Microbe–Heavy Metal Interactions
A special issue of Cells (ISSN 2073-4409).
Deadline for manuscript submissions: 30 September 2024 | Viewed by 2149
Special Issue Editor
Interests: environmental microbiology; community-based participatory research; water and health; waterborne disease
Special Issues, Collections and Topics in MDPI journals
Special Issue Information
Dear Colleagues,
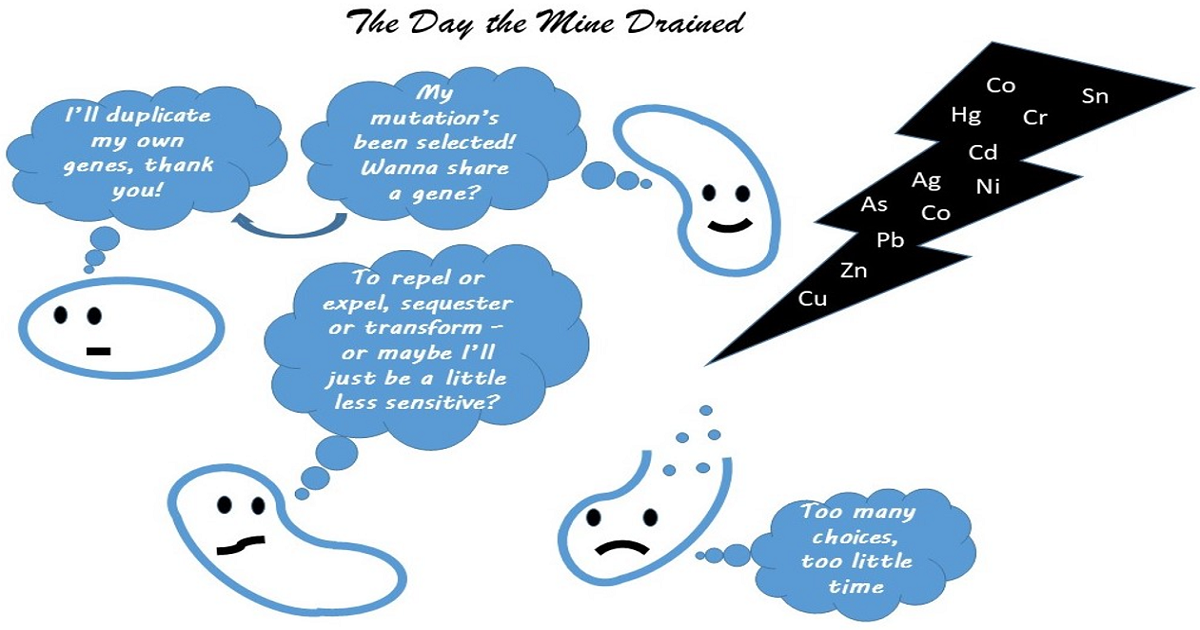
The multitude of ways in which microbes adapt to the presence of heavy metals have been extensively described in the literature. Today’s current focus on “omics” approaches to understand how microbes adapt to harsh environments, such as the presence of heavy metals, is yielding vast amounts of data. However, there are still many unanswered questions, such as what the main adaptive mechanism itself is. The three likely drivers—natural selection, horizontal gene transfer and gene duplication—still need to be defined for specific systems and stressors. There is no doubt that metal resistance in bacteria has significant implications for the fate and transport of heavy metals in the environment, for bioextraction, for bioremediation and for detoxification. In addition to this list, we now add the link between metal resistance and antibiotic resistance that remains to be clearly explained.
This Special Issue will examine the current and anticipated advances in the field, as well as encourage contributions from the cutting-edge omics research that is emerging from many laboratories around the world and advancing, for example, our understanding of the co-selection for antibiotic resistance. We also encourage submissions from the translational research that is driving the fields of bioextraction and bioremediation, in addition to advancing our understanding of the fate of heavy metals in the environment.
Prof. Dr. Timothy E. Ford
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- horizontal gene transfer (HGT)
- gene duplication
- natural selection
- mutation
- heavy metal resistance genes (MRGs)
- antibiotic resistance genes (ARGs)
- acid mine drainage (AMD)
- heavy metals
- plasmids
- mobile genetic elements (MGEs)
- transposons
- extreme environments
- bioremediation






