Heat-Stable Enterotoxin Secretions Assessed via ICP-MS Reveal Iron-Mediated Regulation of Virulence in CFA/I- and CS6-Expressing ETEC Isolates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. ST ELISAs
2.3. ST Activity Assays
2.4. Western Blots
2.5. Patent Mouse Model
2.6. Elemental Analysis
2.7. Real-Time PCR
2.8. Streptomycin Water ETEC Infection Model
2.9. Statistical Analysis
3. Results
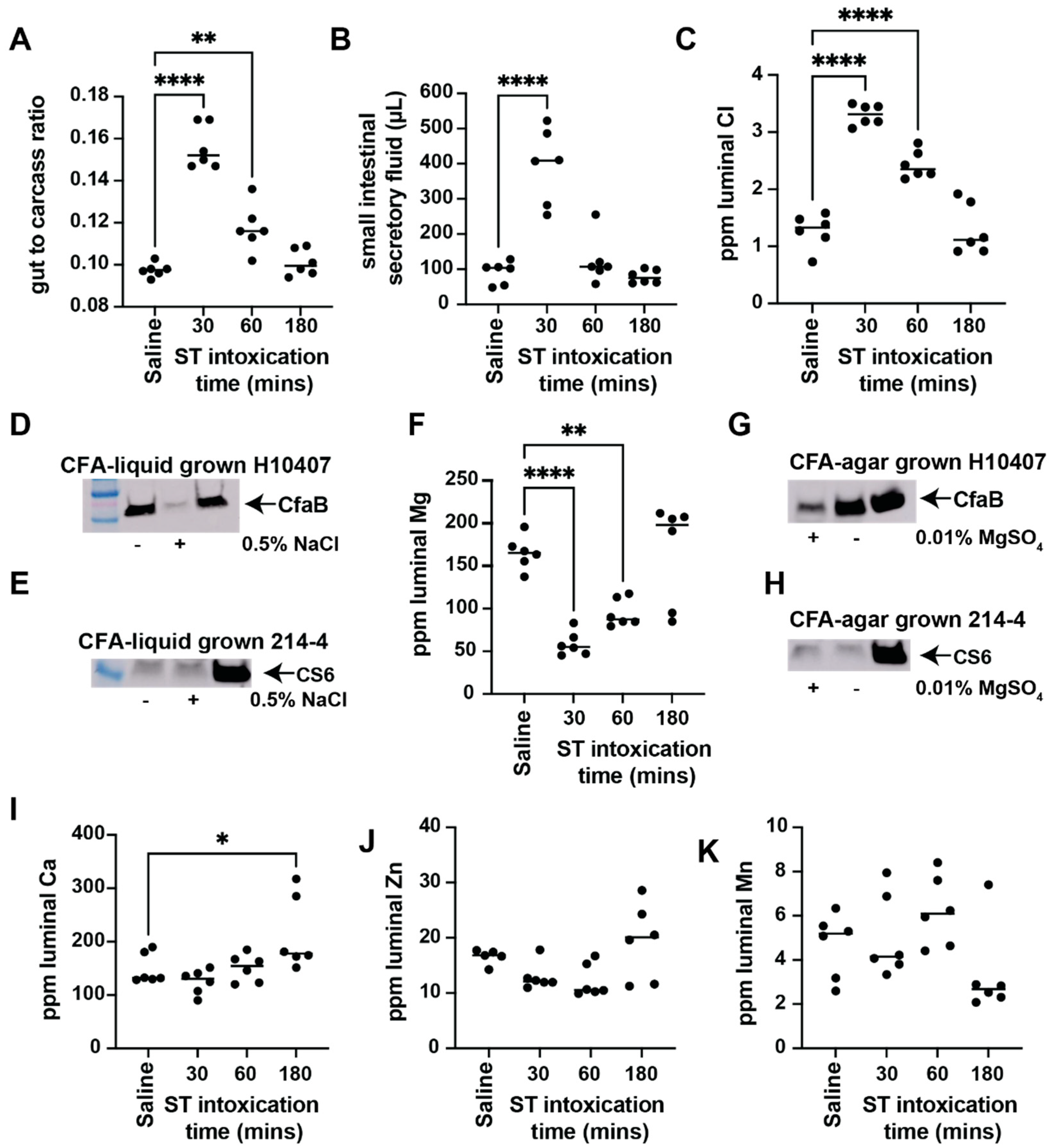
3.1. ST Intoxication Causes Luminal Ion Flux That Affects ETEC Virulence Factor Expression
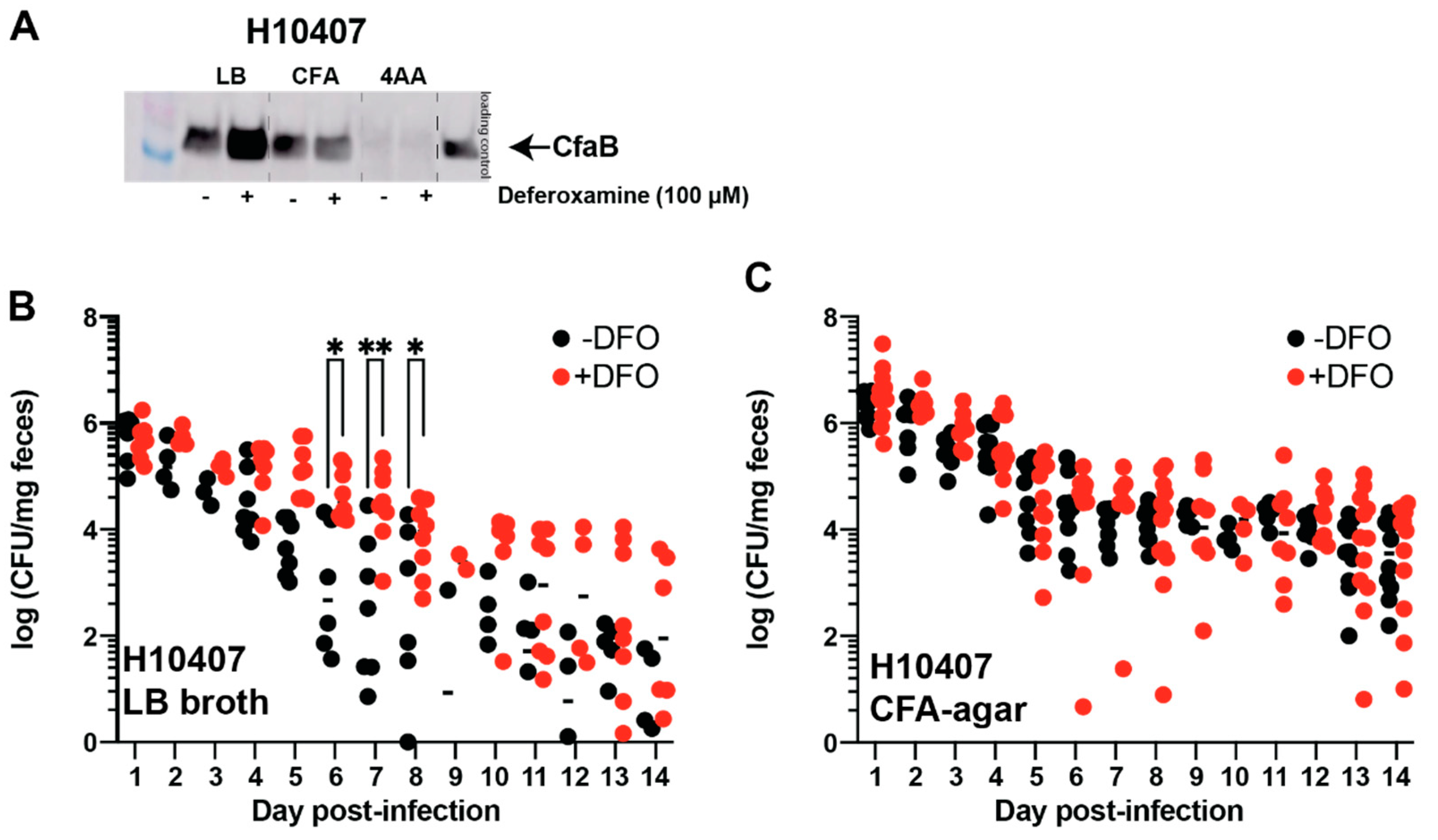
3.2. Deferoxamine Reduces ST Production and Activity in CFA/I+ and CS6+ ST-ETEC Clinical Isolates While Changes in CF Expression Are Isolate-Dependent
3.3. Oral Deferoxamine Treatment Extends ETEC H10407 Colonization
3.4. Oral Deferoxamine Treatment Shortens ETEC 214-4 Colonization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Care and Use Committee
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Platts-Mills, J.A.; Babji, S.; Bodhidatta, L.; Gratz, J.; Haque, R.; Havt, A.; McCormick, B.J.; McGrath, M.; Olortegui, M.P.; Samie, A.; et al. Pathogen-specific burdens of community diarrhoea in developing countries: A multisite birth cohort study (MAL-ED). Lancet Glob. Health 2015, 3, e564–e575. [Google Scholar] [CrossRef]
- Higginson, E.E.; Sayeed, M.A.; Pereira Dias, J.; Shetty, V.; Ballal, M.; Srivastava, S.K.; Willis, I.; Qadri, F.; Dougan, G.; Mutreja, A. Microbiome Profiling of Enterotoxigenic Escherichia coli (ETEC) Carriers Highlights Signature Differences between Symptomatic and Asymptomatic Individuals. mBio 2022, 13, e00157-22. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, J.; Zhang, X.; Bourgeois, A.L.; Harro, C.; Sack, D.A.; Chakraborty, S. Intestinal and systemic inflammation induced by symptomatic and asymptomatic enterotoxigenic E. coli infection and impact on intestinal colonization and ETEC specific immune responses in an experimental human challenge model. Gut Microbes 2021, 13, 1891852. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, D.; Blackwelder, W.C.; Sommerfelt, H.; Wu, Y.; Farag, T.H.; Panchalingam, S.; Biswas, K.; Saha, D.; Hossain, M.J.; Sow, S.O.; et al. Pathogens associated with linear growth faltering in children with diarrhea and impact of antibiotic treatment: The Global Enteric Multicenter Study. J. Infect. Dis. 2021, 224, S848–S855. [Google Scholar] [CrossRef]
- Vidal, R.M.; Muhsen, K.; Tennant, S.M.; Svennerholm, A.M.; Sow, S.O.; Sur, D.; Zaidi, A.K.M.; Faruque, A.S.G.; Saha, D.; Adegbola, R.; et al. Colonization factors among enterotoxigenic Escherichia coli isolates from children with moderate-to-severe diarrhea and from matched controls in the Global Enteric Multicenter Study (GEMS). PLoS Negl. Trop. Dis. 2019, 13, e0007037. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Rao, M.R.; Wierzba, T.F.; Savarino, S.J.; Abu-Elyazeed, R.; El-Ghoreb, N.; Hall, E.R.; Naficy, A.; Abdel-Messih, I.; Frenck, R.W., Jr.; Svennerholm, A.M.; et al. Serologic correlates of protection against enterotoxigenic Escherichia coli diarrhea. J. Infect. Dis. 2005, 191, 562–570. [Google Scholar] [CrossRef]
- Talaat, K.R.; Porter, C.K.; Bourgeois, A.L.; Lee, T.K.; Duplessis, C.A.; Maciel, M., Jr.; Gutierrez, R.L.; DeNearing, B.; Adjoodani, B.; Adkinson, R.; et al. Oral delivery of Hyperimmune bovine serum antibodies against CS6-expressing enterotoxigenic Escherichia coli as a prophylactic against diarrhea. Gut Microbes 2020, 12, 1732852. [Google Scholar] [CrossRef]
- Walker, R.; Kaminski, R.W.; Porter, C.; Choy, R.K.M.; White, J.A.; Fleckenstein, J.M.; Cassels, F.; Bourgeois, L. Vaccines for Protecting Infants from Bacterial Causes of Diarrheal Disease. Microorganisms 2021, 9, 1382. [Google Scholar] [CrossRef]
- Evans, D.J., Jr.; Evans, D.G. Three characteristics associated with enterotoxigenic Escherichia coli isolated from man. Infect. Immun. 1973, 8, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Sack, D.A.; Merson, M.H.; Wells, J.G.; Sack, R.B.; Morris, G.K. Diarrhoea associated with heat-stable enterotoxin-producing strains of Escherichia coli. Lancet 1975, 2, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.M.; Caplan, E.S.; Waterman, D.; Cash, R.A.; Hornick, R.B.; Snyder, M.J. Diarrhea caused by Escherichia coli that produce only heat-stable enterotoxin. Infect. Immun. 1977, 17, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Satterwhite, T.K.; Evans, D.J., Jr.; DuPont, H. Differences in serological responses and excretion patterns of volunteers challenged with enterotoxigenic Escherichia coli with and without the colonization factor antigen. Infect. Immun. 1978, 19, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Hazen, T.H.; Nagaraj, S.; Sen, S.; Permala-Booth, J.; Del Canto, F.; Vidal, R.; Barry, E.M.; Bitoun, J.P.; Chen, W.H.; Tennant, S.M.; et al. Genome and Functional Characterization of Colonization Factor Antigen I- and CS6-Encoding Heat-Stable Enterotoxin-Only Enterotoxigenic Escherichia coli Reveals Lineage and Geographic Variation. mSystems 2019, 4, e00329-18. [Google Scholar] [CrossRef]
- Berberov, E.M.; Zhou, Y.; Francis, D.H.; Scott, M.A.; Kachman, S.D.; Moxley, R.A. Relative importance of heat-labile enterotoxin in the causation of severe diarrheal disease in the gnotobiotic piglet model by a strain of enterotoxigenic Escherichia coli that produces multiple enterotoxins. Infect. Immun. 2004, 72, 3914–3924. [Google Scholar] [CrossRef]
- Allen, K.P.; Randolph, M.M.; Fleckenstein, J.M. Importance of heat-labile enterotoxin in colonization of the adult mouse small intestine by human enterotoxigenic Escherichia coli strains. Infect. Immun. 2006, 74, 869–875. [Google Scholar] [CrossRef]
- Kunin, C.M.; Hua, T.H.; Guerrant, R.L.; Bakaletz, L.O. Effect of salicylate, bismuth, osmolytes, and tetracycline resistance on expression of fimbriae by Escherichia coli. Infect. Immun. 1994, 62, 2178–2186. [Google Scholar] [CrossRef]
- Kiefer, M.C.; Motyka, N.I.; Clements, J.D.; Bitoun, J.P. Enterotoxigenic Escherichia coli Heat-Stable Toxin Increases the Rate of Zinc Release from Metallothionein and Is a Zinc- and Iron-Binding Peptide. mSphere 2020, 5, e00146-20. [Google Scholar] [CrossRef]
- Karjalainen, T.K.; Evans, D.G.; Evans, D.J., Jr.; Graham, D.Y.; Lee, C.H. Iron represses the expression of CFA/I fimbriae of enterotoxigenic E. coli. Microb. Pathog. 1991, 11, 317–323. [Google Scholar] [CrossRef]
- Haines, S.; Arnaud-Barbe, N.; Poncet, D.; Reverchon, S.; Wawrzyniak, J.; Nasser, W.; Renauld-Mongenie, G. IscR Regulates Synthesis of Colonization Factor Antigen I Fimbriae in Response to Iron Starvation in Enterotoxigenic Escherichia coli. J. Bacteriol. 2015, 197, 2896–2907. [Google Scholar] [CrossRef] [Green Version]
- Bhakat, D.; Mondal, I.; Mukhopadhyay, A.K.; Chatterjee, N.S. Iron influences the expression of colonization factor CS6 of enterotoxigenic Escherichia coli. Microbiology 2021, 167, 001089. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hardwidge, P.R. Enterotoxigenic Escherichia coli prevents host NF-kappaB activation by targeting IkappaBalpha polyubiquitination. Infect. Immun. 2012, 80, 4417–4425. [Google Scholar] [CrossRef] [PubMed]
- Motyka, N.I.; Stewart, S.R.; Hollifield, I.E.; Kyllo, T.R.; Mansfield, J.A.; Norton, E.B.; Clements, J.D.; Bitoun, J.P. Elevated Extracellular cGMP Produced after Exposure to Enterotoxigenic Escherichia coli Heat-Stable Toxin Induces Epithelial IL-33 Release and Alters Intestinal Immunity. Infect. Immun. 2021, 89, e00707-20. [Google Scholar] [CrossRef]
- Evans, D.G.; Evans, D.J., Jr.; Tjoa, W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: Correlation with colonization factor. Infect. Immun. 1977, 18, 330–337. [Google Scholar] [CrossRef]
- Alderete, J.F.; Robertson, D.C. Nutrition and enterotoxin synthesis by enterotoxigenic strains of Escherichia coli: Defined medium for production of heat-stable enterotoxin. Infect. Immun. 1977, 15, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Taxt, A.M.; Diaz, Y.; Aasland, R.; Clements, J.D.; Nataro, J.P.; Sommerfelt, H.; Puntervoll, P. Towards rational design of a toxoid vaccine against the heat-stable toxin of Escherichia coli. Infect. Immun. 2016, 84, 1239–1249. [Google Scholar] [CrossRef]
- Motyka, N.I.; Stewart, S.R.; Porretta, C.P.; Hollifield, I.E.; Bauer, D.L.; Bitoun, J.P. Enterotoxigenic Escherichia coli Enterotoxins Regulate Epithelial to Immune Relay of IL-33 and IL-1Ra Cytokines. Infect. Immun. 2022, 90, e0063721. [Google Scholar] [CrossRef]
- Read, L.T.; Hahn, R.W.; Thompson, C.C.; Bauer, D.L.; Norton, E.B.; Clements, J.D. Simultaneous exposure to Escherichia coli heat-labile and heat-stable enterotoxins increases fluid secretion and alters cyclic nucleotide and cytokine production by intestinal epithelial cells. Infect. Immun. 2014, 82, 5308–5316. [Google Scholar] [CrossRef]
- Dorsey, F.C.; Fischer, J.F.; Fleckenstein, J.M. Directed delivery of heat-labile enterotoxin by enterotoxigenic Escherichia coli. Cell. Microbiol. 2006, 8, 1516–1527. [Google Scholar] [CrossRef]
- Sato, T.; Shimonishi, Y. Structural features of Escherichia coli heat-stable enterotoxin that activates membrane-associated guanylyl cyclase. J. Pept. Res. 2004, 63, 200–206. [Google Scholar] [CrossRef]
- Sears, C.L.; Kaper, J.B. Enteric bacterial toxins: Mechanisms of action and linkage to intestinal secretion. Microbiol. Rev. 1996, 60, 167–215. [Google Scholar] [CrossRef] [PubMed]
- Forte, L.R.; Thorne, P.K.; Eber, S.L.; Krause, W.J.; Freeman, R.H.; Francis, S.H.; Corbin, J.D. Stimulation of intestinal Cl- transport by heat-stable enterotoxin: Activation of cAMP-dependent protein kinase by cGMP. Am. J. Physiol. 1992, 263, C607–C615. [Google Scholar] [CrossRef] [PubMed]
- Field, M. Intestinal secretion. Gastroenterology 1974, 66, 1063–1084. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Lin, R.; Avula, L.; Sarker, R.; Yang, J.; Cha, B.; Tse, C.M.; McNamara, G.; Seidler, U.; Waldman, S.; et al. NHERF3 is necessary for Escherichia coli heat-stable enterotoxin-induced inhibition of NHE3: Differences in signaling in mouse small intestine and Caco-2 cells. Am. J. Physiol. Cell Physiol. 2019, 317, C737–C748. [Google Scholar] [CrossRef]
- Utech, M.; Bruwer, M.; Nusrat, A. Tight junctions and cell-cell interactions. Methods Mol. Biol. 2006, 341, 185–195. [Google Scholar] [CrossRef]
- Choi, E.Y.; Kim, E.C.; Oh, H.M.; Kim, S.; Lee, H.J.; Cho, E.Y.; Yoon, K.H.; Kim, E.A.; Han, W.C.; Choi, S.C.; et al. Iron chelator triggers inflammatory signals in human intestinal epithelial cells: Involvement of p38 and extracellular signal-regulated kinase signaling pathways. J. Immunol. 2004, 172, 7069–7077. [Google Scholar] [CrossRef]
- McHugh, J.P.; Rodríguez-Quinoñes, F.; Abdul-Tehrani, H.; Svistunenko, D.A.; Poole, R.K.; Cooper, C.E.; Andrews, S.C. Global iron-dependent gene regulation in Escherichia coli. A new mechanism for iron homeostasis. J. Biol. Chem. 2003, 278, 29478–29486. [Google Scholar] [CrossRef]
- Agoro, R.; Taleb, M.; Quesniaux, V.F.J.; Mura, C. Cell iron status influences macrophage polarization. PLoS ONE 2018, 13, e0196921. [Google Scholar] [CrossRef]
- Liu, Y.; Han, R.; Wang, J.; Yang, P.; Wang, F.; Yang, B. Magnesium Sensing Regulates Intestinal Colonization of Enterohemorrhagic Escherichia coli O157:H7. mBio 2020, 11, e02470-20. [Google Scholar] [CrossRef]
- Booth, I.W.; Milla, P.J.; Harries, J.T. The effects of magnesium on ion transport in short-circuited rabbit terminal ileum. Clin. Sci. 1984, 66, 465–471. [Google Scholar] [CrossRef]
- Pitari, G.M.; Lin, J.E.; Shah, F.J.; Lubbe, W.J.; Zuzga, D.S.; Li, P.; Schulz, S.; Waldman, S.A. Enterotoxin preconditioning restores calcium-sensing receptor-mediated cytostasis in colon cancer cells. Carcinogenesis 2008, 29, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lin, J.E.; Snook, A.E.; Waldman, S.A. ST-Producing E. coli Oppose Carcinogen-Induced Colorectal Tumorigenesis in Mice. Toxins 2017, 9, 279. [Google Scholar] [CrossRef]
- Levine, M.M.; Nalin, D.R.; Hoover, D.L.; Bergquist, E.J.; Hornick, R.B.; Young, C.R. Immunity to enterotoxigenic Escherichia coli. Infect. Immun. 1979, 23, 729–736. [Google Scholar] [CrossRef]
- Jabara, H.H.; Boyden, S.E.; Chou, J.; Ramesh, N.; Massaad, M.J.; Benson, H.; Bainter, W.; Fraulino, D.; Rahimov, F.; Sieff, C.; et al. A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nat. Genet. 2016, 48, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Swann, J.W.; Koneva, L.A.; Regan-Komito, D.; Sansom, S.N.; Powrie, F.; Griseri, T. IL-33 promotes anemia during chronic inflammation by inhibiting differentiation of erythroid progenitors. J. Exp. Med. 2020, 217, e20200164. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Basatemur, G.; Scott, I.C.; Chiarugi, D.; Clement, M.; Harrison, J.; Jugdaohsingh, R.; Yu, X.; Newland, S.A.; Jolin, H.E.; et al. Interleukin-33 Signaling Controls the Development of Iron-Recycling Macrophages. Immunity 2020, 52, 782–793 e785. [Google Scholar] [CrossRef]
- Rivera-Chavez, F.; Mekalanos, J.J. Cholera toxin promotes pathogen acquisition of host-derived nutrients. Nature 2019, 572, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Crofts, A.A.; Giovanetti, S.M.; Rubin, E.J.; Poly, F.M.; Gutierrez, R.L.; Talaat, K.R.; Porter, C.K.; Riddle, M.S.; DeNearing, B.; Brubaker, J.; et al. Enterotoxigenic E. coli virulence gene regulation in human infections. Proc. Natl. Acad. Sci. USA 2018, 115, E8968–E8976. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Tumala, B.; Vickers, T.J.; Alvarado, D.; Ciorba, M.A.; Bhuiyan, T.R.; Qadri, F.; Singer, B.B.; Fleckenstein, J.M. CEACAMs serve as toxin-stimulated receptors for enterotoxigenic Escherichia coli. Proc. Natl. Acad. Sci. USA 2020, 117, 29055–29062. [Google Scholar] [CrossRef]
- Moon, J.; Barry, E.M. Sequence variations in the ETEC CS6 operon affect transcript and protein expression. Virulence 2021, 12, 2659–2669. [Google Scholar] [CrossRef]
- Bodero, M.D.; Munson, G.P. Cyclic AMP receptor protein-dependent repression of heat-labile enterotoxin. Infect. Immun. 2009, 77, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Vidal, M.; Pardo, M.; Torres, A.; Kruger, E.; Farfán, M.; O’Ryan, M.; Luo, Q.; Fleckenstein, J.; Del Canto, F.; et al. Characterization of enterotoxigenic Escherichia coli strains isolated from the massive multi-pathogen gastroenteritis outbreak in the Antofagasta region following the Chilean earthquake, 2010. Infect. Genet. Evol. 2017, 52, 26–29. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hollifield, I.E.; Motyka, N.I.; Stewart, S.R.; Blyth, M.D.; Fernando, K.A.; Clement, K.L.; Bitoun, J.P. Heat-Stable Enterotoxin Secretions Assessed via ICP-MS Reveal Iron-Mediated Regulation of Virulence in CFA/I- and CS6-Expressing ETEC Isolates. Cells 2023, 12, 567. https://doi.org/10.3390/cells12040567
Hollifield IE, Motyka NI, Stewart SR, Blyth MD, Fernando KA, Clement KL, Bitoun JP. Heat-Stable Enterotoxin Secretions Assessed via ICP-MS Reveal Iron-Mediated Regulation of Virulence in CFA/I- and CS6-Expressing ETEC Isolates. Cells. 2023; 12(4):567. https://doi.org/10.3390/cells12040567
Chicago/Turabian StyleHollifield, Ian E., Natalya I. Motyka, Sydney R. Stewart, Michelle D. Blyth, Kaylynn A. Fernando, Kristen L. Clement, and Jacob P. Bitoun. 2023. "Heat-Stable Enterotoxin Secretions Assessed via ICP-MS Reveal Iron-Mediated Regulation of Virulence in CFA/I- and CS6-Expressing ETEC Isolates" Cells 12, no. 4: 567. https://doi.org/10.3390/cells12040567




