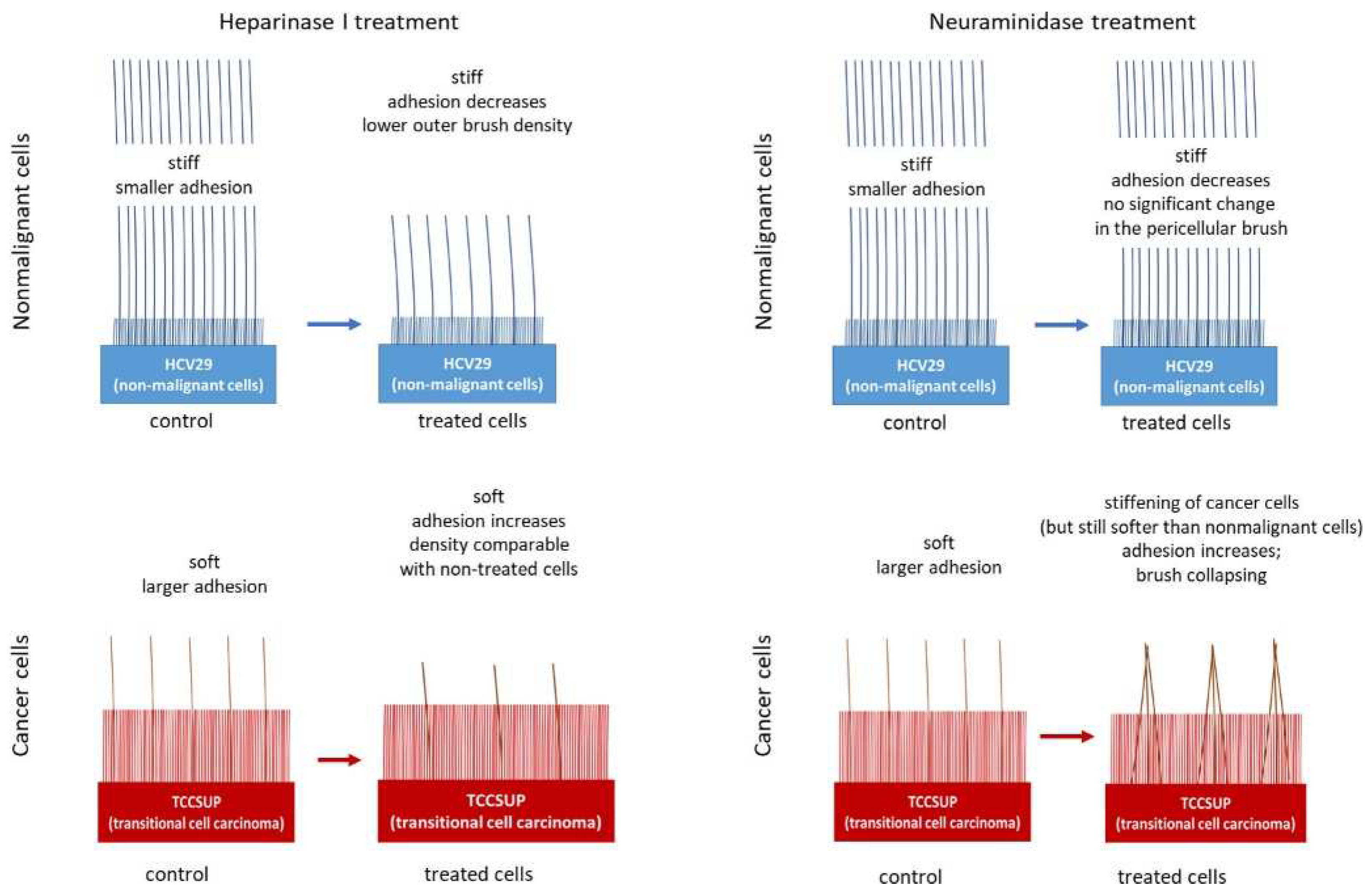
| Bladder epithelial nonmalignant (HCV29) and cancerous (TCCSUP) cells | Cancer | Nanomechanics, brush model | The differences in the structures of the pericellular layers of nonmalignant and cancerous cells and their changes after enzymatic treatment | [63] |
| Brain microvascular endothelial cells (bEnd.3) | Endothelial dysfunction in sepsis | Nanomechanics, mechanical spring model | Restoration of glycocalyx thickness after Sulodexide treatment in cells pretreated with lipopolysaccharides | [64] |
| EA.hy926 endothelial cells and A549 lung carcinoma cells | Cancer and diabetes | Nanomechanics, brush model | Incubation in metformin leads to a significant increase in glycocalyx density and the length of endothelial cells and has almost no effect on lung carcinoma cells | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolesov, D.; Astakhova, A.; Galdobina, M.; Moskovtsev, A.; Kubatiev, A.; Sokolovskaya, A.; Ukrainskiy, L.; Morozov, S. Scanning Probe Microscopy Techniques for Studying the Cell Glycocalyx. Cells 2023, 12, 2778. https://doi.org/10.3390/cells12242778
Kolesov D, Astakhova A, Galdobina M, Moskovtsev A, Kubatiev A, Sokolovskaya A, Ukrainskiy L, Morozov S. Scanning Probe Microscopy Techniques for Studying the Cell Glycocalyx. Cells. 2023; 12(24):2778. https://doi.org/10.3390/cells12242778
Chicago/Turabian StyleKolesov, Dmitry, Anna Astakhova, Maria Galdobina, Alexey Moskovtsev, Aslan Kubatiev, Alisa Sokolovskaya, Leonid Ukrainskiy, and Sergey Morozov. 2023. "Scanning Probe Microscopy Techniques for Studying the Cell Glycocalyx" Cells 12, no. 24: 2778. https://doi.org/10.3390/cells12242778





