Lipid-Lowering Drug Gemfibrozil Protects Mice from Tay-Sachs Disease via Peroxisome Proliferator-Activated Receptor α
Abstract
:1. Introduction
2. Materials and Methods
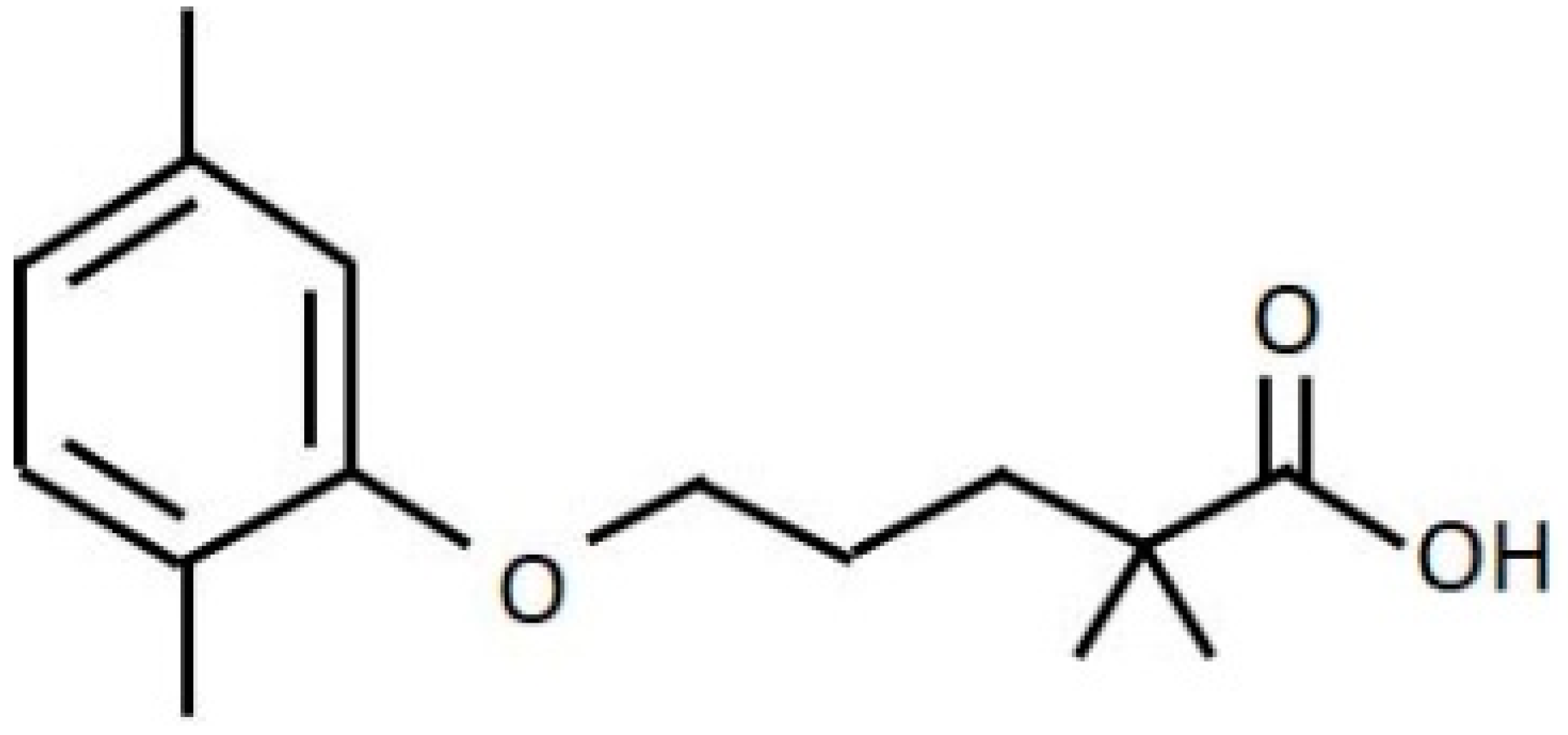
2.1. Reagents
2.2. Animals
2.3. Gemfibrozil (GFB) Treatment
2.4. Western Blotting
2.5. Immunohistochemistry
2.6. PAS and H&E Staining
2.7. Open Field Test
2.8. Rotarod
2.9. Gait Analysis
2.10. Survival Assay
2.11. Statistics
3. Results
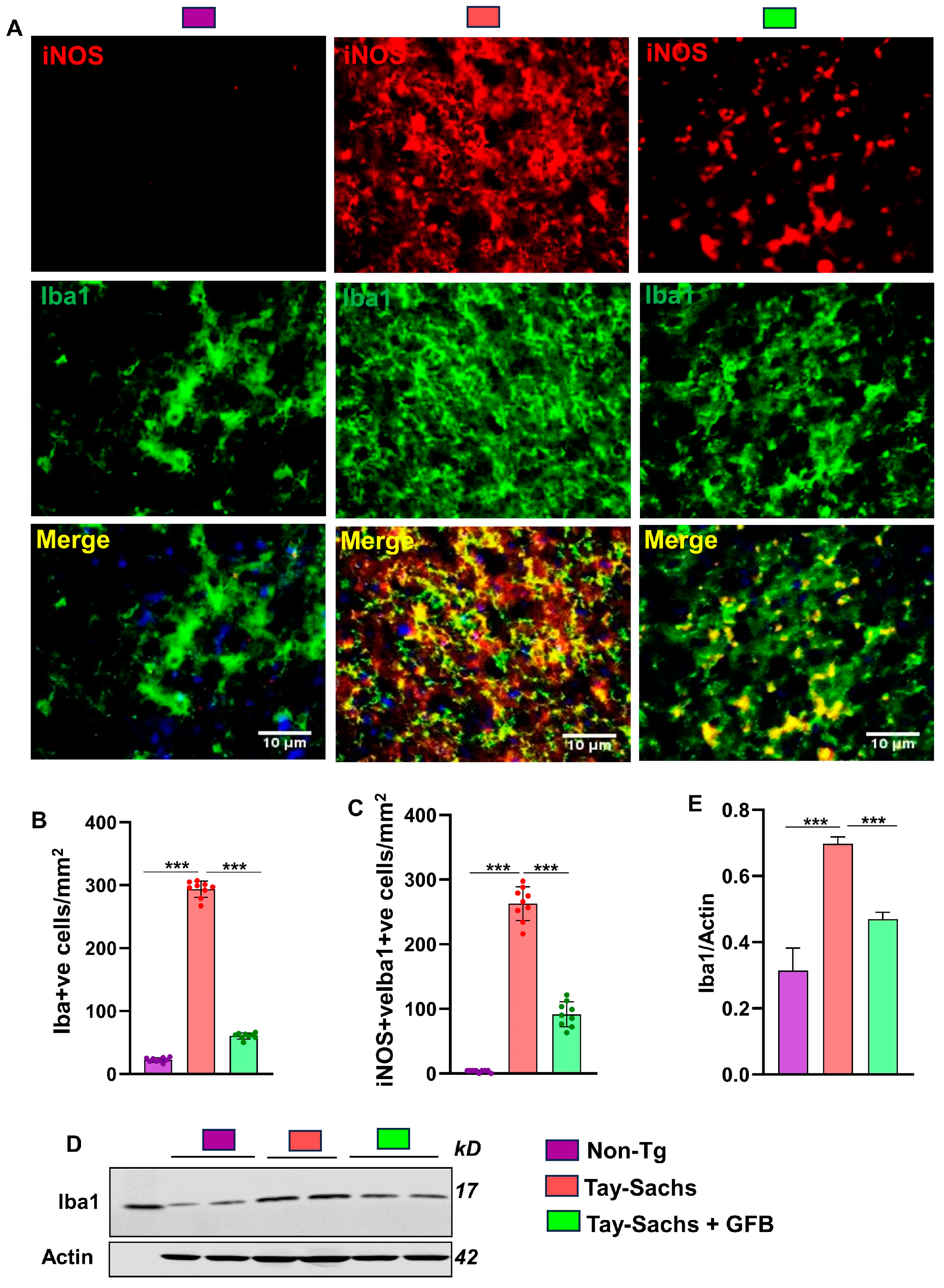
3.1. Oral Administration of GFB Prevents Activation of Astrocytes and Microglia in the Motor Cortex of Tay-Sachs Mice
3.2. Treatment with GFB Attenuates Neuronal Apoptosis and Reduces Glycoconjugates in Tay-Sachs Mice
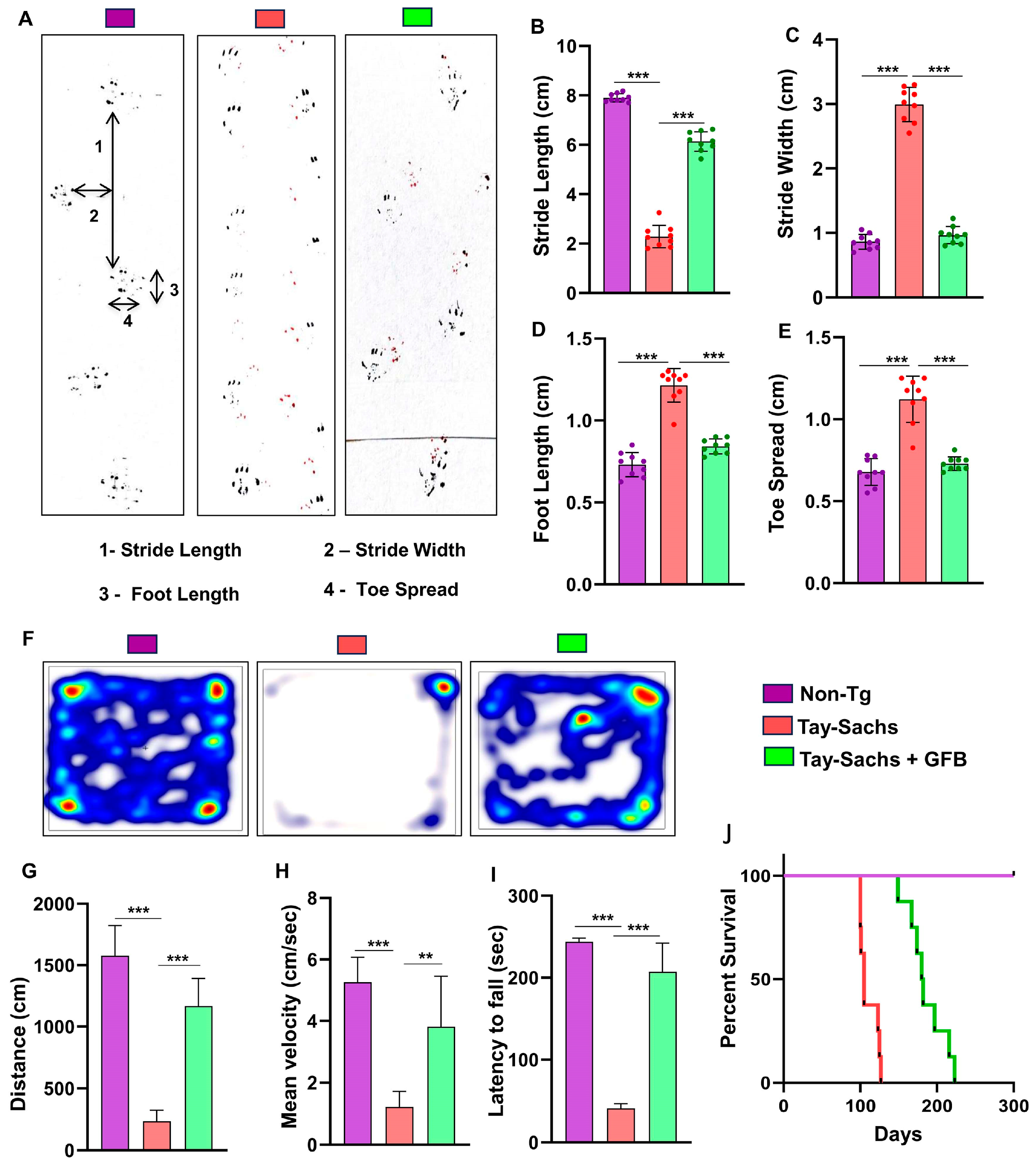
3.3. Oral Administration of GFB Improves Gait, Alleviates Motor Deficits, and Increases Survival in Tay-Sachs Mice
3.4. Oral GFB Induces the Activation of PPARα in Tay-Sachs Mice
3.5. GFB Attenuates Glycoconjugate Materials in Tay-Sachs Mice via PPARα
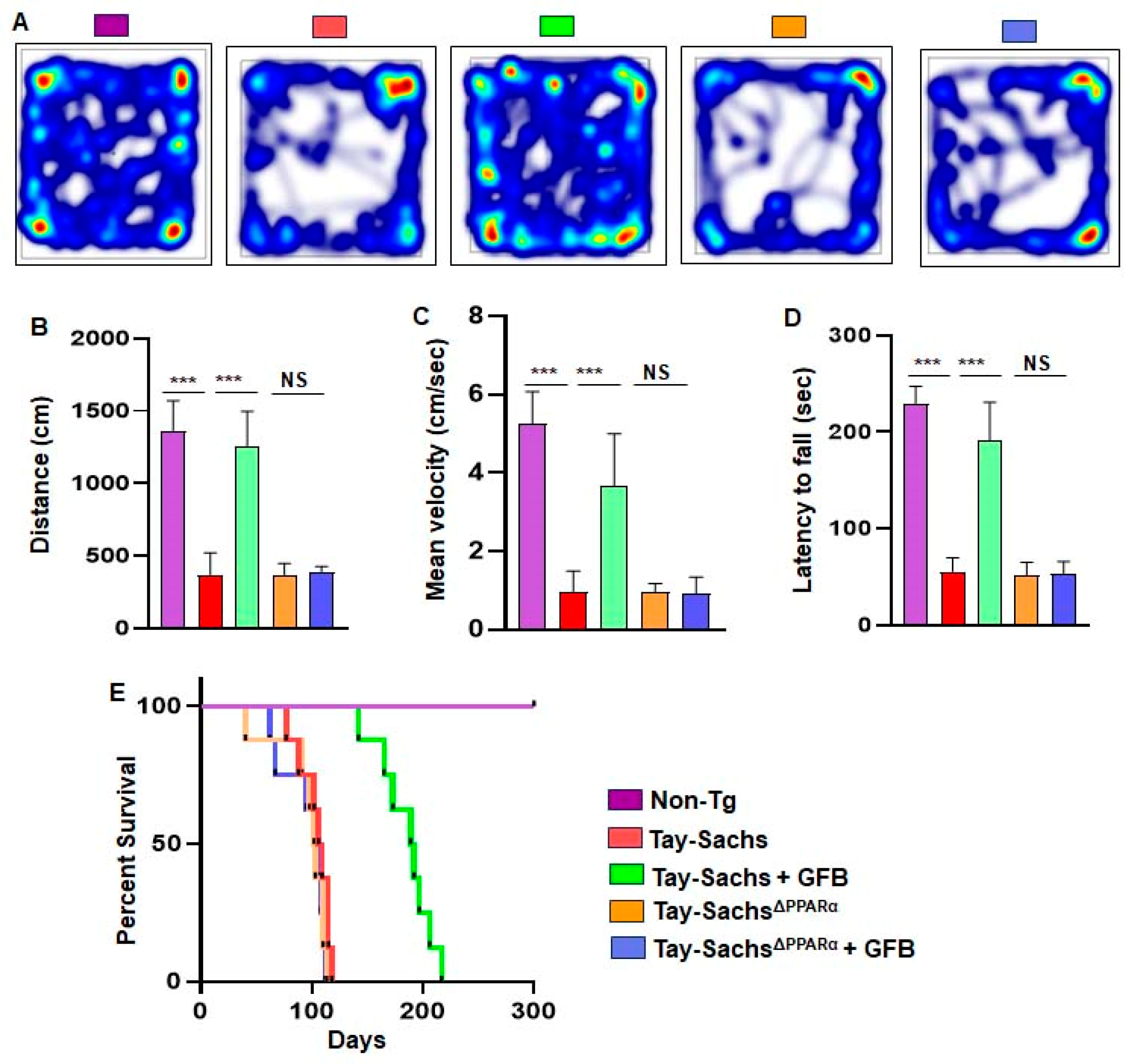
3.6. Oral Administration of GFB Improves Motor Deficits and Increases Survival in Tay-Sachs Mice via PPARα
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cachon-Gonzalez, M.B.; Zaccariotto, E.; Cox, T.M. Genetics and Therapies for GM2 Gangliosidosis. Curr. Gene Ther. 2018, 18, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.R.; Gahl, W.A. Lysosomal storage diseases. Transl. Sci. Rare Dis. 2017, 2, 1–71. [Google Scholar] [CrossRef] [PubMed]
- Lew, R.M.; Burnett, L.; Proos, A.L.; Delatycki, M.B. Tay-Sachs disease: Current perspectives from Australia. Appl. Clin. Genet. 2015, 8, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, R.; Pereira Lda, V. The frequency of Tay-Sachs disease causing mutations in the Brazilian Jewish population justifies a carrier screening program. Sao. Paulo Med. J. 2001, 119, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Stenson, P.D.; Mort, M.; Ball, E.V.; Shaw, K.; Phillips, A.; Cooper, D.N. The Human Gene Mutation Database: Building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum. Genet. 2014, 133, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Hogan, E.L.; Pahan, K. Ceramide and neurodegeneration: Susceptibility of neurons and oligodendrocytes to cell damage and death. J. Neurol. Sci. 2009, 278, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Assi, E.; Cazzato, D.; De Palma, C.; Perrotta, C.; Clementi, E.; Cervia, D. Sphingolipids and brain resident macrophages in neuroinflammation: An emerging aspect of nervous system pathology. Clin. Dev. Immunol. 2013, 2013, 309302. [Google Scholar] [CrossRef] [PubMed]
- Virgolini, M.J.; Feliziani, C.; Cambiasso, M.J.; Lopez, P.H.; Bollo, M. Neurite atrophy and apoptosis mediated by PERK signaling after accumulation of GM2-ganglioside. Biochim Biophys. Acta Mol. Cell Res. 2019, 1866, 225–239. [Google Scholar] [CrossRef]
- Benner, E.J.; Mosley, R.L.; Destache, C.J.; Lewis, T.B.; Jackson-Lewis, V.; Gorantla, S.; Nemachek, C.; Green, S.R.; Przedborski, S.; Gendelman, H.E. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 9435–9440. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Demir, S.A.; Timur, Z.K.; Ates, N.; Martinez, L.A.; Seyrantepe, V. GM2 ganglioside accumulation causes neuroinflammation and behavioral alterations in a mouse model of early onset Tay-Sachs disease. J. Neuroinflammation 2020, 17, 277. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Roy, A.; Matras, J.; Brahmachari, S.; Gendelman, H.E.; Pahan, K. Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson’s disease. J. Neurosci. 2009, 29, 13543–13556. [Google Scholar] [CrossRef] [PubMed]
- Jana, M.; Pahan, K. Gemfibrozil, a lipid lowering drug, inhibits the activation of primary human microglia via peroxisome proliferator-activated receptor beta. Neurochem. Res. 2012, 37, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Pahan, K.; Jana, M.; Liu, X.; Taylor, B.S.; Wood, C.; Fischer, S.M. Gemfibrozil, a lipid-lowering drug, inhibits the induction of nitric-oxide synthase in human astrocytes. J. Biol. Chem. 2002, 277, 45984–45991. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Storer, P.D.; Chavis, J.A.; Racke, M.K.; Drew, P.D. Agonists for the peroxisome proliferator-activated receptor-alpha and the retinoid X receptor inhibit inflammatory responses of microglia. J. Neurosci. Res. 2005, 81, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Raha, S.; Ghosh, A.; Dutta, D.; Patel, D.R.; Pahan, K. Activation of PPARalpha enhances astroglial uptake and degradation of beta-amyloid. Sci. Signal. 2021, 14, eabg4747. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Jana, M.; Majumder, M.; Mondal, S.; Roy, A.; Pahan, K. Selective targeting of the TLR2/MyD88/NF-kappaB pathway reduces alpha-synuclein spreading in vitro and in vivo. Nat. Commun. 2021, 12, 5382. [Google Scholar] [CrossRef]
- Dutta, D.; Paidi, R.K.; Raha, S.; Roy, A.; Chandra, S.; Pahan, K. Treadmill exercise reduces alpha-synuclein spreading via PPARalpha. Cell Rep. 2022, 40, 111058. [Google Scholar] [CrossRef]
- Patel, D.; Roy, A.; Kundu, M.; Jana, M.; Luan, C.H.; Gonzalez, F.J.; Pahan, K. Aspirin binds to PPARalpha to stimulate hippocampal plasticity and protect memory. Proc. Natl. Acad. Sci. USA 2018, 115, E7408–E7417. [Google Scholar] [CrossRef]
- Paidi, R.K.; Jana, M.; Mishra, R.K.; Dutta, D.; Pahan, K. Selective Inhibition of the Interaction between SARS-CoV-2 Spike S1 and ACE2 by SPIDAR Peptide Induces Anti-Inflammatory Therapeutic Responses. J. Immunol. 2021, 207, 2521–2533. [Google Scholar] [CrossRef]
- Mondal, S.; Kundu, M.; Jana, M.; Roy, A.; Rangasamy, S.B.; Modi, K.K.; Wallace, J.; Albalawi, Y.A.; Balabanov, R.; Pahan, K. IL-12 p40 monomer is different from other IL-12 family members to selectively inhibit IL-12Rbeta1 internalization and suppress EAE. Proc. Natl. Acad. Sci. USA 2020, 117, 21557–21567. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, S.B.; Jana, M.; Roy, A.; Corbett, G.T.; Kundu, M.; Chandra, S.; Mondal, S.; Dasarathi, S.; Mufson, E.J.; Mishra, R.K.; et al. Selective disruption of TLR2-MyD88 interaction inhibits inflammation and attenuates Alzheimer’s pathology. J. Clin. Investig. 2018, 128, 4297–4312. [Google Scholar] [CrossRef] [PubMed]
- Brahmachari, S.; Fung, Y.K.; Pahan, K. Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J. Neurosci. 2006, 26, 4930–4939. [Google Scholar] [CrossRef] [PubMed]
- Kuil, L.E.; Lopez Marti, A.; Carreras Mascaro, A.; van den Bosch, J.C.; van den Berg, P.; van der Linde, H.C.; Schoonderwoerd, K.; Ruijter, G.J.G.; van Ham, T.J. Hexb enzyme deficiency leads to lysosomal abnormalities in radial glia and microglia in zebrafish brain development. Glia 2019, 67, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- van der Kant, R.; Goldstein, L.S.B.; Ossenkoppele, R. Amyloid-beta-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 2020, 21, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Idol, R.A.; Wozniak, D.F.; Fujiwara, H.; Yuede, C.M.; Ory, D.S.; Kornfeld, S.; Vogel, P. Neurologic abnormalities in mouse models of the lysosomal storage disorders mucolipidosis II and mucolipidosis III gamma. PLoS ONE 2014, 9, e109768. [Google Scholar] [CrossRef]
- Flotte, T.R.; Cataltepe, O.; Puri, A.; Batista, A.R.; Moser, R.; McKenna-Yasek, D.; Douthwright, C.; Gernoux, G.; Blackwood, M.; Mueller, C.; et al. AAV gene therapy for Tay-Sachs disease. Nat. Med. 2022, 28, 251–259. [Google Scholar] [CrossRef]
- Yamanaka, S.; Johnson, M.D.; Grinberg, A.; Westphal, H.; Crawley, J.N.; Taniike, M.; Suzuki, K.; Proia, R.L. Targeted disruption of the Hexa gene results in mice with biochemical and pathologic features of Tay-Sachs disease. Proc. Natl. Acad. Sci. USA 1994, 91, 9975–9979. [Google Scholar] [CrossRef]
- Tropak, M.B.; Yonekawa, S.; Karumuthil-Melethil, S.; Thompson, P.; Wakarchuk, W.; Gray, S.J.; Walia, J.S.; Mark, B.L.; Mahuran, D. Construction of a hybrid beta-hexosaminidase subunit capable of forming stable homodimers that hydrolyze GM2 ganglioside in vivo. Mol. Methods Clin. Dev. 2016, 3, 15057. [Google Scholar] [CrossRef]
- Jacobs, J.F.; Willemsen, M.A.; Groot-Loonen, J.J.; Wevers, R.A.; Hoogerbrugge, P.M. Allogeneic BMT followed by substrate reduction therapy in a child with subacute Tay-Sachs disease. Bone Marrow Transpl. 2005, 36, 925–926. [Google Scholar] [CrossRef]
- Bembi, B.; Marchetti, F.; Guerci, V.I.; Ciana, G.; Addobbati, R.; Grasso, D.; Barone, R.; Cariati, R.; Fernandez-Guillen, L.; Butters, T.; et al. Substrate reduction therapy in the infantile form of Tay-Sachs disease. Neurology 2006, 66, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Stepien, K.M.; Lum, S.H.; Wraith, J.E.; Hendriksz, C.J.; Church, H.J.; Priestman, D.; Platt, F.M.; Jones, S.; Jovanovic, A.; Wynn, R. Haematopoietic Stem Cell Transplantation Arrests the Progression of Neurodegenerative Disease in Late-Onset Tay-Sachs Disease. JIMD Rep. 2018, 41, 17–23. [Google Scholar] [CrossRef]
- Gonzalez, R.; Hamblin, M.H.; Lee, J.P. Neural Stem Cell Transplantation and CNS Diseases. CNS Neurol Disord Drug Targets 2016, 15, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Desnick, R.J.; Kaback, M.M. Future perspectives for Tay-Sachs disease. Adv. Genet. 2001, 44, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Pahan, K. Lipid-lowering drugs. Cell Mol. Life Sci 2006, 63, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J.; Peters, J.M.; Cattley, R.C. Mechanism of action of the nongenotoxic peroxisome proliferators: Role of the peroxisome proliferator-activator receptor alpha. J. Natl. Cancer Inst. 1998, 90, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.K.; Mannaerts, G.P. Peroxisomal lipid metabolism. Annu Rev. Nutr. 1994, 14, 343–370. [Google Scholar] [CrossRef]
- Braun, L.; Mile, V.; Schaff, Z.; Csala, M.; Kardon, T.; Mandl, J.; Banhegyi, G. Induction and peroxisomal appearance of gulonolactone oxidase upon clofibrate treatment in mouse liver. FEBS Lett 1999, 458, 359–362. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Solovyeva, V.V.; Shaimardanova, A.A.; Chulpanova, D.S.; Kitaeva, K.V.; Chakrabarti, L.; Rizvanov, A.A. New Approaches to Tay-Sachs Disease Therapy. Front. Physiol 2018, 9, 1663. [Google Scholar] [CrossRef]
- Vitner, E.B.; Platt, F.M.; Futerman, A.H. Common and uncommon pathogenic cascades in lysosomal storage diseases. J. Biol. Chem. 2010, 285, 20423–20427. [Google Scholar] [CrossRef] [PubMed]
- Rasband, M.N. Glial Contributions to Neural Function and Disease. Mol. Cell Proteom. 2016, 15, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Pelled, D.; Lloyd-Evans, E.; Riebeling, C.; Jeyakumar, M.; Platt, F.M.; Futerman, A.H. Inhibition of calcium uptake via the sarco/endoplasmic reticulum Ca2+-ATPase in a mouse model of Sandhoff disease and prevention by treatment with N-butyldeoxynojirimycin. J. Biol. Chem. 2003, 278, 29496–29501. [Google Scholar] [CrossRef] [PubMed]
- Sano, R.; Annunziata, I.; Patterson, A.; Moshiach, S.; Gomero, E.; Opferman, J.; Forte, M.; d’Azzo, A. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol. Cell 2009, 36, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Jana, M.; Modi, K.; Gonzalez, F.J.; Sims, K.B.; Berry-Kravis, E.; Pahan, K. Activation of peroxisome proliferator-activated receptor alpha induces lysosomal biogenesis in brain cells: Implications for lysosomal storage disorders. J. Biol. Chem. 2015, 290, 10309–10324. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Su, L.Y.; Li, G.; Yang, J.; Liu, Q.; Yang, L.X.; Zhang, D.F.; Zhou, H.; Xu, M.; Fan, Y.; et al. Activation of PPARA-mediated autophagy reduces Alzheimer disease-like pathology and cognitive decline in a murine model. Autophagy 2020, 16, 52–69. [Google Scholar] [CrossRef]
- Ghosh, A.; Pahan, K. Gemfibrozil, a lipid-lowering drug, induces suppressor of cytokine signaling 3 in glial cells: Implications for neurodegenerative disorders. J. Biol. Chem. 2012, 287, 27189–27203. [Google Scholar] [CrossRef]
- Corbett, G.T.; Roy, A.; Pahan, K. Gemfibrozil, a lipid-lowering drug, upregulates IL-1 receptor antagonist in mouse cortical neurons: Implications for neuronal self-defense. J. Immunol. 2012, 189, 1002–1013. [Google Scholar] [CrossRef]
- Ghosh, A.; Rangasamy, S.B.; Modi, K.K.; Pahan, K. Gemfibrozil, food and drug administration-approved lipid-lowering drug, increases longevity in mouse model of late infantile neuronal ceroid lipofuscinosis. J. Neurochem. 2017, 141, 423–435. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raha, S.; Dutta, D.; Paidi, R.K.; Pahan, K. Lipid-Lowering Drug Gemfibrozil Protects Mice from Tay-Sachs Disease via Peroxisome Proliferator-Activated Receptor α. Cells 2023, 12, 2791. https://doi.org/10.3390/cells12242791
Raha S, Dutta D, Paidi RK, Pahan K. Lipid-Lowering Drug Gemfibrozil Protects Mice from Tay-Sachs Disease via Peroxisome Proliferator-Activated Receptor α. Cells. 2023; 12(24):2791. https://doi.org/10.3390/cells12242791
Chicago/Turabian StyleRaha, Sumita, Debashis Dutta, Ramesh K. Paidi, and Kalipada Pahan. 2023. "Lipid-Lowering Drug Gemfibrozil Protects Mice from Tay-Sachs Disease via Peroxisome Proliferator-Activated Receptor α" Cells 12, no. 24: 2791. https://doi.org/10.3390/cells12242791
APA StyleRaha, S., Dutta, D., Paidi, R. K., & Pahan, K. (2023). Lipid-Lowering Drug Gemfibrozil Protects Mice from Tay-Sachs Disease via Peroxisome Proliferator-Activated Receptor α. Cells, 12(24), 2791. https://doi.org/10.3390/cells12242791





