Therapeutic Cell Repopulation of the Liver: From Fetal Rat Cells to Synthetic Human Tissues
Abstract
1. Introduction
2. Cell Transplantation Models and Donor Cell Candidates
2.1. Major Cell Transplantation Models
2.2. Various Cell Sources for Transplantation
3. Rat Fetal Liver Cell Transplantation
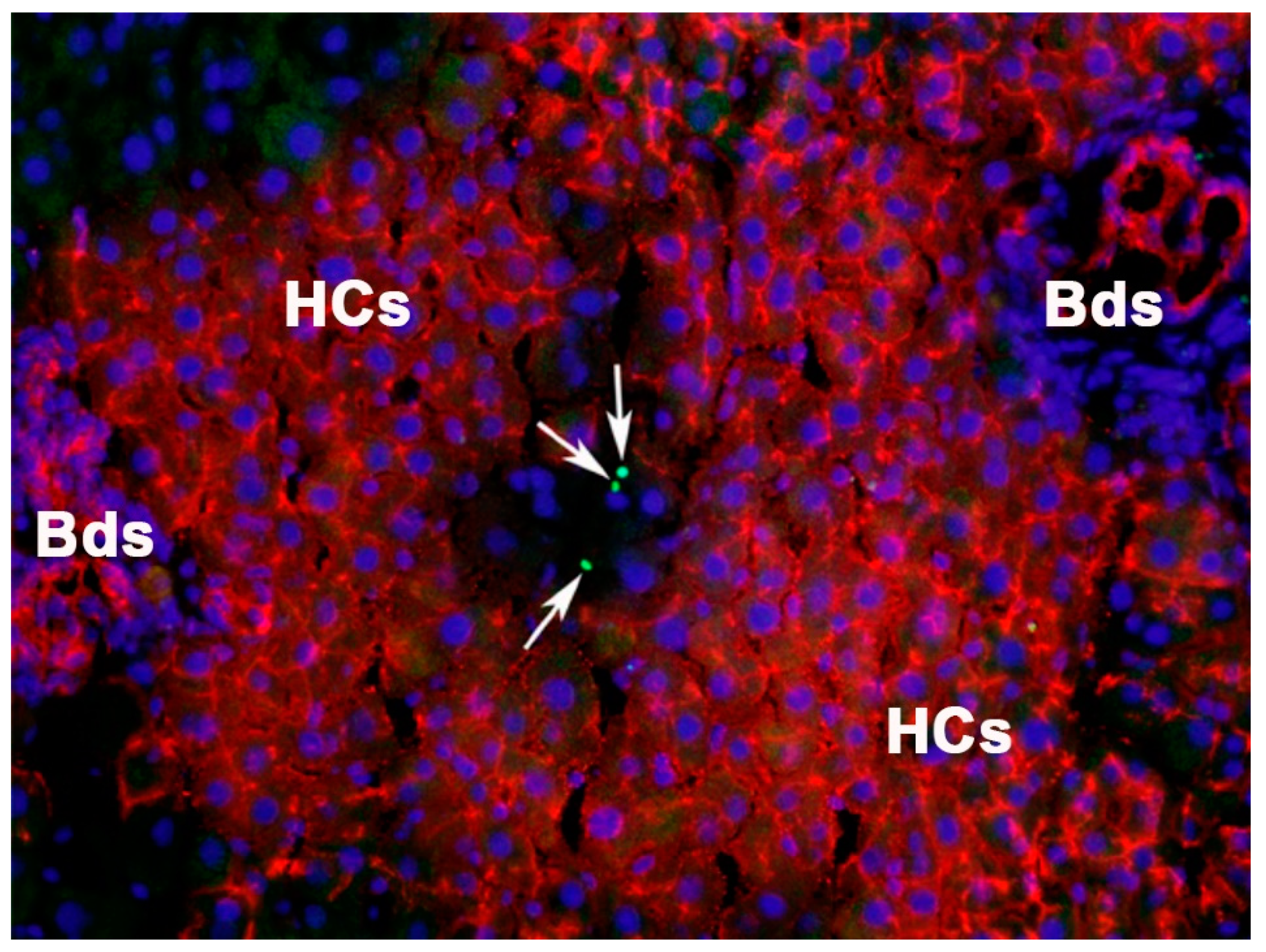
3.1. Repopulation by Hepatic Fetal Liver Stem/Progenitor Cells in a Normal Liver
3.2. Cell Competition Drives Liver Repopulation
3.3. Replacement of Functional Tissue Mass in Diseased Livers
4. Human iPSC-Derived Cells and Application for Human Liver Diseases
4.1. Human iPSC-Derived Hepatocytes, Challenges and Opportunities
4.2. Multilineage Human Fetal Liver Organoids and Their Therapeutic Benefits
5. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Kang, L.I.; Mars, W.M.; Michalopoulos, G.K. Signals and cells involved in regulating liver regeneration. Cells 2012, 1, 1261–1292. [Google Scholar] [CrossRef]
- Xu, J.; Murphy, S.L.; Kochanek, K.D.; Bastian, B.A. Deaths: Final data for 2013. Natl. Vital Stat. Rep. 2016, 64, 1–119. [Google Scholar]
- Cárdenas, A.; Ginès, P. Management of patients with cirrhosis awaiting liver transplantation. Gut 2011, 60, 412–421. [Google Scholar] [CrossRef]
- Available online: http://optn.transplant.hrsa.gov (accessed on 25 January 2023).
- Gramignoli, R.; Vosough, M.; Kannisto, K.; Srinivasan, R.C.; Strom, S.C. Clinical hepatocyte transplantation: Practical limits and possible solutions. Eur. Surg. Res. 2015, 54, 162–177. [Google Scholar] [CrossRef]
- Rhim, J.A.; Sandgren, E.P.; Degen, J.L.; Palmiter, R.D.; Brinster, R.L. Replacement of diseased mouse liver by hepatic cell transplantation. Science 1994, 263, 1149–1152. [Google Scholar] [CrossRef] [PubMed]
- Overturf, K.; Al-Dhalimy, M.; Tanguay, R.; Brantly, M.; Ou, C.N.; Finegold, M.; Grompe, M. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat. Genet. 1996, 12, 266–273. [Google Scholar] [CrossRef]
- Laconi, E.; Oren, R.; Mukhopadhyay, D.K.; Hurston, E.; Laconi, S.; Pani, P.; Dabeva, M.D.; Shafritz, D.A. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am. J. Pathol. 1998, 153, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Guha, C.; Sharma, A.; Gupta, S.; Alfieri, A.; Gorla, G.R.; Gagandeep, S.; Sokhi, R.; Roy-Chowdhury, N.; Tanaka, K.E.; Vikram, B.; et al. Amelioration of radiation-induced liver damage in partially hepatectomized rats by hepatocyte transplantation. Cancer Res. 1999, 59, 5871–5874. [Google Scholar] [PubMed]
- Jirtle, R.L.; Biles, C.; Michalopoulos, G. Morphologic and histochemical analysis of hepatocytes transplanted into syngeneic hosts. Am. J. Pathol. 1980, 101, 115–126. [Google Scholar]
- Sandhu, J.S.; Petkov, P.M.; Dabeva, M.D.; Shafritz, D.A. Stem cell properties and repopulation of the rat liver by fetal liver epithelial progenitor cells. Am. J. Pathol. 2001, 159, 1323–1334. [Google Scholar] [CrossRef]
- Oertel, M.; Menthena, A.; Dabeva, M.D.; Shafritz, D.A. Cell competition leads to a high level of normal liver reconstitution by transplanted fetal liver stem/progenitor cells. Gastroenterology 2006, 130, 507–520. [Google Scholar] [CrossRef]
- Sandgren, E.P.; Palmiter, R.D.; Heckel, J.L.; Daugherty, C.C.; Brinster, R.L.; Degen, J.L. Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell 1991, 66, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shao, Y.; Li, L.; Tian, F.; Cen, J.; Chen, X.; Hu, D.; Zhou, Y.; Xie, W.; Zheng, Y.; et al. Efficient liver repopulation of transplanted hepatocyte prevents cirrhosis in a rat model of hereditary tyrosinemia type I. Sci. Rep. 2016, 6, 31460. [Google Scholar] [CrossRef]
- Chen, H.; Harding, C.O.; Kaiser, R.A.; Nyberg, S.L.; Lillegard, J.B. Autologous gene and cell therapy provides safe and long-term curative therapy in a large pig model of hereditary tyrosinemia type 1. Cell Transplant. 2019, 28, 79–88. [Google Scholar]
- Witek, R.P.; Fisher, S.H.; Petersen, B.E. Monocrotaline, an alternative to retrorsine-based hepatocyte transplantation in rodents. Cell Transplant. 2005, 14, 41–47. [Google Scholar] [CrossRef]
- Petersen, J.; Dandri, M.; Gupta, S.; Rogler, C.E. Liver repopulation with xenogenic hepatocytes in B and T cell-deficient mice leads to chronic hepadnavirus infection and clonal growth of hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 1998, 95, 310–315. [Google Scholar] [CrossRef]
- Mercer, D.; Schiller, D.; Elliott, J.F.; Douglas, D.N.; Hao, C.; Rinfret, A.; Addison, W.R.; Fischer, K.P.; Churchill, T.A.; Lakey, J.R.T.; et al. Hepatitis C virus replication in mice with chimeric human livers. Nat. Med. 2001, 7, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Haridass, D.; Yuan, Q.; Becker, P.D.; Cantz, T.; Iken, M.; Rothe, M.; Narain, N.; Bock, M.; Nörder, M.; Legrand, N.; et al. Repopulation efficiencies of adult hepatocytes, fetal liver progenitor cells, and embryonic stem cell-derived hepatic cells in albumin-promoter-enhancer urokinase-type plasminogen activator mice. Am. J. Pathol. 2009, 175, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Azuma, H.; Paulk, N.; Ranade, A.; Dorrell, C.; Al-Dhalimy, M.; Ellis, E.; Strom, S.; Kay, M.A.; Finegold, M.; Grompe, M. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat. Biotechnol. 2007, 25, 903–910. [Google Scholar] [CrossRef]
- Wilson, E.M.; Bial, J.; Tarlow, B.; Bial, G.; Jensen, B.; Greiner, D.L.; Brehm, M.A.; Grompe, M. Extensive double humanization of both liver and hematopoiesis in FRGN mice. Stem Cell Res. 2014, 13, 404–412. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, X.; Zhang, L.; Li, X.; Zhang, Y.; Wu, K.; Chen, Y.; Cao, J.; Hou, W.; Zhang, J.; et al. A Chimeric humanized mouse model by engrafting the human induced pluripotent stem cell-derived hepatocyte-like cell for the chronic hepatitis B virus infection. Front. Microbiol. 2018, 9, 908. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kawai, K.; Mitsui, T.; Taniguchi, K.; Monnai, M.; Wakui, M.; Ito, M.; Suematsu, M.; Peltz, G.; Nakamura, M.; et al. The reconstituted ‘humanized liver’ in TK-NOG mice is mature and functional. Biochem. Biophys. Res. Commun. 2011, 405, 405–410. [Google Scholar] [CrossRef]
- Maulhardt, H.A.; Hylle, L.; Frost, M.V.; Tornio, A.; Dafoe, S.; Drummond, L.; Quinn, D.I.; Kamat, A.M.; diZerega, G.S. Local injection of submicron particle docetaxel is associated with tumor eradication, reduced systemic toxicity and an immunologic response in uro-oncologic xenografts. Cancers 2019, 11, 577. [Google Scholar] [CrossRef]
- Farber, E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3′-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956, 16, 142–148. [Google Scholar]
- Sells, M.A.; Katyal, S.L.; Shinozuka, H.; Estes, L.W.; Sell, S.; Lombardi, B. Isolation of oval cells and transitional cells from the livers of rats fed the carcinogen DL-ethionine. J. Natl. Cancer Inst. 1981, 66, 355–362. [Google Scholar] [PubMed]
- Lemire, J.M.; Shiojiri, N.; Fausto, N. Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am. J. Pathol. 1991, 139, 535–552. [Google Scholar] [PubMed]
- Wang, X.; Foster, M.; Al-Dhalimy, M.; Lagasse, E.; Finegold, M.; Grompe, M. The origin and liver repopulating capacity of murine oval cells. Proc. Natl. Acad. Sci. USA 2003, 100 (Suppl. 1), 11881–11888. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Witek, R.P.; Lu, Y.; Choi, Y.-K.; Zheng, D.; Jorgensen, M.; Li, C.; Flotte, T.R.; Petersen, B.E. Ex vivo transduced liver progenitor cells as a platform for gene therapy in mice. Hepatology 2004, 40, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Yovchev, M.I.; Grozdanov, P.N.; Zhou, H.; Racherla, H.; Guha, C.; Dabeva, M.D. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology 2008, 47, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.E.; Bowen, W.C.; Patrene, K.D.; Mars, W.M.; Sullivan, A.K.; Murase, N.; Boggs, S.S.; Greenberger, J.S.; Goff, J.P. Bone marrow as a potential source of hepatic oval cells. Science 1999, 284, 1168–1170. [Google Scholar] [CrossRef] [PubMed]
- Theise, N.D.; Badve, S.; Saxena, R.; Henegariu, O.; Sell, S.; Crawford, J.M.; Krause, D.S. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology 2000, 31, 235–240. [Google Scholar] [CrossRef]
- Lagasse, E.; Connors, H.; Al-Dhalimy, M.; Reitsma, M.; Dohse, M.; Osborne, L.; Wang, X.; Finegold, M.; Weissman, I.L.; Grompe, M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 2000, 6, 1229–1234. [Google Scholar] [CrossRef]
- Wang, X.; Willenbring, H.; Akkari, Y.; Torimaru, Y.; Foster, M.; Al-Dhalimy, M.; Lagasse, E.; Finegold, M.; Olson, S.; Grompe, M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 2003, 422, 897–901. [Google Scholar] [CrossRef]
- Vassilopoulos, G.; Wang, P.-R.; Russell, D.W. Transplanted bone marrow regenerates liver by cell fusion. Nature 2003, 422, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Willenbring, H.; Bailey, A.S.; Foster, M.; Akkari, Y.; Dorrell, C.; Olson, S.; Finegold, M.; Fleming, W.H.; Grompe, M. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat. Med. 2004, 10, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Menthena, A.; Deb, N.; Oertel, M.; Grozdanov, P.N.; Sandhu, J.; Shah, S.; Guha, C.; Shafritz, D.A.; Dabeva, M.D. Bone marrow progenitors are not the source of expanding oval cells in injured liver. Stem Cells 2004, 22, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Danet, G.H.; Luongo, J.L.; Butler, G.; Lu, M.M.; Tenner, A.J.; Simon, M.C.; Bonnet, D.A. C1qRp defines a new human stem cell population with hematopoietic and hepatic potential. Proc. Natl. Acad. Sci. USA 2002, 99, 10441–10445. [Google Scholar] [CrossRef]
- Newsome, P.N.; Johannessen, I.; Boyle, S.; Dalakas, E.; Mcaulay, K.A.; Samuel, K.; Rae, F.; Forrester, L.; Turner, M.L.; Hayes, P.C.; et al. Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology 2003, 124, 1891–18900. [Google Scholar] [CrossRef]
- Kollet, O.; Shivtiel, S.; Chen, Y.Q.; Suriawinata, J.; Thung, S.N.; Dabeva, M.D.; Kahn, J.; Spiegel, A.; Dar, A.; Samira, S.; et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J. Clin. Investig. 2003, 112, 160–169. [Google Scholar] [CrossRef]
- Jiang, Y.; Jahagirdar, B.N.; Reinhardt, R.L.; Schwartz, R.E.; Keene, C.D.; Ortiz-Gonzalez, X.R.; Reyes, M.; Lenvik, T.; Lund, T.; Blackstad, M.; et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002, 418, 41–49. [Google Scholar] [CrossRef]
- Sharma, A.D.; Cantz, T.; Richter, R.; Eckert, K.; Henschler, R.; Wilkens, L.; Jochheim-Richter, A.; Arseniev, L.; Ott, M. Human cord blood stem cells generate human cytokeratin 18-negative hepatocyte-like cells in injured mouse liver. Am. J. Pathol. 2005, 167, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Aurich, I.; Mueller, L.P.; Aurich, H.; Luetzkendorf, J.; Tisljar, K.; Dollinger, M.M.; Schormann, W.; Walldorf, J.; Hengstler, J.G.; Fleig, W.E.; et al. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut 2007, 56, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Brulport, M.; Schormann, W.; Bauer, A.; Hermes, M.; Elsner, C.; Hammersen, F.J.; Beerheide, W.; Spitkovsky, D.; Härtig, W.; Nussler, A.; et al. Fate of extrahepatic human stem and precursor cells after transplantation into mouse livers. Hepatology 2007, 46, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Campard, D.; Lysy, P.A.; Najimi, M.; Sokal, E.M. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology 2008, 134, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Sgodda, M.; Aurich, H.; Kleist, S.; Aurich, I.; König, S.; Dollinger, M.M.; Fleig, W.E.; Christ, B. Hepatocyte differentiation of mesenchymal stem cells from rat peritoneal adipose tissue in vitro and in vivo. Exp. Cell Res. 2007, 313, 2875–2886. [Google Scholar] [CrossRef]
- Aurich, H.; Sgodda, M.; Kaltwasser, P.; Vetter, M.; Weise, A.; Liehr, T.; Brulport, M.; Hengstler, J.G.; Dollinger, M.M.; Fleig, W.E.; et al. Hepatocyte differentiation of mesenchymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut 2009, 58, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, F.S. Stem cell therapies for liver failure and cirrhosis. J. Hepatol. 2013, 59, 183–835. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Nelson, E.D.; Abu Rmilah, A.A.; Amiot, B.P.; Nyberg, S.L. Stem cell-related studies and stem cell-based therapies in liver diseases. Cell Transplant. 2019, 28, 1116–1122. [Google Scholar] [CrossRef]
- Liu, P.; Mao, Y.; Xie, Y.; Wei, J.; Yao, J. Stem cells for treatment of liver fibrosis/cirrhosis: Clinical progress and therapeutic potential. Stem Cell Res. Ther. 2022, 13, 356. [Google Scholar] [CrossRef]
- Miki, T.; Lehmann, T.; Cai, H.; Stolz, D.B.; Strom, S.C. Stem cell characteristics of amniotic epithelial cells. Stem Cells 2005, 23, 1549–1559. [Google Scholar] [CrossRef]
- Marongiu, F.; Gramignoli, R.; Dorko, K.; Miki, T.; Ranade, A.R.; Serra, M.P.; Doratiotto, S.; Sini, M.; Sharma, S.; Mitamura, K.; et al. Hepatic differentiation of amniotic epithelial cells. Hepatology 2011, 53, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Skvorak, K.J.; Dorko, K.; Marongiu, F.; Tahan, V.; Hansel, M.C.; Gramignoli, R.; Gibson, K.M.; Strom, S.C. Placental stem cell correction of murine intermediate maple syrup urine disease. Hepatology 2013, 57, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Manuelpillai, U.; Lourensz, D.; Vaghjiani, V.; Tchongue, J.; Lacey, D.; Tee, J.Y.; Murthi, P.; Chan, J.; Hodge, A.; Sievert, W. Human amniotic epithelial cell transplantation induces markers of alternative macrophage activation and reduces established hepatic fibrosis. PLoS ONE 2012, 7, e38631. [Google Scholar] [CrossRef]
- Alhomrani, M.; Correia, J.; Zavou, M.; Leaw, B.; Kuk, N.; Xu, R.; Saad, M.I.; Hodge, A.; Greening, D.W.; Lim, R.; et al. The human amnion epithelial cell secretome decreases hepatic fibrosis in mice with chronic liver fibrosis. Front. Pharmacol. 2017, 8, 748. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.S.; Yanuaria, L.; Parducho, K.M.R.; Garcia, I.M.; Varghese, B.A.; Grubbs, B.H.; Miki, T. Liver-directed human amniotic epithelial cell transplantation improves systemic disease phenotype in Hurler syndrome mouse model. Stem Cells Transl. Med. 2017, 6, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef]
- Hamazaki, T.; Iiboshi, Y.; Oka, M.; Papst, P.J.; Meacham, A.M.; Zon, L.I.; Terada, N. Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Lett. 2001, 497, 15–19. [Google Scholar] [CrossRef]
- Heo, J.; Factor, V.M.; Uren, T.; Takahama, Y.; Lee, J.-S.; Major, M.; Feinstone, S.M.; Thorgeirsson, S.S. Hepatic precursors derived from murine embryonic stem cells contribute to regeneration of injured liver. Hepatology 2006, 44, 1478–1486. [Google Scholar] [CrossRef]
- Gouon-Evans, V.; Boussemart, L.; Gadue, P.; Nierhoff, D.; Koehler, C.I.; Kubo, A.; Shafritz, D.A.; Keller, G. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat. Biotechnol. 2006, 24, 1402–1411. [Google Scholar] [CrossRef]
- Basma, H.; Soto–Gutiérrez, A.; Yannam, G.R.; Liu, L.; Ito, R.; Yamamoto, T.; Ellis, E.; Carson, S.D.; Sato, S.; Chen, Y.; et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology 2009, 136, 990–999. [Google Scholar] [CrossRef]
- Leduc, E.H.; Wilson, J.W. Production of transplantable hepatomas by intrasplenic implantation of normal liver in the mouse. J. Natl. Cancer Inst. 1963, 30, 85–99. [Google Scholar] [PubMed]
- Ebata, H.; Mito, M. Intrasplenic fetal rat hepatic tissue isotransplantation. Transplantation 1985, 39, 77–79. [Google Scholar] [PubMed]
- Suzuki, A.; Zheng, Y.-W.; Kondo, R.; Kusakabe, M.; Takada, Y.; Fukao, K.; Nakauchi, H.; Taniguchi, H. Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology 2000, 32, 1230–1239. [Google Scholar] [CrossRef]
- Suzuki, A.; Zheng, Y.-W.; Kaneko, S.; Onodera, M.; Fukao, K.; Nakauchi, H.; Taniguchi, H. Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. Cell Biol. 2002, 156, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Tanimizu, N.; Nishikawa, M.; Saito, H.; Tsujimura, T.; Miyajima, A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J. Cell Sci. 2003, 116, 1775–1786. [Google Scholar] [CrossRef]
- Nierhoff, D.; Ogawa, A.; Oertel, M.; Chen, Y.-Q.; Shafritz, D.A. Purification and characterization of mouse fetal liver epithelial cells with high in vivo repopulation capacity. Hepatology 2005, 42, 130–139. [Google Scholar] [CrossRef]
- Kubota, H.; Reid, L.M. Clonogenic hepatoblasts, common precursors for hepatocytic and biliary lineages, are lacking classical major histocompatibility complex class I antigen. Proc. Natl. Acad. Sci. USA 2000, 97, 12132–12137. [Google Scholar] [CrossRef]
- Suzuki, A.; Zheng, Y.-W.; Fukao, K.; Nakauchi, H.; Taniguchi, H. Liver repopulation by c-Met-positive stem/progenitor cells isolated from the developing rat liver. Hepatogastroenterology 2004, 51, 423–426. [Google Scholar]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Y.; Wang, X.; Zhang, W.; Sauer, V.; Chang, C.J.; Han, B.; Tchaikovskaya, T.; Avsar, Y.; Tafaleng, E.; et al. Amelioration of Hyperbilirubinemia in Gunn Rats after Transplantation of Human Induced Pluripotent Stem Cell-Derived Hepatocytes. Stem Cell Rep. 2015, 5, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, M.; Grimm, A.A.; Willenbring, H. Assessing the therapeutic potential of lab-made hepatocytes. Hepatology 2016, 64, 287–294. [Google Scholar] [CrossRef]
- Zhu, S.; Rezvani, M.; Harbell, J.; Mattis, A.N.; Wolfe, A.R.; Benet, L.Z.; Willenbring, H.; Ding, S. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature 2014, 508, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, J.; Jia, J.; Song, N.; Xiang, C.; Xu, J.; Hou, Z.; Su, X.; Liu, B.; Jiang, T.; et al. Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell Stem Cell 2014, 14, 394–403. [Google Scholar] [CrossRef]
- Thompson, N.L.; Hixson, D.C.; Callanan, H.; Panzica, M.; Flanagan, D.; Faris, R.A.; Hong, W.J.; Hartel-Schenk, S.; Doyle, D. A Fischer rat substrain deficient in dipeptidyl peptidase IV activity makes normal steady-state RNA levels and an altered protein. Use as a liver-cell transplantation model. Biochem. J. 1991, 273, 497–502. [Google Scholar] [CrossRef]
- Rajvanshi, P.; Kerr, A.; Bhargava, K.K.; Burk, R.D.; Gupta, S. Studies of liver repopulation using the dipeptidyl peptidase IV-deficient rat and other rodent recipients: Cell size and structure relationships regulate capacity for increased transplanted hepatocyte mass in the liver lobule. Hepatology 1996, 23, 482–496. [Google Scholar] [CrossRef]
- Oertel, M.; Menthena, A.; Chen, Y.; Teisner, B.; Jensen, C.H.; Shafritz, D.A. Purification of fetal liver stem/progenitor cells containing all the repopulating potential for the normal adult rat liver. Gastroenterology 2008, 134, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Floridon, C.; Jensen, C.H.; Thorsen, P.; Nielsen, O.; Sunde, L.; Westergaard, J.G.; Thomsen, S.G.; Teisner, B. Does fetal antigen 1 (FA1) identify cells with regenerative, endocrine and neuroendocrine potentials? A study of FA1 in embryonic, fetal, and placental tissue and in maternal circulation. Differentiation 2000, 66, 49–59. [Google Scholar] [CrossRef]
- Jensen, C.H.; Jauho, E.I.; Santoni-Rugiu, E.; Holmskov, U.; Teisner, B.; Tygstrup, N.; Bisgaard, H.C. Transit-amplifying ductular (oval) cells and their hepatocytic progeny are characterized by a novel and distinctive expression of delta-like protein/preadipocyte factor 1/fetal antigen 1. Am. J. Pathol. 2004, 164, 1347–1359. [Google Scholar] [CrossRef]
- Tanimizu, N.; Tsujimura, T.; Takahide, K.; Kodama, T.; Nakamura, K.; Miyajima, A. Expression of Dlk/Pref-1 defines a subpopulation in the oval cell compartment of rat liver. Gene Expr. Patterns 2004, 5, 209–218. [Google Scholar] [CrossRef]
- Oertel, M.; Menthena, A.; Chen, Y.Q.; Shafritz, D.A. Comparison of hepatic properties and transplantation of Thy-1+ and Thy-1- cells isolated from ED14 rat fetal liver. Hepatology 2007, 46, 1236–1245. [Google Scholar] [CrossRef]
- Fiegel, H.C.; Park, J.J.; Lioznov, M.V.; Martin, A.; Jaeschke-Melli, S.; Kaufmann, P.M.; Fehse, B.; Zander, A.R.; Kluth, D. Characterization of cell types during rat liver development. Hepatology 2003, 37, 148–154. [Google Scholar] [CrossRef]
- Masson, N.M.; Currie, I.S.; Terrace, J.D.; Garden, O.J.; Parks, R.W.; Ross, J.A. Hepatic progenitor cells in human fetal liver express the oval cell marker Thy-1. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G45–G54. [Google Scholar] [CrossRef]
- Menthena, A.; Koehler, C.I.; Sandhu, J.S.; Yovchev, M.I.; Hurston, E.; Shafritz, D.A.; Oertel, M. Activin A, p15INK4b signaling, and cell competition promote stem/progenitor cell repopulation of livers in aging rats. Gastroenterology 2011, 140, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Oertel, M.; Menthena, A.; Chen, Y.; Shafritz, D.A. Properties of cryopreserved fetal liver stem/progenitor cells that exhibit long-term repopulation of the normal rat liver. Stem Cells 2006, 24, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Shaik, M.V.; Parveen, N.; Rajendraprasad, A.; Aleem, M.A.; Habeeb, M.A.; Srinivas, G.; Raj, T.A.; Tiwari, S.K.; Kumaresan, K.; et al. Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplant. 2010, 19, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Gridelli, B.; Vizzini, G.; Pietrosi, G.; Luca, A.; Spada, M.; Gruttadauria, S.; Cintorino, D.; Amico, G.; Chinnici, C.; Miki, T.; et al. Efficient human fetal liver cell isolation protocol based on vascular perfusion for liver cell-based therapy and case report on cell transplantation. Liver Transplant. 2012, 18, 226–237. [Google Scholar] [CrossRef]
- Cardinale, V.; Carpino, G.; Gentile, R.; Napoletano, C.; Rahimi, H.; Franchitto, A.; Semeraro, R.; Nuti, M.; Onori, P.; Berloco, P.B.; et al. Transplantation of human fetal biliary tree stem/progenitor cells into two patients with advanced liver cirrhosis. BMC Gastroenterol. 2014, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Pietrosi, G.; Vizzini, G.; Gerlach, J.; Chinnici, C.; Luca, A.; Amico, G.; D’Amato, M.; Conaldi, P.G.; Petri, S.L.; Spada, M.; et al. Phases I–II matched case-control study of human fetal liver cell transplantation for treatment of chronic liver disease. Cell Transplant. 2015, 24, 1627–1638. [Google Scholar] [CrossRef]
- Vimalesvaran, S.; Nulty, J.; Dhawan, A. Cellular Therapies in Pediatric Liver Diseases. Cells 2022, 11, 2483. [Google Scholar] [CrossRef]
- Morata, G.; Ripoll, P. Minutes: Mutants of drosophila autonomously affecting cell division rate. Dev. Biol. 1975, 42, 211–221. [Google Scholar] [CrossRef]
- Bowling, S.; Lawlor, K.; Rodríguez, T.A. Cell competition: The winners and losers of fitness selection. Development 2019, 146, dev167486. [Google Scholar] [CrossRef]
- Li, W.; Baker, N.E. Engulfment is required for cell competition. Cell 2007, 129, 1215–1225. [Google Scholar] [CrossRef]
- Lolo, F.N.; Casas-Tintó, S.; Moreno, E. Cell competition time line: Winners kill losers, which are extruded and engulfed by hemocytes. Cell Rep. 2012, 2, 526–539. [Google Scholar] [CrossRef]
- Morata, G.; Ballesteros-Arias, L. Developmental Biology. Death to the losers. Science 2014, 346, 1181–1182. [Google Scholar] [CrossRef] [PubMed]
- Amoyel, M.; Bach, E.A. Cell competition: How to eliminate your neighbours. Development 2014, 141, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Haridoss, S.; Yovchev, M.I.; Schweizer, H.; Megherhi, S.; Beecher, M.; Locker, J.; Oertel, M. Activin A is a prominent autocrine regulator of hepatocyte growth arrest. Hepatol. Commun. 2017, 1, 852–870. [Google Scholar] [CrossRef]
- Yasuda, H.; Mine, T.; Shibata, H.; Eto, Y.; Hasegawa, Y.; Takeuchi, T.; Asano, S.; Kojima, I. Activin A: An autocrine inhibitor of initiation of DNA synthesis in rat hepatocytes. J. Clin. Investig. 1993, 92, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Schwall, R.H.; Robbins, K.; Jardieu, P.; Chang, L.; Lai, C.; Terrell, T.G. Activin induces cell death in hepatocytes in vivo and in vitro. Hepatology 1993, 18, 347–356. [Google Scholar] [CrossRef]
- Ichikawa, T.; Zhang, Y.Q.; Kogure, K.; Hasegawa, Y.; Takagi, H.; Mori, M.; Kojima, I. Transforming growth factor beta and activin tonically inhibit DNA synthesis in the rat liver. Hepatology 2001, 34, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Yovchev, M.I.; Xue, Y.; Shafritz, D.A.; Locker, J.; Oertel, M. Repopulation of the fibrotic/cirrhotic rat liver by transplanted hepatic stem/progenitor cells and mature hepatocytes. Hepatology 2014, 59, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Yovchev, M.I.; Lee, E.J.; Rodriguez-Silva, W.; Locker, J.; Oertel, M. Biliary obstruction promotes multilineage differentiation of hepatic stem cells. Hepatol. Commun. 2019, 3, 1137–1150. [Google Scholar] [CrossRef]
- Yovchev, M.I.; Locker, J.; Oertel, M. Biliary fibrosis drives liver repopulation and phenotype transition of transplanted hepatocytes. J. Hepatol. 2016, 64, 1348–1357. [Google Scholar] [CrossRef]
- Michalopoulos, G.K.; Bowen, W.C.; Mulè, K.; Lopez-Talavera, J.C.; Mars, W. Hepatocytes undergo phenotypic transformation to biliary epithelium inorganoid cultures. Hepatology 2002, 36, 278–283. [Google Scholar] [CrossRef]
- Michalopoulos, G.K.; Barua, L.; Bowen, W.C. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology 2005, 41, 535–544. [Google Scholar] [CrossRef]
- Nagoshi, S. Osteopontin: Versatile modulator of liver diseases. Hepatol. Res. 2014, 44, 22–30. [Google Scholar] [CrossRef]
- Sugai, K.; Sumida, M.; Shofuda, T.; Yamaguchi, R.; Tamura, T.; Kohzuki, T.; Abe, T.; Shibata, R.; Kamata, Y.; Ito, S.; et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Regen. Ther. 2021, 18, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ir.fatetherapeutics.com/news-releases/news-release-details/fate-therapeutics-announces-positive-interim-clinical-data-its (accessed on 25 January 2023).
- Available online: https://medicalxpress.com/news/2020-05-japan-newborn-liver-stem-cells.html (accessed on 25 January 2023).
- Graffmann, N.; Scherer, B.; Adjaye, J. In vitro differentiation of pluripotent stem cells into hepatocyte like cells—Basic principles and current progress. Stem Cell Res 2022, 61, 102763. [Google Scholar] [CrossRef]
- Yao, J.; Yu, Y.; Nyberg, S.L. Induced pluripotent stem cells for the treatment of liver diseases: Novel concepts. Cells Tissues Organs 2022, 211, 368–384. [Google Scholar] [CrossRef]
- Cahan, P.; Cacchiarelli, D.; Dunn, S.J.; Hemberg, M.; de Sousa Lopes, S.M.C.; Morris, S.A.; Rackham, O.J.L.; Del Sol, A.; Wells, C.A. Computational Stem Cell Biology: Open Questions and Guiding Principles. Cell Stem Cell 2021, 28, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Alavi, A.; Ebrahimkhani, M.R.; Bar-Joseph, Z. Computational tools for analyzing single-cell data in pluripotent cell differentiation studies. Cell Rep. Methods 2021, 1, 100087. [Google Scholar] [CrossRef]
- Velazquez, J.J.; LeGraw, R.; Moghadam, F.; Tan, Y.; Kilbourne, J.; Maggiore, J.C.; Hislop, J.; Liu, S.; Cats, D.; Chuva de Sousa Lopes, S.M.; et al. Gene regulatory network analysis and engineering directs development and vascularization of multilineage human liver organoids. Cell Syst. 2021, 12, 41–55. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, R.; Cardinale, V.; Carpino, G.; Gentile, R.; Napoli, C.; Venere, R.; Gatto, M.; Brunelli, R.; Gaudio, E.; Alvaro, D. The fetal liver as cell source for the regenerative medicine of liver and pancreas. Ann. Transl. Med. 2013, 1, 13. [Google Scholar] [PubMed]
- Guye, P.; Ebrahimkhani, M.R.; Kipniss, N.; Velazquez, J.J.; Schoenfeld, E.; Kiani, S.; Griffith, L.G.; Weiss, R. Genetically engineering self-organization of human pluripotent stem cells into a liver bud-like tissue using Gata6. Nat. Commun. 2016, 7, 10243. [Google Scholar] [CrossRef]
- Velazquez, J.J.; Su, E.; Cahan, P.; Ebrahimkhani, M.R. Programming morphogenesis through systems and synthetic biology. Trends Biotechnol. 2018, 36, 415–429. [Google Scholar] [CrossRef]
- Shiota, G.; Itaba, N. Progress in stem cell-based therapy for liver disease. Hepatol. Res. 2017, 47, 127–141. [Google Scholar] [CrossRef]
- Alwahsh, S.M.; Rashidi, H.; Hay, D.C. Liver cell therapy: Is this the end of the beginning? Cell. Mol. Life Sci. 2018, 75, 1307–1324. [Google Scholar] [PubMed]
- Iansante, V.; Chandrashekran, A.; Dhawan, A. Cell-based liver therapies: Past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170229. [Google Scholar] [CrossRef]
- Ridola, L.; Bragazzi, M.C.; Cardinale, V.; Carpino, G.; Gaudio, E.; Alvaro, D. Cholangiocytes: Cell transplantation. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1516–1523. [Google Scholar] [CrossRef]
- Tricot, T.; De Boeck, J.; Verfaillie, C. Alternative cell sources for liver parenchyma repopulation: Where do we stand? Cells 2020, 9, 566. [Google Scholar] [CrossRef]
- Marongiu, F.; Laconi, E. Cell competition in liver carcinogenesis. World J. Hepatol. 2020, 12, 475–484. [Google Scholar] [CrossRef]
- Pasciu, D.; Montisci, S.; Greco, M.; Doratiotto, S.; Pitzalis, S.; Pani, P.; Laconi, S.; Laconi, E. Aging is associated with increased clonogenic potential in rat liver in vivo. Aging Cell 2006, 5, 373–377. [Google Scholar] [CrossRef]
- Yovchev, M.; Jaber, F.L.; Lu, Z.; Patel, S.; Locker, J.; Rogler, L.E.; Murray, J.W.; Sudol, M.; Dabeva, M.D.; Zhu, L.; et al. Experimental model for successful liver cell therapy by lenti TTR-YapERT2 transduced hepatocytes with tamoxifen control of Yap subcellular location. Sci. Rep. 2016, 6, 19275. [Google Scholar] [CrossRef]
- Peterson, E.A.; Polgar, Z.; Devakanmalai, G.S.; Li, Y.; Jaber, F.L.; Zhang, W.; Wang, X.; Iqbal, N.J.; Murray, J.W.; Roy-Chowdhury, N.; et al. Genes and pathways promoting long-Term liver repopulation by ex vivo hYAP-ERT2 transduced hepatocytes and treatment of jaundice in Gunn rats. Hepatol. Commun. 2018, 3, 129–146. [Google Scholar] [CrossRef]
- Dhawan, A.; Puppi, J.; Hughes, R.D.; Mitry, R.R. Human hepatocyte transplantation: Current experience and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 288–298. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafritz, D.A.; Ebrahimkhani, M.R.; Oertel, M. Therapeutic Cell Repopulation of the Liver: From Fetal Rat Cells to Synthetic Human Tissues. Cells 2023, 12, 529. https://doi.org/10.3390/cells12040529
Shafritz DA, Ebrahimkhani MR, Oertel M. Therapeutic Cell Repopulation of the Liver: From Fetal Rat Cells to Synthetic Human Tissues. Cells. 2023; 12(4):529. https://doi.org/10.3390/cells12040529
Chicago/Turabian StyleShafritz, David A., Mo R. Ebrahimkhani, and Michael Oertel. 2023. "Therapeutic Cell Repopulation of the Liver: From Fetal Rat Cells to Synthetic Human Tissues" Cells 12, no. 4: 529. https://doi.org/10.3390/cells12040529
APA StyleShafritz, D. A., Ebrahimkhani, M. R., & Oertel, M. (2023). Therapeutic Cell Repopulation of the Liver: From Fetal Rat Cells to Synthetic Human Tissues. Cells, 12(4), 529. https://doi.org/10.3390/cells12040529





