Abstract
Temperature has a significant effect on all physiological processes of animals. Suitable temperatures promote responsiveness, movement, metabolism, growth, and reproduction in animals, whereas extreme temperatures can cause injury or even death. Thus, thermosensation is important for survival in all animals. However, mechanisms regulating thermosensation remain unexplored, mostly because of the complexity of mammalian neural circuits. The fruit fly Drosophila melanogaster achieves a desirable body temperature through ambient temperature fluctuations, sunlight exposure, and behavioral strategies. The availability of extensive genetic tools and resources for studying Drosophila have enabled scientists to unravel the mechanisms underlying their temperature preference. Over the past 20 years, Drosophila has become an ideal model for studying temperature-related genes and circuits. This review provides a comprehensive overview of our current understanding of thermosensation and temperature preference in Drosophila. It encompasses various aspects, such as the mechanisms by which flies sense temperature, the effects of internal and external factors on temperature preference, and the adaptive strategies employed by flies in extreme-temperature environments. Understanding the regulating mechanisms of thermosensation and temperature preference in Drosophila can provide fundamental insights into the underlying molecular and neural mechanisms that control body temperature and temperature-related behavioral changes in other animals.
1. Introduction
The physiological processes of all organisms are significantly affected by temperature. The ability to sense, respond, and adapt to external environmental threats is the most critical factor that has evolved to protect the existence of organisms [1]. Suitable temperatures can affect animal responsiveness, movement, metabolism, growth, and reproduction, whereas extreme temperatures can be injurious and potentially lethal. Thermosensation is important not only for endotherms but also for ectotherms. Thermosensation is one of the most ancient sensory processes in all animals. The thermal information is generated by thermoreceptors and transported to animal’s brain. The translation of thermal energy into electrical signal is mediated by these thermoreceptors that are sensitive to a specific range of temperatures [2]. Understanding the mechanisms involved in regulating body temperature (Tb) in different environments is critical for survival. Physiological factors, including morphology, heat adaptation, sex, and age, affect Tb regulation [3]. Brown adipose tissue (BAT), shivering, and the constriction of blood vessels play important roles in the defense against cold temperatures. Conversely, during hot temperatures, thermogenesis decreases, sweat production increases, and blood vessels dilate [4]. Animal behavior also plays a crucial role in Tb control. The most basic thermoregulatory behavior involves seeking cold or warmth. Animals move between microenvironments in their habitats to alter the rate of heat loss or absorption. Besides basic thermoregulatory behaviors, more complex strategies, such as making nests or burrows, have been employed to establish a suitable temperature environment [4].
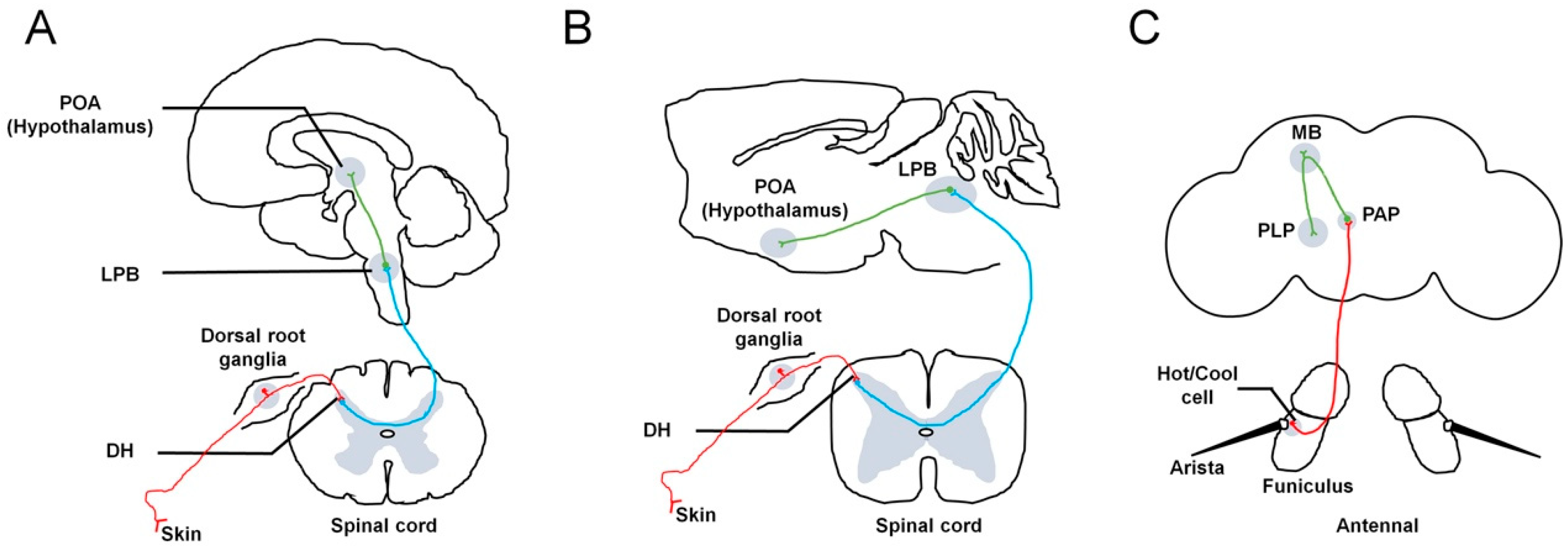
In mammals, environmental temperature is sensed by primary somatosensory neurons in the skin. These neurons subsequently relay this information to the dorsal horn (DH) of the spinal cord. The DH contains neurons projecting to the lateral parabrachial nucleus (LPB) of the brainstem. Finally, third-order neurons located within the LPB project to the preoptic area (POA) of the hypothalamus (Figure 1A,B). In addition, the activation of LPB neurons can be induced by changes in skin temperature. The external lateral subdivision of the LPB (LPBel) receives cold-temperature information from the DH and transmits it to the median preoptic area (MnPO). LPBel neurons are necessary for cold-defense responses, such as shivering and BAT thermogenesis. In contrast, the dorsal subdivision of the LPB (LPBd) receives hot-temperature information from the DH and predominantly transmits it to the MnPO. LPBd neurons are necessary for hot-defense responses, such as cutaneous vasoconstriction and inhibition of BAT thermogenesis [3,4,5].
Figure 1.
Neuronal pathways involved in thermosensation in different species. In humans (A) and rodents (B), temperature information from the skin is sensed by primary somatosensory neurons, whose cell bodies are located in the dorsal root ganglia. Subsequently, these neurons relay this information to the DH of the spinal cord (red lines). The DH contains neurons projecting to the LPB in the brainstem (blue lines). Finally, third-order neurons located within the LPB project to the POA of the hypothalamus (green line). Both warm- and cold-activated LPB neurons send projections to the POA of the hypothalamus. (C) In flies, the external sensors, called hot or cool cells, are located at the base of the aristae and in the sacculus region of the third antennal segment. All cool and hot cells project to a central region called the PAP to form synapses and connect with downstream PNs to convey temperature information (red line). The thermal PNs (green line) further transfer the received information to higher brain centers, including the MB and PLP. DH: dorsal horn. LPB: lateral parabrachial nucleus. POA: preoptic area. TRPP: transient receptor potential polycystin. PAP: proximal antennal protocerebrum. MB: mushroom body. PLP: posterior lateral protocerebrum. PNs: projection neurons.
Drosophila melanogaster can achieve a desirable Tb through ambient temperature fluctuations, sunlight exposure, and behavioral strategies [6,7,8,9,10]. The availability of powerful tools for genetic manipulations has allowed scientists to understand detailed molecular mechanisms and neuronal circuits that regulate thermosensation and temperature-preference behaviors in Drosophila. Since the prominent signaling pathways that regulate neuronal functions in mammals are highly conserved in Drosophila, scientists have been using this model organism to study thermosensation. Temperature-related behaviors in Drosophila include temperature preference rhythm (TPR), the temperature preference of aged flies, and feeding-state-dependent temperature preference. This review discusses current findings regarding the genes, circuits, and molecular mechanisms of thermosensation and temperature-related behaviors in Drosophila.
2. Thermosensation in Drosophila
2.1. The Molecular Basis of Thermosensation
Most ion channels belong to the transient receptor potential (TRP) family. TRP is conserved across species, from humans to fruit flies. In mammals, these channels can be classified into more than fifty subtypes, which are further divided into seven subfamilies based on the homology of their amino acid sequences. These subfamilies include vanilloid (TRPV1-6), canonical (TRPC1-7), melastatin (TRPM1-8), non-mechanoreceptor potential C (NOMP-like, TRPN1), long TRP ankyrin (TRPA1), polycystins (TRPP1-5), and mucolipins (TRPML1-3) [11,12,13,14,15,16,17]. TRP channels exhibit diverse modes of activation through temperature, mechanical, and chemical stimuli, thereby facilitating the modulation of cellular functions via an inward cation current [18,19]. Consequently, the TRP family regulates a wide range of physiological processes, such as responses to environmental stimuli and functions related to vision, hearing, taste, and thermosensation [20]. In mammals, TRPV1-4 receptors are responsible for receiving heat stimuli, whereas TRPA1 and TRPM8 receptors are specialized in detecting cold temperatures. While TRPV1-4 receptors operate within the innocuous temperature range, it is noteworthy that TRPA1, TRPV1, and TRPV2 are capable of functioning within the noxious temperature range [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Furthermore, a previous study showed that TRPM3 receptors serve as sensors for detecting nociceptive heat while TRPM2 receptors serve as warmth sensors in mice [39,40,41].
Manning et al. first demonstrated the existence of TRP channels in Drosophila while investigating a spontaneous mutation that exhibited a transient response to prolonged intense light. This mutation was initially named “type A” [42]. Subsequently, it was found that this mutant, referred to as “transient receptor potential” or TRP is defective for light transmission [43]. TRP channels play a role in temperature sensing in Drosophila. In addition to thirteen TRP channels, Drosophila also possesses four TRPA homologs: dTrpA1, painless, pyrexia, and water witch [44]. In terms of amino acid identity, dTrpA1 is 32% identical to and 54% comparable with its ortholog in mammals [45]. The dTRPA1 channel is expressed in several classes of peripheral sensory neurons and numerous groups of central neurons [46,47,48]. painless is expressed in the larval peripheral nervous system [49] and multiple regions of the adult brain, such as the mushroom body (MB), ellipsoid body (EB), projection neurons (PNs) of olfaction, and the pars intercerebrails (PI) [50,51,52,53]. pyrexia is ubiquitously expressed along the dendrites of a subset of peripheral nervous system neurons [54]. water witch is expressed in neurons associated with specialized sensory hairs located in the third segment of the antenna [55,56]. These neurons extend to the regions of the central nervous system that are involved in the perception of mechanical stimuli [57,58]. The functionality of water witch is essential for the detection of humid air [57]. In contrast to mammalian TrpA1, dTrpA1, painless, and pyrexia have no role in regulating cold avoidance. However, dTrpA1 is also recognized as a receptor for detecting warmth [46,59,60,61]. Additionally, temperatures over 38 and 42 °C can be detected by painless and pyrexia, respectively [49,54,61,62,63].
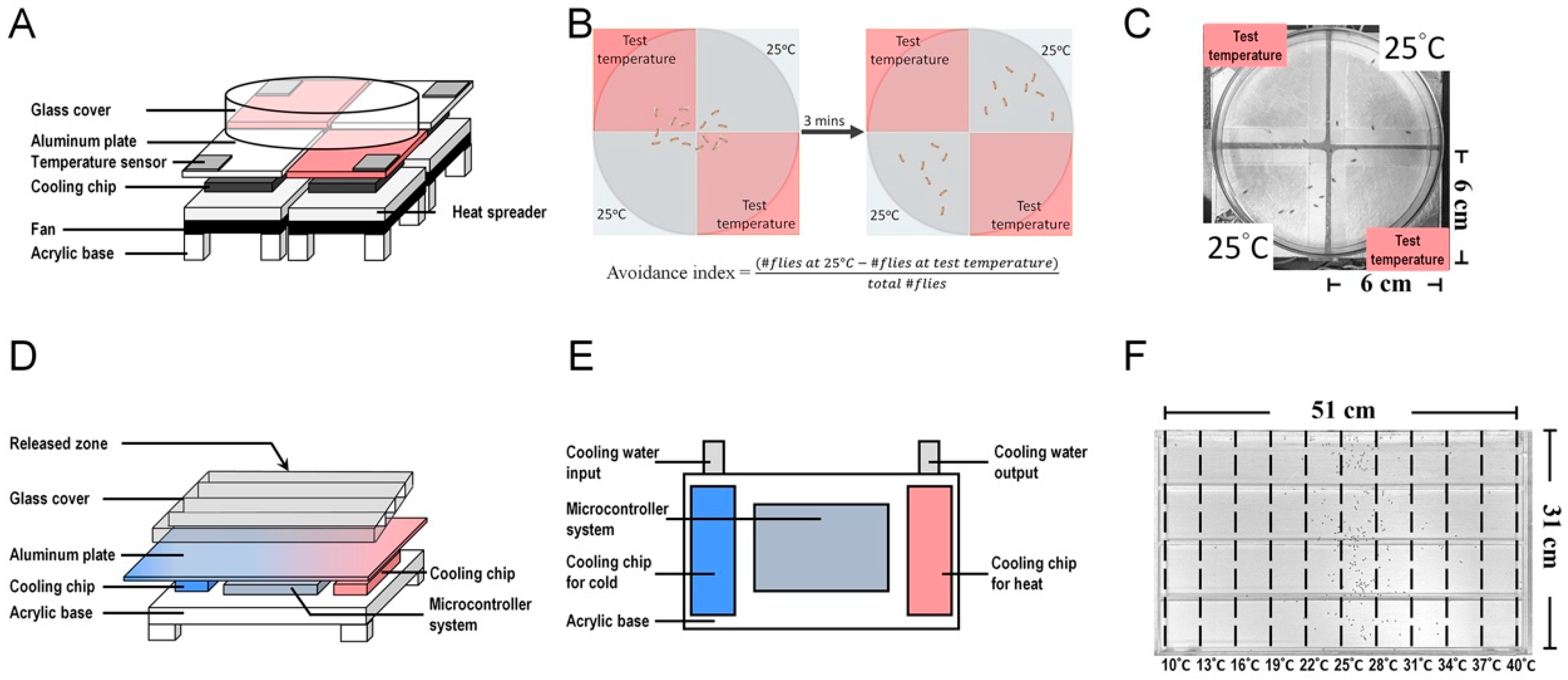
Gallio et al. identified brv1, brv2, and brv3 as potential receptors for cold stimuli [38]. The brivido genes encode channels homologous to the mammalian TRP channels [38,64]. These channels are specifically found in neurons that are sensitive to cold temperatures, capable of detecting drops in temperature up to 11 °C. These channels are not expressed in neurons that respond to heat and can detect temperatures exceeding 25 °C. Notably, these genes are co-expressed in the same sensory neurons, suggesting a potential collaborative role in enhancing the perception of cold stimuli. The elimination of brv1 or brv2 results in a diminished neuronal response to temperature decline in calcium imaging experiments, indicating their essential role in cold sensing. Furthermore, brv1/2/3 genes are also crucial for cold avoidance in the two-choice behavior assay, where flies are faced with the decision of selecting between 25 °C and a lower temperature [38]. The two-choice behavior assay is used to identify temperature avoidance or temperature preference in Drosophila and involves a two-choice thermoelectric device. In this assay, the avoidance index is calculated by comparing the fly behavior at an experimental temperature to that at a constant temperature (25 °C) (Figure 2A–C).
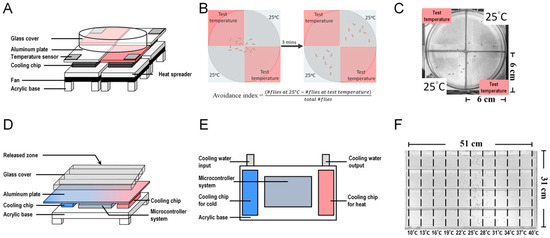
Figure 2.
Thermoelectric devices used for temperature-related behavioral analysis in Drosophila. (A) The two-choice thermoelectric device consists of a microcontroller system, temperature sensors, aluminum plates, cooling chips, heat spreaders, and a glass cover. Pulse-width modulation (PWM) is used to control the operating voltage of the thermoelectric cooling module, which controls the temperature of the aluminum plate ranging from 15 to 35 °C. A 6 cm × 6 cm aluminum sheet is placed on each thermoelectric cooling module to increase conduction velocity. For heat dissipation, a heat spreader with an aluminum plate and a fan is placed below each cooling chip. Four thermoelectric cooling modules are arranged to form an arena, and a temperature sensor is added to each thermoelectric cooling module. (B,C) Diagram illustrating the two-choice assay behavior. Three minutes after the flies are placed in the arena, they seek a comfortable temperature environment through temperature avoidance. Flies determine their Tp by comparing a series of experimental temperatures with a reference temperature (25 °C) [65]. (D) The temperature gradient device contains an aluminum plate, which is equipped with an array of thermoelectric chips at one side for the cold source and another array of thermoelectric chips at the other side for the hot source. An electro-couple thermometer and multiple temperature sensors are set within the aluminum plate to detect and monitor the plate temperature via a microcontroller system. Water (20 °C) flows around the thermoelectric cooling chips, which supply the cold source to dissipate the accumulated heat produced by the chips. (E) Top view of the temperature gradient device below the aluminum plate. (F) Drosophila temperature preference behavior assay. The aluminum plate is divided into 10 sectors, each with a temperature gradient ranging from 10 to 40 °C. Thirty minutes after the flies enter the chamber, they settle at their preferred temperature locations. Flies avoid extreme temperatures and determine their Tp through a balance between cold- and warm-avoidance behaviors [66].
It has been shown that the presence of additional receptor proteins besides TRP channels is responsible for temperature sensitivity. In Drosophila larvae, the role of the photoreceptor rhodopsin, a canonical G protein-coupled receptor in thermosensory neurons, remains unclear. However, recent studies have indicated that it might play a role in temperature responses [67]. In adult Drosophila, the gustatory receptor, GR28B(D), plays a role in detecting high temperatures [68]. Invertebrates possess seven transmembrane proteins known as gustatory receptors [69]. Gustatory receptors, along with olfactory receptors, constitute a distinct gene family and have been extensively studied because of their involvement in the chemoreception of sweet and bitter taste, food odors, carbon dioxide, and various other substances [69,70,71]. A previous study showed that GR28B(D) is expressed in hot cells, the neurons responsible for thermosensation. Furthermore, when GR28B(D) is ectopically expressed in different cell types, it confers the ability to sense temperature, particularly warmth. These findings strongly indicate that GR28B(D) functions as a sensor for detecting warmth [68]. Although GR28B(D) and dTrpA1(B) mediate different forms of thermotactic behaviors and have different expression patterns, it can be postulated that these receptors function as sensors for high temperatures. Although dTrpA1 is expressed in anterior cell (AC) neurons of the anterior brain, GR28B(D) is expressed in peripheral hot cells located at the base of the aristae. GR28B(D) is necessary for rapid negative thermotaxis, whereas dTrpA1 is necessary for slow thermotaxis on a shallow and broad thermal gradient [46,68,72,73].
The ionotropic receptors (IR) IR21a and IR25a, members of the ionotropic glutamate receptor (iGluR) family, have also been implicated in thermosensation [74,75]. Invertebrate IRs commonly act as the receptors for various acids and amines. Although the specific chemoreceptor function of IR21a has not been identified yet, it is conserved in insects, indicating its potential involvement in other sensory modalities [76,77]. The most conserved insect IR across all species is IR25a. In Drosophila, IR25a is expressed in various classes of chemosensory neurons with distinct chemical characteristics. Additionally, IR25a acts as a co-receptor that combines with other stimulus-specific IRs to generate chemoreceptors with different specificities [74,78,79]. It has been shown that both IR21a and IR25a are required for mediating larval dorsal organ ganglion (DOG) responses to cold and regulating cold-avoidance behavior. By ectopically expressing IR21a, it has been shown that IR25a-dependent cold sensitivity is involved, indicating that these two receptors collaborate for cold perception [75] (Table 1).

Table 1.
Molecules involved in thermosensation in Drosophila.
2.2. The Neuronal Basis of Thermosensation
Drosophila senses temperatures through numerous temperature-sensing neurons. During the larval stage, the anterior tip of the larval head contains neurons that are specifically responsible for temperature detection. The cell bodies of these temperature-sensing neurons are located in two ganglia: the DOG and the terminal organ ganglion (TOG) [70,75,80,81,82,83]. Dendritic projections of these neurons extend towards two anatomical structures located beneath the larval body wall, known as terminal organs and dorsal organs, which have the potential to gather external temperature information [84]. Particularly, the responses of DOG and TOG to cold temperatures (below 25 °C) have been observed. DOG expresses ionotropic cold receptors IR21a and IR25a, which exhibit decreased activity as the temperature rises [75]. It has been found that larval cold-avoidance behavior ceases when the temperature-sensing DOG neurons are deactivated. However, the inhibition of TOG did not yield the same results. Both DOG and TOG project to the antennal lobes and establish connections with secondary neurons, resembling the olfactory neurons present in the antennal lobes of both adults and larvae [85]. Furthermore, neurons present in the lateral body wall also show sensitivity to temperature within the range of 10–40 °C. Notably, most of these neurons are stimulated by increasing temperatures and their activity is suppressed by decreasing temperatures [82].
In adult Drosophila, both peripheral and internal neurons are present and can detect temperature changes. Peripheral neurons, specifically primary sensory neurons, are responsible for sensing increases (hot) or decreases (cold) in temperatures. Among the primary sensory neurons located in the antennae, three are sensitive to hot temperatures, and three are sensitive to cold temperatures. Additionally, at the base of each arista, three hot-sensing neurons express the gustatory receptor, GR28B(D) [68]. Furthermore, in the sacculus of the third antennal segment and at the base of the aristae, three cold-sensing neurons express brv1 [38] (Figure 1C). However, a more recent study indicated that the presence of brv1 may not be necessary for temperature sensitivity in aristal cool cells [86]. Instead, thermosensation in cool cells is mediated by IR21a, IR25a, and IR93a [86]. Collectively, these six neurons function together to perceive temperatures. There is an anti-inhibition effect between hot-sensing and cold-sensing neurons. Hot-sensing neurons are suppressed by cold stimuli, whereas cold-sensing neurons are suppressed by hot stimuli. Consequently, the removal of hot or cold stimuli can have opposing effects, resulting in the perception of cold or hot sensations [38,87]. These primary sensory neurons that detect temperature project to the proximal antennal protocerebrum (PAP), where they form synapses with downstream PNs to facilitate further processing. It is important to highlight that the targets of these primary sensory neurons differ within peripheral afferent pathways. This observation suggests that the processing of hot and cold information occurs independently [38] (Figure 1C). Besides peripheral neurons located on the surface, additional internal neurons can also detect temperature. Within the anterior region of the brain, AC neurons are sensitive to warmth and express dTrpA1. These dTRPA1-expressing neurons extend their projections to the PAP, similar to the peripheral primary sensory neurons mentioned previously [46].
A specific group of secondary neurons, known as thermosensory PNs, in the brain is responsible for receiving signals from both hot and cold thermoreceptors [88]. These PNs have dendrites located in the proximal antennal protocerebrum and their axons extend to higher brain regions. One type of PN, known as warm-PNs, is activated by an increase in temperature and suppressed by a decrease in temperature. Warm-PNs exhibit a stronger response to rapid increases in temperatures than to slow increases in temperatures and have a capacity for adaptation to sustained temperature increases. Two distinct types of PNs, fast-cool and slow-cool, have been reported in response to cooling and are inhibited by warming stimuli. Fast-cool PNs display a significant adaptation to sustained temperature decreases and demonstrate higher responsiveness to rapid cooling than to slow cooling. In contrast, slow-cool-PNs exhibit little adaptation to prolonged temperature decreases and respond similarly to both slow and fast cooling. Fast-cool PNs project to the lateral protocerebrum, whereas slow-cool PNs project to the MB. Furthermore, the two types of cool PNs encode different cool inputs. Fast-cool PNs receive inputs from cool cells in the arista, whereas slow-cool PNs primarily receive inputs from cool cells in the sacculus, with weaker inputs from cool cells in the arista. Additionally, two PNs, known as called warm-cool PNs, extend to the lateral protocerebrum. These neurons exhibit a rapid response to changes in temperature, particularly fast changes, whereas their response to sustained stimuli is strongly adaptive [88].
A specific subset of dopaminergic neurons (DANs) upstream of the MB in the brain exhibits responsiveness to cooling stimuli. The MB is a large brain structure comprising approximately 2000 MB neurons (MBn), known as Kenyon cells, in each brain hemisphere. MBn can be further classified into γ, αβ, and α′β′ MBn based on the distribution of their axons [89]. There are two clusters of DANs: the protocerebral anterior media (PAM), whose axons project to the MBn horizontal lobes, and the protocerebral posterior lateral 1 (PPL1), whose axons project to the MBn vertical lobes [90,91,92]. These DANs regulate behavior by modulating MB neuronal activity through four types of dopamine receptors: Dop1R1 (also known as dD1 or DUMB), Dop1R2 (also known as DAMB), Dop2R (also known as DD2R), and DopEcR (noncanonical DA/ecdysteroid receptor) [90,91,92]. There are 21 types of MB output neurons (MBONs) that elaborate on the segregated dendritic arbors along the parallel axons of Kenyon cells. The MBn lobes are subdivided into 15 distinct compartments containing the segregated dendrite arbors of MBONs [91]. PPL1 neurons display primary phasic responses to cooling, with a preference for the initial decrease in temperature [93]. While there is currently no direct evidence supporting the notion that these neurons receive inputs from the previously described thermal sensory neurons or thermal PNs, experiments involving the removal of antennae have demonstrated a reduction in the response of PPL1 neurons in the γ2α′1 region of the MB when temperature is decreased. Additionally, the removal of both antennae and maxillary palps has been found to diminish the responses of DANs in both the γ1pedc and γ2α′1 regions of the MB lobe. These findings suggest that cold-responsive DANs may receive inputs from thermal sensory neurons located in the antennae and that thermal sensors also exist in the maxillary palps [93] (Table 2).

Table 2.
Genes and circuits involved in thermosensation in Drosophila.
3. Temperature-Related Behavior in Drosophila
Drosophila is an ectotherm, and its preferred temperature (Tp) closely matches its selected environmental temperature [94]. Behavioral strategies for thermoregulation play a pivotal role in maintaining metabolism, energy storage, survival, temperature homeostasis, and responses to extreme environments [95,96]. Tp is not constant for adult flies or larvae. They can vary according to circadian rhythms, aging, and feeding status [56,66,94,97,98,99]. Furthermore, the acute avoidance of extreme temperatures is crucial for preventing or minimizing damage to flies. In this context, we will discuss temperature preference behavior under different physiological conditions and thermal nociception behavior.
3.1. Temperature Preference in Aged Drosophila
Aging is a universal biological phenomenon experienced by all living organisms. Several animal models have demonstrated a decline in physiological functions with age, including reproductive functions, muscle strength, cognitive impairment, and age-related diseases [100]. Several studies have used animal models such as rodents, Drosophila, and Caenorhabditis elegans to characterize physiological changes during aging [101]. In rats, age-dependent changes in thermoregulation have been linked to a decrease in the number of dopaminergic neurons. Specifically, the brain’s levels of the key enzyme tyrosine hydroxylase (TH), which plays a crucial role in dopamine synthesis, significantly decreases in rats during aging. This decline in TH levels leads to a decreased resistance to cold stress [102,103].
The concept of a temperature gradient device was developed in 1922 [84]. Researchers have modified the temperature gradient devices according to their experiments or to overcome mechanical obstacles. Sokabe et al. developed a continuous linear gradient by placing black aluminum plates on aluminum blocks, and each plate was set to a distinct temperature using circulating water from a bath [104]. The test plate was divided into 2 cm wide zones ranging from 18 to 28 °C with a gradient of 2 °C. During the experiment, 10–20 min after releasing approximately 150 larvae flies between the 22 and 24 °C zone, the distribution of larvae was calculated as follows: (number of larvae in a given 2 cm zone)/(total number of larvae in six zones) × 100%. However, the late third-instar larvae began to climb on the surface to escape from the food and prepare for pupation. To avoid the juxtaposition of this zone with the edge of the plate and test whether the selection of the 18 °C zone was strong, the authors created a temperature gradient in which the 18 °C zone was in the center and the warmer zones radiated out symmetrically on both sides [104]. Using the temperature gradient assays mentioned above, Sokabe et al. reported that the second-instar and early third-instar larvae (48 and 72 h after egg laying) prefer 24 °C, but this preference shifts to stronger biases for 18–19 °C in mid- and late-third-instar larvae (96 and 120 h after egg laying) [104]. The authors also showed that the rodopsin1 (rh1) null mutant (ninaEI17) larvae were impaired in the 18 °C selection at the mid-third-instar stage, but not in the late-third-instar stage [104]. This is consistent with a study by Shen et al. which showed that third-instar larvae prefer to stay at 18 °C through the thermosensory signaling pathway, which is light-dependent and depends on the guanine nucleotide-binding protein (G protein, Gq), phospholipase C (PLC), and the transient receptor potential TRPA1 channel [67]. Through behavioral assays of rhodopsin (rh) gene mutant screening, Sokabe et al. showed that both rh5 and rh6 are required for Tp in late-third-instar larvae [104]. Furthermore, rh5 and rh6 expression in dTrpA1-positive neurons in the brain and periphery induces the Gq/PLC/TRPA1 signaling cascade for thermotaxis in a light-independent manner in late-third-instar larvae [104]. However, whether rhodopsin acts as a direct thermosensor in Drosophila remains unclear.
Tyrrell et al. identified additional molecular mechanisms underlying the Tp [105]. Unlike the devices mentioned above, the authors utilized another larval temperature gradient assay for their study. In this assay, the authors placed an aluminum plate on the top of an ice bath on the right side and a hot plate on the left side set to approximately 70 °C. An agar gel was placed in the middle of the aluminum plate. This setup generated a continuous linear gradient from 13 to 31 °C, divided into 1 cm wide zones with 1 °C intervals. During the experiment, 10–15 min after releasing 20–35 larvae in the 22 °C zone, the larval distribution was calculated as follows: (number of larvae in the temperature zone)/(total number of larvae) × 100% [105]. Using a larval temperature gradient assay, the authors found that larvae sought a lower temperature during the late-third-instar stage, which is consistent with the study by Sokabe et al. Next, by silencing the dorsal organ cool cells (DOCCs) using a synaptic neurotransmitter blocker tetanus toxin light chain (TNT), the authors found that DOCCs are critical for cold avoidance in the early third-instar, but not in the late-third-instar stage [105]. Calcium imaging data further indicated that DOCCs exhibit reduced cooling responses in the late-third-instar stage [105]. These results suggest that the cool responses of DOCCs are significantly reduced during the late-third-instar stage, which enabled the larvae to remain at a lower temperature. Finally, through immunostaining and behavioral assays, the authors found that in early third-instar larvae, IR21a, IR25a, and IR93a are highly expressed in DOCCs, which led to an increase in cold avoidance [105]. In contrast, in late-third-instar larvae, IR21a, IR25a, and IR93a showed lower expressions in DOCCs and consequently, a reduced cold avoidance [105].
In contrast to larvae, few studies have addressed the age-dependent changes in adult flies. A study by Shih et al. revealed that adult fruit flies prefer to stay at lower temperatures and the MB is involved in regulating age-dependent temperature preference changes [66]. In this study, a temperature gradient device was used for the behavioral assay (Figure 2D–F). Shih et al. identified a microcircuit, the MB β and β′ system, that regulates Tp as flies age [66] (Figure 3A). First, using a behavioral assay, the authors found that flies preferred lower temperatures during aging. They also found that TH-immunopositive signals in the tip of the β′ lobe were strongest among all MB regions in young flies (7 days after eclosion) but significantly decreased in aged flies (21 days after eclosion) [66]. Live brain calcium imaging also demonstrated that the β′ lobe and β lobe strongly responded to cold stimuli [66]. Next, through silencing the neural activity, the authors found that the β′ system, including PAM-β′2, α′β′ MBn, and MBON-β′2mp, and the β system, including PAM-β1/β2, αβ MBn, and MBON-β2β′2a, are all involved in cold avoidance in young flies. In aged flies, PAM-β′2, α′β′ MBn, and MBON-β′2mp exhibited a decreased response to cold stimuli and the activity of these neurons is not required for cold avoidance [66]. These results suggest that both β and β′ systems are required in young flies to establish normal cold avoidance and set the Tp. However, in aged flies, TH expression is decreased in PAM-β′2 which leads to a significant reduction in the dopaminergic modulation in the β′ system. Therefore, the β system takes over the regulation to play a major role in cold avoidance in aged flies [66]. Several studies have suggested that when flies are exposed to low-temperature environments, their metabolic rates decrease, potentially increasing their longevity [106,107]. This may explain why older flies prefer lower temperatures.
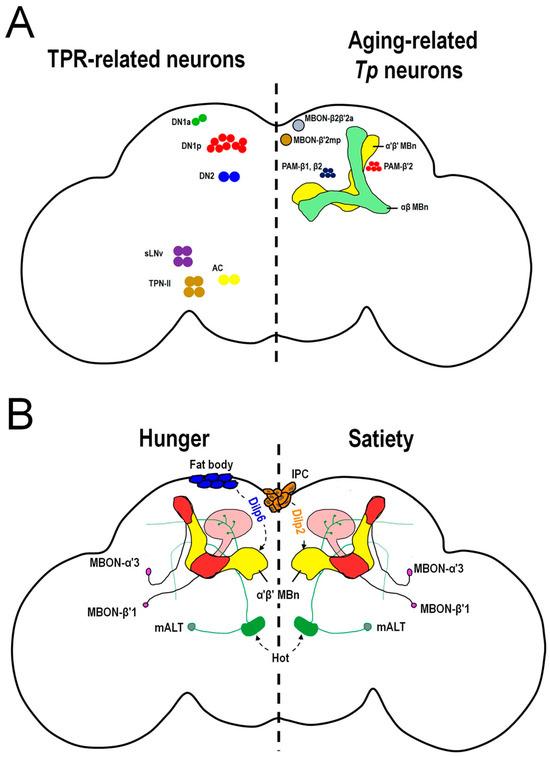
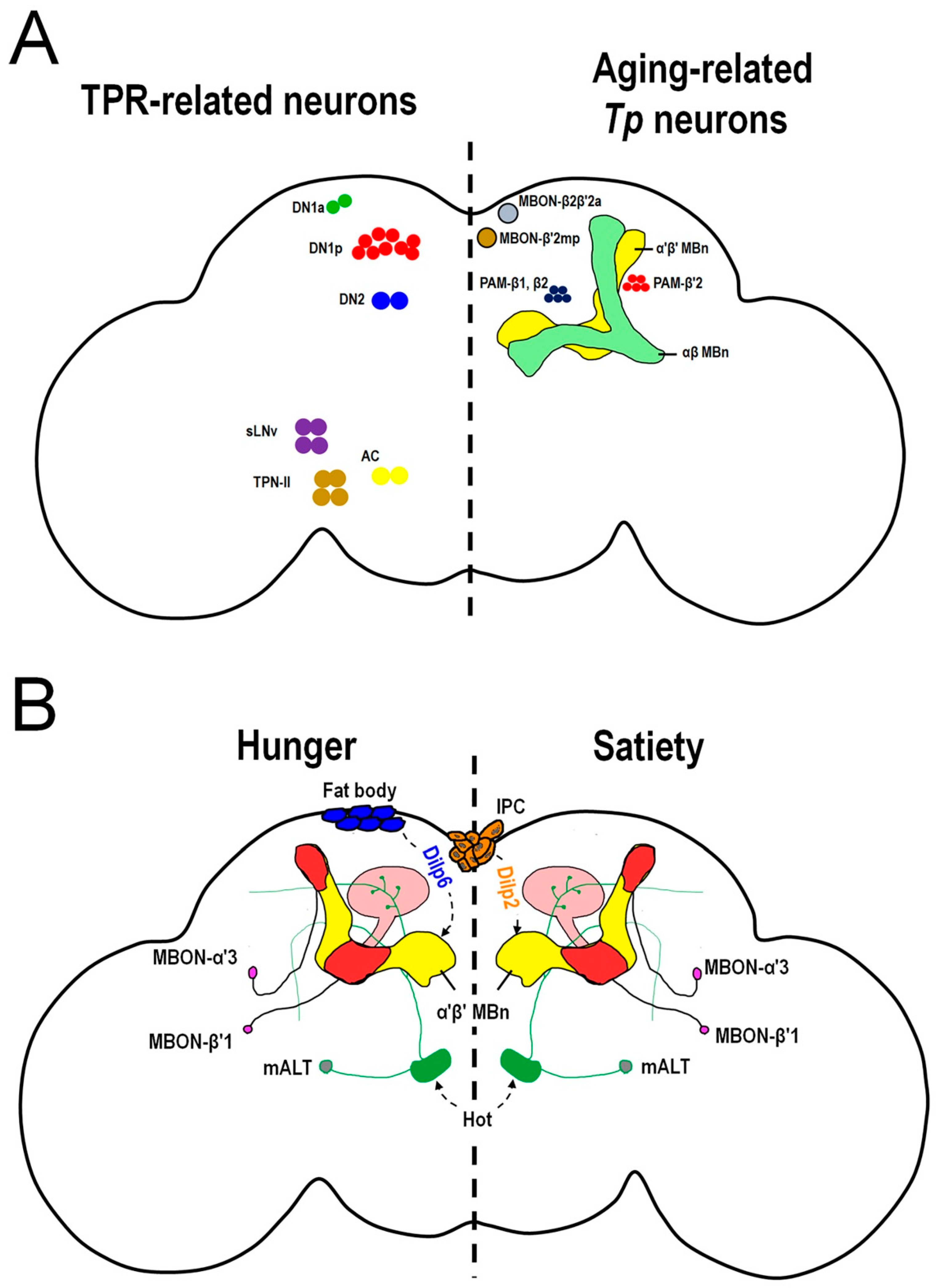
Figure 3.
Neurons involved in temperature-related behaviors in Drosophila. (A) The TPR-related neurons (left panel) and aging-related Tp neurons (right panel) in the fly brain regulate Tp. In the left panel, there are four sets of brain microcircuits that are involved in TPR within different time zones. During daytime, two microcircuits regulate TPR, one is DN2 (blue color) > DN1p (red color) and the other is TPN-II (brown color) > DN1a (green color). During the night, DN2s (blue color) receive the DH31 ligand via PDFR to regulate TPR. In predawn, heat stimulates AC (yellow color) > sLNv (purple color) > DN2 (blue color) to regulate TPR. In the right panel, there are two brain circuits regulating aging-related Tp. In young flies, both the β′ system including the PAM-β′2 (red color) > α′β′ MBn (yellow color) > MBON-β′2mp (brown color) and β system including the PAM-β1, β2 (blue color) > αβ MBn (green color) >MBON-β2β′2a (gray color), contribute to normal Tp. However, in aged flies, the dopamine levels in PAM-β′2 are decreased which reduces the cold responses in α′β′ MBn and MBON-β′2mp. Consequently, aged flies prefer to stay at lower temperatures. The β system still functions in aged flies and is responsible for partial cold-avoidance behaviors. (B) The hot stimulus is conveyed through cholinergic mALT neurons (fiber: green color, soma: gray color) to α′β′ MBn dendrites and the calyx (pink color region). The α′β′ MBn (yellow color) activity is positively correlated with hot-avoidance behavior (HAB). In the food-sated state, Dilp2 is secreted from IPCs (orange color) to inhibit the hot response of α′β′ MBn by inducing the PI3K/AKT signaling pathway which causes a weak HAB. Conversely, Dilp6 is released from the fat body (blue color) to inhibit the hot response of α′β′ MBn by inducing the Ras/ERK signaling pathway. The inhibition efficiency of Ras/ERK signaling in the hungry state is not as strong as that of PI3K/AKT signaling; consequently, flies exhibit strong hot responses in α′β′ MBn during the hungry state which contributes to a strong HAB. The MBON-α′3 and MBON-β′1 (red color) play the roles of transmitting the integrated information from α′β′ MBn for proper HAB during different feeding states. TPR: temperature preference rhythm. DN1a: anterior dorsal neurons. DN1p: posterior dorsal neurons. DN2: dorsal neurons. sLNv: small ventrolateral neurons. TPN-II: second-order thermosensory projection neurons. AC: anterior cell. PAM: protocerebral anterior media. MBON: mushroom body output neuron. IPC: insulin-producing cell. Dilp: Drosophila insulin-like peptide.
3.2. Temperature Preference Rhythm
Body temperature rhythm (BTR) is a phenomenon in which the body temperature fluctuates over 24 h. BTR is highly relevant in maintaining homeostasis by regulating metabolic energy and sleep [108,109,110] (Figure 3A). At least 300 studies have addressed BTR in various species [110]. In humans, the core body temperature (CBT) and distal skin temperature (DST) serve as marker rhythms [111,112,113,114]. Both are subjected to numerous environmental and physiological influences that mask their rhythms, including physical activity, body position, light exposure, environmental temperature, and sleep [115,116,117,118,119,120,121,122,123]. CBT is approximately 37 °C in healthy humans, with nearly 1 °C sinusoidal circadian fluctuations. In mammals, the body temperature is controlled by a biological clock. The suprachiasmatic nucleus (SCN) in the brain controls all biological rhythms via the BMAL1:CLOCK heterodimer loop, which regulates clock-controlled genes [124,125,126,127,128].
Both mammals and ectotherms generate BTR through behavioral strategies to regulate body temperature [110,129]. Drosophila exhibits a robust temperature preference rhythm (TPR) through which flies select a suitable environmental temperature to set their body temperature [130,131]. The TPR increases during the day, peaks in the evening, and decreases at night [130,131], suggesting that Drosophila TPR is similar to the BTR [130,131]. Drosophila TRP is divided into four time zones in a day: daytime zeitgeber time (ZT1-ZT12), night-onset (ZT9-ZT12, ZT13-ZT15), dark time (ZT12-ZT24), and predawn (ZT22-ZT24) [131].
In molecular terms, two basic helix-loop-helix (bHLH) regulator proteins, the clock (Clk) and cycle (cyc), act as rhythm-driving factors by directly activating the transcription of many genes, including period (per) and timeless (tim) [132]. PER and TIM contribute to a negative feedback loop in the molecular cycle, thereby regulating circadian rhythms. per and tim mRNA levels peak in the evening, while protein levels reach their highest levels around the daybreak. PER and TIM undergo several post-translational modifications that critically influence their stability and ability to accumulate and affect their function. Over individual time courses, PER and TIM enter the nucleus and terminate the daily actions of CLK and CYC during transcriptional activation. The subsequent degradation of TIM and PER allows for the resumption of CLK-CYC-mediated transcriptional activation, starting another daily cycle [132,133,134].
At the neuronal circuit level, clock neurons in the Drosophila brain are divided into two groups: the lateral neurons (LN) and the dorsal neurons (DNs) [135]. Traditionally, LNs are considered key for controlling daily patterns of rest and activity [132,135,136,137]. LN can be further divided into three groups: large ventrolateral neurons (lLNv), small ventrolateral neurons (sLNv), and dorsolateral neurons (LNd) [135]. In contrast, the DN is thought to play subtle roles in modulating circadian rhythmicity [132,138]. Similar to LN, DN can be divided into three recognized groups: DN1, DN2, and DN3 [132]. These two groups of clock neurons control physiological functions and behavior; however, the interaction between these groups remains largely unknown. Additionally, a class II G-protein-coupled receptor, the pigment-dispersing factor receptor (PDFR), and its ligand (PDF) are critical for synchronizing circadian clocks and are required for robust circadian locomotor activity in Drosophila [139,140].
Kaneko et al. showed that the circadian clock genes per and tim are involved in regulating Drosophila TPR [130]. Daytime TPR is particularly prominent and exhibits robust and reproducible features controlled by clock genes. Furthermore, PER expression in DN2 neurons is necessary for daily TPR, and the PER protein is sufficient to regulate daytime TPR and does not affect locomotor activity [130]. Subsequently, Chen et al. identified two microcircuits involved in the regulation of the daytime TPR. One contains DN2 and posterior DN1 (DN1p), while the other contains anterior DN1 (DN1a). Chen et al. found that silencing DN2 neuronal activity or disrupting the clock gene tim in DN2 affects daytime TPR [141]. Furthermore, DN1p regulates daytime TPR independently of circadian genes. This suggests that DN1p is essential for increasing Tp but does not require a functional clock for TPR [141]. Live calcium brain imaging and GFP Reconstitution Across Synaptic Partners (GRASP) experiments have shown that DN2 activates DN1p to elevate daytime Tp and set the temperature setpoint during the daytime [141]. Additionally, DN1a is part of a DN2-independent circuit for daytime TPR, potentially integrating environmental signals such as temperature, light, and sleep information [141]. In a recent study by Alpert et al., the authors proposed that a subset of second-order thermosensory PNs, the TPN-II neurons, which are cold-processing neurons, projects to DN1a [142]. TPN-II could be upstream of DN1a; however, the exact role of TPN-II in TPR remains unclear [131].
Drosophila Tp is highest in the evening (ZT9-12) and decreases dramatically during the transition from daytime to night (ZT13-15) [130]. Goda et al. identified a microcircuit that controls night-onset TPR [143]. PDFR is a neuropeptide receptor that synchronizes with the circadian clock and is involved in TRP. Goda et al. found that the Tp of pdfr mutant flies increased during the daytime but decreased only by 0.8 °C at night-onset while wildtype flies exhibited a robust Tp decrease of 1.5–2 °C [143]. Furthermore, by rescuing pdfr in DN2 in a pdfr mutant background, the authors found that PDFR expression in DN2 neurons is sufficient for night-onset TPR [143]. Subsequently, it has been shown that PDF and diuretic hormone 31 (DH31) can bind to PDFR [144]. The rescue experiment with membrane-tethered DH31 (t-DH31) in DN2 under the DH31 mutant background demonstrates that DH31, but not PDF, regulates night-onset TPR [143]. Taken together, these studies confirmed that DH31–PDFR signaling in DN2 specifically regulates the decrease in Tp at night.
In the predawn phase (ZT22-ZT24), Drosophila Tp matches that of the early daytime (ZT1-ZT3) [130]. Tang et al. revealed that a microcircuit, AC-sLNv-DN2, is responsible for predawn TPR regulation [145]. First, by silencing neural activity and disrupting the clock gene in LNv, a PDF-positive neuron, the authors showed that LNv activity is necessary for the predawn TPR and Tp [145]. GRASP and live calcium brain imaging data suggest that sLNv is upstream of DN2 neurons and there are more synaptic contacts between sLNv and DN2, specifically before dawn (ZT22-24) [145]. Additionally, the neuronal functions of AC neurons as warmth sensors control the TPB and transmit temperature signals to sLNv, which activates sLNv-DN2 and sets the Tp before dawn [145]. In summary, these studies in Drosophila have uncovered the fundamental regulatory mechanisms governing TPR.
3.3. Feeding State-Dependent Temperature Preference
Starvation induces significant physiological changes in mammals [146,147,148,149,150]. In humans, starvation leads to a reduction in body mass, glucose levels, lipid content, and temperature [150,151,152,153]. It has been shown that hungry rats exhibit lower body mass, Tb, and blood sugar levels compared to sated rats [154,155,156,157]. In mice, hunger induces changes in the total cytochrome oxidase activity, GDP binding, and UCP1 protein levels, resulting in a lower Tb [158,159]. In Drosophila, there are significant Tp differences between sated and hungry flies. Umezaki et al. showed that flies prefer to stay in a lower-temperature environment when starved (~23 °C) compared to sated flies (~25 °C) [97].
The most well-known mechanism for transmitting satiety/hunger signals in adult flies is the Drosophila insulin-like peptide (Dilp). There are eight types of Dilps and one type of insulin receptor (InR) [160]. Previous studies have shown that Dilps are released from different organs in the larval or adult stages and modulate development, growth, reproduction, metabolism, stress resistance, lifespan, and cognitive functions [160]. In the adult fly brain, 14 insulin-producing cells (IPCs) are located in the pars intercerebralis (PI) and express Dilp2, Dilp3, and Dilp5 [160,161,162]. Moreover, Dilp6 is released from the fat body and suppresses Dilp2 secretion via IPCs [163]. Dilp2 and Dilp6 play key roles in delivering satiety/hunger signals in adult fly brains [163,164,165]. The Dilps–dInR interaction activates multiple signaling pathways, including the PI3K/AKT and Ras/ERK pathways, collectively known as the insulin/insulin-like growth factor signaling (IIS) pathway [166,167,168]. In the PI3K/AKT pathway, signal transduction is initiated when DILPs bind to insulin receptors (InR), triggering receptor activation through autophosphorylation. This leads to the further downstream phosphorylation of CHICO, a major substrate of InR in Drosophila. CHICO then binds to PI3K and induces its activation via phosphorylation at the membrane. PI3K activity is suppressed by phosphatase and tensin homolog (PTEN), which hydrolyzes PIP3 to PIP2 [166,167,168]. The accumulation of PIP3 leads to the phosphorylation and activation of protein kinases, such as Drosophila phosphoinositide-dependent kinase-1(dPDK1) and AKT, at the plasma membrane. AKT phosphorylates several metabolism-regulating proteins, including Drosophila p70 ribosomal S6 kinase (dS6K), forkhead transcription factor (FOXO), and tuberous sclerosis complex (TSC) [166,168]. In the Ras/ERK signaling pathway, DILPs bind to InR, leading to the activation of InR through autophosphorylation, which further phosphorylates downstream targets, such as Shc, initiating the MEK1/2-MAPK cascade. This cascade is typically initiated by Ras activation, which further transmits the signal by recruiting and activating MAP3K-tier Raf at the plasma membrane through phosphorylation. Raf then phosphorylates Dsor1, which, in turn, phosphorylates ERK/rolled serine/threonine kinase (MAPK). ERK is an enzyme with multiple substrates, including transcription factors and regulators of the cytoskeleton and development [169,170,171].
Two neuronal circuits modulate Tp in Drosophila under different feeding conditions. One is AC-based and the other is an MB-based neuronal circuit [65,97]. Umezaki et al. found that flies prefer low temperatures during the hunger state [97]. Using Dilps null mutants and RNAi-mediated silencing of dilp6 in the fat body, the authors showed that Dilp6 released from the fat body is critical for starvation-induced reduction in Tp. It has been shown that TRPA1 in AC neurons is sufficient for controlling TPB [46]. Umezaki et al. further demonstrated that AC neurons are key regulators of starvation-induced reduction in Tp. Through behavioral experiments using RNAi or dominant-negative mutants of InR, chico, PI3K, and PTEN, Umezaki et al. showed that the InR signaling pathway in AC neurons plays a role in starvation-induced reduction in Tp [97]. Taken together, these results suggest that insulin signaling is an important mediator of starvation-induced thermoregulation via Dilp6-dInR signaling in AC neurons [97].
Chiang et al. recently demonstrated the relationship between satiety/hunger and temperature signals within MB-based neuronal circuits [65] (Figure 3B). The authors found that the feeding state significantly affects hot-avoidance behavior (HAB) in Drosophila. Behavioral assays revealed that hungry flies exhibit a strong HAB but not cold-avoidance behavior compared to sated flies. The authors also showed that MBn activity is critical for HAB, and α′β′ MBn is required for HAB in both sated and hungry states, while αβ and γ MBn are required for HAB only during the sated state [65]. Furthermore, the authors showed that Dilp2 is secreted from IPCs, which induces the PI3K/AKT signaling in α′β′ MBn that strongly suppresses the heat response in sated flies. Conversely, during the hungry state, Dilp2 secretion from IPCs is significantly reduced. Instead, Dilp6 is released from the fat body, which triggers the Ras/ERK signaling pathway in α′β′ MBn that shows a weak suppression of heat response in the hungry state. These results indicate that distinct insulin modulations regulate Drosophila HAB under different feeding conditions. Moreover, the modulated hot signals within the α′β′ MBn are further transmitted through MBON-α′3 and MBON-β′1 to execute proper HAB in both feeding states. These behavioral changes in HAB are crucial for the survival of flies. One hypothesis is that, under starvation conditions, flies conserve energy by decreasing their body temperature to reduce the rate of metabolism and become more sensitive to hot stimuli. Therefore, HAB should be increased to avoid hot environments in the hungry state. In contrast, flies become less sensitive to heat and exhibit reduced HAB, which allows them to approach higher temperatures, thereby increasing their metabolic rates after feeding [95,96].
3.4. Thermal Nociception Behavior
Nociception refers to the manner through which organisms react to painful or noxious stimuli that possess the capacity to inflict harm on tissues. This serves as a protective mechanism allowing animals to perceive and evade potentially detrimental stimuli in their environment. When animals encounter noxious heat that causes pain, it is known as thermal nociception. Mice exhibit licking or jumping behaviors in response to noxious heat stimuli [172]. Zebrafish larvae also exhibit increased locomotor activity when exposed to such stimuli [173,174,175]. C. elegans reacts with a withdrawal reflex in response to noxious temperatures [176]. Similarly, Drosophila larvae exhibit stereotyped escape behavior (rolling response), and adult Drosophila displays a rapid jump response when subjected to noxious heat stimuli [53,177,178].
Researchers have used various techniques to measure the responses of Drosophila larvae and adults to toxic heat. There are two primary approaches for testing how larvae react to noxious heat. An example of a well-known experiment involves placing a group of fly larvae in a Petri dish and touching them with a heated soldering iron at 46 °C. Normal larvae respond immediately by rolling [49,179]. Alternatively, larvae can be placed in a Petri dish with agar to create a film of water that allows them to move freely. The dish is then placed on a hot plate, and the rolling response of larvae is recorded at different temperatures [180]. To replicate jumping reflexes, researchers devised a method in which flies are suspended from an electric hot plate using a nylon rope. The flies are then dropped onto the plate and their jumping times are closely observed and recorded [53]. In another assay, to create the desired conditions, adult flies are placed in a specially designed incubator with bottom heating. The incubator maintains a floor temperature of 46 °C. This higher temperature discourages wild-type flies from coming into direct contact with this surface and encourages them to stay in the upper region where the temperature is around 31 °C [181]. The third method involves the integration of heat avoidance and light-seeking behavior of adult flies. This technique uses a vertical transparent apparatus equipped with a heatable metal ring and a lamp positioned at the center and the top. Flies are introduced into this apparatus. Mutant flies with diminished nociceptive behavior are attracted toward the light source and pass through the heated aluminum ring, whereas wild-type flies with intact receptors do not exhibit this behavior [182,183]. Flies respond faster to higher-intensity thermal stimuli because the delay in the jump response is inversely correlated with the thermal stimulus intensity [53].
Several pain-related genes are associated with thermal nociception. TRP channels, the largest family of noxious channels involved in pain, are best known for their functions in nociceptive perception [17]. The heat-activated channel dTRPA1 was first discovered in flies [59]. It is triggered in response to elevated temperatures, chemically reactive substances, and downstream intracellular signaling pathways [45,184,185,186]. dTRPA1 is involved in both larval and adult thermal pain [186,187]. At 46 °C, adult control flies respond relatively quickly to dangerous heat, whereas dTrpA1 mutant flies respond more slowly and are much less able to feel heat [186]. When the temperature is below 40 °C, fly larvae begin to roll around noxiously, and the frequency of this behavior increases sharply until the temperature reaches 33 °C [187]. The dTRPA1 channel, whose activity changes in response to the rate of temperature change, is essential for all thermal nociceptive actions mentioned above.
The painless gene, which belongs to the TRPA channel family, plays a significant role in temperature nociception in Drosophila [49,53]. In larval and adult flies, painless is a molecular sensor for thermal stimulation [188]. Studies have shown that the painless mutant lacks the normal rolling behavior exhibited by wild-type larvae within one second of contact with the heated probe above 40 °C [49]. painless is necessary for thermal nociception in adult flies because painless mutant adults display behavioral defects in the hot plate assay and are unable to jump quickly to escape the noxious heat. This behavioral defect can be rescued by ectopically expressing painless [53]. The multidendritic sensory neurons fire more frequently when the temperature is higher than 38 °C, whereas the painless mutants do not show this trend [53,189]. In addition, painless plays a role in several neural processes in flies such as chemical and mechanical nociception [49,190], larval social behavior [191], and sexual receptivity in virgin females [51].
According to recent studies, Epi neurons respond directly to noxious heat and are necessary for reducing the response to thermal nociception. Epi neurons in the brain exhibit bilateral symmetry. These neurons extend their dendrites to innervate various regions such as the OL, LH, and areas surrounding the MBn. Additionally, the axons of Epi neurons project to segments of the ventral nerve cord (VNC), including the prothoracic, metathoracic, mesothoracic, and abdominal ganglia [192]. The percentage of jumps and the average jump latency is significantly increased and decreased, respectively, by inhibiting Epi neurons. The TRP channel pain is essential for Epi neurons to detect harmful heat levels, and mutations in TRP channels lead to an increase in thermal nociception [192]. The neuropeptide allatostatin C (AstC), released by Epi neurons, is necessary for the inhibition of thermal nociception [192,193]. AstC is similar to the mammalian neuropeptide somatostatin, which suppresses pain induced by heat [193,194]. Reducing AstC expression in Epi neurons significantly increases thermal nociception [192]. Drosophila has two AstC receptors: AstC-R1 and AstC-R2 [195,196]. Only AstC-R1 mutant flies exhibit increased thermal nociception [192] (Table 3).

Table 3.
Neuronal mechanisms in temperature related behaviors in Drosophila.
4. Conclusions
Drosophila is an important model system for studying genetics, physiology, neurobiology, and evolutionary biology [197]. The short life span, ease of maintenance in the laboratory, and the availability of powerful genetic tools make it possible for scientists to uncover the underlying mechanisms of animal behavior, including thermosensation and thermoregulation. In this review, we comprehensively discussed how flies sense temperature, how circadian rhythms affect their temperature preferences, and how flies respond to temperatures in different internal states. We further summarized the molecules involved in Drosophila hot- and cold-temperature sensations (Table 1). Furthermore, we provided an overview of the primary thermosensory neurons in the peripheral nervous system that express temperature-sensitive proteins and convey temperature signals to secondary neurons (Table 2). Finally, we briefly summarized the genes and neuronal circuits involved in thermoregulation under various conditions (Table 3). Revealing the brain circuits and molecules involved in temperature-related behaviors in Drosophila will help in further understanding the principles of thermosensation, and pave the way for understanding the underlying mechanisms of temperature sensation in other animal species.
Author Contributions
Conceptualization, M.-H.C., Y.-C.L. and C.-L.W.; writing—original draft preparation, M.-H.C., Y.-C.L. and C.-L.W.; review and editing, T.W. and C.-L.W.; funding acquisition, C.-L.W.; supervision, C.-L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the National Science and Technology Council, grant number 112-2311-B-182-002-MY3 and Chang Gung Memorial Hospital, grant numbers CMRPD1M0301-3, CMRPD1M0761-3, and BMRPC75 to C.-L.W.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the editor and the reviewers for their editorial assistance and valuable comments. We thank the members of C.-L.W’s laboratory for their critical comments on this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lim, C.L. Fundamental Concepts of Human Thermoregulation and Adaptation to Heat: A Review in the Context of Global Warming. Int. J. Environ. Res. Public. Health 2020, 17, 7795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Molecular sensors and modulators of thermoreception. Channels 2015, 9, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Cramer, M.N.; Gagnon, D.; Laitano, O.; Crandall, C.G. Human temperature regulation under heat stress in health, disease, and injury. Physiol. Rev. 2022, 102, 1907–1989. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.L.; Knight, Z.A. Regulation of Body Temperature by the Nervous System. Neuron 2018, 98, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Madden, C.J.; Morrison, S.F. Central nervous system circuits that control body temperature. Neurosci. Lett. 2019, 696, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.F. The Hot-Blooded Insects—Strategies and Mechanisms of Thermoregulation—Heinrich, B. Science 1993, 260, 1155–1156. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, B.; Garrity, P.A. Temperature sensation in Drosophila. Curr. Opin. Neurobiol. 2015, 34, 8–13. [Google Scholar] [CrossRef]
- Garrity, P.A.; Goodman, M.B.; Samuel, A.D.; Sengupta, P. Running hot and cold: Behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes. Dev. 2010, 24, 2365–2382. [Google Scholar] [CrossRef]
- Huey, R.B.; Hertz, P.E.; Sinervo, B. Behavioral drive versus behavioral inertia in evolution: A null model approach. Am. Nat. 2003, 161, 357–366. [Google Scholar] [CrossRef]
- Stevenson, R.D. The Relative Importance of Behavioral and Physiological Adjustments Controlling Body Temperature in Terrestrial Ectotherms. Am. Nat. 1985, 126, 362–386. [Google Scholar] [CrossRef]
- Clapham, D.E.; Runnels, L.W.; Strubing, C. The TRP ion channel family. Nat. Rev. Neurosci. 2001, 2, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Voets, T.; Peters, J. TRP channels in disease. Sci. STKE 2005, 2005, re8. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.J.; Sweet, T.B.; Clapham, D.E. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 2010, 62, 381–404. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Appendino, G.; Owsianik, G. The transient receptor potential channel TRPA1: From gene to pathophysiology. Pflug. Arch. 2012, 464, 425–458. [Google Scholar] [CrossRef] [PubMed]
- Li, H. TRP Channel Classification. In Transient Receptor Potential Canonical Channels and Brain Diseases; Springer: Dordrecht, The Netherlands, 2017; Volume 976, pp. 1–8. [Google Scholar] [CrossRef]
- Bamps, D.; Vriens, J.; de Hoon, J.; Voets, T. TRP Channel Cooperation for Nociception: Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 655–677. [Google Scholar] [CrossRef]
- He, J.; Li, B.; Han, S.; Zhang, Y.; Liu, K.; Yi, S.; Liu, Y.; Xiu, M. Drosophila as a Model to Study the Mechanism of Nociception. Front. Physiol. 2022, 13, 854124. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient receptor potential cation channels in disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef]
- Gees, M.; Owsianik, G.; Nilius, B.; Voets, T. TRP channels. Compr. Physiol. 2012, 2, 563–608. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Guler, A.D.; Lee, H.; Iida, T.; Shimizu, I.; Tominaga, M.; Caterina, M. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 2002, 22, 6408–6414. [Google Scholar] [CrossRef] [PubMed]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP channel that senses cold stimuli and menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Reeve, A.J.; Andersson, D.A.; Moqrich, A.; Earley, T.J.; Hergarden, A.C.; Story, G.M.; Colley, S.; Hogenesch, J.B.; McIntyre, P.; et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef] [PubMed]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Smith, G.D.; Gunthorpe, M.J.; Kelsell, R.E.; Hayes, P.D.; Reilly, P.; Facer, P.; Wright, J.E.; Jerman, J.C.; Walhin, J.P.; Ooi, L.; et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002, 418, 186–190. [Google Scholar] [CrossRef]
- Xu, H.; Ramsey, I.S.; Kotecha, S.A.; Moran, M.M.; Chong, J.A.; Lawson, D.; Ge, P.; Lilly, J.; Silos-Santiago, I.; Xie, Y.; et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002, 418, 181–186. [Google Scholar] [CrossRef]
- Jordt, S.E.; McKemy, D.D.; Julius, D. Lessons from peppers and peppermint: The molecular logic of thermosensation. Curr. Opin. Neurobiol. 2003, 13, 487–492. [Google Scholar] [CrossRef]
- Lee, H.; Iida, T.; Mizuno, A.; Suzuki, M.; Caterina, M.J. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J. Neurosci. 2005, 25, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.J.; Murray, A.N.; Spencer, K.S.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307, 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Colburn, R.W.; Lubin, M.L.; Stone, D.J., Jr.; Wang, Y.; Lawrence, D.; D’Andrea, M.R.; Brandt, M.R.; Liu, Y.; Flores, C.M.; Qin, N. Attenuated cold sensitivity in TRPM8 null mice. Neuron 2007, 54, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Murray, A.N.; Mathur, J.; Earley, T.J.; Petrus, M.J.; Patapoutian, A. TRPM8 is required for cold sensation in mice. Neuron 2007, 54, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Kwan, K.Y.; Glazer, J.M.; Corey, D.P.; Rice, F.L.; Stucky, C.L. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J. Neurosci. 2009, 29, 4808–4819. [Google Scholar] [CrossRef] [PubMed]
- Gallio, M.; Ofstad, T.A.; Macpherson, L.J.; Wang, J.W.; Zuker, C.S. The coding of temperature in the Drosophila brain. Cell 2011, 144, 614–624. [Google Scholar] [CrossRef]
- Vriens, J.; Owsianik, G.; Hofmann, T.; Philipp, S.E.; Stab, J.; Chen, X.; Benoit, M.; Xue, F.; Janssens, A.; Kerselaers, S.; et al. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron 2011, 70, 482–494. [Google Scholar] [CrossRef]
- Song, K.; Wang, H.; Kamm, G.B.; Pohle, J.; Reis, F.C.; Heppenstall, P.; Wende, H.; Siemens, J. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 2016, 353, 1393–1398. [Google Scholar] [CrossRef]
- Tan, C.H.; McNaughton, P.A. The TRPM2 ion channel is required for sensitivity to warmth. Nature 2016, 536, 460–463. [Google Scholar] [CrossRef]
- Cosens, D.J.; Manning, A. Abnormal electroretinogram from a Drosophila mutant. Nature 1969, 224, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Minke, B.; Wu, C.; Pak, W.L. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature 1975, 258, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.A.; Montell, C. Drosophila TRP channels and animal behavior. Life Sci. 2013, 92, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Bellemer, A. Thermotaxis, circadian rhythms, and TRP channels in Drosophila. Temperature 2015, 2, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Hamada, F.N.; Rosenzweig, M.; Kang, K.; Pulver, S.R.; Ghezzi, A.; Jegla, T.J.; Garrity, P.A. An internal thermal sensor controlling temperature preference in Drosophila. Nature 2008, 454, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Pulver, S.R.; Panzano, V.C.; Chang, E.C.; Griffith, L.C.; Theobald, D.L.; Garrity, P.A. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 2010, 464, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, Y.; Akitake, B.; Woodward, O.M.; Guggino, W.B.; Montell, C. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc. Natl. Acad. Sci. USA 2010, 107, 8440–8445. [Google Scholar] [CrossRef] [PubMed]
- Tracey, W.D., Jr.; Wilson, R.I.; Laurent, G.; Benzer, S. Painless, a Drosophila gene essential for nociception. Cell 2003, 113, 261–273. [Google Scholar] [CrossRef]
- Sakai, T.; Watanabe, K.; Ohashi, H.; Sato, S.; Inami, S.; Shimada, N.; Kitamoto, T. Insulin-producing cells regulate the sexual receptivity through the painless TRP channel in Drosophila virgin females. PLoS ONE 2014, 9, e88175. [Google Scholar] [CrossRef]
- Sakai, T.; Sato, S.; Ishimoto, H.; Kitamoto, T. Significance of the centrally expressed TRP channel painless in Drosophila courtship memory. Learn. Mem. 2012, 20, 34–40. [Google Scholar] [CrossRef]
- Wang, K.; Guo, Y.; Wang, F.; Wang, Z. Drosophila TRPA channel painless inhibits male-male courtship behavior through modulating olfactory sensation. PLoS ONE 2011, 6, e25890. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Cang, C.L.; Liu, X.F.; Peng, Y.Q.; Ye, Y.Z.; Zhao, Z.Q.; Guo, A.K. Thermal nociception in adult Drosophila: Behavioral characterization and the role of the painless gene. Genes Brain Behav. 2006, 5, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, Y.; Lee, J.; Bang, S.; Hyun, S.; Kang, J.; Hong, S.T.; Bae, E.; Kaang, B.K.; Kim, J. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat. Genet. 2005, 37, 305–310. [Google Scholar] [CrossRef]
- Yao, C.A.; Ignell, R.; Carlson, J.R. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J. Neurosci. 2005, 25, 8359–8367. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, O.; Benzer, S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc. Natl. Acad. Sci. USA 1996, 93, 6079–6084. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Wang, R.; Yin, C.; Dong, Q.; Hing, H.; Kim, C.; Welsh, M.J. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature 2007, 450, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Homberg, U.; Christensen, T.A.; Hildebrand, J.G. Structure and function of the deutocerebrum in insects. Annu. Rev. Entomol. 1989, 34, 477–501. [Google Scholar] [CrossRef]
- Rosenzweig, M.; Brennan, K.M.; Tayler, T.D.; Phelps, P.O.; Patapoutian, A.; Garrity, P.A. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005, 19, 419–424. [Google Scholar] [CrossRef]
- Rosenzweig, M.; Kang, K.; Garrity, P.A. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2008, 105, 14668–14673. [Google Scholar] [CrossRef]
- Xiao, R.; Xu, X.Z.S. Temperature Sensation: From Molecular Thermosensors to Neural Circuits and Coding Principles. Annu. Rev. Physiol. 2021, 83, 205–230. [Google Scholar] [CrossRef]
- Li, K.; Gong, Z. Feeling Hot and Cold: Thermal Sensation in Drosophila. Neurosci. Bull. 2017, 33, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Platt, M.D.; Lagnese, C.M.; Leslie, J.R.; Hamada, F.N. Temperature integration at the AC thermosensory neurons in Drosophila. J. Neurosci. 2013, 33, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Montell, C.; Birnbaumer, L.; Flockerzi, V.; Bindels, R.J.; Bruford, E.A.; Caterina, M.J.; Clapham, D.E.; Harteneck, C.; Heller, S.; Julius, D.; et al. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell 2002, 9, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.H.; Lin, Y.C.; Chen, S.F.; Lee, P.S.; Fu, T.F.; Wu, T.; Wu, C.L. Independent insulin signaling modulators govern hot avoidance under different feeding states. PLoS Biol. 2023, 21, e3002332. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.W.; Wu, C.L.; Chang, S.W.; Liu, T.H.; Lai, J.S.; Fu, T.F.; Fu, C.C.; Chiang, A.S. Parallel circuits control temperature preference in Drosophila during ageing. Nat. Commun. 2015, 6, 7775. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.L.; Kwon, Y.; Adegbola, A.A.; Luo, J.; Chess, A.; Montell, C. Function of rhodopsin in temperature discrimination in Drosophila. Science 2011, 331, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Bronk, P.; Chang, E.C.; Lowell, A.M.; Flam, J.O.; Panzano, V.C.; Theobald, D.L.; Griffith, L.C.; Garrity, P.A. A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature 2013, 500, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Silbering, A.F.; Benton, R. Ionotropic and metabotropic mechanisms in chemoreception: ‘Chance or design’? EMBO Rep. 2010, 11, 173–179. [Google Scholar] [CrossRef]
- Vosshall, L.B.; Stocker, R.F. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 2007, 30, 505–533. [Google Scholar] [CrossRef]
- Thorne, N.; Chromey, C.; Bray, S.; Amrein, H. Taste perception and coding in Drosophila. Curr. Biol. 2004, 14, 1065–1079. [Google Scholar] [CrossRef]
- Kang, K.; Panzano, V.C.; Chang, E.C.; Ni, L.; Dainis, A.M.; Jenkins, A.M.; Regna, K.; Muskavitch, M.A.; Garrity, P.A. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature 2011, 481, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, V.; Story, G.M.; Peier, A.M.; Petrus, M.J.; Lee, V.M.; Hwang, S.W.; Patapoutian, A.; Jegla, T. Opposite thermosensor in fruitfly and mouse. Nature 2003, 423, 822–823. [Google Scholar] [CrossRef] [PubMed]
- Croset, V.; Rytz, R.; Cummins, S.F.; Budd, A.; Brawand, D.; Kaessmann, H.; Gibson, T.J.; Benton, R. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 2010, 6, e1001064. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Klein, M.; Svec, K.V.; Budelli, G.; Chang, E.C.; Ferrer, A.J.; Benton, R.; Samuel, A.D.; Garrity, P.A. The Ionotropic Receptors IR21a and IR25a mediate cool sensing in Drosophila. eLife 2016, 5, e13254. [Google Scholar] [CrossRef] [PubMed]
- Silbering, A.F.; Rytz, R.; Grosjean, Y.; Abuin, L.; Ramdya, P.; Jefferis, G.S.; Benton, R. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J. Neurosci. 2011, 31, 13357–13375. [Google Scholar] [CrossRef] [PubMed]
- Benton, R.; Vannice, K.S.; Gomez-Diaz, C.; Vosshall, L.B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 2009, 136, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Rytz, R.; Croset, V.; Benton, R. Ionotropic receptors (IRs): Chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem. Mol. Biol. 2013, 43, 888–897. [Google Scholar] [CrossRef]
- Abuin, L.; Bargeton, B.; Ulbrich, M.H.; Isacoff, E.Y.; Kellenberger, S.; Benton, R. Functional architecture of olfactory ionotropic glutamate receptors. Neuron 2011, 69, 44–60. [Google Scholar] [CrossRef]
- Klein, M.; Afonso, B.; Vonner, A.J.; Hernandez-Nunez, L.; Berck, M.; Tabone, C.J.; Kane, E.A.; Pieribone, V.A.; Nitabach, M.N.; Cardona, A.; et al. Sensory determinants of behavioral dynamics in Drosophila thermotaxis. Proc. Natl. Acad. Sci. USA 2015, 112, E220–E229. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Dahanukar, A.; Weiss, L.A.; Carlson, J.R. Molecular and cellular organization of the taste system in the Drosophila larva. J. Neurosci. 2011, 31, 15300–15309. [Google Scholar] [CrossRef]
- Liu, L.; Yermolaieva, O.; Johnson, W.A.; Abboud, F.M.; Welsh, M.J. Identification and function of thermosensory neurons in Drosophila larvae. Nat. Neurosci. 2003, 6, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Stocker, R.F. The organization of the chemosensory system in Drosophila melanogaster: A review. Cell Tissue Res. 1994, 275, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Dillon, M.E.; Wang, G.; Garrity, P.A.; Huey, R.B. Review: Thermal preference in Drosophila. J. Therm. Biol. 2009, 34, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Asahina, K.; Louis, M.; Piccinotti, S.; Vosshall, L.B. A circuit supporting concentration-invariant odor perception in Drosophila. J. Biol. 2009, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Budelli, G.; Ni, L.; Berciu, C.; van Giesen, L.; Knecht, Z.A.; Chang, E.C.; Kaminski, B.; Silbering, A.F.; Samuel, A.; Klein, M.; et al. Ionotropic Receptors Specify the Morphogenesis of Phasic Sensors Controlling Rapid Thermal Preference in Drosophila. Neuron 2019, 101, 738–747.e733. [Google Scholar] [CrossRef] [PubMed]
- Schepers, R.J.; Ringkamp, M. Thermoreceptors and thermosensitive afferents. Neurosci. Biobehav. Rev. 2009, 33, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.W.; Mazor, O.; Wilson, R.I. Thermosensory processing in the Drosophila brain. Nature 2015, 519, 353–357. [Google Scholar] [CrossRef]
- Aso, Y.; Grubel, K.; Busch, S.; Friedrich, A.B.; Siwanowicz, I.; Tanimoto, H. The mushroom body of adult Drosophila characterized by GAL4 drivers. J. Neurogenet. 2009, 23, 156–172. [Google Scholar] [CrossRef]
- Mao, Z.; Davis, R.L. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: Anatomical and physiological heterogeneity. Front. Neural Circuits 2009, 3, 5. [Google Scholar] [CrossRef]
- Aso, Y.; Hattori, D.; Yu, Y.; Johnston, R.M.; Iyer, N.A.; Ngo, T.T.; Dionne, H.; Abbott, L.F.; Axel, R.; Tanimoto, H.; et al. The neuronal architecture of the mushroom body provides a logic for associative learning. eLife 2014, 3, e04577. [Google Scholar] [CrossRef]
- Karam, C.S.; Jones, S.K.; Javitch, J.A. Come Fly with Me: An overview of dopamine receptors in Drosophila melanogaster. Basic. Clin. Pharmacol. Toxicol. 2020, 126 (Suppl. 6), 56–65. [Google Scholar] [CrossRef] [PubMed]
- Tomchik, S.M. Dopaminergic neurons encode a distributed, asymmetric representation of temperature in Drosophila. J. Neurosci. 2013, 33, 2166a–2176a. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Hamada, F.N. Drosophila Temperature Preference Rhythms: An Innovative Model to Understand Body Temperature Rhythms. Int. J. Mol. Sci. 2019, 20, 1988. [Google Scholar] [CrossRef] [PubMed]
- Khazaeli, A.A.; Van Voorhies, W.; Curtsinger, J.W. Longevity and metabolism in Drosophila melanogaster: Genetic correlations between life span and age-specific metabolic rate in populations artificially selected for long life. Genetics 2005, 169, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Berrigan, D.; Partridge, L. Influence of temperature and activity on the metabolic rate of adult Drosophila melanogaster. Comp. Biochem. Physiol. A Physiol. 1997, 118, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Umezaki, Y.; Hayley, S.E.; Chu, M.L.; Seo, H.W.; Shah, P.; Hamada, F.N. Feeding-State-Dependent Modulation of Temperature Preference Requires Insulin Signaling in Drosophila Warm-Sensing Neurons. Curr. Biol. 2018, 28, 779–787.e773. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.H.; Ohba, S. Temperature preferences of eleven Drosophila species from Japan—The relationship between preferred temperature and some ecological character istics in their natural habitats. Zool. Sci. 1984, 1, 631–640. [Google Scholar]
- Yoshii, T.; Sakamoto, M.; Tomioka, K. A temperature-dependent timing mechanism is involved in the circadian system that drives locomotor rhythms in the fruit fly Drosophila melanogaster. Zoolog Sci. 2002, 19, 841–850. [Google Scholar] [CrossRef]
- Melzer, D.; Pilling, L.C.; Ferrucci, L. The genetics of human ageing. Nat. Rev. Genet. 2020, 21, 88–101. [Google Scholar] [CrossRef]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef]
- Tumer, N.; Larochelle, J.S. Tyrosine hydroxylase expression in rat adrenal medulla: Influence of age and cold. Pharmacol. Biochem. Behav. 1995, 51, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Algeri, S.; Calderini, G.; Lomuscio, G.; Vantini, G.; Toffano, G.; Ponzio, F. Changes with age in rat central monoaminergic system responses to cold stress. Neurobiol. Aging 1982, 3, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Sokabe, T.; Chen, H.C.; Luo, J.; Montell, C. A Switch in Thermal Preference in Drosophila Larvae Depends on Multiple Rhodopsins. Cell Rep. 2016, 17, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, J.J.; Wilbourne, J.T.; Omelchenko, A.A.; Yoon, J.; Ni, L. Ionotropic Receptor-dependent cool cells control the transition of temperature preference in Drosophila larvae. PLoS Genet. 2021, 17, e1009499. [Google Scholar] [CrossRef] [PubMed]
- Molon, M.; Dampc, J.; Kula-Maximenko, M.; Zebrowski, J.; Molon, A.; Dobler, R.; Durak, R.; Skoczowski, A. Effects of Temperature on Lifespan of Drosophila melanogaster from Different Genetic Backgrounds: Links between Metabolic Rate and Longevity. Insects 2020, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Salerian, A.J.; Saleri, N.G. Cooler biologically compatible core body temperatures may prolong longevity and combat neurodegenerative disorders. Med. Hypotheses 2006, 66, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Baker, F.C.; Waner, J.I.; Vieira, E.F.; Taylor, S.R.; Driver, H.S.; Mitchell, D. Sleep and 24 hour body temperatures: A comparison in young men, naturally cycling women and women taking hormonal contraceptives. J. Physiol. 2001, 530, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Minors, D.S.; Waterhouse, J.M. Effects upon circadian rhythmicity of an alteration to the sleep-wake cycle: Problems of assessment resulting from measurement in the presence of sleep and analysis in terms of a single shifted component. J. Biol. Rhythms 1988, 3, 23–40. [Google Scholar] [CrossRef]
- Refinetti, R. Circadian rhythmicity of body temperature and metabolism. Temperature 2020, 7, 321–362. [Google Scholar] [CrossRef]
- Gradisar, M.; Lack, L. Relationships between the circadian rhythms of finger temperature, core temperature, sleep latency, and subjective sleepiness. J. Biol. Rhythm. 2004, 19, 157–163. [Google Scholar] [CrossRef]
- Sarabia, J.A.; Rol, M.A.; Mendiola, P.; Madrid, J.A. Circadian rhythm of wrist temperature in normal-living subjects A candidate of new index of the circadian system. Physiol. Behav. 2008, 95, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Mormont, M.C.; Langouet, A.M.; Claustrat, B.; Bogdan, A.; Marion, S.; Waterhouse, J.; Touitou, Y.; Levi, F. Marker rhythms of circadian system function: A study of patients with metastatic colorectal cancer and good performance status. Chronobiol. Int. 2002, 19, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, E.J.W. More than a marker: Interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities. Chronobiol. Int. 2000, 17, 313–354. [Google Scholar] [CrossRef]
- Krauchi, K.; Wirz-Justice, A. Circadian clues to sleep onset mechanisms. Neuropsychopharmacology 2001, 25, S92–S96. [Google Scholar] [CrossRef] [PubMed]
- Krauchi, K. The human sleep-wake cycle reconsidered from a thermoregulatory point of view. Physiol. Behav. 2007, 90, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Cajochen, C.; Zeitzer, J.M.; Czeisler, C.A.; Dijk, D.J. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav. Brain Res. 2000, 115, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Reilly, T.; Waterhouse, J. Circadian aspects of body temperature regulation in exercise. J. Therm. Biol. 2009, 34, 161–170. [Google Scholar] [CrossRef]
- Scheer, F.A.J.L.; van Doornen, L.J.P.; Buijs, R.M. Light and diurnal cycle affect human heart rate: Possible role for the circadian pacemaker. J. Biol. Rhythm. 1999, 14, 202–212. [Google Scholar] [CrossRef]
- Wakamura, T.; Tokura, H. Circadian rhythm of rectal temperature in humans under different ambient temperature cycles. J. Therm. Biol. 2002, 27, 439–447. [Google Scholar] [CrossRef]
- Waterhouse, J.; Edwards, B.; Mugarza, J.; Flemming, R.; Minors, D.; Calbraith, D.; Williams, G.; Atkinson, G.; Reilly, T. Purification of masked temperature data from humans: Some preliminary observations on a comparison of the use of an activity diary, wrist actimetry, and heart rate monitoring. Chronobiol. Int. 1999, 16, 461–475. [Google Scholar] [CrossRef]
- Krauchi, K.; Cajochen, C.; Wirz-Justice, A. Thermophysiologic aspects of the three-process-model of sleepiness regulation (vol 24, pg 287, 2005). Clin. Sport. Med. 2005, 24, 979. [Google Scholar] [CrossRef]
- Martinez-Nicolas, A.; Ortiz-Tudela, E.; Rol, M.A.; Madrid, J.A. Uncovering Different Masking Factors on Wrist Skin Temperature Rhythm in Free-Living Subjects. PLoS ONE 2013, 8, e61142. [Google Scholar] [CrossRef] [PubMed]
- Coiffard, B.; Diallo, A.B.; Mezouar, S.; Leone, M.; Mege, J.L. A Tangled Threesome: Circadian Rhythm, Body Temperature Variations, and the Immune System. Biology 2021, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Refinetti, R. The circadian rhythm of body temperature. Front. Biosci. 2010, 15, 564–594. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.M.; Bellet, M.M.; Sassone-Corsi, P.; O’Neill, L.A. Circadian clock proteins and immunity. Immunity 2014, 40, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Rosenwasser, A.M.; Turek, F.W. Neurobiology of Circadian Rhythm Regulation. Sleep. Med. Clin. 2015, 10, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.J.; Firth, B.T.; Belan, I. Circadian rhythms of locomotor activity and temperature selection in sleepy lizards, Tiliqua rugosa. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2007, 193, 695–701. [Google Scholar] [CrossRef]
- Kaneko, H.; Head, L.M.; Ling, J.; Tang, X.; Liu, Y.; Hardin, P.E.; Emery, P.; Hamada, F.N. Circadian rhythm of temperature preference and its neural control in Drosophila. Curr. Biol. 2012, 22, 1851–1857. [Google Scholar] [CrossRef]
- Goda, T.; Umezaki, Y.; Hamada, F.N. Molecular and Neural Mechanisms of Temperature Preference Rhythm in Drosophila melanogaster. J. Biol. Rhythms 2023, 38, 326–340. [Google Scholar] [CrossRef]
- Nitabach, M.N.; Taghert, P.H. Organization of the Drosophila circadian control circuit. Curr. Biol. 2008, 18, R84–R93. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Saez, L.; Young, M.W. PER-TIM interactions in living Drosophila cells: An interval timer for the circadian clock. Science 2006, 311, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Shafer, O.T.; Rosbash, M.; Truman, J.W. Sequential Nuclear Accumulation of the Clock Proteins Period and Timeless in the Pacemaker Neurons of Drosophila melanogaster. J. Neurosci. 2002, 22, 5946–5954. [Google Scholar] [CrossRef] [PubMed]
- Taghert, P.H.; Shafer, O.T. Mechanisms of clock output in the Drosophila circadian pacemaker system. J. Biol. Rhythms 2006, 21, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Vosshall, L.B.; Young, M.W. Circadian rhythms in Drosophila can be driven by period expression in a restricted group of central brain cells. Neuron 1995, 15, 345–360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frisch, B.; Hardin, P.E.; Hamblen-Coyle, M.J.; Rosbash, M.; Hall, J.C. A promoterless period gene mediates behavioral rhythmicity and cyclical per expression in a restricted subset of the drosophila nervous system. Neuron 1994, 12, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Dushay, M.S.; Rosbash, M.; Hall, J.C. The disconnected visual system mutations in Drosophila melanogaster drastically disrupt circadian rhythms. J. Biol. Rhythms 1989, 4, 1–27. [Google Scholar] [CrossRef]
- Hyun, S.; Lee, Y.; Hong, S.-T.; Bang, S.; Paik, D.; Kang, J.; Shin, J.; Lee, J.; Jeon, K.; Hwang, S.; et al. Drosophila GPCR Han Is a Receptor for the Circadian Clock Neuropeptide PDF. Neuron 2005, 48, 267–278. [Google Scholar] [CrossRef]
- Taghert, P.H.; Nitabach, M.N. Peptide neuromodulation in invertebrate model systems. Neuron 2012, 76, 82–97. [Google Scholar] [CrossRef]
- Chen, S.C.; Tang, X.; Goda, T.; Umezaki, Y.; Riley, A.C.; Sekiguchi, M.; Yoshii, T.; Hamada, F.N. Dorsal clock networks drive temperature preference rhythms in Drosophila. Cell Rep. 2022, 39, 110668. [Google Scholar] [CrossRef]
- Alpert, M.H.; Frank, D.D.; Kaspi, E.; Flourakis, M.; Zaharieva, E.E.; Allada, R.; Para, A.; Gallio, M. A Circuit Encoding Absolute Cold Temperature in Drosophila. Curr. Biol. 2020, 30, 2275–2288.e2275. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Tang, X.; Umezaki, Y.; Chu, M.L.; Kunst, M.; Nitabach, M.N.N.; Hamada, F.N. Drosophila DH31 Neuropeptide and PDF Receptor Regulate Night-Onset Temperature Preference. J. Neurosci. 2016, 36, 11739–11754. [Google Scholar] [CrossRef] [PubMed]
- Mertens, I.; Vandingenen, A.; Johnson, E.C.; Shafer, O.T.; Li, W.; Trigg, J.S.; De Loof, A.; Schoofs, L.; Taghert, P.H. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron 2005, 48, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Roessingh, S.; Hayley, S.E.; Chu, M.L.; Tanaka, N.K.; Wolfgang, W.; Song, S.; Stanewsky, R.; Hamada, F.N. The role of PDF neurons in setting the preferred temperature before dawn in Drosophila. eLife 2017, 6, e23206. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; BroŽEk, J.; Henschel, A.; Mickelsen, O.; Taylor, H.L.; Simonson, E.; Skinner, A.S.; Wells, S.M.; Drummond, J.C.; Wilder, R.M.; et al. The Biology of Human Starvation; University of Minnesota Press: Minneapolis, MN, USA, 1950; Volume I. [Google Scholar]
- Piccione, G.; Caola, G.; Refinetti, R. Circadian modulation of starvation-induced hypothermia in sheep and goats. Chronobiol. Int. 2002, 19, 531–541. [Google Scholar] [CrossRef] [PubMed]
- McCue, M.D. Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hung, C.C.; Randall, D.J. The comparative physiology of food deprivation: From feast to famine. Annu. Rev. Physiol. 2006, 68, 223–251. [Google Scholar] [CrossRef]
- Bicego, K.C.; Barros, R.C.H.; Branco, L.G.S. Physiology of temperature regulation: Comparative aspects. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 147, 616–639. [Google Scholar] [CrossRef]
- Young, V.R.; Scrimshaw, N.S. The physiology of starvation. Sci. Am. 1971, 225, 14–21. [Google Scholar] [CrossRef]
- Owen, O.E.; Smalley, K.J.; D’Alessio, D.A.; Mozzoli, M.A.; Dawson, E.K. Protein, fat, and carbohydrate requirements during starvation: Anaplerosis and cataplerosis. Am. J. Clin. Nutr. 1998, 68, 12–34. [Google Scholar] [CrossRef]
- Owen, O.E.; Reichard, G.A., Jr.; Patel, M.S.; Boden, G. Energy metabolism in feasting and fasting. Adv. Exp. Med. Biol. 1979, 111, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.W.; Foster, D.O. Starvation-induced changes in metabolic rate, blood flow, and regional energy expenditure in rats. Can. J. Physiol. Pharmacol. 1986, 64, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Cherel, Y.; Robin, J.P.; Heitz, A.; Calgari, C.; Le Maho, Y. Relationships between lipid availability and protein utilization during prolonged fasting. J. Comp. Physiol. B 1992, 162, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.N.; Lowell, B.; Belur, E.; Ruderman, N.B. Sites of protein conservation and loss during starvation: Influence of adiposity. Am. J. Physiol. 1984, 246, E383–E390. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K. How Rats Economize: Energy Loss in Starvation. Physiol. Zool. 1977, 50, 331–362. [Google Scholar] [CrossRef]
- Desautels, M. Mitochondrial thermogenin content is unchanged during atrophy of BAT of fasting mice. Am. J. Physiol. 1985, 249, E99–E106. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, I.; Schreiber, R.; Prokesch, A. Regulation of thermogenic adipocytes during fasting and cold. Mol. Cell Endocrinol. 2020, 512, 110869. [Google Scholar] [CrossRef]
- Nassel, D.R.; Kubrak, O.I.; Liu, Y.T.; Luo, J.N.; Lushchak, O.V. Factors that regulate insulin producing cells and their output in Drosophila. Front. Physiol. 2013, 4, 252. [Google Scholar] [CrossRef]
- Brogiolo, W.; Stocker, H.; Ikeya, T.; Rintelen, F.; Fernandez, R.; Hafen, E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001, 11, 213–221. [Google Scholar] [CrossRef]
- Cao, C.; Brown, M.R. Localization of an insulin-like peptide in brains of two flies. Cell Tissue Res. 2001, 304, 317–321. [Google Scholar] [CrossRef]
- Bai, H.; Kang, P.; Tatar, M. Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin-like peptide-2 from the brain. Aging Cell 2012, 11, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Geminard, C.; Rulifson, E.J.; Leopold, P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009, 10, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, S.R.; Pathak, H.; Rehman, N.; Fernandes, J.; Vishnu, S.; Varghese, J. Insulin signalling elicits hunger-induced feeding in Drosophila. Dev. Biol. 2020, 459, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, R.S. Genetic analysis of insulin signaling in Drosophila. Trends Endocrinol. Metab. 2002, 13, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.L.; Cline, H.T. Insulin receptor signaling in the development of neuronal structure and function. Neural Dev. 2010, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Semaniuk, U.; Piskovatska, V.; Strilbytska, O.; Strutynska, T.; Burdyliuk, N.; Vaiserman, A.; Bubalo, V.; Storey, K.B.; Lushchak, O. Drosophila insulin-like peptides: From expression to functions—A review. Entomol. Exp. Appl. 2021, 169, 195–208. [Google Scholar] [CrossRef]
- Shilo, B.Z. The regulation and functions of MAPK pathways in Drosophila. Methods 2014, 68, 151–159. [Google Scholar] [CrossRef]
- Wassarman, D.A.; Therrien, M.; Rubin, G.M. The Ras signaling pathway in Drosophila. Curr. Opin. Genet. Dev. 1995, 5, 44–50. [Google Scholar] [CrossRef]
- Lim, B.; Dsilva, C.J.; Levario, T.J.; Lu, H.; Schupbach, T.; Kevrekidis, I.G.; Shvartsman, S.Y. Dynamics of Inductive ERK Signaling in the Drosophila Embryo. Curr. Biol. 2015, 25, 1784–1790. [Google Scholar] [CrossRef]
- Lee, D.E.; Kim, S.J.; Zhuo, M. Comparison of behavioral responses to noxious cold and heat in mice. Brain Res. 1999, 845, 117–121. [Google Scholar] [CrossRef]
- Malafoglia, V.; Bryant, B.; Raffaeli, W.; Giordano, A.; Bellipanni, G. The zebrafish as a model for nociception studies. J. Cell Physiol. 2013, 228, 1956–1966. [Google Scholar] [CrossRef] [PubMed]
- Malafoglia, V.; Colasanti, M.; Raffaeli, W.; Balciunas, D.; Giordano, A.; Bellipanni, G. Extreme thermal noxious stimuli induce pain responses in zebrafish larvae. J. Cell Physiol. 2014, 229, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Prober, D.A.; Zimmerman, S.; Myers, B.R.; McDermott, B.M., Jr.; Kim, S.H.; Caron, S.; Rihel, J.; Solnica-Krezel, L.; Julius, D.; Hudspeth, A.J.; et al. Zebrafish TRPA1 channels are required for chemosensation but not for thermosensation or mechanosensory hair cell function. J. Neurosci. 2008, 28, 10102–10110. [Google Scholar] [CrossRef] [PubMed]
- Wittenburg, N.; Baumeister, R. Thermal avoidance in Caenorhabditis elegans: An approach to the study of nociception. Proc. Natl. Acad. Sci. USA 1999, 96, 10477–10482. [Google Scholar] [CrossRef] [PubMed]
- Boivin, J.C.; Zhu, J.; Ohyama, T. Nociception in fruit fly larvae. Front. Pain Res. 2023, 4, 1076017. [Google Scholar] [CrossRef] [PubMed]
- Hwang, R.Y.; Zhong, L.; Xu, Y.; Johnson, T.; Zhang, F.; Deisseroth, K.; Tracey, W.D. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr. Biol. 2007, 17, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Tenedini, F.; Hoyer, N.; Kutschera, F.; Soba, P. Assaying Thermo-nociceptive Behavior in Drosophila Larvae. Bio Protoc. 2018, 8, e2737. [Google Scholar] [CrossRef]
- Oswald, M.; Rymarczyk, B.; Chatters, A.; Sweeney, S.T. A novel thermosensitive escape behavior in Drosophila larvae. Fly 2011, 5, 304–306. [Google Scholar] [CrossRef][Green Version]
- Neely, G.G.; Hess, A.; Costigan, M.; Keene, A.C.; Goulas, S.; Langeslag, M.; Griffin, R.S.; Belfer, I.; Dai, F.; Smith, S.B.; et al. A genome-wide Drosophila screen for heat nociception identifies alpha2delta3 as an evolutionarily conserved pain gene. Cell 2010, 143, 628–638. [Google Scholar] [CrossRef]
- Aldrich, B.T.; Kasuya, J.; Faron, M.; Ishimoto, H.; Kitamoto, T. The amnesiac gene is involved in the regulation of thermal nociception in Drosophila melanogaster. J. Neurogenet. 2010, 24, 33–41. [Google Scholar] [CrossRef]
- Benzer, S. Behavioral mutants of Drosophila isolated by Countercurrent Distribution. Proc. Natl. Acad. Sci. USA 1967, 58, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Boonen, B.; Startek, J.B.; Milici, A.; Lopez-Requena, A.; Beelen, M.; Callaerts, P.; Talavera, K. Activation of Drosophila melanogaster TRPA1 Isoforms by Citronellal and Menthol. Int. J. Mol. Sci. 2021, 22, 997. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Shim, H.S.; Wang, X.; Montell, C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat. Neurosci. 2008, 11, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Neely, G.G.; Keene, A.C.; Duchek, P.; Chang, E.C.; Wang, Q.P.; Aksoy, Y.A.; Rosenzweig, M.; Costigan, M.; Woolf, C.J.; Garrity, P.A.; et al. TrpA1 regulates thermal nociception in Drosophila. PLoS ONE 2011, 6, e24343. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Shen, W.L.; Montell, C. TRPA1 mediates sensation of the rate of temperature change in Drosophila larvae. Nat. Neurosci. 2017, 20, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.B. Sensation is painless. Trends Neurosci. 2003, 26, 643–645. [Google Scholar] [CrossRef]
- Sokabe, T.; Tominaga, M. A temperature-sensitive TRP ion channel, Painless, functions as a noxious heat sensor in fruit flies. Commun. Integr. Biol. 2009, 2, 170–173. [Google Scholar] [CrossRef]
- Al-Anzi, B.; Tracey, W.D., Jr.; Benzer, S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr. Biol. 2006, 16, 1034–1040. [Google Scholar] [CrossRef]
- Xu, J.; Sornborger, A.T.; Lee, J.K.; Shen, P. Drosophila TRPA channel modulates sugar-stimulated neural excitation, avoidance and social response. Nat. Neurosci. 2008, 11, 676–682. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Thakur, D.; Mack, J.; Spina, A.; Montell, C. Alleviation of thermal nociception depends on heat-sensitive neurons and a TRP channel in the brain. Curr. Biol. 2023, 33, 2397–2406.e2396. [Google Scholar] [CrossRef]
- Sizemore, T.R.; Carlson, J.R. Pain: The agony and the AstC. Curr. Biol. 2023, 33, R695–R697. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Polgar, E.; Solinski, H.J.; Mishra, S.K.; Tseng, P.Y.; Iwagaki, N.; Boyle, K.A.; Dickie, A.C.; Kriegbaum, M.C.; Wildner, H.; et al. Circuit dissection of the role of somatostatin in itch and pain. Nat. Neurosci. 2018, 21, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Birgul, N.; Weise, C.; Kreienkamp, H.J.; Richter, D. Reverse physiology in drosophila: Identification of a novel allatostatin-like neuropeptide and its cognate receptor structurally related to the mammalian somatostatin/galanin/opioid receptor family. EMBO J. 1999, 18, 5892–5900. [Google Scholar] [CrossRef] [PubMed]
- Lenz, C.; Williamson, M.; Grimmelikhuijzen, C.J. Molecular cloning and genomic organization of a second probable allatostatin receptor from Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2000, 273, 571–577. [Google Scholar] [CrossRef]
- Kornberg, T.B.; Krasnow, M.A. The Drosophila genome sequence: Implications for biology and medicine. Science 2000, 287, 2218–2220. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).