Resveratrol’s Impact on the Chondrogenic Reagents’ Effects in Cell Sheet Cultures of Wharton’s Jelly-Derived MSCs
Abstract
:1. Introduction
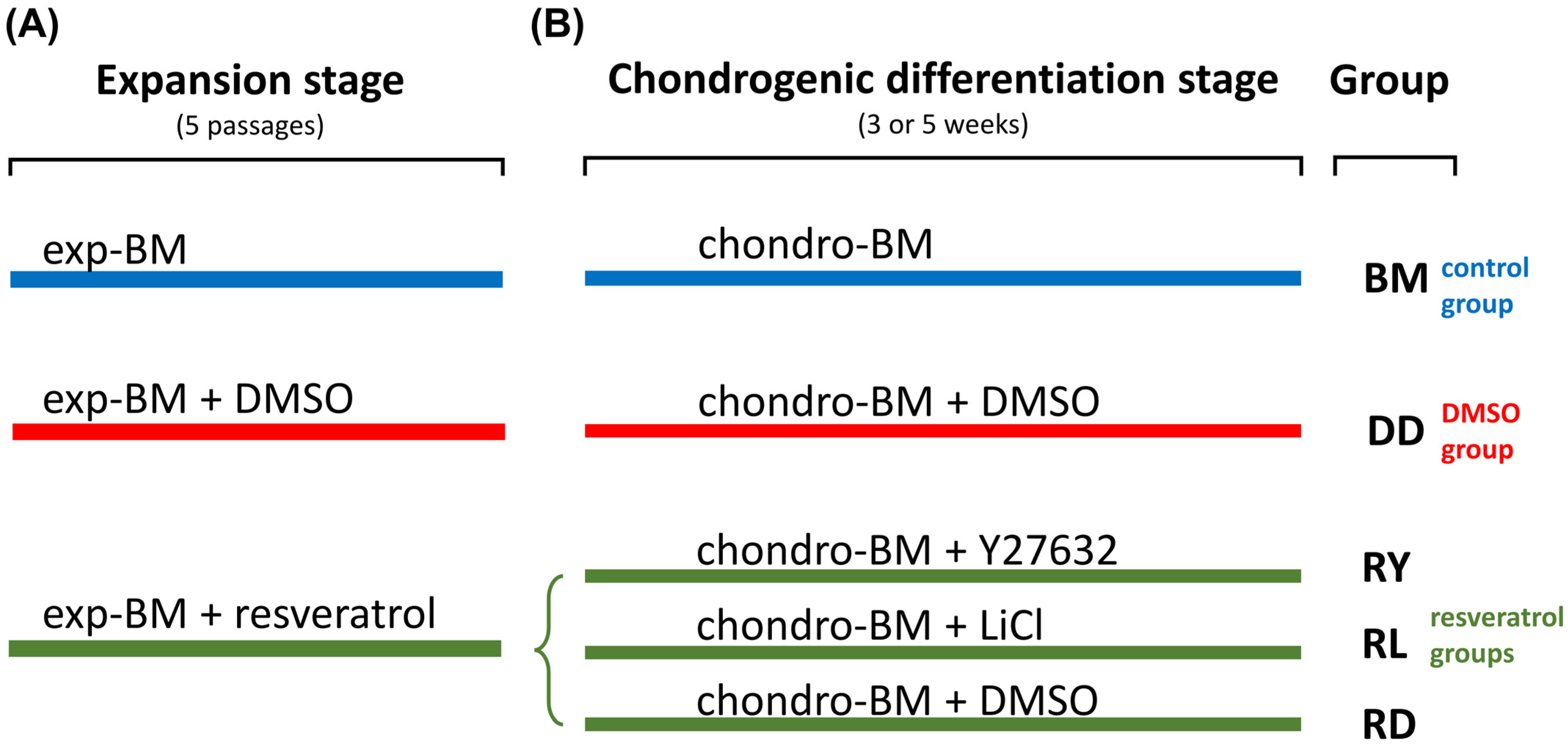
2. Materials and Methods
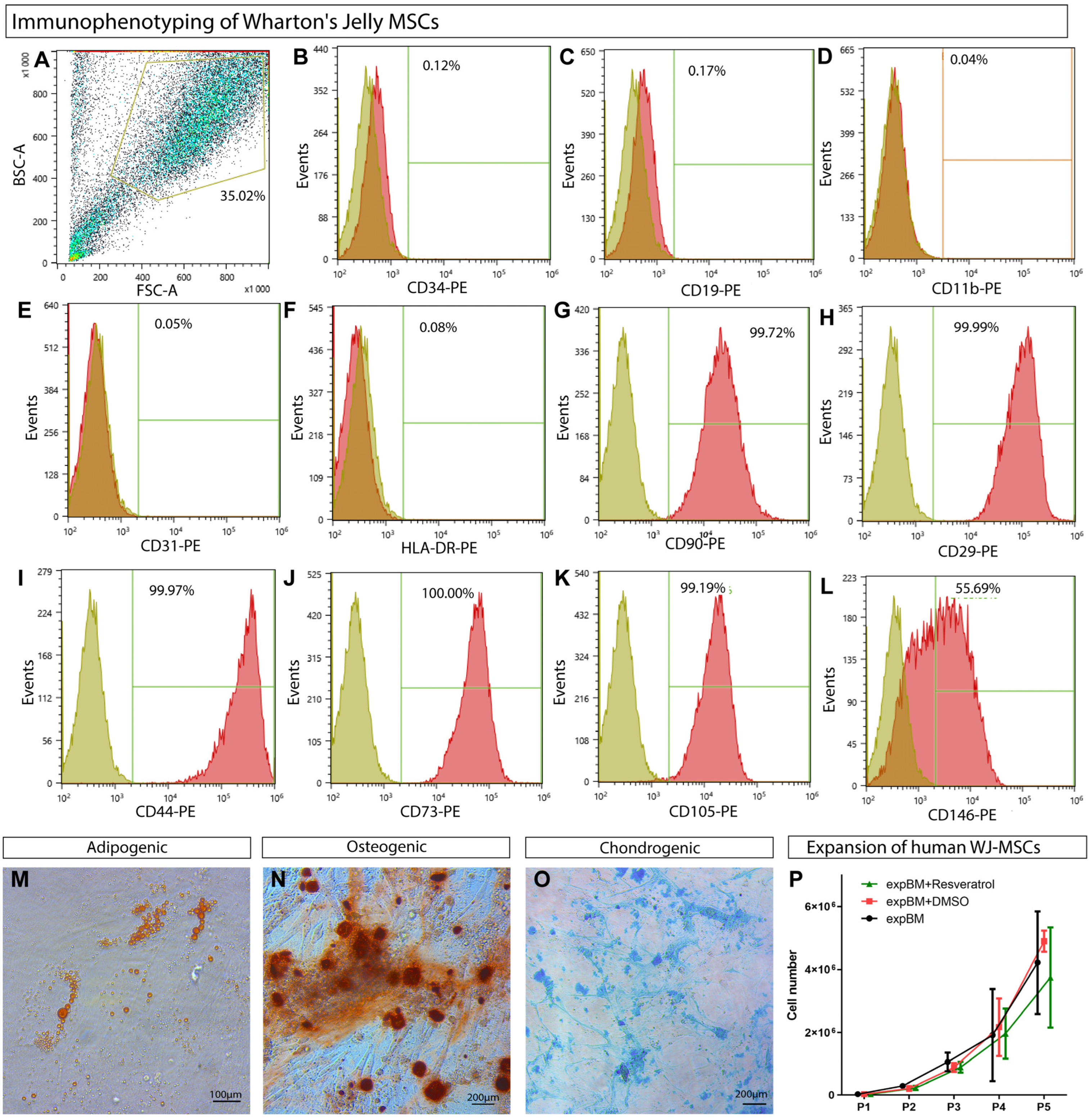
2.1. Human WJ-MSC Isolation
2.2. Compliance with the Established Minimal Criteria for MSCs
2.3. Preparation of Synthetic P(NIPAM-co-NtBA)-Based Matrices
2.4. Cell Culture Techniques and the Formation of Cell Sheets
2.5. Animals
2.6. Acute Knee Injury Model
2.7. Sample Preparation
2.8. Immunofluorescence and Histology Staining
2.9. Gene Expression Analysis
2.10. Statistical Analysis
3. Results
3.1. WJ-MSCs’ Phenotypic Characterization
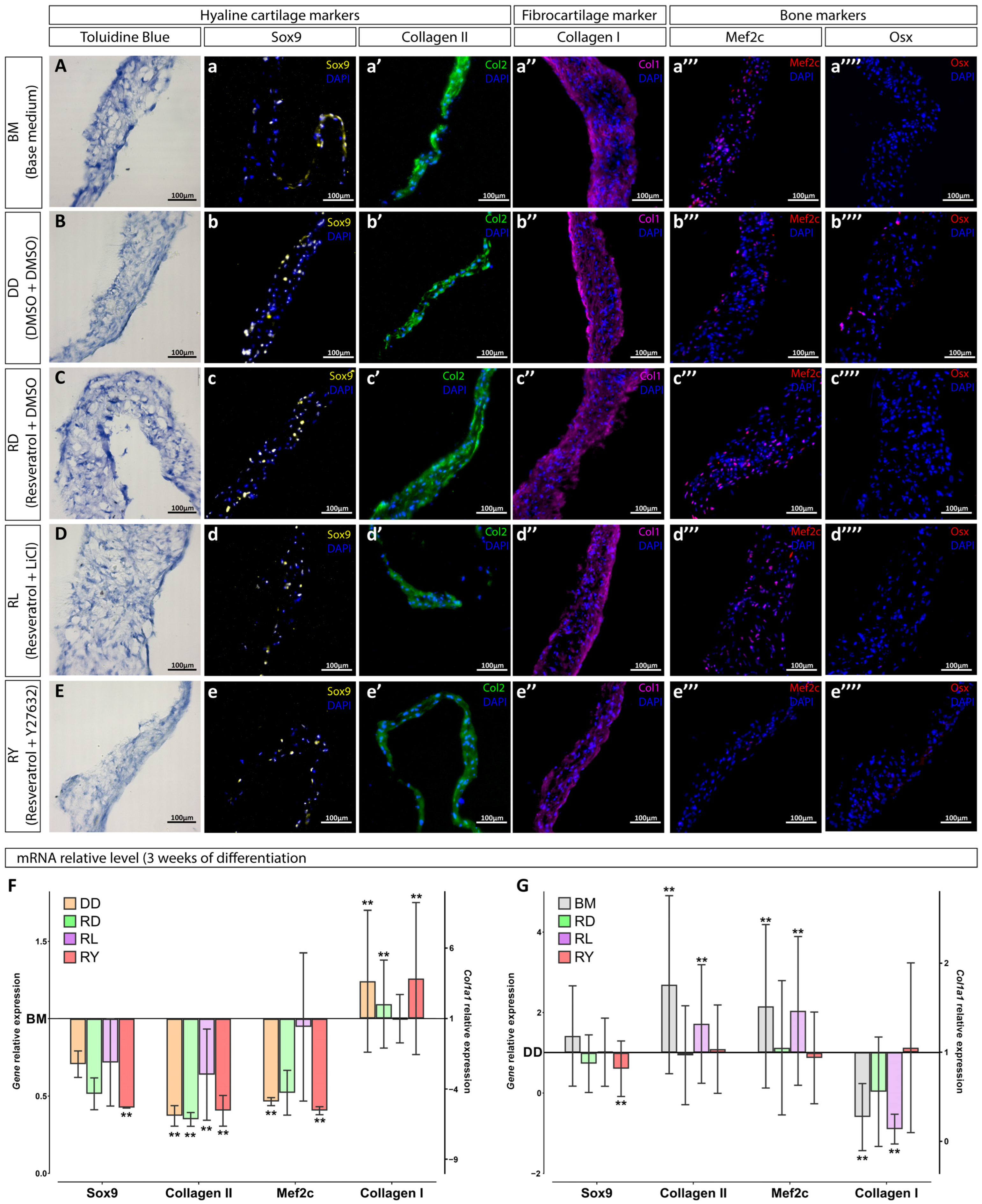
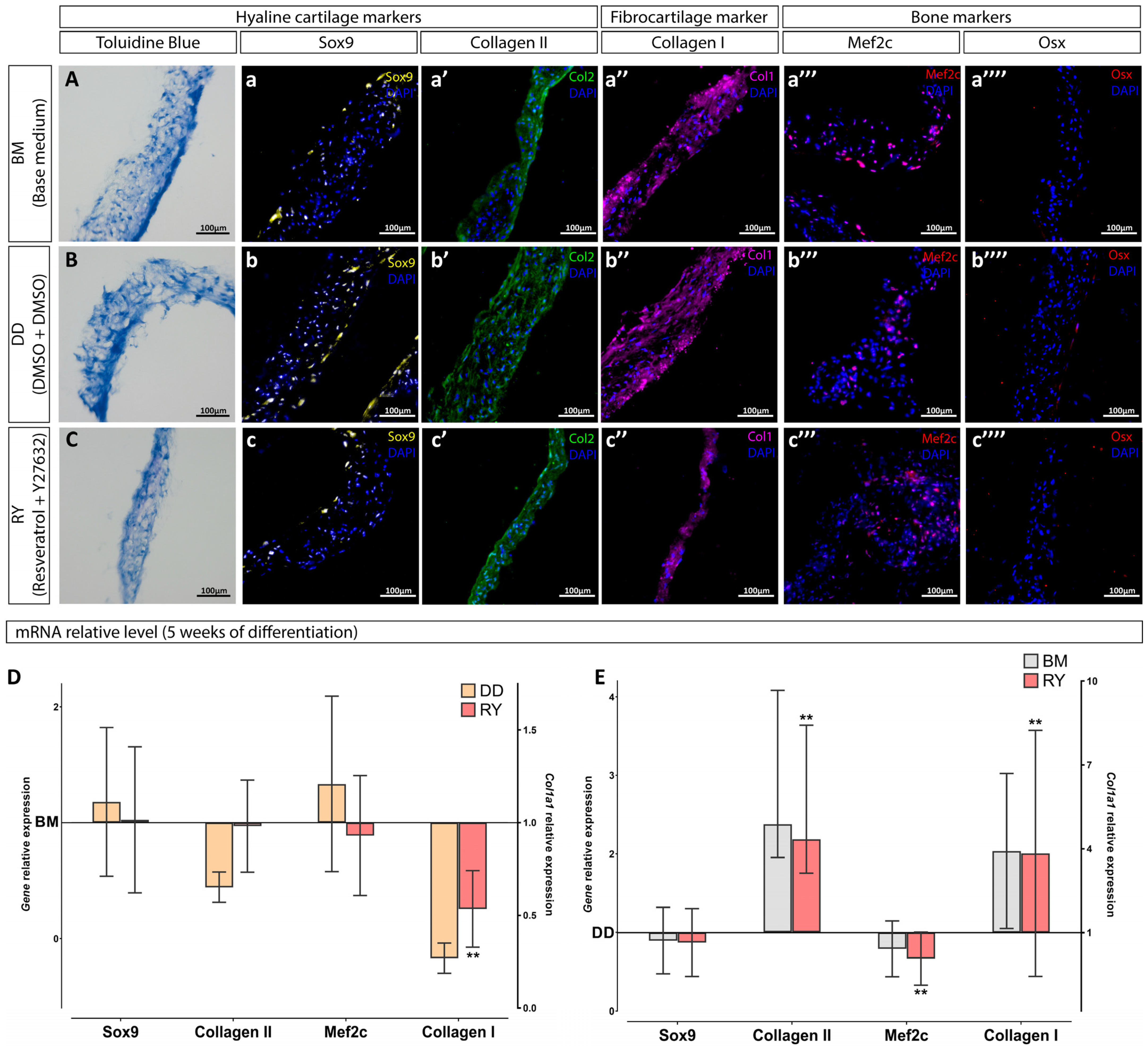
3.2. Assessment of the Differentiation Grade of Cell Sheets after 3 and 5 Weeks of Culturing in the Chondrogenic Medium
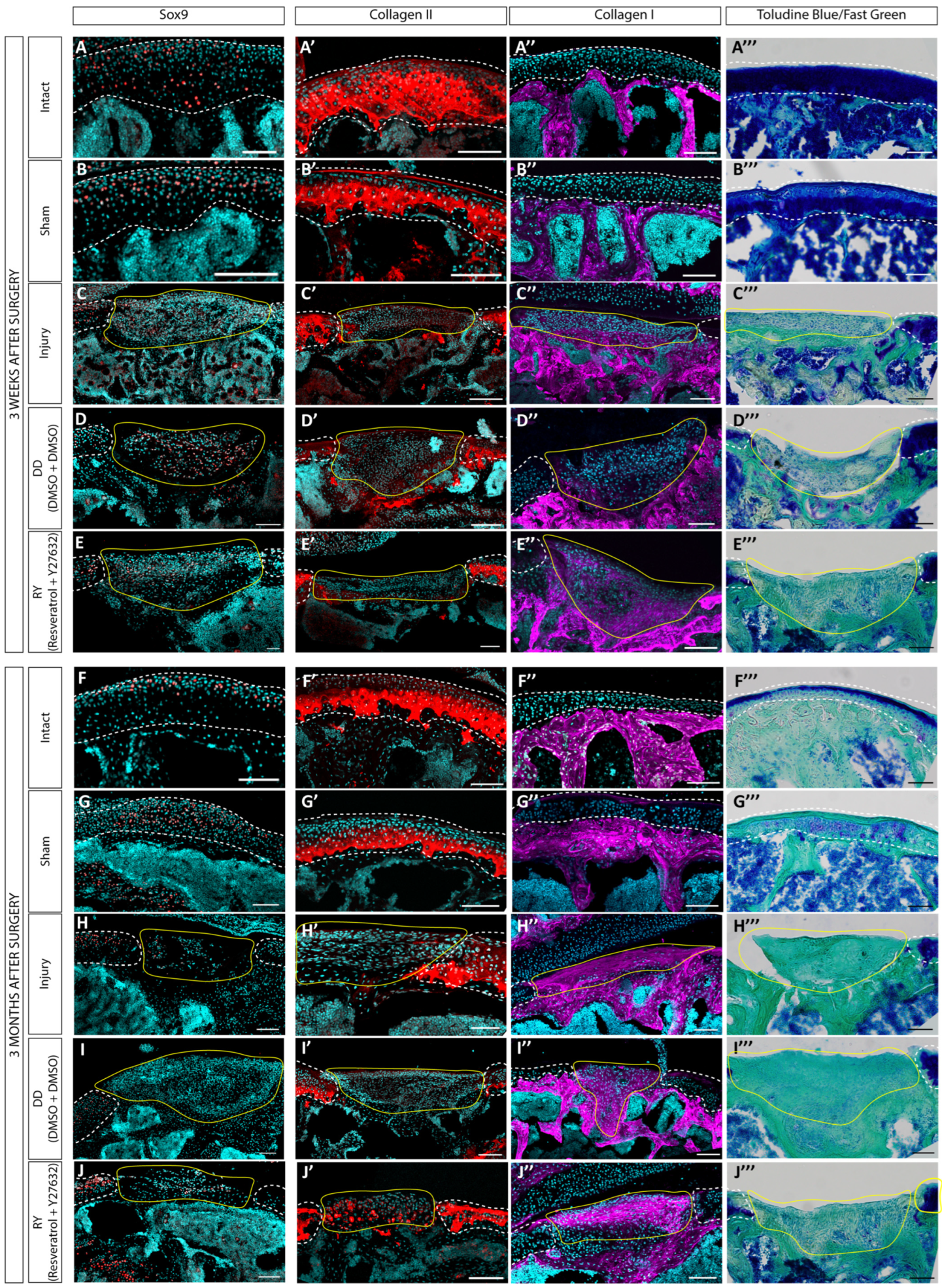
3.3. Estimation of the Regenerative Abilities of hWJ-MSC Sheets in a Rat Model of a Cartilage Defect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marino, L.; Castaldi, M.A.; Rosamilio, R.; Ragni, E.; Vitolo, R.; Fulgione, C.; Castaldi, S.G.; Serio, B.; Bianco, R.; Guida, M.; et al. Mesenchymal stem cells from the Wharton’s jelly of the human umbilical cord: Biological properties and therapeutic potential. Int. J. Stem Cells 2019, 12, 218–226. [Google Scholar] [CrossRef]
- El Omar, R.; Beroud, J.; Stoltz, J.F.; Menu, P.; Velot, E.; Decot, V. Umbilical cord mesenchymal stem cells: The new gold standard for mesenchymal stem cell-based therapies? Tissue Eng. Part B Rev. 2014, 20, 523–544. [Google Scholar] [CrossRef]
- Chen, M.Y.; Lie, P.C.; Li, Z.L.; Wei, X. Endothelial differentiation of Wharton’s jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp. Hematol. 2009, 37, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Kitada, M.; Wakao, S.; Dezawa, M. Mesenchymal stem cells and umbilical cord as sources for schwann cell differentiation: Their potential in peripheral nerve repair. Open Tissue Eng. Regen. Med. J. 2011, 4, 54–63. [Google Scholar] [CrossRef]
- Shi, Q.; Gao, J.; Jiang, Y.; Sun, B.; Lu, W.; Su, M.; Xu, Y.; Yang, X.; Zhang, Y. Differentiation of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells into endometrial cells. Stem Cell Res. Ther. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Theerakittayakorn, K.; Somredngan, S.; Ngernsoungnern, A.; Ngernsoungnern, P.; Sritangos, P.; Ketudat-Cairns, M.; Imsoonthornruksa, S.; Assawachananont, J.; Keeratibharat, N.; et al. Signaling Pathways Impact on Induction of Corneal Epithelial-like Cells Derived from Human Wharton’s Jelly Mesenchymal Stem Cells. Int. J. Mol. Sci. 2022, 23, 3078. [Google Scholar] [CrossRef] [PubMed]
- Musiał-Wysocka, A.; Kot, M.; Sułkowski, M.; Badyra, B.; Majka, M. Molecular and functional verification of wharton’s jelly mesenchymal stem cells (WJ-MSCs) pluripotency. Int. J. Mol. Sci. 2019, 20, 1807. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.N.; Fong, C.Y.; Subramanian, A.; Liu, W.; Feng, Y.; Choolani, M.; Biswas, A.; Rajapakse, J.C.; Bongso, A. Human Wharton’s Jelly Mesenchymal Stem Cells Show Unique Gene Expression Compared with Bone Marrow Mesenchymal Stem Cells Using Single-Cell RNA-Sequencing. Stem Cells Dev. 2019, 28, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Tian, G.; Yang, Z.; Gao, X.; Wang, F.; Li, J.; Tian, Z.; Huang, B.; Wei, F.; Sang, X.; et al. Enhancement of acellular cartilage matrix scaffold by Wharton’s jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact. Mater. 2021, 6, 2711–2728. [Google Scholar] [CrossRef]
- Zheng, K.; Ma, Y.; Chiu, C.; Pang, Y.; Gao, J.; Zhang, C.; Du, D. Co-culture pellet of human Wharton’s jelly mesenchymal stem cells and rat costal chondrocytes as a candidate for articular cartilage regeneration: In vitro and in vivo study. Stem Cell Res. Ther. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Li, Z.; Bi, Y.; Wu, Q.; Chen, C.; Zhou, L.; Qi, J.; Xie, D.; Song, H.; Han, Y.; Qu, P.; et al. A composite scaffold of Wharton’s jelly and chondroitin sulphate loaded with human umbilical cord mesenchymal stem cells repairs articular cartilage defects in rat knee. J. Mater. Sci. Mater. Med. 2021, 32, 36. [Google Scholar] [CrossRef]
- Sadlik, B.; Jaroslawski, G.; Puszkarz, M.; Blasiak, A.; Oldak, T.; Gladysz, D.; Whyte, G.P. Cartilage Repair in the Knee Using Umbilical Cord Wharton’s Jelly–Derived Mesenchymal Stem Cells Embedded Onto Collagen Scaffolding and Implanted Under Dry Arthroscopy. Arthrosc. Tech. 2018, 7, e57–e63. [Google Scholar] [CrossRef] [PubMed]
- Eslaminejad, M.B.; Karimi, N.; Shahhoseini, M. Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells treated by GSK-3 inhibitors. Histochem. Cell Biol. 2013, 140, 623–633. [Google Scholar] [CrossRef]
- Tanthaisong, P.; Imsoonthornruksa, S.; Ngernsoungnern, A.; Ngernsoungnern, P.; Ketudat-Cairns, M.; Parnpai, R. Enhanced chondrogenic differentiation of human umbilical cord wharton’s jelly derived mesenchymal stem cells by GSK-3 Inhibitors. PLoS ONE 2017, 12, e0168059. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.S.; Melton, D.A. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 1996, 93, 8455–8459. [Google Scholar] [CrossRef]
- Wu, G.; Huang, H.; Abreu, J.G.; He, X. Inhibition of GSK3 phosphorylation of β-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS ONE 2009, 3, e4046. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, E.; Furumatsu, T.; Kanazawa, T.; Tamura, M.; Ozaki, T. ROCK inhibitor prevents the dedifferentiation of human articular chondrocytes. Biochem. Biophys. Res. Commun. 2012, 420, 124–129. [Google Scholar] [CrossRef]
- Gegg, C.; Yang, F. The Effects of ROCK Inhibition on Mesenchymal Stem Cell Chondrogenesis Are Culture Model Dependent. Tissue Eng. Part A 2020, 26, 130–139. [Google Scholar] [CrossRef]
- Piltti, J.; Bygdell, J.; Fernández-Echevarría, C.; Marcellino, D.; Lammi, M.J. Rho-kinase inhibitor Y-27632 and hypoxia synergistically enhance chondrocytic phenotype and modify S100 protein profiles in human chondrosarcoma cells. Sci. Rep. 2017, 7, 3708. [Google Scholar] [CrossRef]
- Wang, K.-C.; Egelhoff, T.T.; Caplan, A.I.; Welter, J.F.; Baskaran, H. ROCK Inhibition Promotes the Development of Chondrogenic Tissue by Improved Mass Transport. Tissue Eng. Part A 2018, 24, 1218–1227. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Cantó, C. The molecular targets of resveratrol. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Lee, K.-M.; Ryu, S.B.; Park, Y.J.; Hwang, Y.G.; Baek, D.; Choi, Y.; Park, K.H.; Park, K.D.; Lee, J.W. Enhanced articular cartilage regeneration with SIRT1-activated MSCs using gelatin-based hydrogel. Cell Death Dis. 2018, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, G.; Jalili, C.; Pazhouhi, M.; Khazaei, M. Resveratrol effect on adipose-derived stem cells differentiation to chondrocyte in three-dimensional culture. Adv. Pharm. Bull. 2020, 10, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Peltz, L.; Gomez, J.; Marquez, M.; Alencastro, F.; Atashpanjeh, N.; Quang, T.; Bach, T.; Zhao, Y. Resveratrol exerts dosage and duration dependent effect on human mesenchymal stem cell development. PLoS ONE 2012, 7, e37162. [Google Scholar] [CrossRef] [PubMed]
- Gorkun, A.A.; Shpichka, A.I.; Zurina, I.M.; Koroleva, A.V.; Kosheleva, N.V.; Nikishin, D.A.; Butnaru, D.V.; Timashev, P.S.; Repin, V.S.; Saburina, I.N. Angiogenic potential of spheroids from umbilical cord and adipose-derived multipotent mesenchymal stromal cells within fibrin gel. Biomed. Mater. 2018, 13, 044108. [Google Scholar] [CrossRef]
- Widholz, B.; Tsitlakidis, S.; Reible, B.; Moghaddam, A.; Westhauser, F. Pooling of Patient-Derived Mesenchymal Stromal Cells Reduces Inter-Individual Confounder-Associated Variation without Negative Impact on Cell Viability, Proliferation and Osteogenic Differentiation. Cells 2019, 8, 633. [Google Scholar] [CrossRef] [PubMed]
- Thej, C.; Balasubramanian, S.; Rengasamy, M.; Walvekar, A.; Swamynathan, P.; Raj, S.S.; Shahani, P.; Siddikuzzaman; Kolkundkar, U.; Seetharam, R.N.; et al. Human bone marrow-derived, pooled, allogeneic mesenchymal stromal cells manufactured from multiple donors at different times show comparable biological functions in vitro, and in vivo to repair limb ischemia. Stem Cell Res. Ther. 2021, 12, 279. [Google Scholar] [CrossRef]
- Willer, H.; Spohn, G.; Morgenroth, K.; Thielemann, C.; Elvers-Hornung, S.; Bugert, P.; Delorme, B.; Giesen, M.; Schmitz-Rixen, T.; Seifried, E.; et al. Pooled human bone marrow-derived mesenchymal stromal cells with defined trophic factors cargo promote dermal wound healing in diabetic rats by improved vascularization and dynamic recruitment of M2-like macrophages. Front. Immunol. 2022, 13, 976511. [Google Scholar] [CrossRef]
- Kuci, Z.; Bonig, H.; Kreyenberg, H.; Bunos, M.; Jauch, A.; Janssen, J.W.G.; Kifi, M.; Michel, K.; Eising, B.; Lucchini, G.; et al. Mesenchymal stromal cells from pooled mononuclear cells of multiple bone marrow donors as rescue therapy in pediatric severe steroid-refractory graft-versus-host disease: A multicenter survey. Haematologica 2016, 101, 985–994. [Google Scholar] [CrossRef]
- Frolova, A.; Ksendzov, E.; Kostjuk, S.; Efremov, Y.; Solovieva, A.; Rochev, Y.; Timashev, P.; Kotova, S. Thin Thermoresponsive Polymer Films for Cell Culture: Elucidating an Unexpected Thermal Phase Behavior by Atomic Force Microscopy. Langmuir 2021, 37, 11386–11396. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, G.K.; Presniakova, V.S.; Efremov, Y.M.; Kotova, S.L.; Frolova, A.A.; Kostjuk, S.V.; Rochev, Y.A.; Timashev, P.S. Preparation and Characterization of Thermoresponsive Polymer Scaffolds Based on Poly(N-isopropylacrylamide-co-N-tert-butylacrylamide) for Cell Culture. Technologies 2023, 11, 145. [Google Scholar] [CrossRef]
- Nash, M.E.; Fan, X.; Carroll, W.M.; Gorelov, A.V.; Barry, F.P.; Shaw, G.; Rochev, Y.A. Thermoresponsive Substrates used for the Expansion of Human Mesenchymal Stem Cells and the Preservation of Immunophenotype. Stem Cell Rev. Rep. 2013, 9, 148–157. [Google Scholar] [CrossRef]
- Komiya, Y.; Habas, R. Wnt Secretion and Extra-Cellular Regulators. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef]
- Baumans, V.; Brain, P.F.; Brugére, H.; Clausing, P.; Jeneskog, T.; Perretta, G. Pain and distress in laboratory rodents and lagomorphs. Lab. Anim. 1994, 28, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Han, X.; Liu, J.; Chen, L.; Lei, Y.; Chen, K.; Si, J.; Wang, T.-Y.; Zhou, H.; Zhao, X.; et al. Single-cell Transcriptomic Analysis Reveals the Cellular Heterogeneity of Mesenchymal Stem Cells. Genom. Proteom. Bioinf. 2022, 20, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Zhang, X.; Qi, L.; Zhou, J.; Liu, K. Isolation, identification, and comparison of cartilage stem progenitor/cells from auricular cartilage and perichondrium. Am. J. Transl. Res. 2016, 8, 732–741. [Google Scholar]
- Rakic, R.; Bourdon, B.; Demoor, M.; Maddens, S.; Saulnier, N.; Galéra, P. Differences in the intrinsic chondrogenic potential of equine umbilical cord matrix and cord blood mesenchymal stromal/stem cells for cartilage regeneration. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Guo, W.; Wang, M.; Hao, C.; Gao, S.; Zhang, X.; Li, X.; Chen, M.; Jing, X.; et al. Human umbilical cord Wharton’s jelly mesenchymal stem cells combined with an acellular cartilage extracellular matrix scaffold improve cartilage repair compared with microfracture in a caprine model. Osteoarthr. Cartil. 2018, 26, 954–965. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Harkness, L.; Zaher, W.; Ditzel, N.; Isa, A.; Kassem, M. CD146/MCAM defines functionality of human bone marrow stromal stem cell populations. Stem Cell Res. Ther. 2016, 7, 4. [Google Scholar] [CrossRef]
- Sultan, S.; Alalmie, A.; Noorwali, A.; Alyamani, A.; Shaabad, M.; Alfakeeh, S.; Bahmaid, A.; Ahmed, F.; Pushparaj, P.; Kalamegam, G. Resveratrol promotes chondrogenesis of human Wharton’s jelly stem cells in a hyperglycemic state by modulating the expression of inflammation-related cytokines. All Life 2020, 13, 577–586. [Google Scholar] [CrossRef]
- Cavalli, E.; Fisch, P.; Formica, F.A.; Gareus, R.; Linder, T.; Applegate, L.A.; Zenobi-Wong, M. A comparative study of cartilage engineered constructs in immunocompromised, humanized and immunocompetent mice. J. Immunol. Regen. Med. 2018, 2, 36–46. [Google Scholar] [CrossRef]
- Griesemer, A.; Yamada, K.; Sykes, M. Xenotransplantation: Immunological hurdles and progress toward tolerance. Immunol. Rev. 2014, 258, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Bartoli-Leonard, F.; Wilkinson, F.L.; Langford-Smith, A.W.W.; Alexander, M.Y.; Weston, R. The Interplay of SIRT1 and Wnt Signaling in Vascular Calcification. Front. Cardiovasc. Med. 2018, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Wang, G.; Beier, F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J. Biol. Chem. 2005, 280, 11626–11634. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C. Wnt signaling in cartilage development. In Cartilage: Volume 1: Physiology and Development; Springer: Cham, Switzerland, 2016; pp. 229–252. [Google Scholar] [CrossRef]
- Huang, X.; Zhong, L.; Hendriks, J.; Post, J.N.; Karperien, M. The effects of the WNT-signaling modulators BIO and PKF118-310 on the chondrogenic differentiation of human mesenchymal stem cells. Int. J. Mol. Sci. 2018, 19, 561. [Google Scholar] [CrossRef] [PubMed]
- Schizas, N.P.; Zafeiris, C.; Neri, A.A.; Anastasopoulos, P.P.; Papaioannou, N.A.; Dontas, I.A. Inhibition versus activation of canonical Wnt-signaling, to promote chondrogenic differentiation of Mesenchymal Stem Cells. A review. Orthop. Rev. 2021, 13, 1–9. [Google Scholar] [CrossRef]
- Costa, L.A.; Eiro, N.; Fraile, M.; Gonzalez, L.O.; Saá, J.; Garcia-Portabella, P.; Vega, B.; Schneider, J.; Vizoso, F.J. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: Implications for further clinical uses. Cell. Mol. Life Sci. 2021, 78, 447–467. [Google Scholar] [CrossRef]
- Dunn, C.M.; Kameishi, S.; Grainger, D.W.; Okano, T. Strategies to address mesenchymal stem/stromal cell heterogeneity in immunomodulatory profiles to improve cell-based therapies. Acta Biomater. 2021, 133, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Xue, X.; Sun, J.; Wu, Q.; Wang, H.; Guo, Y.; Sun, B. The promising effects of transplanted umbilical cord mesenchymal stem cells on the treatment in traumatic brain injury. J. Craniofac. Surg. 2018, 29, 1689–1692. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, J.; Shao, X.; Luo, E.; Yu, L. DAPT inhibits the chondrogenesis of human umbilical cord mesenchymal stem cells. Open Life Sci. 2015, 10, 395–408. [Google Scholar] [CrossRef]
- De La Vega, F.M.; Mendoza-Figueroa, T. Dimethyl sulfoxide enhances lipid synthesis and secretion by long-term cultures of adult rat hepatocytes. Biochimie 1991, 73, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, F.A.; Garner, B.; Ball, G.E.; Rae, C. Modulation of brain metabolism by very low concentrations of the commonly used drug delivery vehicle dimethyl sulfoxide (DMSO). J. Neurosci. Res. 2008, 86, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Sainz, B.; Corcoran, P.; Uprichard, S.; Jeong, H. Characterization of increased drug metabolism activity in dimethyl sulfoxide (DMSO)-treated Huh7 hepatoma cells. Xenobiotica 2009, 39, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Thaler, R.; Spitzer, S.; Karlic, H.; Klaushofer, K.; Varga, F. DMSO is a strong inducer of DNA hydroxymethylation in pre-osteoblastic MC3T3-E1 cells. Epigenetics 2012, 7, 635–651. [Google Scholar] [CrossRef]
- Kurenkova, A.D.; Romanova, I.A.; Kibirskiy, P.D.; Timashev, P.; Medvedeva, E.V. Strategies to Convert Cells into Hyaline Cartilage: Magic Spells for Adult Stem Cells. Int. J. Mol. Sci. 2022, 23, 11169. [Google Scholar] [CrossRef]
- Farrell, M.J.; Fisher, M.B.; Huang, A.H.; Shin, J.I.; Farrell, K.M.; Mauck, R.L. Functional properties of bone marrow-derived MSC-based engineered cartilage are unstable with very long-term in vitro culture. J. Biomech. 2014, 47, 2173–2182. [Google Scholar] [CrossRef]
- Li, M.; Yin, H.; Yan, Z.; Li, H.; Wu, J.; Wang, Y.; Wei, F.; Tian, G.; Ning, C.; Li, H.; et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. 2022, 140, 23–42. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurenkova, A.D.; Presniakova, V.S.; Mosina, Z.A.; Kibirskiy, P.D.; Romanova, I.A.; Tugaeva, G.K.; Kosheleva, N.V.; Vinogradov, K.S.; Kostjuk, S.V.; Kotova, S.L.; et al. Resveratrol’s Impact on the Chondrogenic Reagents’ Effects in Cell Sheet Cultures of Wharton’s Jelly-Derived MSCs. Cells 2023, 12, 2845. https://doi.org/10.3390/cells12242845
Kurenkova AD, Presniakova VS, Mosina ZA, Kibirskiy PD, Romanova IA, Tugaeva GK, Kosheleva NV, Vinogradov KS, Kostjuk SV, Kotova SL, et al. Resveratrol’s Impact on the Chondrogenic Reagents’ Effects in Cell Sheet Cultures of Wharton’s Jelly-Derived MSCs. Cells. 2023; 12(24):2845. https://doi.org/10.3390/cells12242845
Chicago/Turabian StyleKurenkova, Anastasiia D., Viktoria S. Presniakova, Zlata A. Mosina, Pavel D. Kibirskiy, Irina A. Romanova, Gilyana K. Tugaeva, Nastasia V. Kosheleva, Kirill S. Vinogradov, Sergei V. Kostjuk, Svetlana L. Kotova, and et al. 2023. "Resveratrol’s Impact on the Chondrogenic Reagents’ Effects in Cell Sheet Cultures of Wharton’s Jelly-Derived MSCs" Cells 12, no. 24: 2845. https://doi.org/10.3390/cells12242845






