Development and Validation of the HIV-CARDIO-PREDICT Score to Estimate the Risk of Cardiovascular Events in HIV-Infected Patients
Abstract
1. Introduction
2. Methods
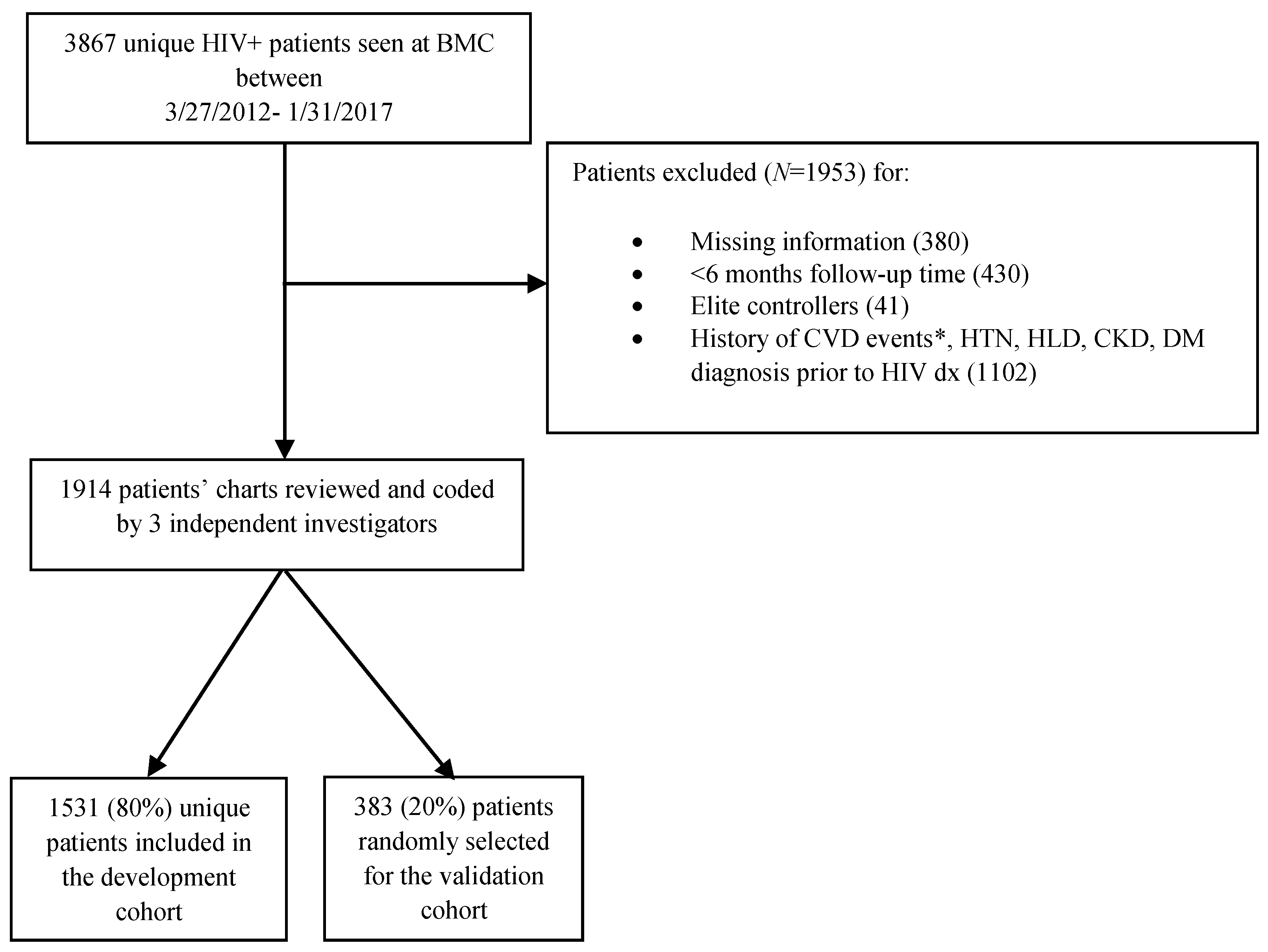
2.1. Study Design, Data Source, and Processing
2.2. Potential Predictors
2.3. Outcome
2.4. Variable Selection and Score Construction
2.5. Statistical Analysis
2.6. Assessment of Accuracy, Development of a Point System-Based Calculator, and Model Validation
3. Results
3.1. Baseline Patient Characteristics
3.2. Predictive Model
3.3. Estimating 10-Year Risk of Developing a CVE Using a Point System-Based Calculator
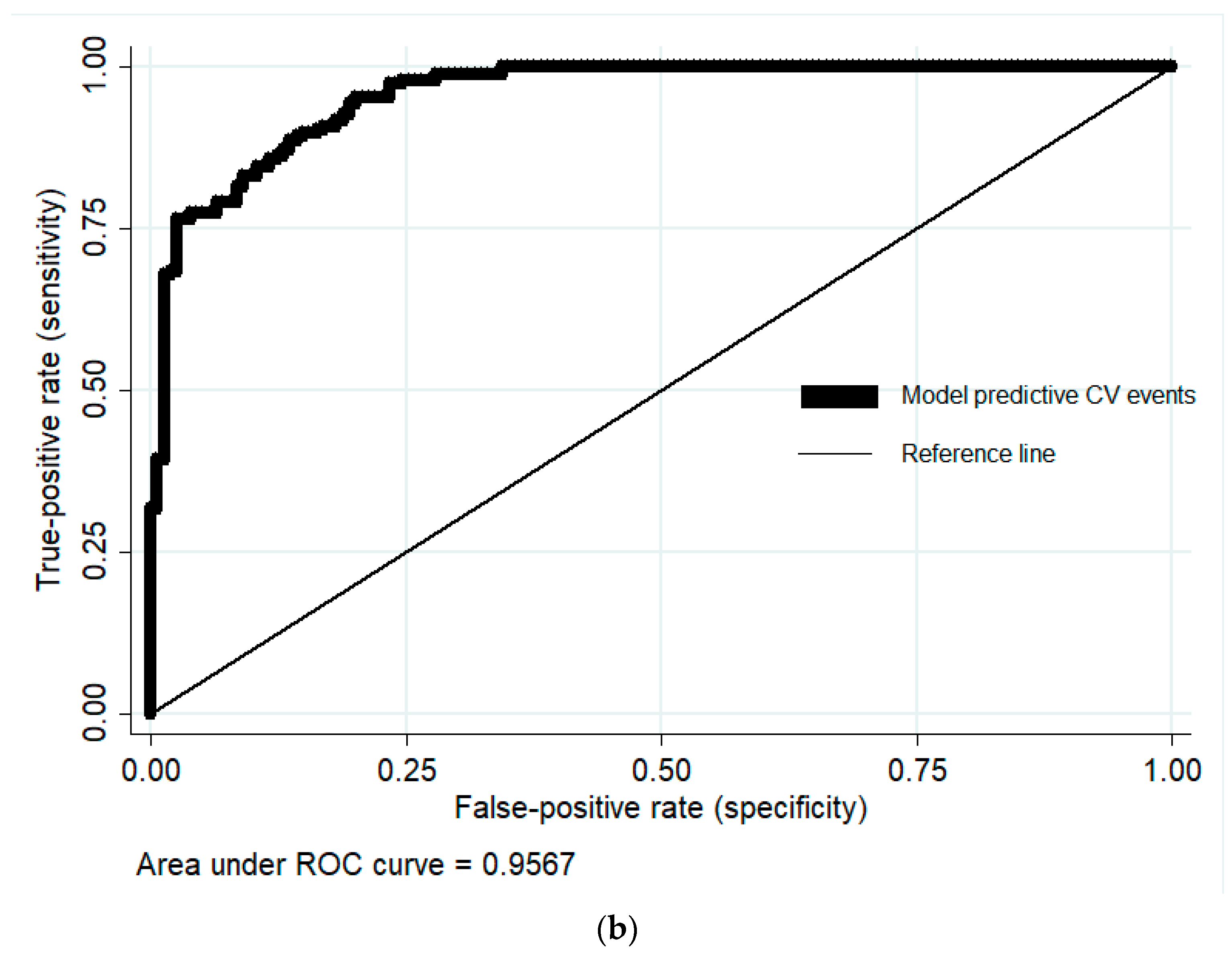
3.4. Score Validation
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosures
Abbreviations
References
- Collaboration, A.T.C. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: Collaborative analysis of 13 HIV cohort studies. Clin. Infect. Dis. 2010, 50, 1387–1396. [Google Scholar] [CrossRef]
- Mocroft, A.; Reiss, P.; Gasiorowski, J.; Ledergerber, B.; Kowalska, J.; Chiesi, A.; Gatell, J.; Rakhmanova, A.; Johnson, M.; Kirk, O.; et al. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J. Acquir. Immune Defic. Syndr. 2010, 55, 262–270. [Google Scholar] [CrossRef]
- Feinstein, M.J.; Bahiru, E.; Achenbach, C.; Longenecker, C.T.; Hsue, P.; So-Armah, K.; Freiberg, M.S.; Lloyd-Jones, D.M. Patterns of Cardiovascular Mortality for HIV-Infected Adults in the United States: 1999 to 2013. Am. J. Cardiol. 2016, 117, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Rodger, A.J.; Lodwick, R.; Schechter, M.; Deeks, S.; Amin, J.; Gilson, R.; Paredes, R.; Bakowska, E.; Engsig, F.N.; Phillips, A. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS 2013, 27, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.H.; French, A.L.; Benning, L.; Kovacs, A.; Anastos, K.; Young, M.; Minkoff, H.; Hessol, N.A. Causes of death among women with human immunodeficiency virus infection in the era of combination antiretroviral therapy. Am. J. Med. 2002, 113, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Touloumi, G.; Kalpourtzi, N. Cardiovascular risk factors in HIV infected individuals: Comparison with general adult control population in Greece. PLOS ONE 2020, 15, e0230730. [Google Scholar] [CrossRef]
- Boccara, F.; Mary-Krause, M.; Potard, V.; Teiger, E.; Lang, S.; Hammoudi, N.; Chauvet, M.; Ederhy, S.; Dufour-Soulat, L.; Ancedy, Y.; et al. HIV Infection and Long-Term Residual Cardiovascular Risk After Acute Coronary Syndrome. J. Am. Heart Assoc. 2020, 9, e017578. [Google Scholar] [CrossRef]
- Kaplan, R.C.; Kingsley, L.A.; Sharrett, A.R.; Li, X.; Lazar, J.; Tien, P.C.; Mack, W.J.; Cohen, M.H.; Jacobson, L.; Gange, S.J. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin. Infect. Dis. 2007, 45, 1074–1081. [Google Scholar] [CrossRef]
- Stein, J.H.; Hsue, P.Y. Inflammation, immune activation, and CVD risk in individuals with HIV infection. JAMA 2012, 308, 405–406. [Google Scholar] [CrossRef]
- Friis-Moller, N.; Reiss, P.; Sabin, C.A.; Weber, R.; Monforte, A.; El-Sadr, W.; Thiebaut, R.; De Wit, S.; Kirk, O.; Fontas, E.; et al. Class of antiretroviral drugs and the risk of myocardial infarction. New Engl. J. Med. 2007, 356, 1723–1735. [Google Scholar] [CrossRef]
- Triant, V.A.; Perez, J.; Regan, S.; Massaro, J.M.; Meigs, J.B.; Grinspoon, S.K.; D’Agostino, R.B., Sr. Cardiovascular Risk Prediction Functions Underestimate Risk in HIV Infection. Circulation 2018, 137, 2203–2214. [Google Scholar] [CrossRef]
- Van Zoest, R.A.; Law, M.; Sabin, C.A.; Vaartjes, I.; Van Der Valk, M.; Arends, J.E.; Reiss, P.; Wit, F.W. Predictive Performance of Cardiovascular Disease Risk Prediction Algorithms in People Living with HIV. J. Acquir. Immune Defic. Syndr. 2019, 81, 562–571. [Google Scholar] [CrossRef]
- Friis-Moller, N.; Ryom, L.; Smith, C.; Weber, R.; Reiss, P.; Dabis, F.; De Wit, S.; Monforte, A.D.; Kirk, O.; Fontas, E.; et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: The Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur. J. Prev. Cardiol. 2016, 23, 214–223. [Google Scholar] [CrossRef]
- Friis-Moller, N.; Sabin, C.A.; Weber, R.; d’Arminio Monforte, A.; El-Sadr, W.M.; Reiss, P.; Thiebaut, R.; Morfeldt, L.; De Wit, S.; Pradier, C.; et al. Combination antiretroviral therapy and the risk of myocardial infarction. New Engl. J. Med. 2003, 349, 1993–2003. [Google Scholar] [CrossRef]
- Friis-Moller, N.; Thiebaut, R.; Reiss, P.; Weber, R.; Monforte, A.D.; De Wit, S.; El-Sadr, W.; Fontas, E.; Worm, S.; Kirk, O.; et al. Predicting the risk of cardiovascular disease in HIV-infected patients: The data collection on adverse effects of anti-HIV drugs study. Eur. J. Prev. Cardiol. 2010, 17, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Paul, A.M.; Lichtenstein, K.A.; Armon, C.; Palella, F.J., Jr.; Skarbinski, J.; Chmiel, J.S.; Hart, R.; Wei, S.C.; Loustalot, F.; Brooks, J.T.; et al. Cardiovascular Disease Risk Prediction in the HIV Outpatient Study. Clin. Infect. Dis. 2016, 63, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Salinas, J.L.; Rentsch, C.; Marconi, V.C.; Tate, J.; Budoff, M.; Butt, A.A.; Freiberg, M.S.; Gibert, C.L.; Goetz, M.B.; Leaf, D.; et al. Baseline, Time-Updated, and Cumulative HIV Care Metrics for Predicting Acute Myocardial Infarction and All-Cause Mortality. Clin. Infect. Dis. 2016, 63, 1423–1430. [Google Scholar] [CrossRef]
- Chow, D.; Thomas, B.S.; Liang, C.Y.; Souza, S.C.; Nakamoto, B.K.; Parikh, N.I.; Shikuma, C. Role of the veterans aging cohort study index in assessing total atherosclerotic burden. Clin. Infect. Dis. 2012, 55, 750–751. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S49–S73. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129 (Suppl. 2), S1–S45. [Google Scholar] [CrossRef]
- Aberg, J.A.; Gallant, J.E.; Ghanem, K.G.; Emmanuel, P.; Zingman, B.S.; Horberg, M.A. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 58, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pareto and Generalized Pareto Distributions; Chotikapanich, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 121–122. [Google Scholar]
- Armstrong, C. JNC8 guidelines for the management of hypertension in adults. Am. Fam. Physician 2014, 90, 503–504. [Google Scholar]
- Ballantyne, C.M.; Grundy, S.M.; Oberman, A.; Kreisberg, R.A.; Havel, R.J.; Frost, P.H.; Haffner, S.M. Hyperlipidemia: Diagnostic and therapeutic perspectives. J. Clin. Endocrinol. Metab. 2000, 85, 2089–2112. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef]
- Stevens, P.E.; Levin, A. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Huffman, M.D.; Karmali, K.N.; Sanghavi, D.M.; Wright, J.S.; Pelser, C.; Gulati, M.; Masoudi, F.A.; Goff, D.C., Jr. Estimating Longitudinal Risks and Benefits From Cardiovascular Preventive Therapies Among Medicare Patients: The Million Hearts Longitudinal ASCVD Risk Assessment Tool: A Special Report From the American Heart Association and American College of Cardiology. Circulation 2017, 135, e793–e813. [Google Scholar] [CrossRef]
- Duncan, M.S.; Freiberg, M.S.; Greevy, R.A., Jr.; Kundu, S.; Vasan, R.S.; Tindle, H.A. Association of Smoking Cessation with Subsequent Risk of Cardiovascular Disease. JAMA 2019, 322, 642–650. [Google Scholar] [CrossRef]
- Hicks, K.A.; Tcheng, J.E.; Bozkurt, B.; Chaitman, B.R.; Cutlip, D.E.; Farb, A.; Fonarow, G.C.; Jacobs, J.P.; Jaff, M.R.; Lichtman, J.H.; et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). Circulation 2015, 132, 302–361. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.M.; Massaro, J.M.; D’Agostino, R.B., Sr. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat. Med. 2004, 23, 1631–1660. [Google Scholar] [CrossRef]
- Arvanitis, M.; Koch, C.M.; Chan, G.G.; Torres-Arancivia, C.; LaValley, M.P.; Jacobson, D.R.; Berk, J.L.; Connors, L.H.; Ruberg, F.L. Identification of Transthyretin Cardiac Amyloidosis Using Serum Retinol-Binding Protein 4 and a Clinical Prediction Model. JAMA Cardiol. 2017, 2, 305–313. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef] [PubMed]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Ryom, L.; Lundgren, J.D.; Ross, M.; Kirk, O.; Law, M.; Morlat, P.; Fontas, E.; Smit, C.; Fux, C.A.; Hatleberg, C.I.; et al. Renal Impairment and Cardiovascular Disease in HIV-Positive Individuals: The D:A:D Study. J. Infect. Dis. 2016, 214, 1212–1220. [Google Scholar] [CrossRef]
- Lichtenstein, K.A.; Armon, C.; Buchacz, K.; Chmiel, J.S.; Buckner, K.; Tedaldi, E.M.; Wood, K.; Holmberg, S.D.; Brooks, J.T. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin. Infect. Dis. 2010, 51, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Mogadam, E.; King, K.; Shriner, K.; Chu, K.; Sondergaard, A.; Young, K.; Naghavi, M.; Kloner, R.A. The association of nadir CD4-T cell count and endothelial dysfunction in a healthy HIV cohort without major cardiovascular risk factors. SAGE Open Med. 2020, 8, 2050312120924892. [Google Scholar] [CrossRef]
- Triant, V.A.; Regan, S.; Lee, H.; Sax, P.E.; Meigs, J.B.; Grinspoon, S.K. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J. Acquir. Immune Defic. Syndr. 2010, 55, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Calmy, A.; Gayet-Ageron, A.; Montecucco, F.; Nguyen, A.; Mach, F.; Burger, F.; Ubolyam, S.; Carr, A.; Ruxungtham, K.; Hirschel, B.; et al. HIV increases markers of cardiovascular risk: Results from a randomized, treatment interruption trial. AIDS 2009, 23, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Lagares, G.; Romero-Sanchez, M.C.; Ruiz-Mateos, E.; Genebat, M.; Ferrando-Martinez, S.; Munoz-Fernandez, M.A.; Pacheco, Y.M.; Leal, M. Long-term suppressive combined antiretroviral treatment does not normalize the serum level of soluble CD14. J. Infect. Dis. 2013, 207, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- French, M.A.; King, M.S.; Tschampa, J.M.; da Silva, B.A.; Landay, A.L. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J. Infect. Dis. 2009, 200, 1212–1215. [Google Scholar] [CrossRef]
- Hsue, P.Y.; Hunt, P.W.; Schnell, A.; Kalapus, S.C.; Hoh, R.; Ganz, P.; Martin, J.N.; Deeks, S.G. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS 2009, 23, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, F.; Lo, J.; Triant, V.A.; Wei, J.; Buzon, M.J.; Fitch, K.V.; Hwang, J.; Campbell, J.H.; Burdo, T.H.; Williams, K.C.; et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 2012, 26, 2409–2412. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, M.; Zanichelli, V.; Serpelloni, G.; Bosco, O.; Malena, M.; Mazzi, R.; Mengoli, C.; Parisi, S.G.; Moyle, G. Abacavir use and cardiovascular disease events: A meta-analysis of published and unpublished data. AIDS 2011, 25, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Andraca-Carrera, E.; Cooper, C.; Miele, P.; Kornegay, C.; Soukup, M.; Marcus, K.A. No association of abacavir use with myocardial infarction: Findings of an FDA meta-analysis. J. Acquir. Immune Defic. Syndr. 2012, 61, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Bavinger, C.; Bendavid, E.; Niehaus, K.; Olshen, R.A.; Olkin, I.; Sundaram, V.; Wein, N.; Holodniy, M.; Hou, N.; Owens, D.K.; et al. Risk of cardiovascular disease from antiretroviral therapy for HIV: A systematic review. PloS ONE 2013, 8, e59551. [Google Scholar] [CrossRef] [PubMed]

| Patients Characteristics | |
|---|---|
| Age, years (mean + /− SD) | 46.1 +/− 11.2 |
| Age at HIV diagnosis, years (mean + /− SD) | 37.1 +/− 10.0 |
| Gender | |
| Female (%) | 654 (42.8) |
| Race | |
| White (%) | 629 (41.1) |
| African American (%) | 649 (42.4) |
| Hispanic/Latino (%) | 201 (13.1) |
| Asian (%) | 52 (3.4) |
| Hypertension (%) | 378 (24.7) |
| Hyperlipidemia (%) | 364 (23.8) |
| Chronic Kidney Disease (%) | 378 (24.7) |
| Diabetes Mellitus (%) | 406 (26.5) |
| Hepatitis C (%) | 301 (19.7) |
| Smoking (%) | 501 (32.7) |
| Substance use disorder (%) | 582 (38.0) |
| Alcohol use disorder (%) | 367 (24.0) |
| Cardiovascular Events | No. of Patients (%) |
| Sudden cardiac death | 51 (3.3) |
| Hospitalization for unstable angina | 21 (1.4) |
| Myocardial infarction | 135 (8.8) |
| Stroke | 51 (3.3) |
| TIA | 55 (3.6) |
| Carotid endarterectomy | 38 (2.5) |
| CABG | 33 (2.2) |
| Total CVD events | 384 (25.1) |
| CD4 count at HIV diagnosis, cells/μL (mean, SD) | 165 (47) |
| CD4% at HIV diagnosis (mean, SD) | 12.2 (6.2) |
| CD4 nadir, cells/μL (mean, SD) | 114.2 (57.4) |
| CD4% nadir (mean, SD) | 9.3 (3.9) |
| Peak HIV Viral Load, copies/mL (mean, SD) | 280,149 (1,216,258) |
| HIV Viral Load at HIV diagnosis, copies/mL (mean, SD) | 210,147 (678,800) |
| Months to control HIV (mean, SD) | 5.7 (5.2) |
| Months from HIV diagnosis to CVD event (mean, SD) | 81.4 (46.2) |
| No. regimens necessary to reach an undetectable status (median, range) | 1 (1–4) |
| Adherence to ARVs (median, range) | 3 (1–4) |
| Abacavir treatment for >6 months (%) | 306 (20.1) |
| Variables Associated with Cardiovascular Events | Coefficient | 95% CI | p-Value |
|---|---|---|---|
| Sex (male) | 3.26 | 2.53–3.99 | <0.001 |
| Race | |||
| White | Reference | ||
| African American | 2.32 | 1.50–3.13 | <0.001 |
| Latino | −0.75 | −1.93–0.42 | 0.207 |
| Asian | −0.62 | −3.60–0.73 | 0.598 |
| Current age | 0.10 | 0.07–0.13 | <0.001 |
| Age at HIV diagnosis | −0.06 | −0.10–(−0.02) | 0.006 |
| Hypertension | 0.87 | 0.20–1.54 | 0.011 |
| Diabetes | 1.25 | 0.61–1.90 | <0.001 |
| Smoking | 1.25 | 0.12–2.39 | 0.030 |
| Hyperlipidemia | 3.82 | 3.03–4.60 | <0.001 |
| Chronic kidney disease | 1.943 | 1.26–2.62 | <0.001 |
| Peak HIV viral load | 2.00 × 10−6 | 9.89 × 10−7–3.00 × 10−6 | <0.001 |
| Nadir CD4 count | −0.02 | −0.03–(−0.02) | <0.001 |
| Risk Factor | Categories | Points |
|---|---|---|
| Sex | Female | 0 |
| Male | 3 | |
| Race | White | 0 |
| African American | 2 | |
| Hypertension | No | 0 |
| Yes | 1 | |
| Diabetes | No | 0 |
| Yes | 1 | |
| Hyperlipidemia | No | 0 |
| Yes | 4 | |
| Chronic Kidney Disease | No | 0 |
| Yes | 2 | |
| Smoking | No | 0 |
| Yes | 1 | |
| Peak HIV Viral Load (copies/mL) | <20,000 | 0 |
| 20,000–99,999 | 1 | |
| 100,000–199,999 | 2 | |
| ≥200,000 | 3 | |
| Current age (years) | 18–30 | 0 |
| 30–39 | 1 | |
| 40–49 | 2 | |
| 50–59 | 3 | |
| 60–69 | 4 | |
| ≥70 | 5 | |
| Age at HIV diagnosis (years) | <24 | 3 |
| 25–44 | 2 | |
| 45–54 | 1 | |
| ≥55 | 0 | |
| Nadir CD4 count (cells/mm3) | <50 | 4 |
| 50–119 | 2 | |
| 120–157 | 1 | |
| ≥158 | 0 |
| Total Points | Risk Estimate (%) |
|---|---|
| 0 | 0.61 |
| 1 | 0.91 |
| 2 | 1.36 |
| 3 | 2.03 |
| 4 | 3.03 |
| 5 | 4.43 |
| 6 | 6.49 |
| 7 | 9.42 |
| 8 | 13.48 |
| 9 | 18.92 |
| 10 | 25.89 |
| 11 | 34.36 |
| 12 | 43.95 |
| 13 | 54.01 |
| 14 | 63.76 |
| 15 | 72.49 |
| 16 | 79.78 |
| 17 | 85.53 |
| 18 | 89.85 |
| 19 | 92.99 |
| 20 | 95.21 |
| 21 | 96.75 |
| 22 | 97.81 |
| 23 | 98.52 |
| 24 | 99.01 |
| 25 | 99.34 |
| 26 | 99.56 |
| 27 | 99.70 |
| 28 | 99.80 |
| 29 | 99.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karanika, S.; Karantanos, T.; Carneiro, H.; Assoumou, S.A. Development and Validation of the HIV-CARDIO-PREDICT Score to Estimate the Risk of Cardiovascular Events in HIV-Infected Patients. Cells 2023, 12, 523. https://doi.org/10.3390/cells12040523
Karanika S, Karantanos T, Carneiro H, Assoumou SA. Development and Validation of the HIV-CARDIO-PREDICT Score to Estimate the Risk of Cardiovascular Events in HIV-Infected Patients. Cells. 2023; 12(4):523. https://doi.org/10.3390/cells12040523
Chicago/Turabian StyleKaranika, Styliani, Theodoros Karantanos, Herman Carneiro, and Sabrina A. Assoumou. 2023. "Development and Validation of the HIV-CARDIO-PREDICT Score to Estimate the Risk of Cardiovascular Events in HIV-Infected Patients" Cells 12, no. 4: 523. https://doi.org/10.3390/cells12040523
APA StyleKaranika, S., Karantanos, T., Carneiro, H., & Assoumou, S. A. (2023). Development and Validation of the HIV-CARDIO-PREDICT Score to Estimate the Risk of Cardiovascular Events in HIV-Infected Patients. Cells, 12(4), 523. https://doi.org/10.3390/cells12040523









