Infrared Laser Effects on Cell Projection Depend on Irradiation Intermittence and Cell Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Expression of Actin-GFP
2.3. Optical Setup
2.4. Laser Stimulations and Live Cell Imaging
2.5. Image Processing and Data Analysis
3. Results
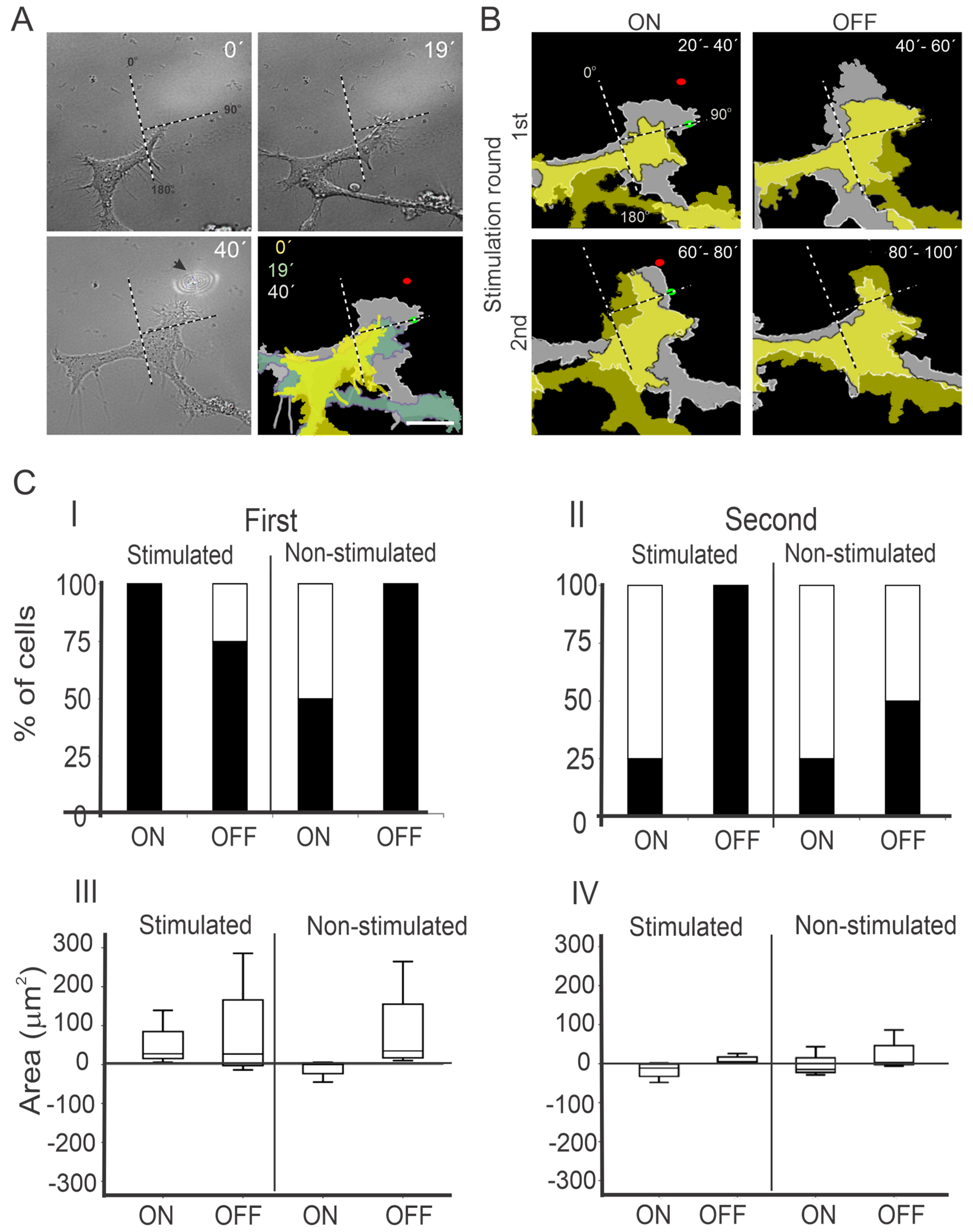
3.1. Intermittent NIR Laser Stimulation on PC12 Cells with a Moving Laser Spot
3.1.1. First NIR Irradiation
3.1.2. Second NIR Irradiation
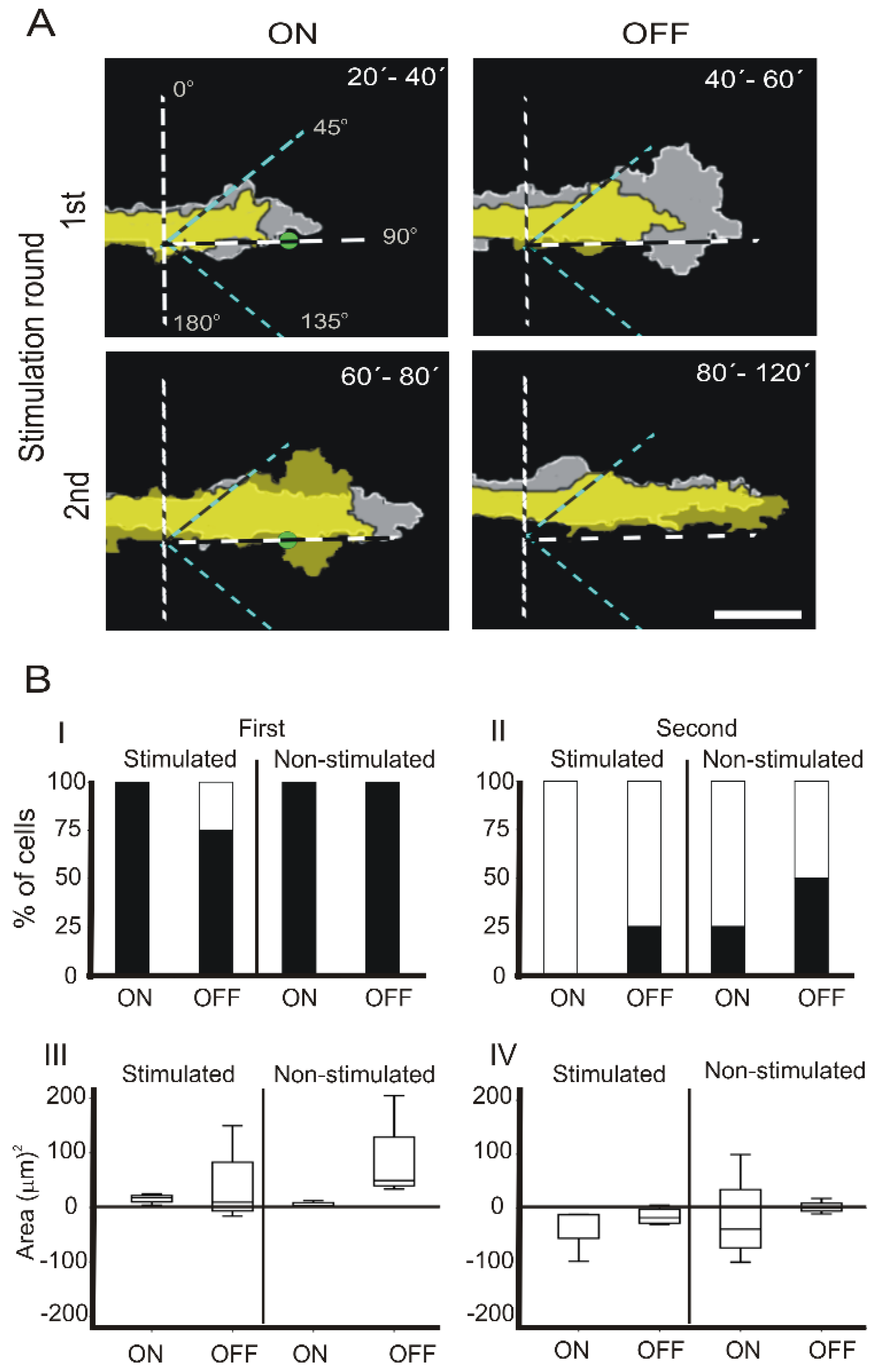
3.2. Intermittent NIR Laser Stimulation on PC12 Cells with a Static Laser Spot
3.2.1. First NIR Irradiation
3.2.2. Second NIR Irradiation
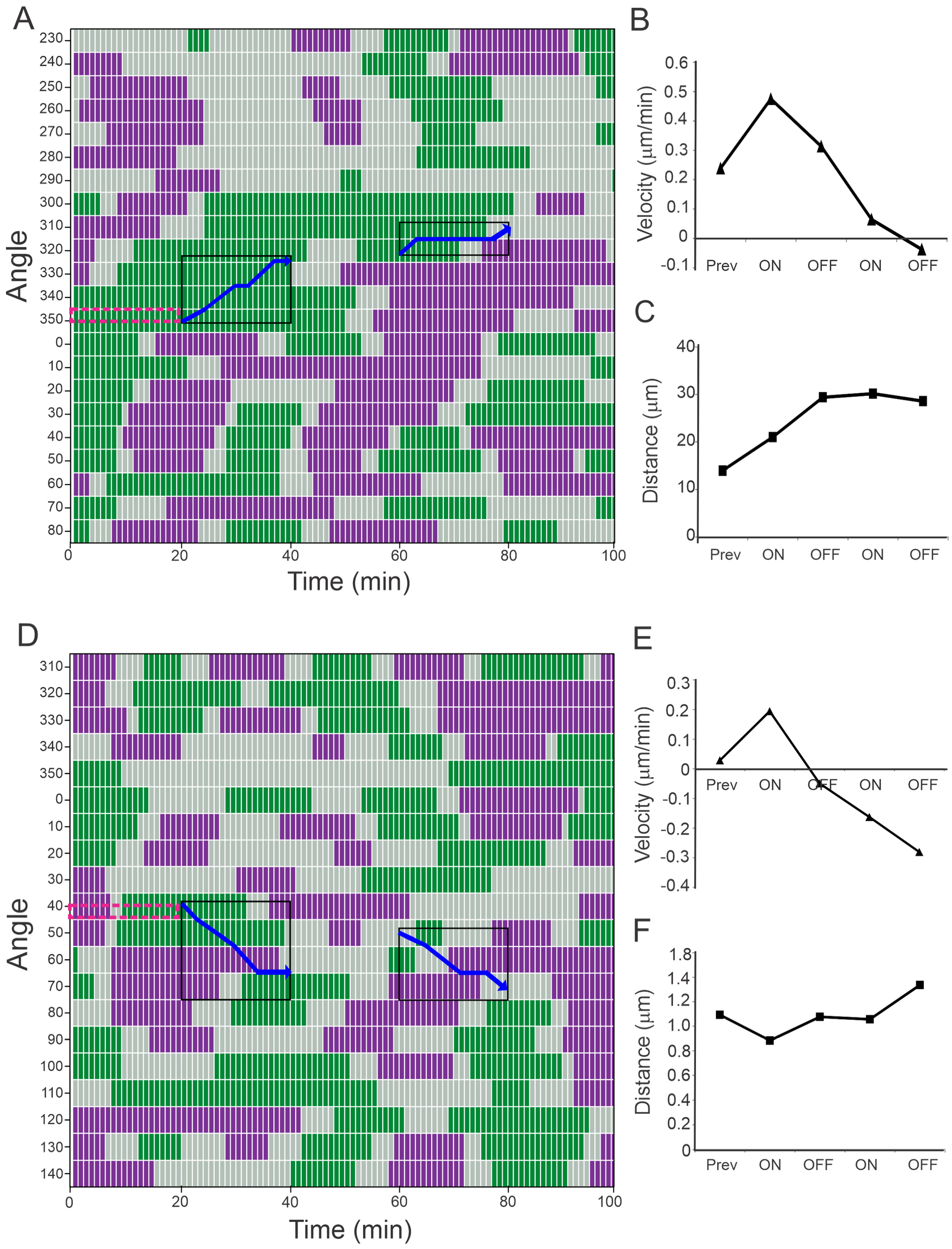
3.3. Laser Stimulation Can Increase the Velocity of Cell Projection
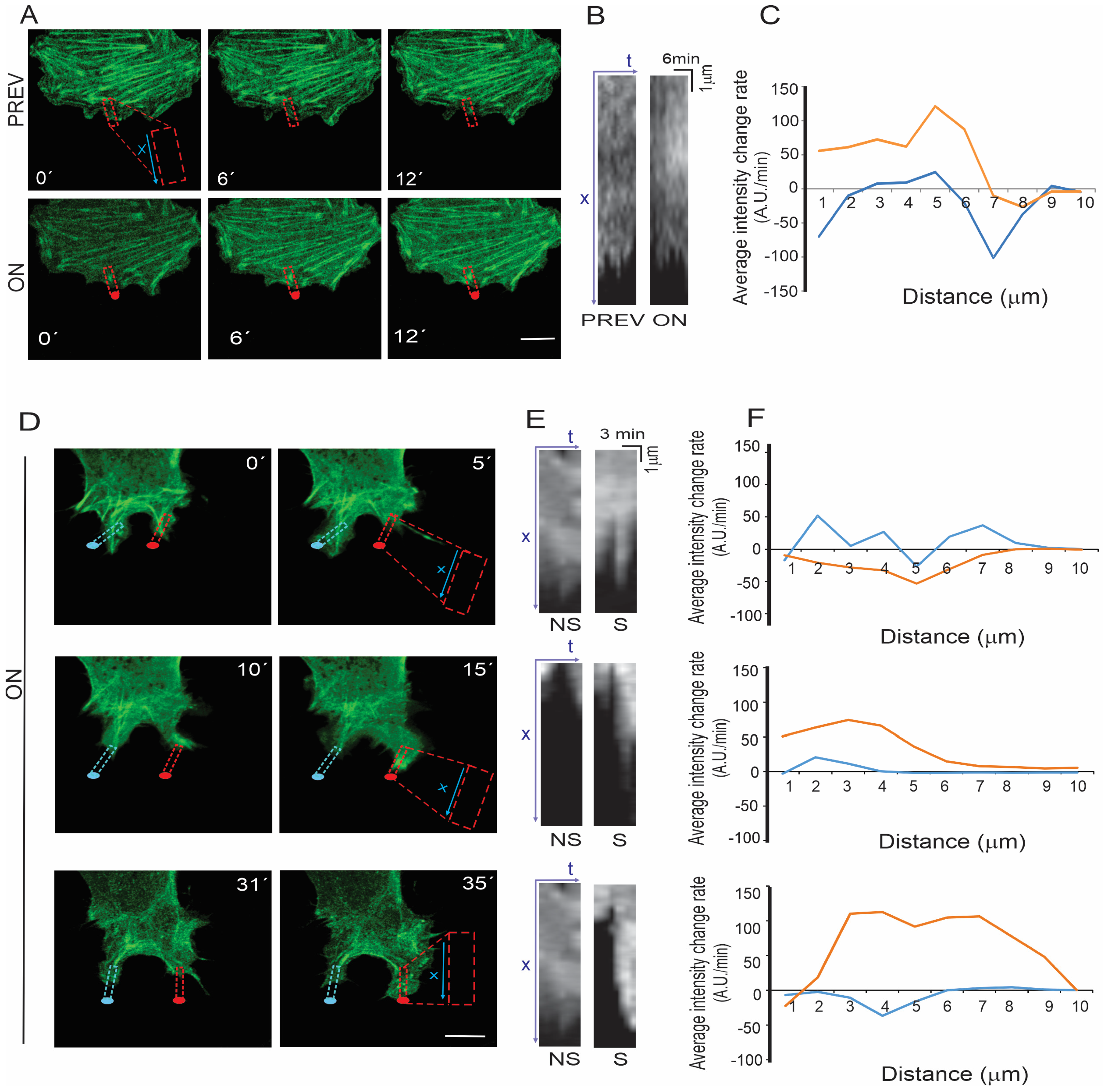
3.4. Effects of Laser Stimulation on Actin Cytoskeleton
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albrecht-Buehler, G. Surface extensions of 3T3 cells towards distant infrared light sources. J. Cell Biol. 1991, 114, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Albrecht-Buehler, G. A long-range attraction between aggregating 3T3 cells mediated by near-infrared light scattering. Proc. Natl. Acad. Sci. USA 2005, 102, 5050–5055. [Google Scholar] [CrossRef] [PubMed]
- Avila, R.; Medina-Villalobos, N.; Tamariz, E.; Chiu, R.; Lopez-Marín, L.M.; Acosta, A.; Castaño, V. Optical tweezers experiments for fibroblast cell growth stimulation. In Proceedings of the Biophotonics: Photonic Solutions for Better Health Care IV, Brussels, Belgium, 14–17 April 2014; Volume 9129, p. 91291U. [Google Scholar] [CrossRef]
- Carnegie, D.J.; Cizmar, T.; Baumgartl, J.; Gunn-Moore, F.J.; Dholakia, K. Automated laser guidance of neuronal growth cones using a spatial light modulator. J. Biophotonics 2009, 2, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Carnegie, D.J.; Stevenson, D.J.; Mazilu, M.; Gunn-Moore, F.; Dholakia, K. Guided neuronal growth using optical line traps. Opt. Express 2008, 16, 10507–10517. [Google Scholar] [CrossRef]
- Ehrlicher, A.; Betz, T.; Stuhrmann, B.; Koch, D.; Milner, V.; Raizen, M.G.; Kas, J. Guiding neuronal growth with light. Proc. Natl. Acad. Sci. USA 2002, 99, 16024–16028. [Google Scholar] [CrossRef]
- Graves, C.E.; McAllister, R.G.; Rosoff, W.J.; Urbach, J.S. Optical neuronal guidance in three-dimensional matrices. J. Neurosci. Methods 2009, 179, 278–283. [Google Scholar] [CrossRef]
- Mohanty, S.K.; Sharma, M.; Panicker, M.M.; Gupta, P.K. Controlled induction, enhancement, and guidance of neuronal growth cones by use of line optical tweezers. Opt. Lett. 2005, 30, 2596–2598. [Google Scholar] [CrossRef]
- Stevenson, D.J.; Lake, T.K.; Agate, B.; Gárcés-Chávez, V.; Dholakia, K.; Gunn-Moore, F. Optically guided neuronal growth at near infrared wavelengths. Opt. Express 2006, 14, 9786–9793. [Google Scholar] [CrossRef]
- Mathew, M.; Amat-Roldan, I.; Andrés, R.; Santos, S.I.; Artigas, D.; Soriano, E.; Loza-Alvarez, P. Signalling effect of NIR pulsed lasers on axonal growth. J. Neurosci. Methods 2010, 186, 196–201. [Google Scholar] [CrossRef]
- Mondal, A.; Black, B.; Kim, Y.-T.; Mohanty, S. Loop formation and self-fasciculation of cortical axon using photonic guidance at long working distance. Sci. Rep. 2014, 4, 6902. [Google Scholar] [CrossRef]
- Black, B.; Vishwakarma, V.; Dhakal, K.; Bhattarai, S.; Pradhan, P.; Jain, A.; Kim, Y.-T.; Mohanty, S. Spatial temperature gradients guide axonal outgrowth. Sci. Rep. 2016, 6, 29876. [Google Scholar] [CrossRef]
- Ebbesen, C.L.; Bruus, H. Analysis of laser-induced heating in optical neuronal guidance. J. Neurosci. Methods 2012, 209, 168–177. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, D.; Sonek, G.; Berns, M.; Chapman, C.; Tromberg, B. Evidence for localized cell heating induced by infrared optical tweezers. Biophys. J. 1995, 68, 2137–2144. [Google Scholar] [CrossRef]
- Oyama, K.; Zeeb, V.; Kawamura, Y.; Arai, T.; Gotoh, M.; Itoh, H.; Itabashi, T.; Suzuki, M.; Ishiwata, S. Triggering of high-speed neurite outgrowth using an optical microheater. Sci. Rep. 2015, 5, 16611. [Google Scholar] [CrossRef]
- Kou, L.; Labrie, D.; Chylek, P. Refractive indices of water and ice in the 0.65- to 25-μm spectral range. Appl. Opt. 1993, 32, 3531–3540. [Google Scholar] [CrossRef]
- Ridley, A.J. Life at the Leading Edge. Cell 2011, 145, 1012–1022. [Google Scholar] [CrossRef]
- Giannone, G.; Dubin-Thaler, B.J.; Döbereiner, H.-G.; Kieffer, N.; Bresnick, A.R.; Sheetz, M.P. Periodic Lamellipodial Contractions Correlate with Rearward Actin Waves. Cell 2004, 116, 431–443. [Google Scholar] [CrossRef]
- Galbraith, C.G.; Yamada, K.; Sheetz, M.P. The relationship between force and focal complex development. J. Cell Biol. 2002, 159, 695–705. [Google Scholar] [CrossRef]
- Gallo, G.; Yee, H.F.; Letourneau, P.C. Actin turnover is required to prevent axon retraction driven by endogenous actomyosin contractility. J. Cell Biol. 2002, 158, 1219–1228. [Google Scholar] [CrossRef] [Green Version]
- Mathew, M.; Santos, S.I.C.O.; Zalvidea, D.; Loza-Alvarez, P. Multimodal optical workstation for simultaneous linear, nonlinear microscopy and nanomanipulation: Upgrading a commercial confocal inverted microscope. Rev. Sci. Instrum. 2009, 80, 073701. [Google Scholar] [CrossRef]
- Matrecitos-Avila, M.; Avila, R.; Pimentel-Domínguez, R.; Cuevas, S.; Tamariz, E.; Loza-Alvarez, P. Focus variation due to near infrared laser in a confocal microscope. Microsc. Res. Tech. 2022, 85, 3431–3438. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.D. A Method for Visualizing Multivariate Time Series Data. J. Stat. Softw. 2008, 25, 1–17. [Google Scholar] [CrossRef]
- Shimada, T.; Toriyama, M.; Uemura, K.; Kamiguchi, H.; Sugiura, T.; Watanabe, N.; Inagaki, N. Shootin1 interacts with actin retrograde flow and L1-CAM to promote axon outgrowth. J. Cell Biol. 2008, 181, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Suter, D.M.; Forscher, P. An emerging link between cytoskeletal dynamics and cell adhesion molecules in growth cone guidance. Curr. Opin. Neurobiol. 1998, 8, 106–116. [Google Scholar] [CrossRef]
- Barnhart, E.L.; Allard, J.; Lou, S.S.; Theriot, J.A.; Mogilner, A. Adhesion-Dependent Wave Generation in Crawling Cells. Curr. Biol. 2016, 27, 27–38. [Google Scholar] [CrossRef]
- Bear, J.E.; Svitkina, T.M.; Krause, M.; Schafer, D.A.; Loureiro, J.J.; Strasser, G.A.; Maly, I.V.; Chaga, O.Y.; Cooper, J.A.; Borisy, G.G.; et al. Antagonism between Ena/VASP Proteins and Actin Filament Capping Regulates Fibroblast Motility. Cell 2002, 109, 509–521. [Google Scholar] [CrossRef]
- Katsuno, H.; Toriyama, M.; Hosokawa, Y.; Mizuno, K.; Ikeda, K.; Sakumura, Y.; Inagaki, N. Actin Migration Driven by Directional Assembly and Disassembly of Membrane-Anchored Actin Filaments. Cell Rep. 2015, 12, 648–660. [Google Scholar] [CrossRef]
- Betz, T.; Koch, D.; Stuhrmann, B.; Ehrlicher, A.; Käs, J. Statistical analysis of neuronal growth: Edge dynamics and the effect of a focused laser on growth cone motility. New J. Phys. 2007, 9, 426. [Google Scholar] [CrossRef]
- Bershadsky, A.D.; Ballestrem, C.; Carramusa, L.; Zilberman, Y.; Gilquin, B.; Khochbin, S.; Alexandrova, A.Y.; Verkhovsky, A.B.; Shemesh, T.; Kozlov, M.M. Assembly and mechanosensory function of focal adhesions: Experiments and models. Eur. J. Cell Biol. 2006, 85, 165–173. [Google Scholar] [CrossRef]
- Maiuri, P.; Rupprecht, J.-F.; Wieser, S.; Ruprecht, V.; Bénichou, O.; Carpi, N.; Coppey, M.; De Beco, S.; Gov, N.; Heisenberg, C.-P.; et al. Actin Flows Mediate a Universal Coupling between Cell Speed and Cell Persistence. Cell 2015, 161, 374–386. [Google Scholar] [CrossRef]
- Kao, Y.-C.; Liao, Y.-C.; Cheng, P.-L.; Lee, C.-H. Neurite regrowth stimulation by a red-light spot focused on the neuronal cell soma following blue light-induced retraction. Sci. Rep. 2019, 9, 18210. [Google Scholar] [CrossRef]
- Emiliani, V.; Entcheva, E.; Hedrich, R.; Hegemann, P.; Konrad, K.R.; Lüscher, C.; Mahn, M.; Pan, Z.-H.; Sims, R.R.; Vierock, J.; et al. Optogenetics for light control of biological systems. Nat. Rev. Methods Prim. 2022, 2, 55. [Google Scholar] [CrossRef]
- Leopold, A.V.; Chernov, K.G.; Shemetov, A.A.; Verkhusha, V.V. Neurotrophin receptor tyrosine kinases regulated with near-infrared light. Nat. Commun. 2019, 10, 1129. [Google Scholar] [CrossRef]
- Bath, D.E.; Stowers, J.R.; Hörmann, D.; Poehlmann, A.; Dickson, B.J.; Straw, A.D. FlyMAD: Rapid thermogenetic control of neuronal activity in freely walking Drosophila. Nat. Methods 2014, 11, 756–762. [Google Scholar] [CrossRef]
- Kolesov, D.V.; Sokolinskaya, E.L.; Lukyanov, K.A.; Bogdanov, A.M. Molecular Tools for Targeted Control of Nerve Cell Electrical Activity. Part I. Acta Nat. 2021, 13, 52–64. [Google Scholar] [CrossRef]
- Kolesov, D.V.; Sokolinskaya, E.L.; Lukyanov, K.A.; Bogdanov, A.M. Molecular Tools for Targeted Control of Nerve Cell Electrical Activity. Part II. Acta Nat. 2021, 13, 17–32. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Villalobos, N.; Avila, R.; Marsal, M.; Andilla, J.; Loza-Álvarez, P.; Ojeda-Ramírez, M.M.; Tamariz, E. Infrared Laser Effects on Cell Projection Depend on Irradiation Intermittence and Cell Activity. Cells 2023, 12, 540. https://doi.org/10.3390/cells12040540
Medina-Villalobos N, Avila R, Marsal M, Andilla J, Loza-Álvarez P, Ojeda-Ramírez MM, Tamariz E. Infrared Laser Effects on Cell Projection Depend on Irradiation Intermittence and Cell Activity. Cells. 2023; 12(4):540. https://doi.org/10.3390/cells12040540
Chicago/Turabian StyleMedina-Villalobos, Norma, Remy Avila, María Marsal, Jordi Andilla, Pablo Loza-Álvarez, Mario Miguel Ojeda-Ramírez, and Elisa Tamariz. 2023. "Infrared Laser Effects on Cell Projection Depend on Irradiation Intermittence and Cell Activity" Cells 12, no. 4: 540. https://doi.org/10.3390/cells12040540
APA StyleMedina-Villalobos, N., Avila, R., Marsal, M., Andilla, J., Loza-Álvarez, P., Ojeda-Ramírez, M. M., & Tamariz, E. (2023). Infrared Laser Effects on Cell Projection Depend on Irradiation Intermittence and Cell Activity. Cells, 12(4), 540. https://doi.org/10.3390/cells12040540






