High Resolution Multiplex Confocal Imaging of the Neurovascular Unit in Health and Experimental Ischemic Stroke
Abstract
1. Introduction
2. Methods
2.1. Animals and Ethical Approval
2.2. Transient Middle Cerebral Artery Occlusion (MCAO) Model
2.3. TTC Staining
2.4. Laser Doppler Blood Flow
2.5. Tissue Processing
2.6. Immunofluorescence Staining
2.7. Confocal Microscopy
2.8. Statistics
3. Results
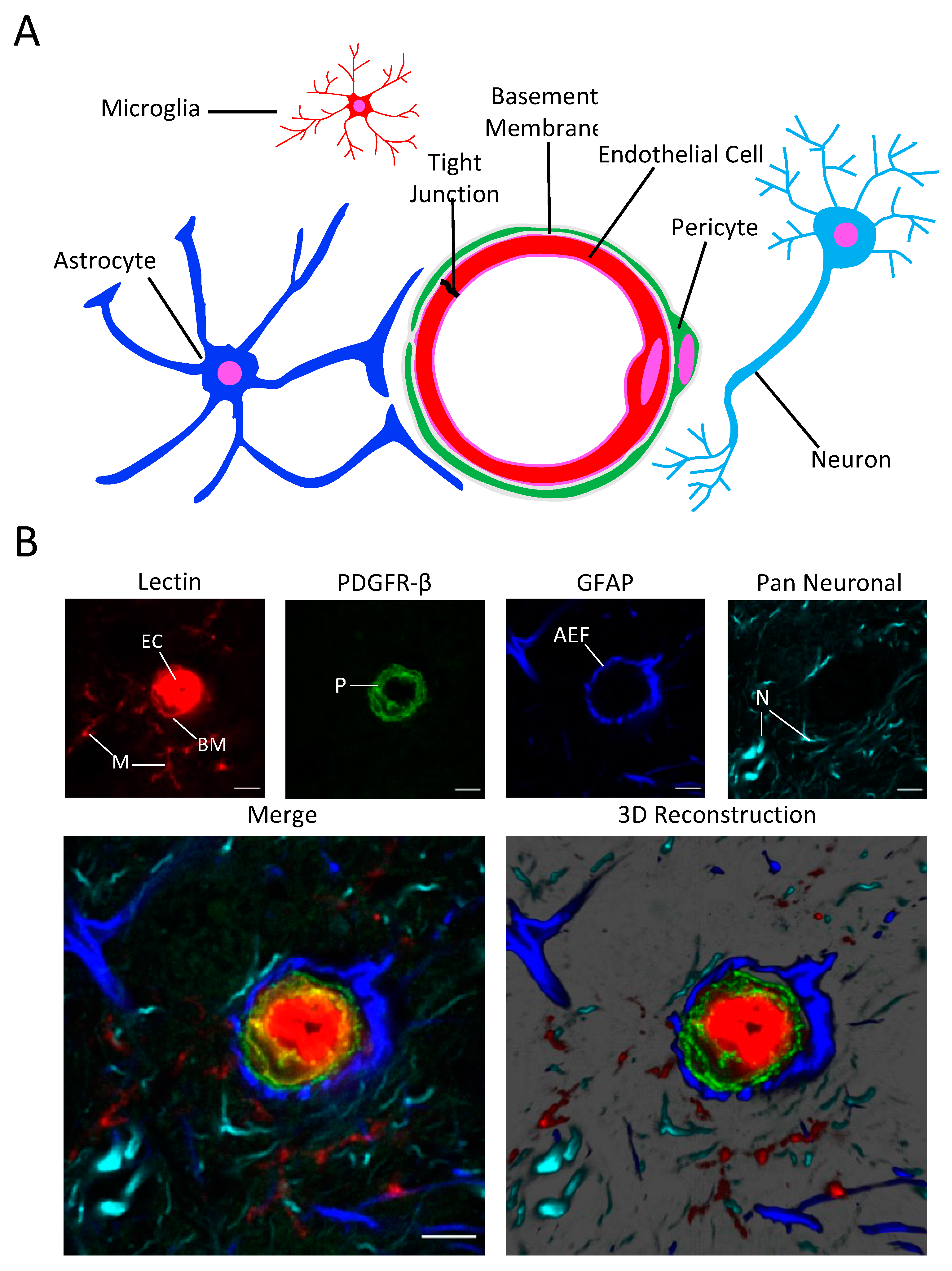
3.1. Fluorescence Staining and Confocal Imaging of the NVU
3.2. Middle Cerebral Artery Occlusion (MCAO) Induces Changes in the NVU
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. Fluid transport in the brain. Physiol. Rev. 2022, 102, 1025–1151. [Google Scholar] [CrossRef]
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The role of brain barriers in fluid movement in the CNS: Is there a ‘glymphatic’ system? Acta Neuropathol. 2018, 135, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; Barrand, M.A. The glymphatic hypothesis: The theory and the evidence. Fluids Barriers CNS 2022, 19, 9. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Yang, J.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Kugler, E.C.; Greenwood, J.; MacDonald, R.B. The “Neuro-Glial-Vascular” Unit: The Role of Glia in Neurovascular Unit Formation and Dysfunction. Front. Cell Dev. Biol. 2021, 9, 732820. [Google Scholar] [CrossRef]
- Butt, A.M.; Jones, H.C.; Abbott, N.J. Electrical resistance across the blood-brain barrier in anaesthetized rats: A developmental study. J. Physiol. 1990, 429, 47–62. [Google Scholar] [CrossRef]
- Reese, T.S.; Karnovsky, M.J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 1967, 34, 207–217. [Google Scholar] [CrossRef]
- McConnell, H.L.; Kersch, C.N.; Woltjer, R.L.; Neuwelt, E.A. The Translational Significance of the Neurovascular Unit. J. Biol. Chem. 2017, 292, 762–770. [Google Scholar] [CrossRef]
- Brzica, H.; Abdullahi, W.; Ibbotson, K.; Ronaldson, P.T. Role of Transporters in Central Nervous System Drug Delivery and Blood-Brain Barrier Protection: Relevance to Treatment of Stroke. J. Cent. Nerv. Syst. Dis. 2017, 9, 1179573517693802. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.I.; Hartmann, D.A.; Underly, R.G.; Berthiaume, A.A.; Bhat, N.R.; Shih, A.Y. Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. J. Cereb. Blood Flow Metab. 2019, 39, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Villasenor, R.; Kuennecke, B.; Ozmen, L.; Ammann, M.; Kugler, C.; Gruninger, F.; Loetscher, H.; Freskgard, P.O.; Collin, L. Region-specific permeability of the blood-brain barrier upon pericyte loss. J. Cereb. Blood Flow Metab. 2017, 37, 3683–3694. [Google Scholar] [CrossRef]
- Hellstrom, M.; Gerhardt, H.; Kalen, M.; Li, X.; Eriksson, U.; Wolburg, H.; Betsholtz, C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J. Cell Biol. 2001, 153, 543–553. [Google Scholar] [CrossRef]
- Hartmann, D.A.; Berthiaume, A.A.; Grant, R.I.; Harrill, S.A.; Koski, T.; Tieu, T.; McDowell, K.P.; Faino, A.V.; Kelly, A.L.; Shih, A.Y. Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat. Neurosci. 2021, 24, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Winkler, E.A.; Sagare, A.P.; Singh, I.; LaRue, B.; Deane, R.; Zlokovic, B.V. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010, 68, 409–427. [Google Scholar] [CrossRef]
- Hall, C.N.; Reynell, C.; Gesslein, B.; Hamilton, N.B.; Mishra, A.; Sutherland, B.A.; O’Farrell, F.M.; Buchan, A.M.; Lauritzen, M.; Attwell, D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature 2014, 508, 55–60. [Google Scholar] [CrossRef]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef]
- Ornelas, S.; Berthiaume, A.A.; Bonney, S.K.; Coelho-Santos, V.; Underly, R.G.; Kremer, A.; Guerin, C.J.; Lippens, S.; Shih, A.Y. Three-dimensional ultrastructure of the brain pericyte-endothelial interface. J. Cereb. Blood Flow Metab. 2021, 41, 2185–2200. [Google Scholar] [CrossRef]
- Brown, L.S.; Foster, C.G.; Courtney, J.M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and Neurovascular Function in the Healthy and Diseased Brain. Front. Cell Neurosci. 2019, 13, 282. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, Z.L.; Norris, E.H.; Strickland, S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat. Commun. 2014, 5, 3413. [Google Scholar] [CrossRef] [PubMed]
- Pivoriunas, A.; Verkhratsky, A. Astrocyte-Endotheliocyte Axis in the Regulation of the Blood-Brain Barrier. Neurochem. Res. 2021, 46, 2538–2550. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Yang, Y.; Ju, W.N.; Wang, X.; Zhang, H.L. Emerging Roles of Astrocytes in Neuro-Vascular Unit and the Tripartite Synapse With Emphasis on Reactive Gliosis in the Context of Alzheimer’s Disease. Front. Cell Neurosci. 2018, 12, 193. [Google Scholar] [CrossRef]
- Heithoff, B.P.; George, K.K.; Phares, A.N.; Zuidhoek, I.A.; Munoz-Ballester, C.; Robel, S. Astrocytes are necessary for blood-brain barrier maintenance in the adult mouse brain. Glia 2021, 69, 436–472. [Google Scholar] [CrossRef] [PubMed]
- van Putten, M.; Fahlke, C.; Kafitz, K.W.; Hofmeijer, J.; Rose, C.R. Dysregulation of Astrocyte Ion Homeostasis and Its Relevance for Stroke-Induced Brain Damage. Int. J. Mol. Sci. 2021, 22, 5679. [Google Scholar] [CrossRef] [PubMed]
- Filosa, J.A.; Morrison, H.W.; Iddings, J.A.; Du, W.; Kim, K.J. Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience 2016, 323, 96–109. [Google Scholar] [CrossRef]
- Gu, X.; Chen, W.; Volkow, N.D.; Koretsky, A.P.; Du, C.; Pan, Y. Synchronized Astrocytic Ca(2+) Responses in Neurovascular Coupling during Somatosensory Stimulation and for the Resting State. Cell Rep. 2018, 23, 3878–3890. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhauser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Jolivel, V.; Bicker, F.; Biname, F.; Ploen, R.; Keller, S.; Gollan, R.; Jurek, B.; Birkenstock, J.; Poisa-Beiro, L.; Bruttger, J.; et al. Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol. 2015, 129, 279–295. [Google Scholar] [CrossRef]
- Bisht, K.; Okojie, K.A.; Sharma, K.; Lentferink, D.H.; Sun, Y.Y.; Chen, H.R.; Uweru, J.O.; Amancherla, S.; Calcuttawala, Z.; Campos-Salazar, A.B.; et al. Capillary-associated microglia regulate vascular structure and function through PANX1-P2RY12 coupling in mice. Nat. Commun. 2021, 12, 5289. [Google Scholar] [CrossRef]
- Haruwaka, K.; Ikegami, A.; Tachibana, Y.; Ohno, N.; Konishi, H.; Hashimoto, A.; Matsumoto, M.; Kato, D.; Ono, R.; Kiyama, H.; et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat. Commun. 2019, 10, 5816. [Google Scholar] [CrossRef] [PubMed]
- Lou, N.; Takano, T.; Pei, Y.; Xavier, A.L.; Goldman, S.A.; Nedergaard, M. Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc. Natl. Acad. Sci. USA 2016, 113, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, P.T.; Davis, T.P. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J. Cereb. Blood Flow Metab. 2020, 40, S6–S24. [Google Scholar] [CrossRef] [PubMed]
- Csaszar, E.; Lenart, N.; Cserep, C.; Kornyei, Z.; Fekete, R.; Posfai, B.; Balazsfi, D.; Hangya, B.; Schwarcz, A.D.; Szabadits, E.; et al. Microglia modulate blood flow, neurovascular coupling, and hypoperfusion via purinergic actions. J. Exp. Med. 2022, 219, e20211071. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; El Khoury, J. Microglia in Health and Disease. Cold Spring Harb. Perspect Biol. 2015, 8, a020560. [Google Scholar] [CrossRef] [PubMed]
- Stanton, J.A.; Williams, E.I.; Betterton, R.D.; Davis, T.P.; Ronaldson, P.T. Targeting organic cation transporters at the blood-brain barrier to treat ischemic stroke in rats. Exp. Neurol. 2022, 357, 114181. [Google Scholar] [CrossRef]
- Barber, P.A.; Hoyte, L.; Colbourne, F.; Buchan, A.M. Temperature-regulated model of focal ischemia in the mouse: A study with histopathological and behavioral outcomes. Stroke 2004, 35, 1720–1725. [Google Scholar] [CrossRef]
- Mathiisen, T.M.; Lehre, K.P.; Danbolt, N.C.; Ottersen, O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia 2010, 58, 1094–1103. [Google Scholar] [CrossRef]
- Shen, X.Y.; Gao, Z.K.; Han, Y.; Yuan, M.; Guo, Y.S.; Bi, X. Activation and Role of Astrocytes in Ischemic Stroke. Front. Cell Neurosci. 2021, 15, 755955. [Google Scholar] [CrossRef]
- Wang, H.; Song, G.; Chuang, H.; Chiu, C.; Abdelmaksoud, A.; Ye, Y.; Zhao, L. Portrait of glial scar in neurological diseases. Int. J. Immunopathol. Pharmacol. 2018, 31, 2058738418801406. [Google Scholar] [CrossRef]
- Brawek, B.; Olmedillas Del Moral, M.; Garaschuk, O. In Vivo Visualization of Microglia Using Tomato Lectin. Methods Mol. Biol. 2019, 2034, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Sampei, S.; Okada, H.; Tomita, H.; Takada, C.; Suzuki, K.; Kinoshita, T.; Kobayashi, R.; Fukuda, H.; Kawasaki, Y.; Nishio, A.; et al. Endothelial Glycocalyx Disorders May Be Associated With Extended Inflammation During Endotoxemia in a Diabetic Mouse Model. Front. Cell Dev. Biol. 2021, 9, 623582. [Google Scholar] [CrossRef] [PubMed]
- Battistella, R.; Kritsilis, M.; Matuskova, H.; Haswell, D.; Cheng, A.X.; Meissner, A.; Nedergaard, M.; Lundgaard, I. Not All Lectins Are Equally Suitable for Labeling Rodent Vasculature. Int. J. Mol. Sci. 2021, 22, 11554. [Google Scholar] [CrossRef]
- Villacampa, N.; Almolda, B.; Gonzalez, B.; Castellano, B. Tomato lectin histochemistry for microglial visualization. Methods Mol. Biol. 2013, 1041, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Acarin, L.; Vela, J.M.; Gonzalez, B.; Castellano, B. Demonstration of poly-N-acetyl lactosamine residues in ameboid and ramified microglial cells in rat brain by tomato lectin binding. J. Histochem. Cytochem. 1994, 42, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.N.; Revelo, N.H.; Seitz, K.J.; Helm, M.S.; Sarkar, D.; Saleeb, R.S.; D’Este, E.; Eberle, J.; Wagner, E.; Vogl, C.; et al. Glyoxal as an alternative fixative to formaldehyde in immunostaining and super-resolution microscopy. EMBO J. 2018, 37, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Sadanandan, J.; Blackburn, S.L.; McBride, D.W.; Dienel, A.; Hong, S.; Zeineddine, H.A.; Thankamani, P.K. Glyoxal Fixation Is Optimal for Immunostaining of Brain Vessels, Pericytes and Blood-Brain Barrier Proteins. Int. J. Mol. Sci. 2022, 23, 7776. [Google Scholar] [CrossRef]
- Hartmann, D.A.; Underly, R.G.; Grant, R.I.; Watson, A.N.; Lindner, V.; Shih, A.Y. Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics 2015, 2, 041402. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, K.K. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Hubbard, J.A.; Hsu, M.S.; Seldin, M.M.; Binder, D.K. Expression of the Astrocyte Water Channel Aquaporin-4 in the Mouse Brain. ASN Neuro 2015, 7, 1–14. [Google Scholar] [CrossRef]
- Dirnagl, U. Pathobiology of injury after stroke: The neurovascular unit and beyond. Ann. N. Y. Acad. Sci. 2012, 1268, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lu, J.; Shao, A.; Zhang, J.H.; Zhang, J. Glial Cells: Role of the Immune Response in Ischemic Stroke. Front. Immunol. 2020, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Klett, F.; Potas, J.R.; Hilpert, D.; Blazej, K.; Radke, J.; Huck, J.; Engel, O.; Stenzel, W.; Genove, G.; Priller, J. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J. Cereb. Blood Flow Metab. 2013, 33, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Arai, K.; Lo, E.H.; Hommel, M. Pathophysiologic cascades in ischemic stroke. Int. J. Stroke 2012, 7, 378–385. [Google Scholar] [CrossRef]
- Pawluk, H.; Wozniak, A.; Grzesk, G.; Kolodziejska, R.; Kozakiewicz, M.; Kopkowska, E.; Grzechowiak, E.; Kozera, G. The Role of Selected Pro-Inflammatory Cytokines in Pathogenesis of Ischemic Stroke. Clin. Interv. Aging 2020, 15, 469–484. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflammation 2019, 16, 142. [Google Scholar] [CrossRef]
- Underly, R.G.; Levy, M.; Hartmann, D.A.; Grant, R.I.; Watson, A.N.; Shih, A.Y. Pericytes as Inducers of Rapid, Matrix Metalloproteinase-9-Dependent Capillary Damage during Ischemia. J. Neurosci. 2017, 37, 129–140. [Google Scholar] [CrossRef]
- Cao, L.; Zhou, Y.; Chen, M.; Li, L.; Zhang, W. Pericytes for Therapeutic Approaches to Ischemic Stroke. Front. Neurosci. 2021, 15, 629297. [Google Scholar] [CrossRef]
- Hatakeyama, M.; Ninomiya, I.; Kanazawa, M. Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen. Res. 2020, 15, 16–19. [Google Scholar] [CrossRef]
- Roth, M.; Enstrom, A.; Aghabeick, C.; Carlsson, R.; Genove, G.; Paul, G. Parenchymal pericytes are not the major contributor of extracellular matrix in the fibrotic scar after stroke in male mice. J. Neurosci. Res. 2020, 98, 826–842. [Google Scholar] [CrossRef]
- Taft, J.R.; Vertes, R.P.; Perry, G.W. Distribution of GFAP+ astrocytes in adult and neonatal rat brain. Int. J. Neurosci. 2005, 115, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, G.; Ohtomo, R.; Takase, H.; Lok, J.; Arai, K. Role of oligodendrocyte-neurovascular unit in white matter repair. Neurosci. Lett. 2018, 684, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Bonney, S.K.; Sullivan, L.T.; Cherry, T.J.; Daneman, R.; Shih, A.Y. Distinct features of brain perivascular fibroblasts and mural cells revealed by in vivo two-photon imaging. J. Cereb. Blood Flow Metab. 2022, 42, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Vanlandewijck, M.; He, L.; Mae, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Lavina, B.; Gouveia, L.; et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef] [PubMed]




| Labeling Reagent (Biological Source) | Target |
|---|---|
| DyLight 649-tomato lectin (tomato) | Endothelial cells, basal lamina, microglia |
| Anti-platelet derived growth factor receptor-β (PDGFR-β) (rabbit) | Pericytes |
| Alexa 488-Aquaporin-4 antibody (mouse) | Astrocyte end-feet |
| Cy3-glial acidic fibrillary protein (GFAP) antibody (mouse) | Astrocytes |
| Alexa 488-Milli-Mark FluoroPan Neuronal Marker or CF555-Milli-Mark Pan Neuronal Marker (mouse) | Neurites |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lochhead, J.J.; Williams, E.I.; Reddell, E.S.; Dorn, E.; Ronaldson, P.T.; Davis, T.P. High Resolution Multiplex Confocal Imaging of the Neurovascular Unit in Health and Experimental Ischemic Stroke. Cells 2023, 12, 645. https://doi.org/10.3390/cells12040645
Lochhead JJ, Williams EI, Reddell ES, Dorn E, Ronaldson PT, Davis TP. High Resolution Multiplex Confocal Imaging of the Neurovascular Unit in Health and Experimental Ischemic Stroke. Cells. 2023; 12(4):645. https://doi.org/10.3390/cells12040645
Chicago/Turabian StyleLochhead, Jeffrey J., Erica I. Williams, Elizabeth S. Reddell, Emma Dorn, Patrick T. Ronaldson, and Thomas P. Davis. 2023. "High Resolution Multiplex Confocal Imaging of the Neurovascular Unit in Health and Experimental Ischemic Stroke" Cells 12, no. 4: 645. https://doi.org/10.3390/cells12040645
APA StyleLochhead, J. J., Williams, E. I., Reddell, E. S., Dorn, E., Ronaldson, P. T., & Davis, T. P. (2023). High Resolution Multiplex Confocal Imaging of the Neurovascular Unit in Health and Experimental Ischemic Stroke. Cells, 12(4), 645. https://doi.org/10.3390/cells12040645








