The Prognostic Long-Term Impact of Chronic Obstructive Pulmonary Disease and Postoperative Mucostasis in Patients with Curatively Resected Non-Small Cell Lung Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Postoperative Follow-Up Schedule
2.2. Data Management
2.3. Statistical Analysis
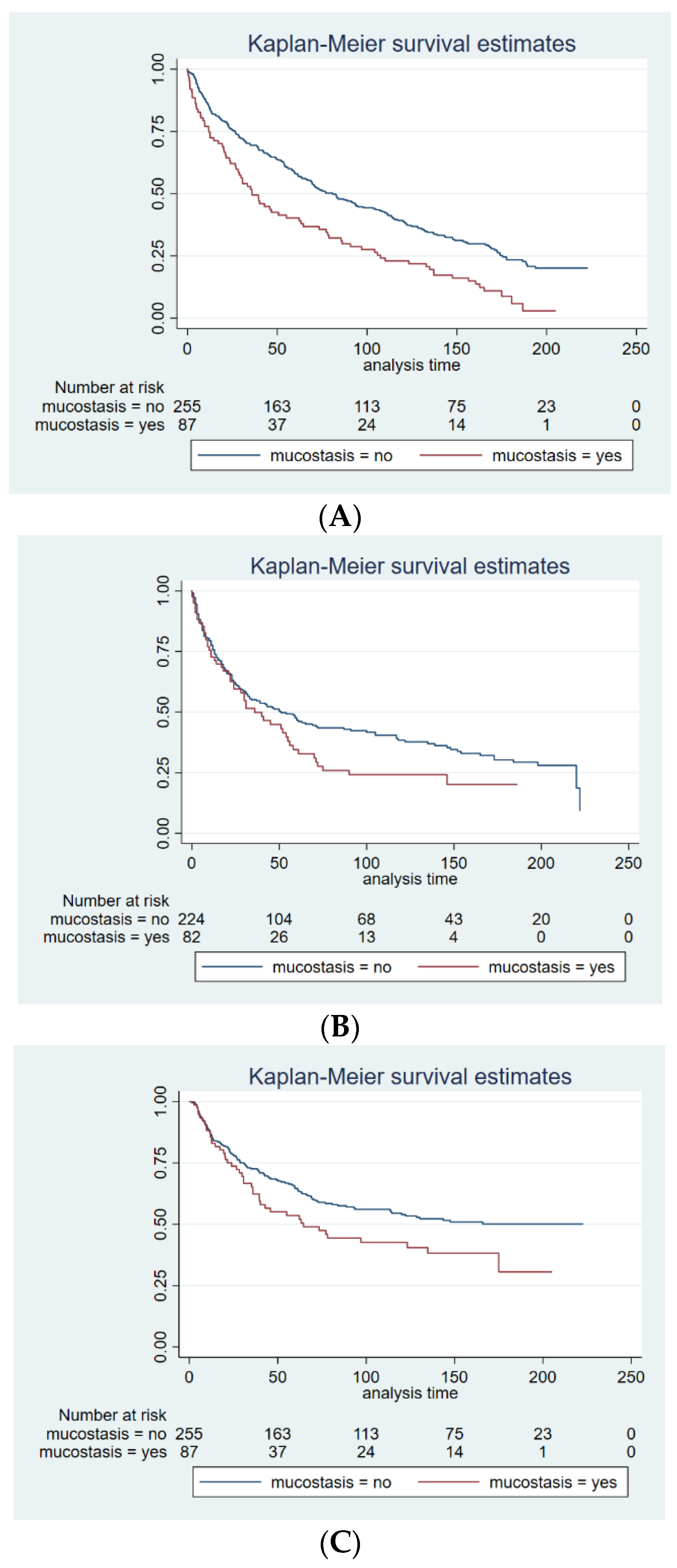
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Lewis, S.Z.; Diekemper, R.; Addrizzo-Harris, D.; Alberts, W.M. Executive summary: Diagnosis and management of lung cancer: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. 5), 7S–37S. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, S.; Ma, S.; Zhang, S. Glasgow prognostic score predicts prognosis of non-small cell lung cancer: A meta-analysis. Springerplus 2016, 5, 43. [Google Scholar] [CrossRef]
- Jiang, A.-G.; Chen, H.-L.; Lu, H.-Y. The relationship between glasgow prognostic score and serum tumor markers in patients with advanced non-small cell lung cancer. BMC Cancer 2015, 15, 386. [Google Scholar] [CrossRef]
- Lindenmann, J.; Fink-Neuboeck, N.; Taucher, V.; Pichler, M.; Posch, F.; Brcic, L.; Smolle, E.; Koter, S.; Smolle, J.; Smolle-Juettner, F.M. Prediction of Postoperative Clinical Outcomes in Resected Stage I Non-Small Cell Lung Cancer Focusing on the Preoperative Glasgow Prognostic Score. Cancers 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 28 April 2022).
- Gardner, L.; Loffredo, C.A.; Langenberg, P.; George, D.M.S.; Deepak, J.; Harris, C.C.; Amr, S. Associations between history of chronic lung disease and non–small cell lung carcinoma in Maryland: Variations by sex and race. Ann. Epidemiol. 2018, 28, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.V.; Rogers, D.F. Novel Therapies to Inhibit Mucus Synthesis and Secretion in Airway Hypersecretory Diseases. Pharmacology 2016, 97, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Adcock, I.M.; Caramori, G.; Barnes, P.J. Chronic Obstructive Pulmonary Disease and Lung Cancer: New Molecular Insights. Respiration 2011, 81, 265–284. [Google Scholar] [CrossRef]
- Taucher, E.; Mykoliuk, I.; Lindenmann, J.; Smolle-Juettner, F.-M. Implications of the Immune Landscape in COPD and Lung Cancer: Smoking Versus Other Causes. Front. Immunol. 2022, 13, 846605. [Google Scholar] [CrossRef]
- Matthys, H.; Vastag, E.; Köhler, D.; Daikeler, G.; Fischer, J. Mucociliary Clearance in Patients with Chronic Bronchitis and Bronchial Carcinoma. Respir. Int. Rev. Thorac. Dis. 1983, 44, 329–337. [Google Scholar] [CrossRef]
- Lim, J.U.; Yeo, C.D.; Rhee, C.K.; Kang, H.S.; Park, C.K.; Kim, J.S.; Kim, J.W.; Kim, S.J.; Yoon, H.K.; Lee, S.H. Comparison of clinical characteristics and overall survival between spirometrically diagnosed chronic obstructive pulmonary disease (COPD) and non-COPD never-smoking stage I-IV non-small cell lung cancer patients. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 929–938. [Google Scholar] [CrossRef]
- Akamine, T.; Tagawa, T.; Shimokawa, M.; Matsubara, T.; Kozuma, Y.; Haratake, N.; Takamori, S.; Toyokawa, G.; Maehara, Y. The prognostic impact of obstructive lung disease on survival of never smokers with resected non-small-cell lung cancer: A comparison with smokers. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 735–743. [Google Scholar] [CrossRef]
- Ginsberg, R.J.; Rubinstein, L.V. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann. Thorac. Surg. 1995, 60, 615–623. [Google Scholar] [CrossRef]
- El-Sherif, A.; Gooding, W.E.; Santos, R.; Pettiford, B.; Ferson, P.F.; Fernando, H.C.; Urda, S.J.; Luketich, J.D.; Landreneau, R.J. Outcomes of sublobar resection versus lo-bectomy for stage I non-small cell lung cancer: A 13-year analysis. Ann. Thorac. Surg. 2006, 82, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Sienel, W.; Dango, S.; Kirschbaum, A.; Cucuruz, B.; Hörth, W.; Stremmel, C.; Passlick, B. Sublobar resections in stage IA non-small cell lung cancer: Segmentectomies result in significantly better cancer-related survival than wedge resections. Eur. J. Cardio-Thorac. Surg. 2008, 33, 728–734. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef]
- Fink-Neuboeck, N.; Lindenmann, J.; Porubsky, C.; Fediuk, M.; Anegg, U.; Maier, A.; Smolle, J.; Lamont, E.; Smolle-Juettner, F.M. Hazards of Recurrence, Second Primary, or Other Tumor at Ten Years After Surgery for Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2020, 21, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.F. Physiology of airway mucus secretion and pathophysiology of hypersecretion. Respir. Care 2007, 52, 1134–1146. [Google Scholar] [PubMed]
- Papi, A.; Casoni, G.; Caramori, G.; Guzzinati, I.; Boschetto, P.; Ravenna, F.; Calia, N.; Petruzzelli, S.; Corbetta, L.; Cavallesco, G.; et al. COPD increases the risk of squamous histological subtype in smokers who develop non-small cell lung carcinoma. Thorax 2004, 59, 679–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engels, E.A. Inflammation in the development of lung cancer: Epidemiological evidence. Expert Rev. Anticancer Ther. 2008, 8, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Lathan, C.S. Lung cancer care: The impact of facilities and area measures. Transl. Lung Cancer Res. 2015, 4, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Parini, S.; Azzolina, D.; Massera, F.; Mastromarino, M.G.; Papalia, E.; Baietto, G.; Curcio, C.; Crisci, R.; Rena, O.; Alloisio, M.; et al. The Overweight Paradox: Impact of Body Mass Index on Patients Undergoing VATS Lobectomy or Segmentectomy. Semin. Thorac. Cardiovasc. Surg. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Zhou, S.; Wang, D.; Zhu, L.; Hou, J.; Tang, J.; Zhao, J.; Zhong, S. Body mass index and mortality in lung cancer patients: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2018, 72, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Berghmans, T.; Paesmans, M.; Meert, A.; Mascaux, C.; Lothaire, P.; Lafitte, J.; Sculier, J. Survival improvement in resectable non-small cell lung cancer with (neo)adjuvant chemotherapy: Results of a meta-analysis of the literature. Lung Cancer 2005, 49, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, D.; Nicolson, M.; Smith, I.; Groen, H.; Dalesio, O.; Goldstraw, P.; Hatton, M.; Hopwood, P.; Manegold, C.; Schramel, F.; et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: Results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 2007, 369, 1929–1937. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, J.; Park, Y.S.; Lee, C.-H.; Lee, S.-M.; Yim, J.-J.; Yoo, C.-G.; Han, S.K.; Kim, Y.W. Impact of Chronic Obstructive Pulmonary Disease on the Mortality of Patients with Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2014, 9, 812–817. [Google Scholar] [CrossRef]
- Ytterstad, E.; Moe, P.C.; Hjalmarsen, A. COPD in primary lung cancer patients: Prevalence and mortality. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 625–636. [Google Scholar] [CrossRef]
- Media, A.S.; Persson, M.; Tajhizi, N.; Weinreich, U.M. Chronic obstructive pulmonary disease and comorbidities’ influence on mortality in non-small cell lung cancer patients. Acta Oncol. 2019, 58, 1102–1106. [Google Scholar] [CrossRef]
- Wu, K.; Wang, J.; Zhao, L.; Wang, P.; Duan, Q. The prognosis of non-small cell lung cancer combined with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Clin. Respir. J. 2020, 14, 389–396. [Google Scholar] [CrossRef]
- Sekine, Y.; Behnia, M.; Fujisawa, T. Impact of COPD on pulmonary complications and on long-term survival of patients un-dergoing surgery for NSCLC. Lung Cancer 2002, 37, 95–101. [Google Scholar] [CrossRef]
- Choi, S.; Park, J. Surgical outcomes and prognosis of non-small-cell lung cancer in patients with chronic lung diseases: A ret-rospective analysis. Eur. J. Cardiothorac. Surg. 2020, 58, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Iachina, M.; Jakobsen, E.; Moller, H.; Lüchtenborg, M.; Mellemgaard, A.; Krasnik, M.; Green, A. The Effect of Different Comorbidities on Survival of Non-small Cells Lung Cancer Patients. Lung 2015, 193, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Yu, X.; Shafer, A.; Wain, J.C.; Christiani, D.C. The Impact of Coexisting COPD on Survival of Patients With Early-Stage Non-small Cell Lung Cancer Undergoing Surgical Resection. Chest 2014, 145, 346–353. [Google Scholar] [CrossRef]
- López-Encuentra, A.; Astudillo, J.; Cerezal, J.; Gonzalez-Aragoneses, F.; Novoa, N.; Sánchez-Palencia, A. Bronchogenic Carcinoma Cooperative Group of the Spanish Society of Pneumology and Thoracic Surgery (GCCB-S). Prognostic value of chronic ob-structive pulmonary disease in 2994 cases of lung cancer. Eur. J. Cardiothorac. Surg. 2005, 27, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Frostad, A.; Søyseth, V.; Haldorsen, T.; Andersen, A.; Gulsvik, A. Impact of respiratory symptoms on lung cancer: 30-year follow-up of an urban population. Lung Cancer 2008, 60, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Denholm, R.; Schüz, J.; Straif, K.; Stücker, I.; Jöckel, K.H.; Brenner, D.R.; De Matteis, S.; Boffetta, P.; Guida, F.; Brüske, I.; et al. Is previous respiratory disease a risk factor for lung cancer? Am. J. Respir. Crit. Care Med. 2014, 190, 549–559. [Google Scholar] [CrossRef] [Green Version]
| All Cases n = 342 | Non-COPD n = 161 (47.1%) | COPD n = 181 (52.9%) | Significance | Non-Mucostasis n = 255 (74.6%) | Mucostasis n = 87 (25.4%) | Significance | Non-Smoker n = 68 (19.9%) | Smoker n = 274 (80.1%) | Significance | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years; mean) | 63.6 ± 9.5 | 63.1 ± 9.7 | 64.1 ± 9.3 | t = −0.98, p = 0.328 | 63.1 ± 9.7 | 65.2 ± 87.8 | t = −1.85, p = 0.0651 | 67.8 ± 9.9 | 62.6 ± 9.1 | t = 4.12, p = 0.000 |
| Weight (kg; mean) | 75.5 ± 14.3 | 74.4 ± 14.4 | 67.5 ± 14.1 | t = −35, p = 1.0.178 | 75.1 ± 14.3 | 76.6 ± 14.4 | t = −0.85, p = 0.3976 | 71.5 ± 11.4 | 76.4 ± 14.8 | t = −2.56, p = 0.011 |
| BMI (kg/m2; mean) | 25.9 ± 4.0 | 25.9 ± 4.0 | 26.0 ± 4.0 | t = −0.26, p = 0.795 | 25.8 ± 3.8 | 26.2 ± 4.5 | t = −0.89, p = 0.3693 | 25.8 ± 3.5 | 25.9 ± 4.1 | t = −0.19, p = 0.849 |
| Gender | ||||||||||
| Male | 225 (65.8%) | 87 (45.0%) | 138 (76.2%) | chi2 = 18.67, p = 0.000 | 160 (62.8%) | 65 (74.7%) | chi2 = 4.13, p = 0.042 | 27 (39.7%) | 198 (72.3%) | chi2 = 25.66, p = 0.000 |
| Female | 117 (34.2%) | 74 (46.0% | 43 (23.8%) | 95 (37.3%) | 22 (25.3%) | 41 (60.3%) | 76 (27.7%) | |||
| Smoking Status | ||||||||||
| No | 68 (19.9%) | 50 (31.1%) | 18 (9.9%) | chi2 = 23.83, p = 0.000 | 58 (22.8%) | 10 (11.5%) | chi2 = 5.15, p = 0.023 | |||
| Yes | 274 (80.1%) | 111 (68.9%) | 163 (90.1%) | 197 (77.3%) | 77 (88.5%) | |||||
| COPD | ||||||||||
| No | 161 (47.1%) | 131 (51.4%) | 30 (34.5%) | chi2 = 7.43, p = 0.006 | 50 (73.5%) | 111 (40.5%) | chi2 = 23.83, p = 0.000 | |||
| Yes | 181 (52.9%) | 124 (48.6%) | 57 (65.5%) | 18 (26.5%) | 163 (59.5%) | |||||
| Mucostasis | ||||||||||
| No | 255 (74.6%) | 131 (81.4%) | 124 (68.5%) | chi2 = 7.42, p = 0.006 | 58 (85.3%) | 197 (71.9%) | chi2 = 5.15, p = 0.023 | |||
| Yes | 87 (25.4%) | 30 (18.6%) | 57 (31.5%) | 10 (14.7%) | 77 (28.1%) |
| All Cases n = 342 | Non-COPD n = 161 (47.1%) | COPD n = 181 (52.9%) | Significance | Non-Mucostasis n = 255 (74.6%) | Mucostasis n = 87 (25.4%) | Significance | Non-Smoker n = 68 (19.9%) | Smoker n = 274 (80.1%) | Significance | |
|---|---|---|---|---|---|---|---|---|---|---|
| Histology | ||||||||||
| Adenocarcinoma | 140 (40.9%) | 79 (49.1%) | 61 (33.7%) | chi2 = 8.34, p = 0.015 | 110 (43.1%) | 30 (34.5%) | chi2 = 3.228, p = 0.199 | 43 (63.2%) | 97 (35.4%) | chi2 = 17.55, p = 0.000 |
| Squamous cell carcinoma | 112 (32.8%) | 46 (28.6%) | 66 (36.5%) | 77 (30.2%) | 35 (40.2%) | 13 (19.1%) | 99 (36.1%) | |||
| Other | 90 (26.3%) | 36 (22.4%) | 54 (29.8%) | 68 (26.7%) | 22 (25.3%) | 12 (17.7%) | 78 (28.5%) | |||
| Grading | ||||||||||
| G1 | 42 (12.3%) | 26 (16.2%) | 16 (8.8%) | chi2 = 5.20, p = 0.074 | 33 (12.9%) | 9 (10.3%) | chi2 = 0.49, p = 0.782 | 12 (17.7%) | 30 (11.0%) | chi2 = 3.49, p = 0.175 |
| G2 | 135 (39.4%) | 65 (40.4%) | 70 (38.7%) | 101 (39.6%) | 34 (39.1%) | 29 (42.7%) | 106 (38.7%) | |||
| G3 | 165 (48.3%) | 70 (43.5%) | 95 (52.5%) | 121 (47.5%) | 44 (50.6%) | 27 (39.7%) | 138 (50.4%) | |||
| Tumor size | ||||||||||
| T0 | 0 (0.9%) | 0 (0.0%) | 3 (1.7%) | chi2 = 7.89, p = 0.096 | 2 (0.8%) | 1 (1.2%) | chi2 = 3.37, p = 0.497 | 0 (0.0%) | 3 (1.1%) | chi2 = 5.36, p = 0.252 |
| T1 | 186 (54.4%) | 94 (58.4%) | 92 (50.8%) | 144 (56.5%) | 42 (48.3%) | 41 (60.3%) | 145 (52.9%) | |||
| T2 | 128 (37.4%) | 52 (32.3%) | 76 (42.0%) | 89 (34.9%) | 39 (44.8%) | 26 (38.2%) | 102 (37.2%) | |||
| T3 | 18 (5.3%) | 10 (6.2%) | 8 (4.4%) | 15 (5.9%) | 3 (3.5%) | 1 (1.5%) | 17 (6.2%) | |||
| T4 | 7 (2.1%) | 5 (3.1%) | 2 (1.1%) | 5 (2.0%) | 2 (2.3%) | 0 (0.0%) | 7 (2.6%) | |||
| Tumor stage | ||||||||||
| 0 | 3 (0.9%) | 0 (0.0%) | 3 (1.7%) | chi2 = 13.19, p = 0.040 | 2 (0.8%) | 1 (1.2%) | chi2 = 3.96, p = 0.682 | 0 (0.0%) | 3 (1.1%) | chi2 = 4.67, p = 0.586 |
| IA | 120 (35.1%) | 64 (39.8%) | 56 (30.9%) | 95 (37.3%) | 25 (28.7%) | 27 (39.7%) | 93 (34.0%) | |||
| IB | 62 (18.1%) | 22 (13.7%) | 40 (22.1%) | 42 (16.5%) | 20 (23.0%) | 15 (22.1%) | 47 (17.2%) | |||
| IIA | 43 (12.6%) | 18 (11.2%) | 25 (13.8%) | 34 (13.3%) | 9 (10.3%) | 8 (11.8%) | 35 (12.8%) | |||
| IIB | 42 (12.3%) | 16 (9.9%) | 26 (14.4%) | 31 (12.2%) | 11 (12.6%) | 6 (8.8%) | 36 (13.1%) | |||
| IIIA | 65 (19.0%) | 36 (22.4%) | 29 (16.0%) | 46 (18.0%) | 19 (21.8%) | 12 (17.7%) | 53 (19.3%) | |||
| IIIB | 7 (2.1%) | 5 (3.1%) | 2 (1.1%) | 5 (1.0%) | 2 (2.3%) | 0 (0.0%) | 7 (2.6%) | |||
| Surgical treatment | ||||||||||
| Bi-, Lobectomy (incl. Sleeve technique) | 315 (92.1%) | 150 (93.2%) | 165 (91.2%) | chi2 = 0.47, p = 0.492 | 234 (91.8%) | 81 (93.1%) | chi2 = 0.16, p = 0.689 | 66 (97.1%) | 249 (90.9%) | chi2 = 2.86, p = 0.091 |
| Pneumonectomy | 27 (7.9%) | 11 (6.8%) | 16 (8.8%) | 21 (8.2%) | 6 (6.9%) | 2 (2.9%) | 25 (9.1%) | |||
| Additional treatment | ||||||||||
| Neo-adjuvant Chemotherapy | 44 (12.9%) | 21 (13.0%) | 23 (12.7%) | chi2 = 0.01, p = 0.926 | 35 (13.7%) | 9 (10.3%) | chi2 = 0.66, p = 0.416 | 4 (5.9%) | 40 (16.6%) | chi2 = 3.69, p = 0.055 |
| Adjuvant Chemotherapy | 93 (27.2%) | 33 (20.5%) | 60 (33.2%) | chi2 = 6.89, p = 0.009 | 73 (28.6%) | 20 (23.0%) | chi2 = 1.04, p = 0.307 | 12 (17.7%) | 81 (29.6%) | chi2 = 3.91, p = 0.048 |
| Adjuvant Chemo-Radiotherapy | 11 (3.2%) | 4 (2.5%) | 7 (3.9%) | chi2 = 0.52, p = 0.469 | 8 (3.1%) | 3 (3.5%) | chi2 = 0.02, p = 0.887 | 2 (2.9%) | 9 (3.3%) | chi2 = 0.02, p = 0.886 |
| Adjuvant Radiotherapy | 34 (9.9%) | 15 (9.3%) | 19 (10.5%) | chi2 = 0.13, p = 0.716 | 24 (9.4%) | 10 (11.5%) | chi2 = 0.31, p = 0.575 | 6 (8.8%) | 28 (10.2%) | chi2 = 0.12, p = 0.731 |
| Cause of Death | Number | Percentage (%) |
|---|---|---|
| NSCLC | 157 | 45.9 |
| Neoplasia other than NSCLC | 22 | 6.4 |
| Other than neoplasia | 66 | 19.3 |
| Chronic cardiac failure | 11 | 3.2 |
| Pneumonia | 9 | 2.6 |
| Myocardial infarction | 8 | 2.3 |
| Renal failure | 7 | 2.0 |
| Stroke | 7 | 2.0 |
| Dementia | 5 | 1.5 |
| Right heart failure | 5 | 1.5 |
| COPD | 3 | 0.9 |
| Decrepitude | 2 | 0.6 |
| Pulmonary embolism | 2 | 0.6 |
| Ileus | 2 | 0.6 |
| Parkinson´s disease | 1 | 0.3 |
| Antibody deficiency syndrome | 1 | 0.3 |
| Multiorgan failure | 1 | 0.3 |
| Peritonitis | 1 | 0.3 |
| Influenza | 1 | 0.3 |
| Total | 245 | 71.6 |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | z | p | Hazard Ratio | z | p | |
| OS | ||||||
| Age | 1.020 ± 0.006 | 2.99 | 0.003 | 1.026 ± 0.007 | 3.67 | 0.000 |
| Weight | 0.994 ± 0.004 | −1.32 | 0.187 | |||
| Bmi | 0.976 ± 0.016 | −1.41 | 0.157 | 0.954 ± 0.015 | −2.80 | 0.005 |
| Female | 0.088 ± 0.109 | −1.63 | 0.103 | |||
| Smoking | 1.075 ± 0.170 | 0.46 | 0.647 | |||
| Copd | 1.013 ± 0.129 | 0.11 | 0.914 | |||
| Mucostasis | 1.672 ± 0.234 | 3.67 | 0.000 | 1.703 ± 0.243 | 3.73 | 0.000 |
| Adenocarcinoma | ref. | |||||
| Squamous cell carcinoma | 1.088 ± 0.164 | 0.56 | 0.575 | |||
| Other | 1.398 ± 0.221 | 2.12 | 0.034 | |||
| Grading | 1.192 ± 0.112 | 1.86 | 0.063 | |||
| pT | 1.464 ± 0.133 | 4.18 | 0.000 | |||
| Stage (UICC) | 1.756 ± 0.139 | 7.08 | 0.000 | 1.679 ± 0.141 | 6.17 | 0.000 |
| Pneumonectomy | 1.682 ± 0.393 | 2.23 | 0.026 | |||
| Neo-adjuvant chemotherapy | 2.243 ± 0.395 | 4.59 | 0.000 | 1.815 ± 0.348 | 3.10 | 0.002 |
| Adjuvant chemotherapy | 0.959 ± 0.139 | −0.28 | 0.778 | |||
| Adjuvant chemo-radiotherapy | 1.135 ± 0.408 | 0.35 | 0.723 | |||
| Adjuvant radiotherapy | 1.406 ± 0.287 | 1.67 | 0.095 | |||
| CSS | ||||||
| Age | 1.008 ± 0.007 | 1.04 | 0.299 | 1.019 ± 0.008 | 2.28 | 0.023 |
| Weight | 0.989 ± 0.005 | −1.91 | 0.056 | |||
| Bmi | 0.966 ± 0.018 | −1.75 | 0.081 | 0.954 ± 0.019 | −2.30 | 0.021 |
| Female | 1.004 ± 0.155 | 0.03 | 0.979 | |||
| Smoking | 0.923 ± 0.164 | −0.45 | 0.655 | |||
| Copd | 0.094 ± 0.133 | −0.74 | 0.458 | |||
| Mucostasis | 1.393 ± 0.237 | 1.95 | 0.052 | 1.588 ± 0.278 | 2.64 | 0.008 |
| Adenocarcinoma | ref. | |||||
| Squamous cell carcinoma | 0.772 ± 0.144 | −1.37 | 0.169 | 0.619 ± 0.119 | −2.49 | 0.013 |
| Other | 1.446 ± 0.254 | 2.10 | 0.036 | 1.213 ± 0.220 | 1.07 | 0.286 |
| Grading | 1.156 ± 0.127 | 1.32 | 0.187 | |||
| pT | 1.438 ± 0.153 | 3.41 | 0.001 | |||
| Stage (UICC) | 1.937 ± 0.177 | 7.22 | 0.000 | 1.873 ± 0.181 | 6.46 | 0.000 |
| Pneumonectomy | 1.424 ± 0.426 | 1.18 | 0.237 | |||
| Neo-adjuvant chemotherapy | 2.664 ± 0.517 | 5.05 | 0.000 | 1.823 ± 0.402 | 2.72 | 0.006 |
| Adjuvant chemotherapy | 1.175 ± 0.191 | 0.99 | 0.323 | |||
| Adjuvant chemo-radiotherapy | 1.560 ± 0.565 | 1.23 | 0.219 | |||
| Adjuvant radiotherapy | 1.841 ± 0.397 | 2.83 | 0.005 | |||
| RFS | ||||||
| Age | 1.001 ± 0.007 | 0.16 | 0.869 | |||
| Weight | 0.987 ± 0.005 | −2.43 | 0.015 | |||
| Bmi | 0.960 ± 0.017 | −2.14 | 0.032 | 0.956 ± 0.017 | −2.36 | 0.018 |
| Female | 1.110 ± 0.162 | 0.72 | 0.472 | |||
| Smoking | 0.909 ± 0.154 | −0.56 | 0.576 | |||
| Copd | 0.944 ± 0.134 | −0.40 | 0.688 | |||
| Mucostasis | 1.371 ± 0.220 | 1.96 | 0.049 | 1.544 ± 0.255 | 2.63 | 0.009 |
| Adenocarcinoma | ref. | |||||
| Squamous cell carcinoma | 0.789 ± 0.137 | −1.36 | 0.174 | 0.627 ± 0.104 | −2.80 | 0.005 |
| Other | 1.333 ± 0.226 | 1.70 | 0.089 | |||
| Grading | 1.123 ± 0.116 | 1.13 | 0.258 | |||
| pT | 1.384 ± 0.137 | 3.27 | 0.001 | |||
| Stage (UICC) | 1.794 ± 0.156 | 6.71 | 0.000 | 1.737 ± 0.160 | 5.99 | 0.000 |
| Pneumonectomy | 1.804 ± 0.471 | 2.26 | 0.024 | |||
| Neo-adjuvant chemotherapy | 2.354 ± 0.451 | 4.46 | 0.000 | 1.637 ± 0.338 | 2.39 | 0.017 |
| Adjuvant chemotherapy | 1.290 ± 0.197 | 1.66 | 0.096 | |||
| Adjuvant chemo-radiotherapy | 1.610 ± 0.550 | 1.40 | 0.163 | |||
| Adjuvant radiotherapy | 1.638 ± 0.345 | 2.34 | 0.019 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindenmann, J.; Fediuk, M.; Fink-Neuboeck, N.; Mykoliuk, I.; Taucher, E.; Pichler, M.; Smolle, J.; Smolle-Juettner, F.M. The Prognostic Long-Term Impact of Chronic Obstructive Pulmonary Disease and Postoperative Mucostasis in Patients with Curatively Resected Non-Small Cell Lung Cancer. Cells 2023, 12, 480. https://doi.org/10.3390/cells12030480
Lindenmann J, Fediuk M, Fink-Neuboeck N, Mykoliuk I, Taucher E, Pichler M, Smolle J, Smolle-Juettner FM. The Prognostic Long-Term Impact of Chronic Obstructive Pulmonary Disease and Postoperative Mucostasis in Patients with Curatively Resected Non-Small Cell Lung Cancer. Cells. 2023; 12(3):480. https://doi.org/10.3390/cells12030480
Chicago/Turabian StyleLindenmann, Joerg, Melanie Fediuk, Nicole Fink-Neuboeck, Iurii Mykoliuk, Elisabeth Taucher, Martin Pichler, Josef Smolle, and Freyja Maria Smolle-Juettner. 2023. "The Prognostic Long-Term Impact of Chronic Obstructive Pulmonary Disease and Postoperative Mucostasis in Patients with Curatively Resected Non-Small Cell Lung Cancer" Cells 12, no. 3: 480. https://doi.org/10.3390/cells12030480





