Prospective Evaluation of CD45RA+/CCR7- Effector Memory T (TEMRA) Cell Subsets in Patients with Primary and Secondary Brain Tumors during Radiotherapy of the Brain within the Scope of the Prospective Glio-CMV-01 Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Cohort
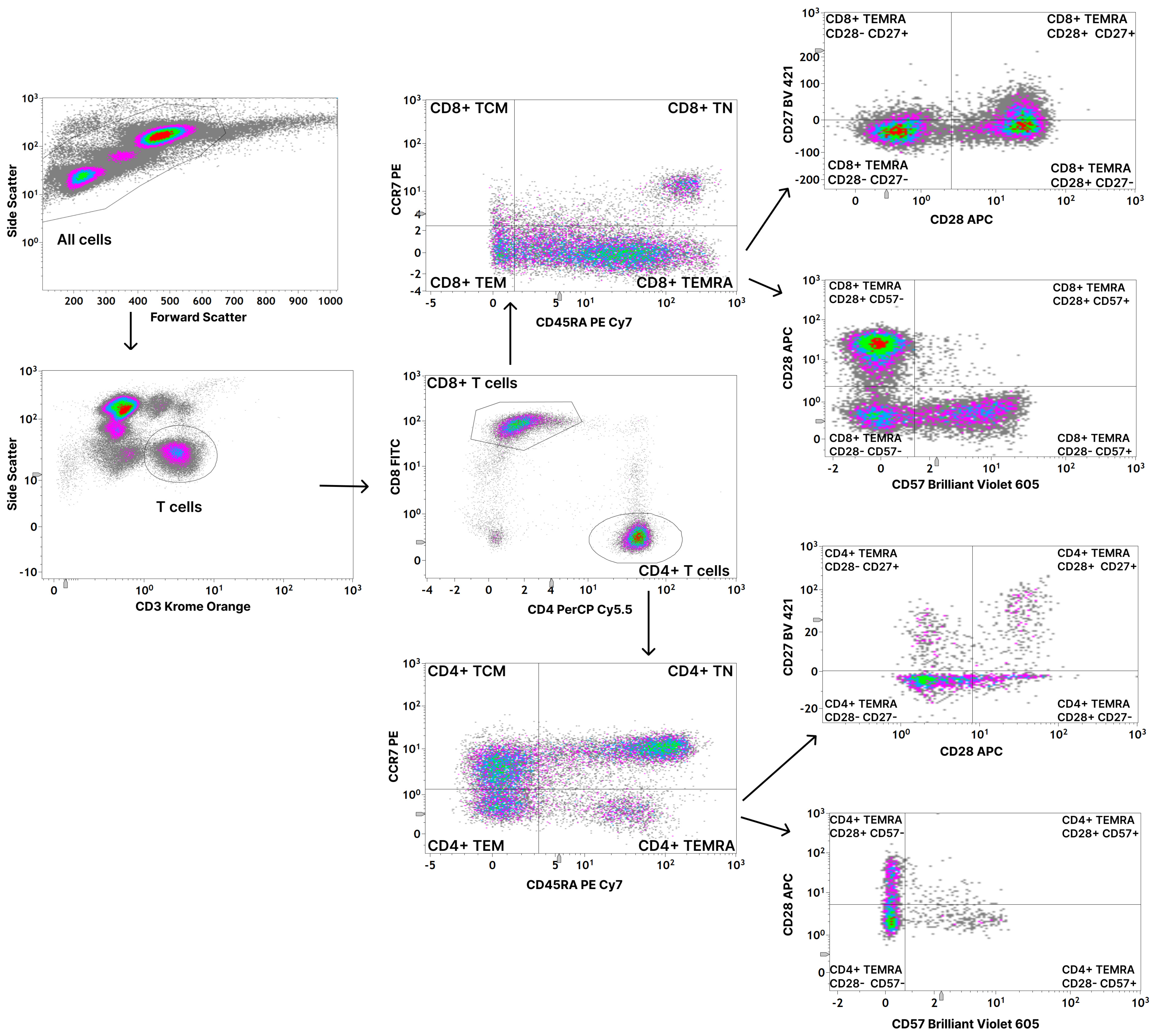
2.3. Flow Cytometric Analysis
2.4. Statistical Analysis
3. Results
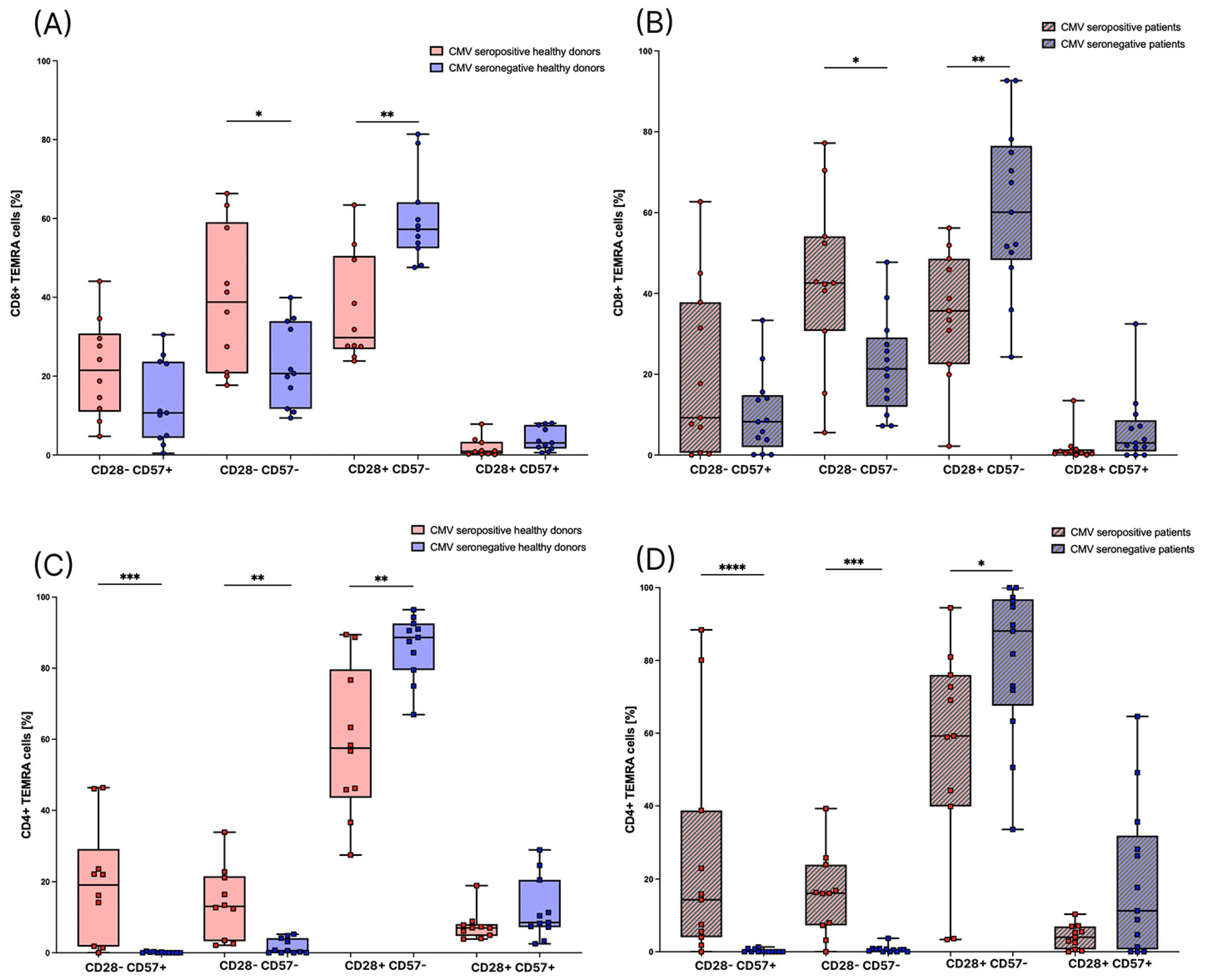
3.1. CMV Seropositive Healthy Individuals Have Significantly Higher Amounts of CD8+ TEMRA Cells
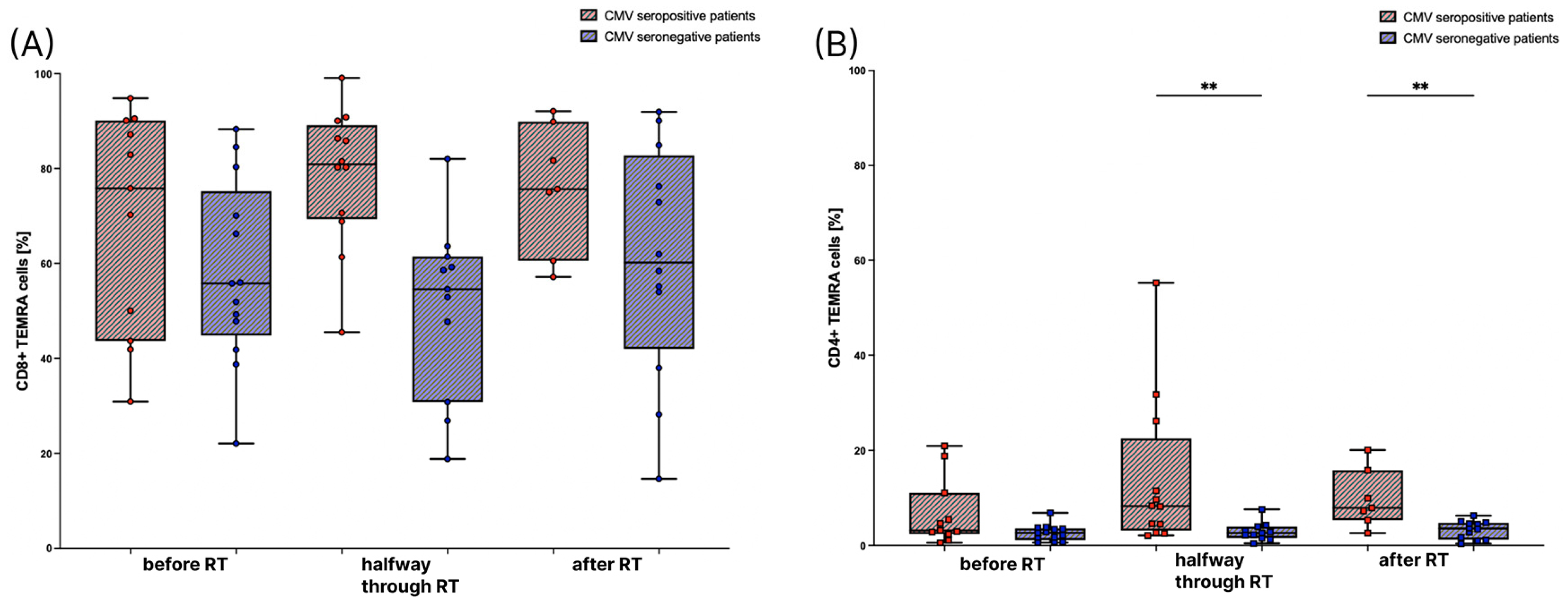
3.2. The Amount of TEMRA Cells during Radiation Therapy
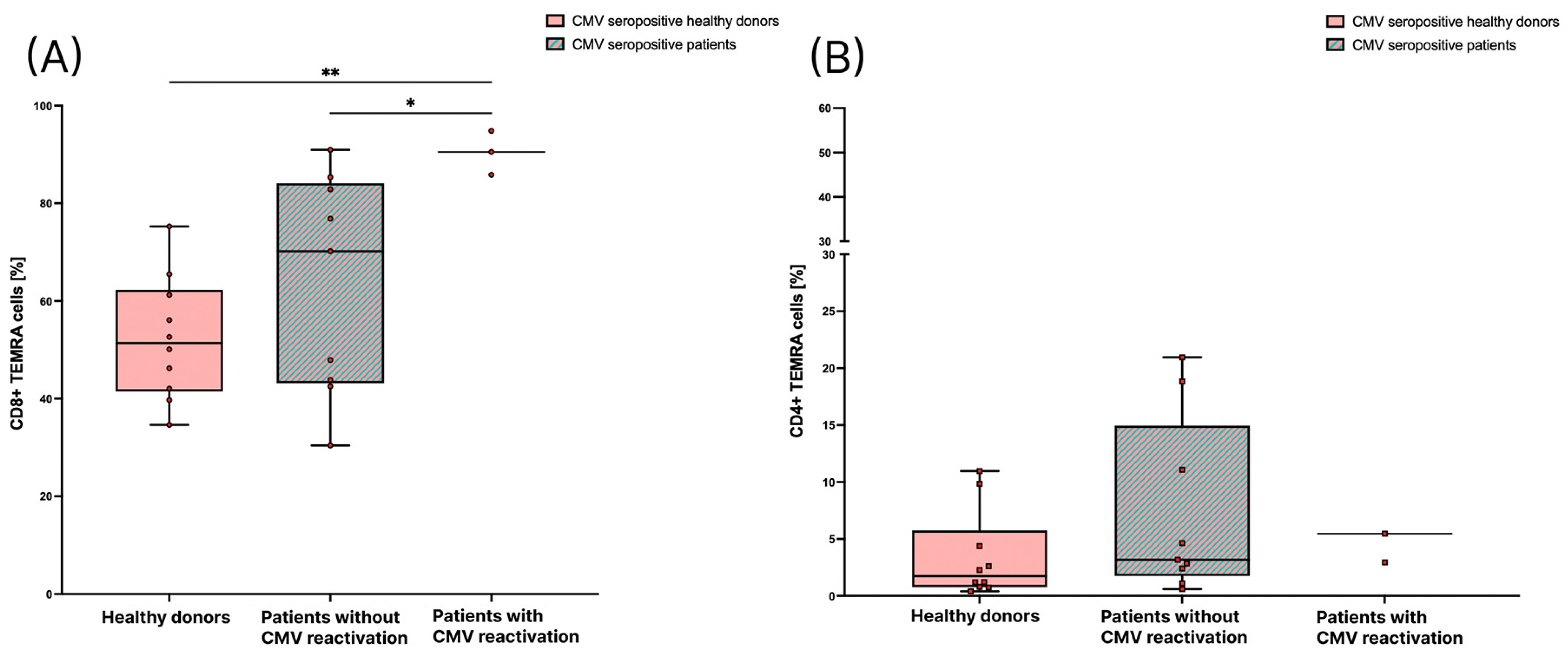
3.3. Patients with CMV Reactivation Have a Significantly Higher Percentage of CD8+ TEMRA Cells
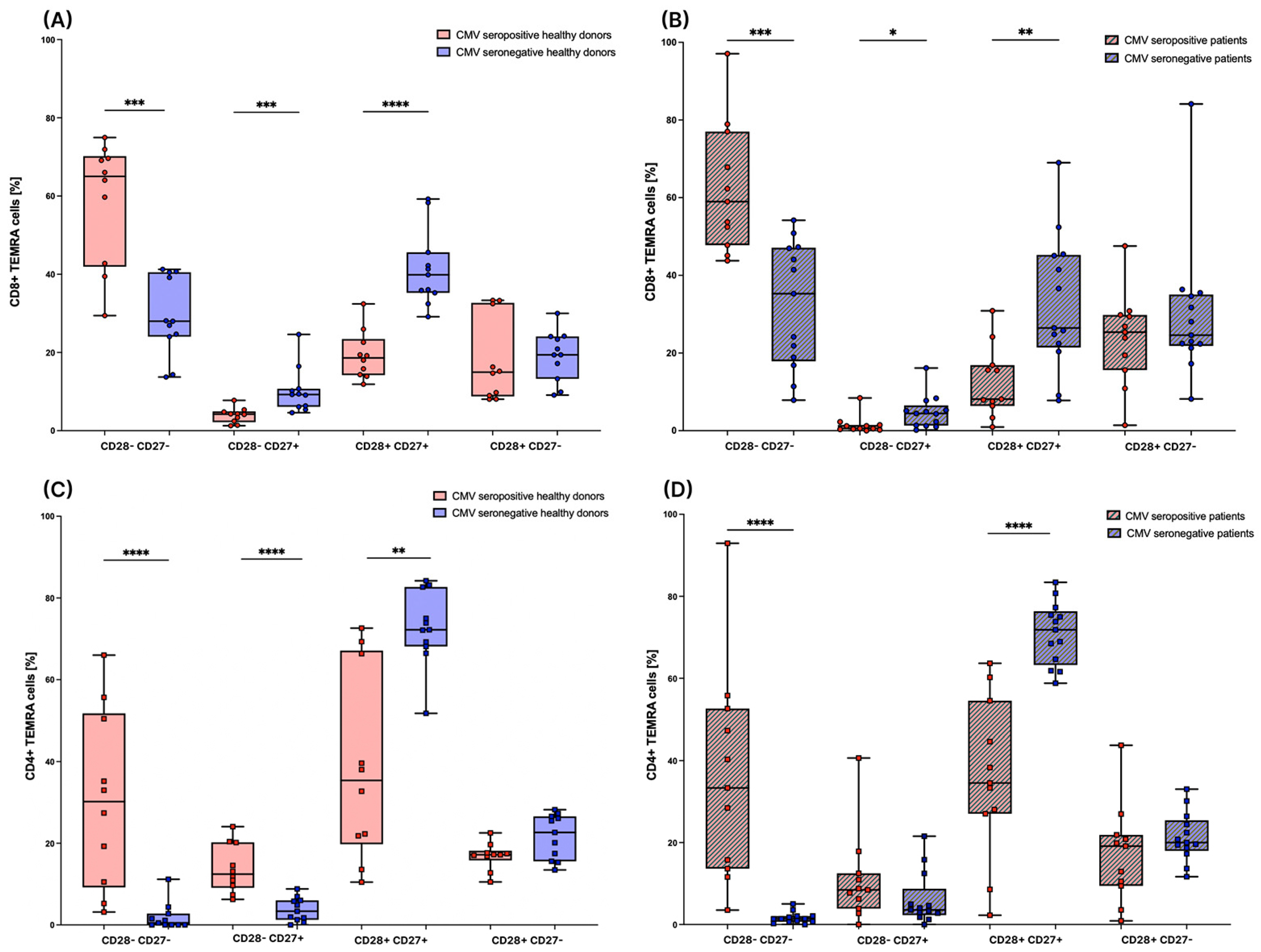
3.4. Subsets of TEMRA Cells Are Strongly Influenced by the CMV Serostatus
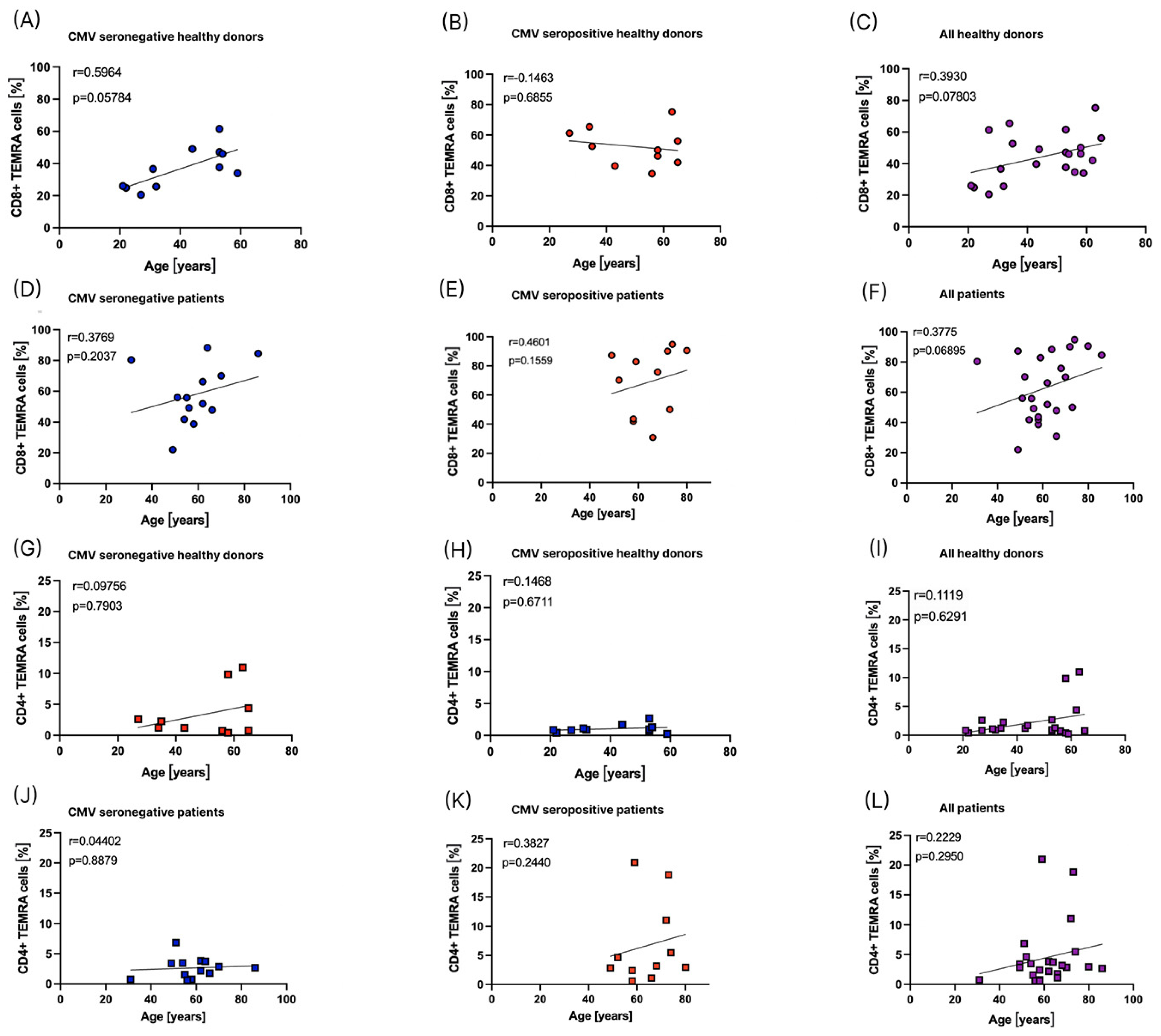
3.5. Influence of Age on the Distribution of TEMRA Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sodeik, B.; Messerle, M.; Schulz, T.F. Herpesviren. In Medizinische Mikrobiologie und Infektiologie; Suerbaum, S., Burchard, G.-D., Kaufmann, S.H.E., Schulz, T.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 723–747. [Google Scholar]
- Goerig, N.L.; Frey, B.; Korn, K.; Fleckenstein, B.; Uberla, K.; Schmidt, M.A.; Dorfler, A.; Engelhorn, T.; Eyupoglu, I.; Ruhle, P.F.; et al. Frequent occurrence of therapeutically reversible CMV-associated encephalopathy during radiotherapy of the brain. Neuro-Oncology 2016, 18, 1664–1672. [Google Scholar] [CrossRef]
- Goerig, N.L.; Frey, B.; Korn, K.; Fleckenstein, B.; Uberla, K.; Schmidt, M.A.; Dorfler, A.; Engelhorn, T.; Eyupoglu, I.; Ruhle, P.F.; et al. Early Mortality of Brain Cancer Patients and its Connection to Cytomegalovirus Reactivation During Radiochemotherapy. Clin. Cancer Res. 2020, 26, 3259–3270. [Google Scholar] [CrossRef] [PubMed]
- Bodensohn, R.; Kaempfel, A.L.; Fleischmann, D.F.; Hadi, I.; Hofmaier, J.; Garny, S.; Reiner, M.; Forbrig, R.; Corradini, S.; Thon, N.; et al. Simultaneous stereotactic radiosurgery of multiple brain metastases using single-isocenter dynamic conformal arc therapy: A prospective monocentric registry trial. Strahlenther. Onkol. 2021, 197, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Rühle, P.F.; Fietkau, R.; Gaipl, U.S.; Frey, B. Development of a Modular Assay for Detailed Immunophenotyping of Peripheral Human Whole Blood Samples by Multicolor Flow Cytometry. Int. J. Mol. Sci. 2016, 17, 1316. [Google Scholar] [CrossRef] [PubMed]
- Donaubauer, A.J.; Rühle, P.F.; Becker, I.; Fietkau, R.; Gaipl, U.S.; Frey, B. One-Tube Multicolor Flow Cytometry Assay (OTMA) for Comprehensive Immunophenotyping of Peripheral Blood. Methods Mol. Biol. 2019, 1904, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Donaubauer, A.J.; Becker, I.; Rühle, P.F.; Fietkau, R.; Gaipl, U.S.; Frey, B. Analysis of the immune status from peripheral whole blood with a single-tube multicolor flow cytometry assay. Methods Enzymol. 2020, 632, 389–415. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G.; Derhovanessian, E.; Larbi, A.; Strindhall, J.; Wikby, A. Cytomegalovirus and human immunosenescence. Rev. Med. Virol. 2009, 19, 47–56. [Google Scholar] [CrossRef]
- Sallusto, F.; Lenig, D.; Förster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef]
- Sallusto, F.; Geginat, J.; Lanzavecchia, A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu. Rev. Immunol. 2004, 22, 745–763. [Google Scholar] [CrossRef]
- Hamann, D.; Baars, P.A.; Rep, M.H.; Hooibrink, B.; Kerkhof-Garde, S.R.; Klein, M.R.; van Lier, R.A. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 1997, 186, 1407–1418. [Google Scholar] [CrossRef]
- Geginat, J.; Lanzavecchia, A.; Sallusto, F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood 2003, 101, 4260–4266. [Google Scholar] [CrossRef]
- Burel, J.G.; Qian, Y.; Lindestam Arlehamn, C.; Weiskopf, D.; Zapardiel-Gonzalo, J.; Taplitz, R.; Gilman, R.H.; Saito, M.; de Silva, A.D.; Vijayanand, P.; et al. An Integrated Workflow To Assess Technical and Biological Variability of Cell Population Frequencies in Human Peripheral Blood by Flow Cytometry. J. Immunol. 2017, 198, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Salumets, A.; Tserel, L.; Rumm, A.P.; Türk, L.; Kingo, K.; Saks, K.; Oras, A.; Uibo, R.; Tamm, R.; Peterson, H.; et al. Epigenetic quantification of immunosenescent CD8(+) TEMRA cells in human blood. Aging Cell 2022, 21, e13607. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.; Ogonek, J.; Varanasi, P.R.; Luther, S.; Bunting, I.; Thomay, K.; Behrens, Y.L.; Mischak-Weissinger, E.; Hambach, L. Human CD8+ CD57- TEMRA cells: Too young to be called “old”. PLoS ONE 2017, 12, e0177405. [Google Scholar] [CrossRef]
- Rufer, N.; Zippelius, A.; Batard, P.; Pittet, M.J.; Kurth, I.; Corthesy, P.; Cerottini, J.C.; Leyvraz, S.; Roosnek, E.; Nabholz, M.; et al. Ex vivo characterization of human CD8+ T subsets with distinct replicative history and partial effector functions. Blood 2003, 102, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Larbi, A.; Derhovanessian, E.; Ozcelik, D.; Naumova, E.; Pawelec, G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun. Ageing 2008, 5, 6. [Google Scholar] [CrossRef]
- Sumpf, B.I.; Freie Universität Berlin. Available online: https://refubium.fu-berlin.de/handle/fub188/5975 (accessed on 3 February 2023).
- Li, L.; Li, X.W.; Ma, C.J.; Wang, L.H.; Yu, F.T.; Yang, S.Y.; Song, S.J.; Tang, Y.X. Accelerated Aging of T-cell Subsets among ART-Naïve HIV-Infected Chinese Men who Have Sex with Men: A Case-control Study. Curr. HIV Res. 2022, 20, 129–136. [Google Scholar] [CrossRef]
- Derhovanessian, E.; Maier, A.B.; Hahnel, K.; Beck, R.; de Craen, A.J.M.; Slagboom, E.P.; Westendorp, R.G.J.; Pawelec, G. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J. Gen. Virol. 2011, 92, 2746–2756. [Google Scholar] [CrossRef]
- Weltevrede, M.; Eilers, R.; de Melker, H.E.; van Baarle, D. Cytomegalovirus persistence and T-cell immunosenescence in people aged fifty and older: A systematic review. Exp. Gerontol. 2016, 77, 87–95. [Google Scholar] [CrossRef]
- Derhovanessian, E.; Larbi, A.; Pawelec, G. Biomarkers of human immunosenescence: Impact of Cytomegalovirus infection. Curr. Opin. Immunol. 2009, 21, 440–445. [Google Scholar] [CrossRef]
- Mengling, V.; Bert, C.; Perrin, R.; Masitho, S.; Weissmann, T.; Mansoorian, S.; Siavooshhaghighi, H.; Janka, R.; Doussin, S.; Habatsch, M.; et al. Implementation of a dedicated 1.5 T MR scanner for radiotherapy treatment planning featuring a novel high-channel coil setup for brain imaging in treatment position. Strahlenther. Onkol. 2021, 197, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Sylwester, A.W.; Mitchell, B.L.; Edgar, J.B.; Taormina, C.; Pelte, C.; Ruchti, F.; Sleath, P.R.; Grabstein, K.H.; Hosken, N.A.; Kern, F.; et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005, 202, 673–685. [Google Scholar] [CrossRef]
- Abana, C.O.; Pilkinton, M.A.; Gaudieri, S.; Chopra, A.; McDonnell, W.J.; Wanjalla, C.; Barnett, L.; Gangula, R.; Hager, C.; Jung, D.K.; et al. Cytomegalovirus (CMV) Epitope-Specific CD4(+) T Cells Are Inflated in HIV(+) CMV(+) Subjects. J. Immunol. 2017, 199, 3187–3201. [Google Scholar] [CrossRef] [PubMed]
- Falcke, S.E.; Rühle, P.F.; Deloch, L.; Fietkau, R.; Frey, B.; Gaipl, U.S. Clinically Relevant Radiation Exposure Differentially Impacts Forms of Cell Death in Human Cells of the Innate and Adaptive Immune System. Int. J. Mol. Sci. 2018, 19, 3574. [Google Scholar] [CrossRef]
- Macaulay, R.; Akbar, A.N.; Henson, S.M. The role of the T cell in age-related inflammation. Age 2013, 35, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.L.; Shu, K.H.; Yang, F.J.; Chou, T.Y.; Chen, P.M.; Lay, F.Y.; Pan, S.Y.; Lin, C.J.; Litjens, N.H.R.; Betjes, M.G.H.; et al. A comprehensive characterization of aggravated aging-related changes in T lymphocytes and monocytes in end-stage renal disease: The iESRD study. Immun. Ageing 2018, 15, 27. [Google Scholar] [CrossRef]
- Jacquemont, L.; Tilly, G.; Yap, M.; Doan-Ngoc, T.M.; Danger, R.; Guérif, P.; Delbos, F.; Martinet, B.; Giral, M.; Foucher, Y.; et al. Terminally Differentiated Effector Memory CD8(+) T Cells Identify Kidney Transplant Recipients at High Risk of Graft Failure. J. Am. Soc. Nephrol. 2020, 31, 876–891. [Google Scholar] [CrossRef]
- Spyridopoulos, I.; Martin-Ruiz, C.; Hilkens, C.; Yadegarfar, M.E.; Isaacs, J.; Jagger, C.; Kirkwood, T.; von Zglinicki, T. CMV seropositivity and T-cell senescence predict increased cardiovascular mortality in octogenarians: Results from the Newcastle 85+ study. Aging Cell 2016, 15, 389–392. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E. T-Zellen. In Medizinische Mikrobiologie und Infektiologie; Suerbaum, S., Burchard, G.-D., Kaufmann, S.H.E., Schulz, T.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 105–122. [Google Scholar]
- Tian, Y.; Babor, M.; Lane, J.; Schulten, V.; Patil, V.S.; Seumois, G.; Rosales, S.L.; Fu, Z.; Picarda, G.; Burel, J.; et al. Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nat. Commun. 2017, 8, 1473. [Google Scholar] [CrossRef]
- Razonable, R.R.; Humar, A. Cytomegalovirus in solid organ transplant recipients—Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13512. [Google Scholar] [CrossRef]

| Factor | Category | CMV Seronegative (n = 11) | CMV Seropositive (n = 10) |
|---|---|---|---|
| Age at start | Mean Range | 40.8 | 50.1 |
| 21–59 | 27–65 | ||
| Gender | Male Female | 2 (18.2%) | 1 (10%) |
| 9 (81.8%) | 9 (90%) |
| Factor | Category | CMV Seronegative (n = 21) | CMV Seropositive (n = 16) |
|---|---|---|---|
| Age at start | Mean Range | 57.9 | 65.1 |
| 27–86 | 38–81 | ||
| Gender | Male Female | 17 (81%) | 8 (50%) |
| 4 (19%) | 8 (50%) | ||
| Tumor disease | High-grade brain tumor (WHO III-IV) Brain metastases | 18 (85.7%) 3 (14.3%) | 13 (81.2%) 3 (18.8%) |
| Radiotherapy (RT) | Whole Brain Radiation Local Radiation | 2 (9.5%) 19 (90.5%) | 2 (12.5%) 14 (87.5%) |
| Single dose of RT | Mean Range | 2.2 Gy 1.8–4 Gy | 2.3 Gy 1.8–4 Gy |
| Cumulative dose of RT | Mean Range | 52.7 Gy 12–60 Gy | 51.7 Gy 30–60 Gy |
| Chemotherapy during RT | No Yes | 4 (19%) 17 (81%) | 3 (18.8%) 13 (81.2%) |
| Immunotherapy during RT | No Yes | 21 (100%) 0 (0%) | 14 (87.5%) 2 (12.5%) |
| Reactivation of CMV | No Yes | 21 (100%) 0 (0%) | 13 (81.2%) 3 (18.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheer, I.; Becker, I.; Schmitter, C.; Semrau, S.; Fietkau, R.; Gaipl, U.S.; Frey, B.; Donaubauer, A.-J. Prospective Evaluation of CD45RA+/CCR7- Effector Memory T (TEMRA) Cell Subsets in Patients with Primary and Secondary Brain Tumors during Radiotherapy of the Brain within the Scope of the Prospective Glio-CMV-01 Clinical Trial. Cells 2023, 12, 516. https://doi.org/10.3390/cells12040516
Scheer I, Becker I, Schmitter C, Semrau S, Fietkau R, Gaipl US, Frey B, Donaubauer A-J. Prospective Evaluation of CD45RA+/CCR7- Effector Memory T (TEMRA) Cell Subsets in Patients with Primary and Secondary Brain Tumors during Radiotherapy of the Brain within the Scope of the Prospective Glio-CMV-01 Clinical Trial. Cells. 2023; 12(4):516. https://doi.org/10.3390/cells12040516
Chicago/Turabian StyleScheer, Ilka, Ina Becker, Charlotte Schmitter, Sabine Semrau, Rainer Fietkau, Udo S. Gaipl, Benjamin Frey, and Anna-Jasmina Donaubauer. 2023. "Prospective Evaluation of CD45RA+/CCR7- Effector Memory T (TEMRA) Cell Subsets in Patients with Primary and Secondary Brain Tumors during Radiotherapy of the Brain within the Scope of the Prospective Glio-CMV-01 Clinical Trial" Cells 12, no. 4: 516. https://doi.org/10.3390/cells12040516
APA StyleScheer, I., Becker, I., Schmitter, C., Semrau, S., Fietkau, R., Gaipl, U. S., Frey, B., & Donaubauer, A.-J. (2023). Prospective Evaluation of CD45RA+/CCR7- Effector Memory T (TEMRA) Cell Subsets in Patients with Primary and Secondary Brain Tumors during Radiotherapy of the Brain within the Scope of the Prospective Glio-CMV-01 Clinical Trial. Cells, 12(4), 516. https://doi.org/10.3390/cells12040516







