Functional Deficiency of Interneurons and Negative BOLD fMRI Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Preparation
2.2. fMRI Data Collection and Analysis
2.3. Stimulus Preparation
2.4. Electrophysiological Recording and Microinjections
2.5. Statistical Analysis
3. Results
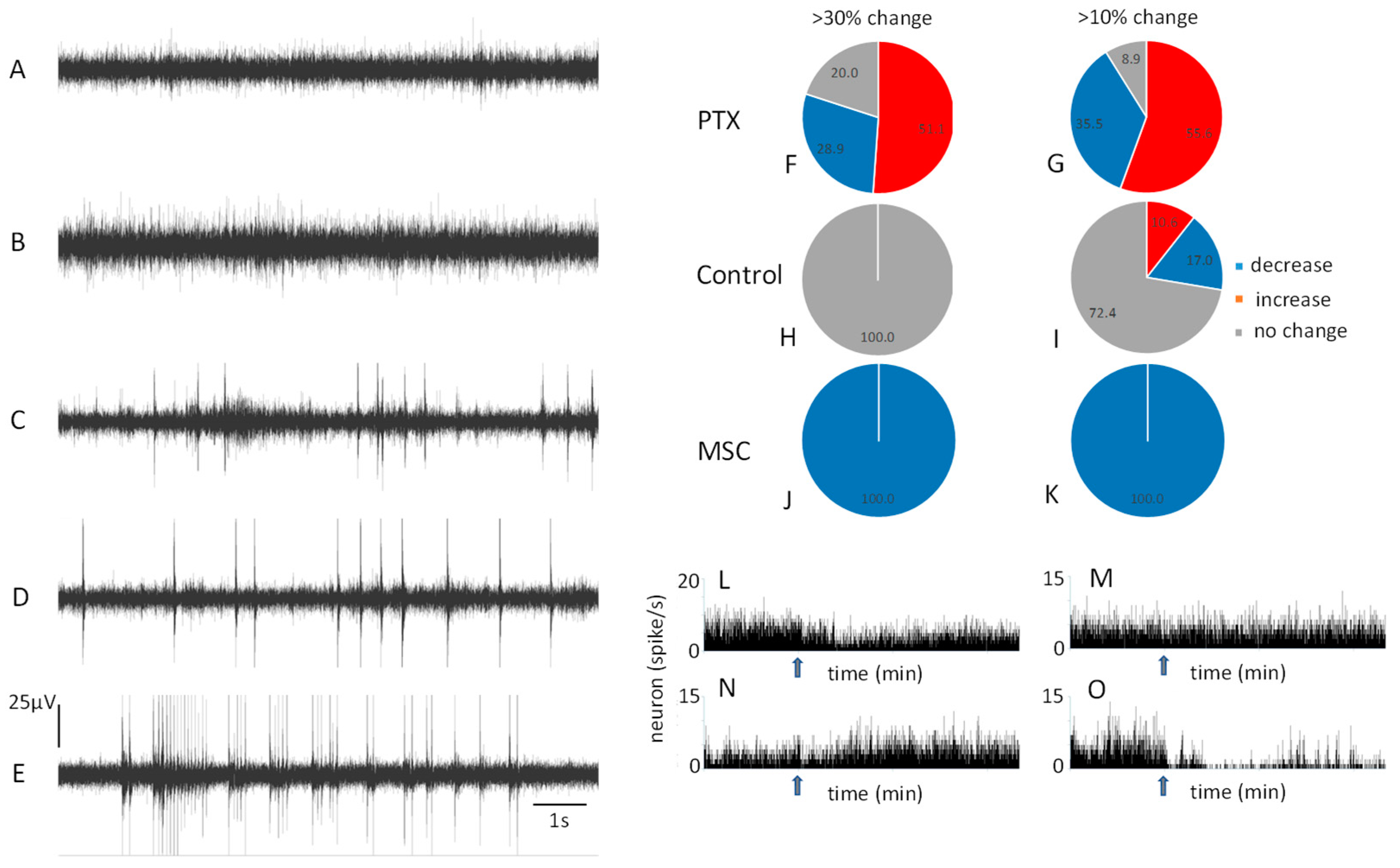
3.1. Baseline Resting Neuronal Activity
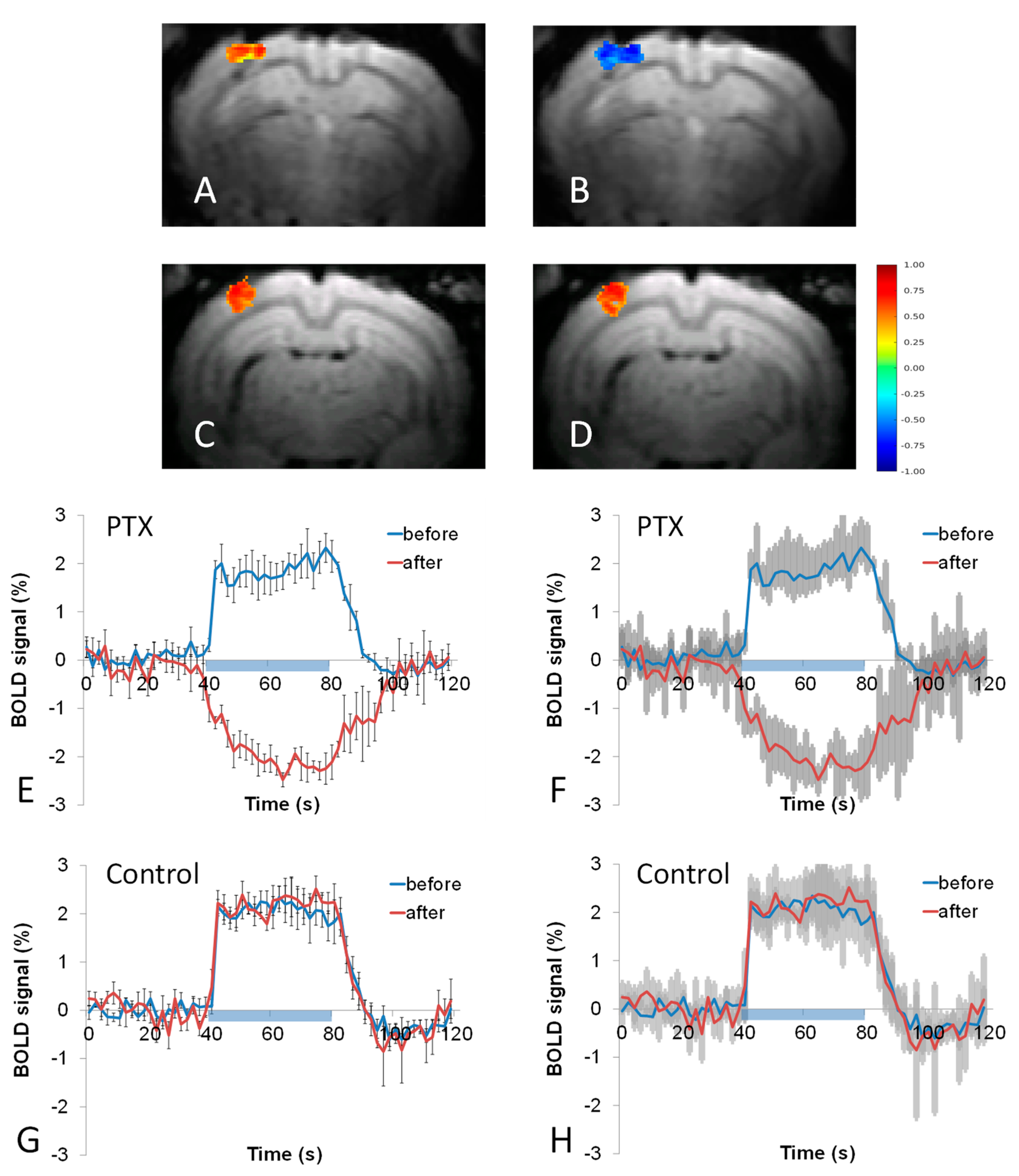
3.2. BOLD fMRI Responses to Whisker Stimulation
3.3. Electrophysiological Responses to Whisker Stimulation
3.4. PO2 Responses to Whisker Stimulation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gascoigne, D.A.; Serdyukova, N.A.; Aksenov, D.P. Early Development of the GABAergic System and the Associated Risks of Neonatal Anesthesia. Int. J. Mol. Sci. 2021, 22, 12951. [Google Scholar] [CrossRef]
- Abbah, J.; Vacher, C.-M.; Goldstein, E.; Li, Z.; Kundu, S.; Talbot, B.; Bhattacharya, S.; Hashimoto-Torii, K.; Wang, L.; Banerjee, P.; et al. Oxidative Stress-Induced Damage to the Developing Hippocampus Is Mediated by GSK3beta. J. Neurosci. 2022, 42, 4812–4827. [Google Scholar] [CrossRef]
- Abbah, J.; Vacher, C.-M.; Goldstein, E.; Li, Z.; Kundu, S.; Talbot, B.; Bhattacharya, S.; Hashimoto-Torii, K.; Wang, L.; Banerjee, P.; et al. Severe intraventricular hemorrhage causes long-lasting structural damage in a preterm rabbit pup model. Pediatr. Res. 2022, 92, 403–414. [Google Scholar]
- Ardalan, M.; Svedin, P.; Baburamani, A.; Supramaniam, V.; Ek, J.; Hagberg, H.; Mallard, C. Dysmaturation of Somatostatin Interneurons Following Umbilical Cord Occlusion in Preterm Fetal Sheep. Front. Physiol. 2019, 10, 563. [Google Scholar] [CrossRef]
- Gascoigne, D.A.; Minhaj, M.M.; Aksenov, D.P. Neonatal Anesthesia and Oxidative Stress. Antioxidants 2022, 11, 787. [Google Scholar] [CrossRef]
- Kaeser, P.S.; Regehr, W.G. Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release. Annu. Rev. Physiol. 2014, 76, 333–363. [Google Scholar] [CrossRef]
- Rubenstein, J.L.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef]
- Calvin, O.L.; Redish, A.D. Global disruption in excitation-inhibition balance can cause localized network dysfunction and Schizophrenia-like context-integration deficits. PLoS Comput. Biol. 2021, 17, e1008985. [Google Scholar] [CrossRef]
- Berg, A.T. Epilepsy, cognition, and behavior: The clinical picture. Epilepsia 2011, 52 (Suppl. S1), 7–12. [Google Scholar] [CrossRef]
- Vaucher, E.; Tong, X.K.; Cholet, N.; Lantin, S.; Hamel, E. GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: A means for direct regulation of local cerebral blood flow. J. Comp. Neurol. 2000, 421, 161–171. [Google Scholar] [CrossRef]
- Fergus, A.; Lee, K.S. GABAergic regulation of cerebral microvascular tone in the rat. J. Cereb. Blood Flow Metab. 1997, 17, 992–1003. [Google Scholar] [CrossRef]
- Tremblay, R.; Lee, S.; Rudy, B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 2016, 91, 260–292. [Google Scholar] [CrossRef]
- Cauli, B.; Tong, X.K.; Rancillac, A.; Serluca, N.; Lambolez, B.; Rossier, J.; Hamel, E. Cortical GABA interneurons in neurovascular coupling: Relays for subcortical vasoactive pathways. J. Neurosci. 2004, 24, 8940–8949. [Google Scholar] [CrossRef]
- Aksenov, D.P.; Li, L.; Miller, M.J.; Wyrwicz, A.M. Role of the inhibitory system in shaping the BOLD fMRI response. Neuroimage 2019, 201, 116034. [Google Scholar] [CrossRef]
- Wyrwicz, A.M.; Chen, N.-K.; Li, L.; Weiss, C.; Disterhoft, J.F. fMRI of visual system activation in the conscious rabbit. Magn. Res. Med. 2000, 44, 474–478. [Google Scholar] [CrossRef]
- Yoo, T.S.; Ackerman, M.J.; Lorensen, W.E.; Schroeder, W.; Chalana, V.; Aylward, S.; Metaxas, D.; Whitaker, R. Engineering and algorithm design for an image processing Api: A technical report on ITK-the Insight Toolkit. Stud. Health Technol. Inf. 2002, 85, 586–592. [Google Scholar]
- Song, X.; Wyrwicz, A.M. Unsupervised spatiotemporal fMRI data analysis using support vector machines. Neuroimage 2009, 47, 204–212. [Google Scholar] [CrossRef]
- Li, L.; Weiss, C.; Talk, A.C.; Disterhoft, J.F.; Wyrwicz, A.M. A MRI-compatible system for whisker stimulation. J. Neurosci. Methods 2012, 205, 305–311. [Google Scholar] [CrossRef]
- Pratt, W. Morphological Image Processing, 2nd ed.; Wiley-Interscience: New York, NY, USA, 1991. [Google Scholar]
- Quiroga, R.Q.; Nadasdy, Z.; Ben-Shaul, Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 2004, 16, 1661–1687. [Google Scholar] [CrossRef]
- Swadlow, H. Efferent neurons and suspected interneurons in S-1 vibrissa cortex of the awake rabbit: Receptive fields and axonal properties. J. Neurophysiol. 1989, 62, 288–308. [Google Scholar] [CrossRef]
- Aksenov, D.P.; Miller, M.J.; Dixon, C.J.; Wyrwicz, A.M. The effect of sevoflurane and isoflurane anesthesia on single unit and local field potentials. Exp. Brain Res. 2019, 237, 1521–1529. [Google Scholar] [CrossRef]
- Aksenov, D.P.; Doubovikov, E.D.; Serdyukova, N.A.; Gascoigne, D.A.; Linsenmeier, R.A.; Drobyshevsky, A. Brain tissue oxygen dynamics while mimicking the functional deficiency of interneurons. Front. Cell Neurosci. 2022, 16, 983298. [Google Scholar] [CrossRef]
- Aksenov, D.P.; Li, L.; Iordanescu, G.; Miller, M.J.; Wyrwicz, A.M. Volume effect of localized injection in functional MRI and electrophysiology. Magn. Reson Med. 2014, 72, 1170–1175. [Google Scholar] [CrossRef]
- Hablitz, J.J. Picrotoxin-induced epileptiform activity in hippocampus: Role of endogenous versus synaptic factors. J. Neurophysiol. 1984, 51, 1011–1027. [Google Scholar] [CrossRef]
- Goense, J.; Merkle, H.; Logothetis, N.K. High-resolution fMRI reveals laminar differences in neurovascular coupling between positive and negative BOLD responses. Neuron 2012, 76, 629–639. [Google Scholar] [CrossRef]
- Moraschi, M.; DiNuzzo, M.; Giove, F. On the origin of sustained negative BOLD response. J. Neurophysiol. 2012, 108, 2339–2342. [Google Scholar] [CrossRef]
- Fracasso, A.; Gaglianese, A.; Vansteensel, M.J.; Aarnoutse, E.J.; Ramsey, N.F.; Dumoulin, S.O.; Petridou, N. FMRI and intra-cranial electrocorticography recordings in the same human subjects reveals negative BOLD signal coupled with silenced neuronal activity. Brain Struct. Funct. 2022, 227, 1371–1384. [Google Scholar] [CrossRef]
- Sten, S.; Lundengård, K.; Witt, S.T.; Cedersund, G.; Elinder, F.; Engström, M. Neural inhibition can explain negative BOLD responses: A mechanistic modelling and fMRI study. Neuroimage 2017, 158, 219–231. [Google Scholar] [CrossRef]
- Mullinger, K.J.; Mayhew, S.D.; Bagshaw, A.P.; Bowtell, R.; Francis, S.T. Evidence that the negative BOLD response is neuronal in origin: A simultaneous EEG-BOLD-CBF study in humans. Neuroimage 2014, 94, 263–274. [Google Scholar] [CrossRef]
- Shmuel, A.; Augath, M.; Oeltermann, A.; Logothetis, N.K. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat. Neurosci. 2006, 9, 569–577. [Google Scholar] [CrossRef]
- Boorman, L.; Kennerley, A.J.; Johnston, D.; Jones, M.; Zheng, Y.; Redgrave, P.; Berwick, J. Negative blood oxygen level dependence in the rat: A model for investigating the role of suppression in neurovascular coupling. J. Neurosci. 2010, 30, 4285–4294. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.V.; Harel, N.; Panesar, J.; Mount, R.J. Blood capillary distribution correlates with hemodynamic-based functional imaging in cerebral cortex. Cereb. Cortex 2002, 12, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Bianciardi, M.; Fukunaga, M.; van Gelderen, P.; de Zwart, J.A.; Duyn, J.H. Negative BOLD-fMRI signals in large cerebral veins. J. Cereb. Blood Flow Metab. 2011, 31, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.P.; Liu, P.; Aslan, S.; King, K.S.; van Osch, M.J.; Lu, H. Physiologic underpinnings of negative BOLD cerebrovascular reactivity in brain ventricles. Neuroimage 2013, 83, 505–512. [Google Scholar] [CrossRef]
- Shih, Y.Y.; Chen, C.C.; Shyu, B.C.; Lin, Z.J.; Chiang, Y.C.; Jaw, F.S.; Chen, Y.Y.; Chang, C. A new scenario for negative functional magnetic resonance imaging signals: Endogenous neurotransmission. J. Neurosci. 2009, 29, 3036–3044. [Google Scholar] [CrossRef]
- Nakata, H.; Domoto, R.; Mizuguchi, N.; Sakamoto, K.; Kanosue, K. Negative BOLD responses during hand and foot movements: An fMRI study. PLoS ONE 2019, 14, e0215736. [Google Scholar] [CrossRef]
- Devor, A.; Tian, P.; Nishimura, N.; Teng, I.C.; Hillman, E.M.; Narayanan, S.N.; Ulbert, I.; Boas, D.A.; Kleinfeld, D.; Dale, A.M. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J. Neurosci. 2007, 27, 4452–4459. [Google Scholar] [CrossRef]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef]
- Hillman, E.M. Coupling mechanism and significance of the BOLD signal: A status report. Annu. Rev. Neurosci. 2014, 37, 161–181. [Google Scholar] [CrossRef]
- Marx, M.; Haas, C.A.; Haussler, U. Differential vulnerability of interneurons in the epileptic hippocampus. Front. Cell Neurosci. 2013, 7, 167. [Google Scholar] [CrossRef]
- Schridde, U.; Khubchandani, M.; Motelow, J.E.; Sanganahalli, B.G.; Hyder, F.; Blumenfeld, H. Negative BOLD with large increases in neuronal activity. Cereb. Cortex 2008, 18, 1814–1827. [Google Scholar] [CrossRef]
- Suarez, A.; Valdés-Hernández, P.A.; Bernal, B.; Dunoyer, C.; Khoo, H.M.; Bosch-Bayard, J.; Riera, J.J. Identification of Negative BOLD Responses in Epilepsy Using Windkessel Models. Front. Neurol. 2021, 12, 659081. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, P.; Yan, F.; Luo, Y.; Zhao, G. Animal Models of Epilepsy: A Phenotype-oriented Review. Aging Dis. 2022, 13, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, A.M.; Niskanen, J.P.; Chamberlain, R.; Huttunen, J.K.; Nissinen, J.; Garwood, M.; Pitkanen, A.; Grohn, O. Simultaneous fMRI and local field potential measurements during epileptic seizures in medetomidine-sedated rats using raser pulse sequence. Magn. Reson. Med. 2010, 64, 1191–1199. [Google Scholar] [CrossRef]
- Lee, L.; Boorman, L.; Glendenning, E.; Christmas, C.; Sharp, P.; Redgrave, P.; Shabir, O.; Bracci, E.; Berwick, J.; Howarth, C. Key Aspects of Neurovascular Control Mediated by Specific Populations of Inhibitory Cortical Interneurons. Cereb. Cortex 2020, 30, 2452–2464. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, G.; Monteverdi, A.; Casali, S.; Laforenza, U.; Gandini Wheeler-Kingshott, C.A.M.; D’Angelo, E.; Mapelli, L. Non-Linear Frequency Dependence of Neurovascular Coupling in the Cerebellar Cortex Implies Vasodilation-Vasoconstriction Competition. Cells 2022, 11, 1047. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, L.; Gagliano, G.; Soda, T.; Laforenza, U.; Moccia, F.; D’Angelo, E.U. Granular Layer Neurons Control Cerebellar Neurovascular Coupling Through an NMDA Receptor/NO-Dependent System. J. Neurosci. 2017, 37, 1340–1351. [Google Scholar] [CrossRef]
- Huo, B.X.; Smith, J.B.; Drew, P.J. Neurovascular coupling and decoupling in the cortex during voluntary locomotion. J. Neurosci. 2014, 34, 10975–10981. [Google Scholar] [CrossRef]
- Nippert, A.R.; Biesecker, K.R.; Newman, E.A. Mechanisms Mediating Functional Hyperemia in the Brain. Neuroscientist 2018, 24, 73–83. [Google Scholar] [CrossRef]
- Girouard, H.; Iadecola, C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J. Appl. Physiol. 2006, 100, 328–335. [Google Scholar] [CrossRef]
- Tarantini, S.; Hertelendy, P.; Tucsek, Z.; Valcarcel-Ares, M.N.; Smith, N.; Menyhart, A.; Farkas, E.; Hodges, E.L.; Towner, R.; Deak, F.; et al. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J. Cereb. Blood Flow Metab. 2015, 35, 1871–1881. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aksenov, D.P.; Li, L.; Serdyukova, N.A.; Gascoigne, D.A.; Doubovikov, E.D.; Drobyshevsky, A. Functional Deficiency of Interneurons and Negative BOLD fMRI Response. Cells 2023, 12, 811. https://doi.org/10.3390/cells12050811
Aksenov DP, Li L, Serdyukova NA, Gascoigne DA, Doubovikov ED, Drobyshevsky A. Functional Deficiency of Interneurons and Negative BOLD fMRI Response. Cells. 2023; 12(5):811. https://doi.org/10.3390/cells12050811
Chicago/Turabian StyleAksenov, Daniil P., Limin Li, Natalya A. Serdyukova, David A. Gascoigne, Evan D. Doubovikov, and Alexander Drobyshevsky. 2023. "Functional Deficiency of Interneurons and Negative BOLD fMRI Response" Cells 12, no. 5: 811. https://doi.org/10.3390/cells12050811
APA StyleAksenov, D. P., Li, L., Serdyukova, N. A., Gascoigne, D. A., Doubovikov, E. D., & Drobyshevsky, A. (2023). Functional Deficiency of Interneurons and Negative BOLD fMRI Response. Cells, 12(5), 811. https://doi.org/10.3390/cells12050811







