Complete Freund’s Adjuvant Induces a Fibroblast-like Synoviocytes (FLS) Metabolic and Migratory Phenotype in Resident Fibroblasts of the Inoculated Footpad at the Earliest Stage of Adjuvant-Induced Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. DNA Microarray and Bioinformatics Analysis
2.3. Histological Analysis
2.4. Statistical Analysis
3. Results
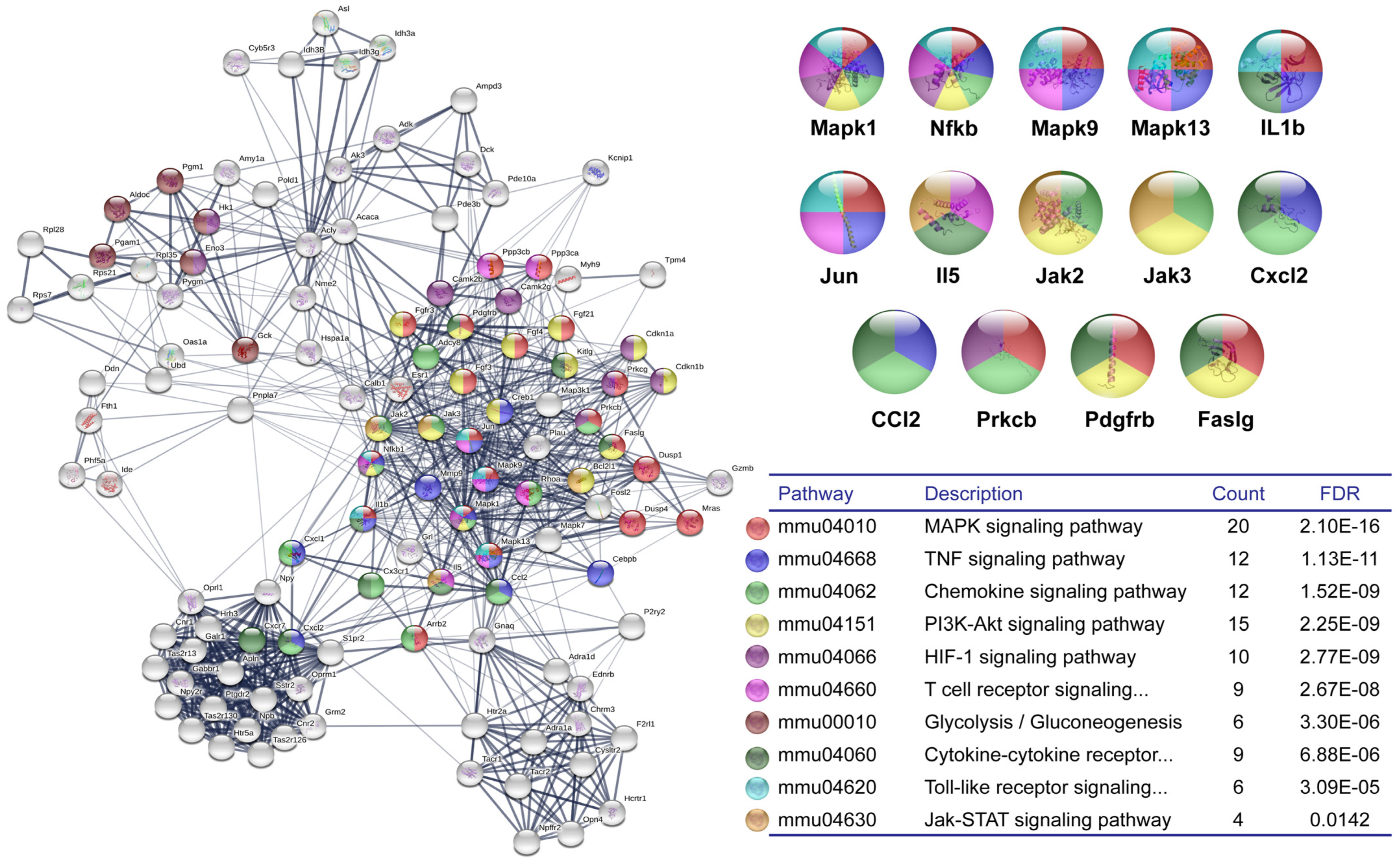
3.1. DNA Microarray and Bioinformatic Analysis
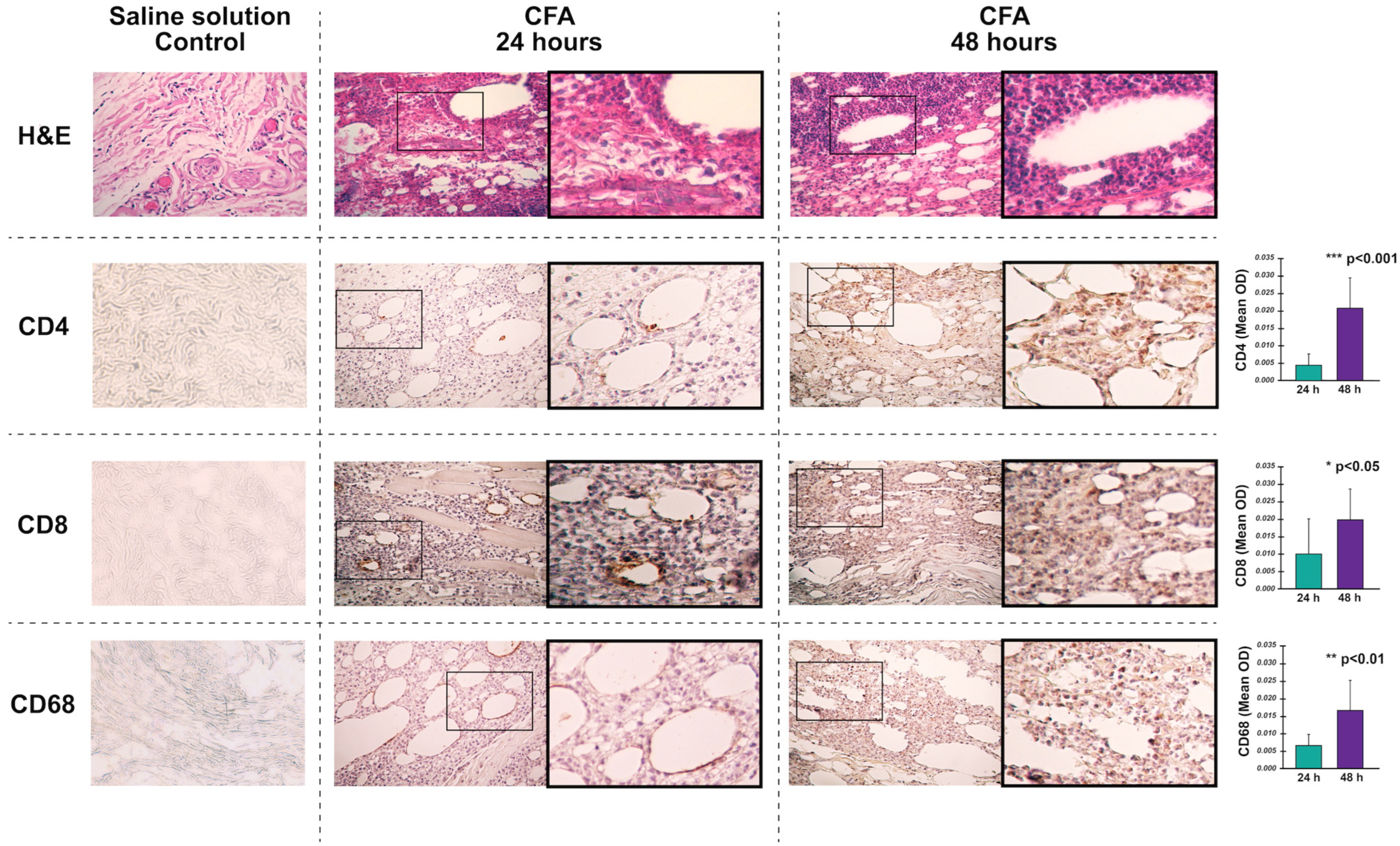
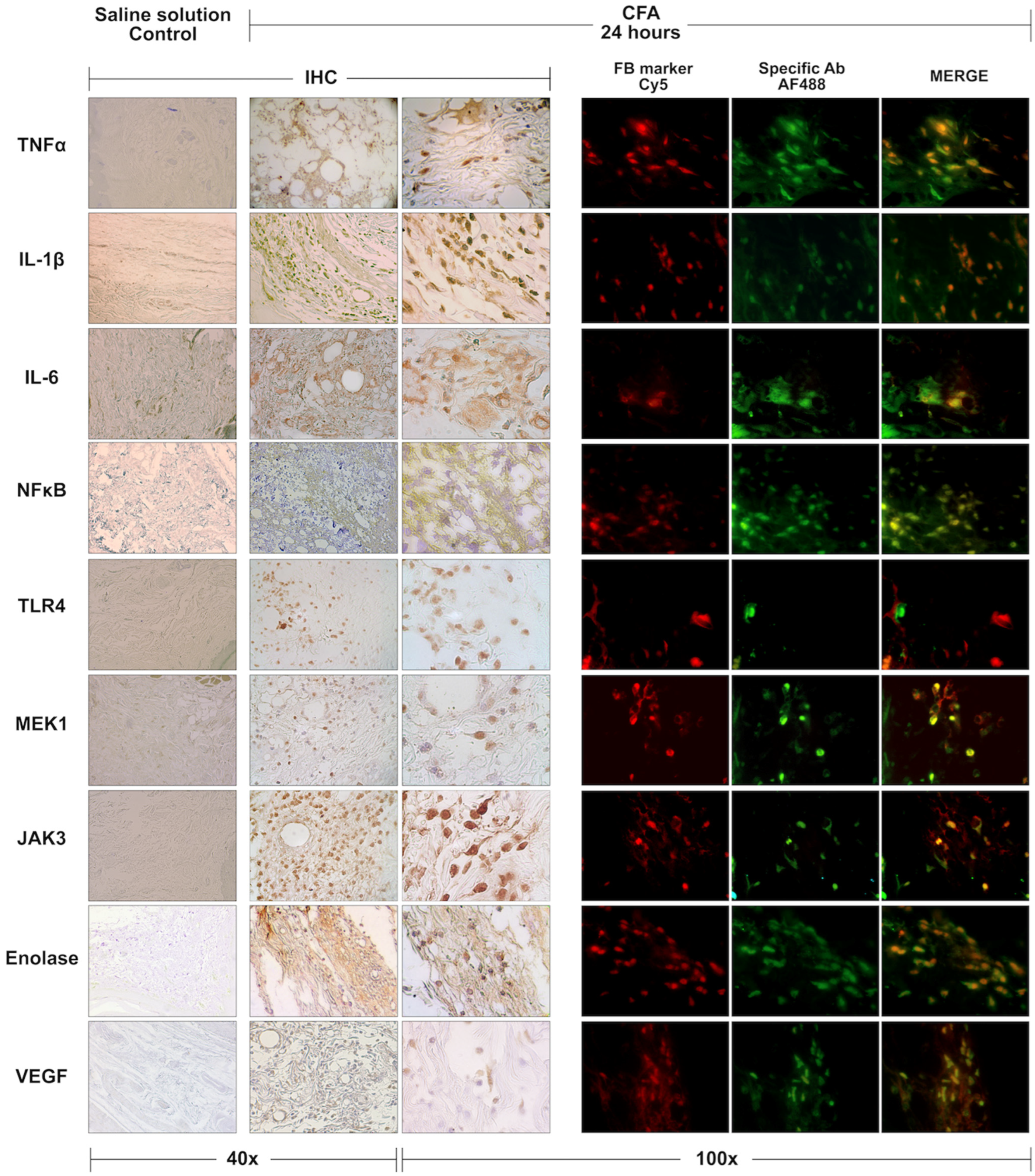
3.2. Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Marsh, L.; Kemble, S.; Reis Nisa, P.; Singh, R.; Croft, A.P. Fibroblast Pathology in Inflammatory Joint Disease. Immunol. Rev. 2021, 302, 163–183. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-Q. Highlights of Strategies Targeting Fibroblasts for Novel Therapies for Rheumatoid Arthritis. Front. Med. 2022, 9, 846300. [Google Scholar] [CrossRef]
- Fearon, U.; Hanlon, M.M.; Floudas, A.; Veale, D.J. Cellular Metabolic Adaptations in Rheumatoid Arthritis and Their Therapeutic Implications. Nat. Rev. Rheumatol. 2022, 18, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Bartok, B.; Firestein, G.S. Fibroblast-like Synoviocytes: Key Effector Cells in Rheumatoid Arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Croft, A.P.; Campos, J.; Jansen, K.; Turner, J.D.; Marshall, J.; Attar, M.; Savary, L.; Wehmeyer, C.; Naylor, A.J.; Kemble, S.; et al. Distinct Fibroblast Subsets Drive Inflammation and Damage in Arthritis. Nature 2019, 570, 246–251. [Google Scholar] [CrossRef]
- Pap, T.; Dankbar, B.; Wehmeyer, C.; Korb-Pap, A.; Sherwood, J. Synovial Fibroblasts and Articular Tissue Remodelling: Role and Mechanisms. Semin. Cell Dev. Biol. 2020, 101, 140–145. [Google Scholar] [CrossRef]
- Lefèvre, S.; Knedla, A.; Tennie, C.; Kampmann, A.; Wunrau, C.; Dinser, R.; Korb, A.; Schnäker, E.-M.; Tarner, I.H.; Robbins, P.D.; et al. Synovial Fibroblasts Spread Rheumatoid Arthritis to Unaffected Joints. Nat. Med. 2009, 15, 1414–1420. [Google Scholar] [CrossRef]
- Németh, T.; Nagy, G.; Pap, T. Synovial Fibroblasts as Potential Drug Targets in Rheumatoid Arthritis, Where Do We Stand and Where Shall We Go? Ann. Rheum. Dis. 2022, 81, 1055–1064. [Google Scholar] [CrossRef]
- Nygaard, G.; Firestein, G.S. Restoring Synovial Homeostasis in Rheumatoid Arthritis by Targeting Fibroblast-like Synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333. [Google Scholar] [CrossRef]
- Sandhu, G.; Thelma, B.K. New Druggable Targets for Rheumatoid Arthritis Based on Insights from Synovial Biology. Front. Immunol. 2022, 13, 834247. [Google Scholar] [CrossRef]
- Filer, A. The Fibroblast as a Therapeutic Target in Rheumatoid Arthritis. Curr. Opin. Pharmacol. 2013, 13, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Bottini, N.; Firestein, G.S. Duality of Fibroblast-like Synoviocytes in RA: Passive Responders and Imprinted Aggressors. Nat. Rev. Rheumatol. 2013, 9, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wei, X.; Jiao, Y.; Bai, Y.; Sam, W.N.; Yan, Q.; Sun, X.; Li, G.; Ma, J.; Wei, W.; et al. STAT3/HIF-1α/Fascin-1 Axis Promotes RA FLSs Migration and Invasion Ability under Hypoxia. Mol. Immunol. 2022, 142, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, J.; Wang, M.; Weng, W.; Chen, Y.; Pan, Y. Rheumatoid Arthritis Fibroblast-like Synoviocytes Maintain Tumor-like Biological Characteristics through CiRS-7-Dependent Regulation of MiR-7. Mol. Biol. Rep. 2022, 49, 8473–8483. [Google Scholar] [CrossRef]
- Pacheco-Tena, C.; González-Chávez, S.A. The Danger Model Approach to the Pathogenesis of the Rheumatic Diseases. J. Immunol. Res. 2015, 2015, 506089. [Google Scholar] [CrossRef]
- Tu, J.; Huang, W.; Zhang, W.; Mei, J.; Zhu, C. Two Main Cellular Components in Rheumatoid Arthritis: Communication Between T Cells and Fibroblast-Like Synoviocytes in the Joint Synovium. Front. Immunol. 2022, 13, 922111. [Google Scholar] [CrossRef]
- Qin, Y.; Cai, M.-L.; Jin, H.-Z.; Huang, W.; Zhu, C.; Bozec, A.; Huang, J.; Chen, Z. Age-Associated B Cells Contribute to the Pathogenesis of Rheumatoid Arthritis by Inducing Activation of Fibroblast-like Synoviocytes via TNF-α-Mediated ERK1/2 and JAK-STAT1 Pathways. Ann. Rheum. Dis. 2022, 81, 1504–1514. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, B.; Sun, X.; Li, H.; Ouyang, X.; Wei, J.; Dai, B.; Zhang, Y.; Li, X. Rheumatoid Arthritis Fibroblast-like Synoviocytes Co-Cultured with PBMC Increased Peripheral CD4+ CXCR5+ ICOS+ T Cell Numbers. Clin. Exp. Immunol. 2017, 190, 384–393. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, D.; Yang, H.; Gao, J.; Zhang, G.; Xu, K.; Zhang, L. Fibroblast-like Synoviocytes in Rheumatoid Arthritis: Surface Markers and Phenotypes. Int. Immunopharmacol. 2021, 93, 107392. [Google Scholar] [CrossRef]
- Jiang, P.; Wei, K.; Chang, C.; Zhao, J.; Zhang, R.; Xu, L.; Jin, Y.; Xu, L.; Shi, Y.; Guo, S.; et al. SFRP1 Negatively Modulates Pyroptosis of Fibroblast-Like Synoviocytes in Rheumatoid Arthritis: A Review. Front. Immunol. 2022, 13, 903475. [Google Scholar] [CrossRef]
- Bustamante, M.F.; Garcia-Carbonell, R.; Whisenant, K.D.; Guma, M. Fibroblast-like Synoviocyte Metabolism in the Pathogenesis of Rheumatoid Arthritis. Arthritis Res. Ther. 2017, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, D.E.; Kaabachi, W.; Sassi, N.; Tarhouni, L.; Rekik, S.; Jemmali, S.; Sehli, H.; Kallel-Sellami, M.; Cheour, E.; Laadhar, L. The Synovial Fluid Fibroblast-like Synoviocyte: A Long-Neglected Piece in the Puzzle of Rheumatoid Arthritis Pathogenesis. Front. Immunol. 2022, 13, 942417. [Google Scholar] [CrossRef] [PubMed]
- Herzog, E.L.; Bucala, R. Fibrocytes in Health and Disease. Exp. Hematol. 2010, 38, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Galligan, C.L.; Fish, E.N. Circulating Fibrocytes Contribute to the Pathogenesis of Collagen Antibody-Induced Arthritis. Arthritis Rheum. 2012, 64, 3583–3593. [Google Scholar] [CrossRef]
- Choudhary, N.; Bhatt, L.K.; Prabhavalkar, K.S. Experimental Animal Models for Rheumatoid Arthritis. Immunopharmacol. Immunotoxicol. 2018, 40, 193–200. [Google Scholar] [CrossRef]
- Fischer, B.D.; Adeyemo, A.; O’Leary, M.E.; Bottaro, A. Animal Models of Rheumatoid Pain: Experimental Systems and Insights. Arthritis Res. Ther. 2017, 19, 146. [Google Scholar] [CrossRef]
- Bordy, R.; Verhoeven, F.; Tournier-Nappey, M.; Wendling, D.; Demougeot, C.; Totoson, P. Methotrexate Did Not Improve Endothelial Function in Rheumatoid Arthritis: A Study in Rats with Adjuvant-Induced Arthritis. Clin. Exp. Rheumatol. 2019, 37, 81–88. [Google Scholar]
- Abdel-Maged, A.E.-S.; Gad, A.M.; Abdel-Aziz, A.K.; Aboulwafa, M.M.; Azab, S.S. Comparative Study of Anti-VEGF Ranibizumab and Interleukin-6 Receptor Antagonist Tocilizumab in Adjuvant-Induced Arthritis. Toxicol. Appl. Pharmacol. 2018, 356, 65–75. [Google Scholar] [CrossRef]
- Wang, Z.-Z.; Liu, F.; Gong, Y.-F.; Huang, T.-Y.; Zhang, X.-M.; Huang, X.-Y. Antiarthritic Effects of Sorafenib in Rats with Adjuvant-Induced Arthritis: Antiarthritic effects of sorafenib. Anat. Rec. 2018, 301, 1519–1526. [Google Scholar] [CrossRef]
- Vidal, B.; Cascão, R.; Finnilä, M.A.J.; Lopes, I.P.; da Glória, V.G.; Saarakkala, S.; Zioupos, P.; Canhão, H.; Fonseca, J.E. Effects of Tofacitinib in Early Arthritis-Induced Bone Loss in an Adjuvant-Induced Arthritis Rat Model. Rheumatology 2018, 57, 1461–1471. [Google Scholar] [CrossRef]
- Stevenson, C.S.; Marshall, L.A.; Morgan, D.W. In Vivo Models of Inflammation; Birkhäuser Verlag: Basel, Switzerland, 2006; ISBN 978-3-7643-7760-1. [Google Scholar]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Kir, J.; Liu, D.; Bryant, D.; Guo, Y.; Stephens, R.; Baseler, M.W.; Lane, H.C.; et al. DAVID Bioinformatics Resources: Expanded Annotation Database and Novel Algorithms to Better Extract Biology from Large Gene Lists. Nucleic Acids Res. 2007, 35, W169–W175. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING Database in 2017: Quality-Controlled Protein–Protein Association Networks, Made Broadly Accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Isserlin, R.; Merico, D.; Voisin, V.; Bader, G.D. Enrichment Map—A Cytoscape App to Visualize and Explore OMICs Pathway Enrichment Results. F1000Research 2014, 3, 141. [Google Scholar] [CrossRef] [PubMed]
- Bader, G.D.; Hogue, C.W.V. An Automated Method for Finding Molecular Complexes in Large Protein Interaction Networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef]
- González-Chávez, S.A.; Pacheco-Tena, C.; Macías-Vázquez, C.E.; Luévano-Flores, E. Assessment of Different Decalcifying Protocols on Osteopontin and Osteocalcin Immunostaining in Whole Bone Specimens of Arthritis Rat Model by Confocal Immunofluorescence. Int. J. Clin. Exp. Pathol. 2013, 6, 1972–1983. [Google Scholar]
- Schiavinato, A.; Przyklenk, M.; Kobbe, B.; Paulsson, M.; Wagener, R. Collagen Type VI Is the Antigen Recognized by the ER-TR7 Antibody. Eur. J. Immunol. 2021, 51, 2345–2347. [Google Scholar] [CrossRef]
- Malhotra, D.; Fletcher, A.L.; Astarita, J.; Lukacs-Kornek, V.; Tayalia, P.; Gonzalez, S.F.; Elpek, K.G.; Chang, S.K.; Knoblich, K.; Hemler, M.E.; et al. Transcriptional Profiling of Stroma from Inflamed and Resting Lymph Nodes Defines Immunological Hallmarks. Nat. Immunol. 2012, 13, 499–510. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING Database in 2021: Customizable Protein–Protein Networks, and Functional Characterization of User-Uploaded Gene/Measurement Sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Falconer, J.; Murphy, A.N.; Young, S.P.; Clark, A.R.; Tiziani, S.; Guma, M.; Buckley, C.D. Review: Synovial Cell Metabolism and Chronic Inflammation in Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 984–999. [Google Scholar] [CrossRef]
- Paunovic, V.; Harnett, M.M. Mitogen-Activated Protein Kinases as Therapeutic Targets for Rheumatoid Arthritis. Drugs 2013, 73, 101–115. [Google Scholar] [CrossRef]
- Hu, L.; Liu, R.; Zhang, L. Advance in Bone Destruction Participated by JAK/STAT in Rheumatoid Arthritis and Therapeutic Effect of JAK/STAT Inhibitors. Int. Immunopharmacol. 2022, 111, 109095. [Google Scholar] [CrossRef] [PubMed]
- Quiñonez-Flores, C.M.; González-Chávez, S.A.; Pacheco-Tena, C. Hypoxia and Its Implications in Rheumatoid Arthritis. J. Biomed. Sci. 2016, 23, 62. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ma, H.; Zhang, H.; Deng, C.; Xin, P. Recent Advances on Signaling Pathways and Their Inhibitors in Rheumatoid Arthritis. Clin. Immunol. Orlando Fla 2021, 230, 108793. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Wang, S.; Liu, F. Therapeutic Potential of Non-Coding RNAs and TLR Signalling Pathways in Rheumatoid Arthritis. Curr. Pharm. Biotechnol. 2021, 22, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Getting, S.J.; Locke, I.C. Regulation of TNF-Induced Osteoclast Differentiation. Cells 2021, 11, 132. [Google Scholar] [CrossRef]
- Li, J.; Tang, R.-S.; Shi, Z.; Li, J.-Q. Nuclear Factor-ΚB in Rheumatoid Arthritis. Int. J. Rheum. Dis. 2020, 23, 1627–1635. [Google Scholar] [CrossRef]
- Stolina, M.; Bolon, B.; Middleton, S.; Dwyer, D.; Brown, H.; Duryea, D.; Zhu, L.; Rohner, A.; Pretorius, J.; Kostenuik, P.; et al. The Evolving Systemic and Local Biomarker Milieu at Different Stages of Disease Progression in Rat Adjuvant-Induced Arthritis. J. Clin. Immunol. 2009, 29, 158–174. [Google Scholar] [CrossRef]
- Yu, H.; Lu, C.; Tan, M.T.; Moudgil, K.D. The Gene Expression Profile of Preclinical Autoimmune Arthritis and Its Modulation by a Tolerogenic Disease-Protective Antigenic Challenge. Arthritis Res. Ther. 2011, 13, R143. [Google Scholar] [CrossRef]
- González-Chávez, S.A.; Quiñonez-Flores, C.M.; Espino-Solís, G.P.; Vázquez-Contreras, J.Á.; Pacheco-Tena, C. Exercise Exacerbates the Transcriptional Profile of Hypoxia, Oxidative Stress and Inflammation in Rats with Adjuvant-Induced Arthritis. Cells 2019, 8, 1493. [Google Scholar] [CrossRef]
- Wei, J.; Liu, J.; Liang, S.; Sun, M.; Duan, J. Low-Dose Exposure of Silica Nanoparticles Induces Neurotoxicity via Neuroactive Ligand–Receptor Interaction Signaling Pathway in Zebrafish Embryos. Int. J. Nanomed. 2020, 15, 4407–4415. [Google Scholar] [CrossRef]
- Gao, D.; Gao, X.; Yang, F.; Wang, Q. Neuroimmune Crosstalk in Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 8158. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonell, R.; Divakaruni, A.S.; Lodi, A.; Vicente-Suarez, I.; Saha, A.; Cheroutre, H.; Boss, G.R.; Tiziani, S.; Murphy, A.N.; Guma, M. Critical Role of Glucose Metabolism in Rheumatoid Arthritis Fibroblast-like Synoviocytes: Fls glycolytic metabolism in rheumatoid arthritis. Arthritis Rheumatol. 2016, 68, 1614–1626. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.G.; Farinon, M.; Sanchez-Lopez, E.; Miyamoto, S.; Guma, M. Fibroblast-Like Synoviocytes Glucose Metabolism as a Therapeutic Target in Rheumatoid Arthritis. Front. Immunol. 2019, 10, 1743. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, M.; Mehrabzadeh, M.; Mahmoudzehi, S.; Mousavi, M.J.; Jamalzehi, S.; Sahebkar, A.; Karami, J. Role of Glucose Metabolism in Aggressive Phenotype of Fibroblast-like Synoviocytes: Latest Evidence and Therapeutic Approaches in Rheumatoid Arthritis. Int. Immunopharmacol. 2020, 89, 107064. [Google Scholar] [CrossRef]
- Quiñonez-Flores, C.M.; González-Chávez, S.A.; Del Río Nájera, D.; Pacheco-Tena, C. Oxidative Stress Relevance in the Pathogenesis of the Rheumatoid Arthritis: A Systematic Review. BioMed Res. Int. 2016, 2016, 6097417. [Google Scholar] [CrossRef]
- López-Armada, M.J.; Fernández-Rodríguez, J.A.; Blanco, F.J. Mitochondrial Dysfunction and Oxidative Stress in Rheumatoid Arthritis. Antioxidants 2022, 11, 1151. [Google Scholar] [CrossRef]
- Hammaker, D.; Nygaard, G.; Kuhs, A.; Ai, R.; Boyle, D.L.; Wang, W.; Firestein, G.S. Joint Location–Specific JAK-STAT Signaling in Rheumatoid Arthritis Fibroblast-like Synoviocytes. ACR Open Rheumatol. 2019, 1, 640–648. [Google Scholar] [CrossRef]
- Xing, X.-W.; Shi, H.-Y.; Liu, S.; Feng, S.-X.; Feng, S.-Q.; Gong, B.-Q. MiR-496/MMP10 Is Involved in the Proliferation of IL-1β-Induced Fibroblast-Like Synoviocytes Via Mediating the NF-ΚB Signaling Pathway. Inflammation 2021, 44, 1359–1369. [Google Scholar] [CrossRef]
- Longman, R.S.; Littman, D.R. The Functional Impact of the Intestinal Microbiome on Mucosal Immunity and Systemic Autoimmunity. Curr. Opin. Rheumatol. 2015, 27, 381–387. [Google Scholar] [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of Intestinal Prevotella Copri Correlates with Enhanced Susceptibility to Arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Quirke, A.-M.; Perry, E.; Cartwright, A.; Kelly, C.; De Soyza, A.; Eggleton, P.; Hutchinson, D.; Venables, P.J. Bronchiectasis Is a Model for Chronic Bacterial Infection Inducing Autoimmunity in Rheumatoid Arthritis. Arthritis Rheumatol. 2015, 67, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Reynisdottir, G.; Karimi, R.; Joshua, V.; Olsen, H.; Hensvold, A.H.; Harju, A.; Engström, M.; Grunewald, J.; Nyren, S.; Eklund, A.; et al. Structural Changes and Antibody Enrichment in the Lungs Are Early Features of Anti-Citrullinated Protein Antibody-Positive Rheumatoid Arthritis. Arthritis Rheumatol. Hoboken NJ 2014, 66, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Demoruelle, M.K.; Weisman, M.H.; Simonian, P.L.; Lynch, D.A.; Sachs, P.B.; Pedraza, I.F.; Harrington, A.R.; Kolfenbach, J.R.; Striebich, C.C.; Pham, Q.N.; et al. Brief Report: Airways Abnormalities and Rheumatoid Arthritis-Related Autoantibodies in Subjects without Arthritis: Early Injury or Initiating Site of Autoimmunity? Arthritis Rheum. 2012, 64, 1756–1761. [Google Scholar] [CrossRef]
- Klareskog, L.; Stolt, P.; Lundberg, K.; Källberg, H.; Bengtsson, C.; Grunewald, J.; Rönnelid, J.; Harris, H.E.; Ulfgren, A.-K.; Rantapää-Dahlqvist, S.; et al. A New Model for an Etiology of Rheumatoid Arthritis: Smoking May Trigger HLA-DR (Shared Epitope)-Restricted Immune Reactions to Autoantigens Modified by Citrullination. Arthritis Rheum. 2006, 54, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, C.; Kobayashi, T.; Ito, S.; Sugita, N.; Murasawa, A.; Ishikawa, H.; Tabeta, K. Association among Periodontitis Severity, Anti-Agalactosyl Immunoglobulin G Titer, and the Disease Activity of Rheumatoid Arthritis. J. Periodontal Res. 2021, 56, 702–709. [Google Scholar] [CrossRef]
- Li, Y.; Guo, R.; Oduro, P.K.; Sun, T.; Chen, H.; Yi, Y.; Zeng, W.; Wang, Q.; Leng, L.; Yang, L.; et al. The Relationship Between Porphyromonas Gingivalis and Rheumatoid Arthritis: A Meta-Analysis. Front. Cell. Infect. Microbiol. 2022, 12, 956417. [Google Scholar] [CrossRef]
- Pischon, N.; Pischon, T.; Kröger, J.; Gülmez, E.; Kleber, B.-M.; Bernimoulin, J.-P.; Landau, H.; Brinkmann, P.-G.; Schlattmann, P.; Zernicke, J.; et al. Association among Rheumatoid Arthritis, Oral Hygiene, and Periodontitis. J. Periodontol. 2008, 79, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Køster, D.; Egedal, J.H.; Lomholt, S.; Hvid, M.; Jakobsen, M.R.; Müller-Ladner, U.; Eibel, H.; Deleuran, B.; Kragstrup, T.W.; Neumann, E.; et al. Phenotypic and Functional Characterization of Synovial Fluid-Derived Fibroblast-like Synoviocytes in Rheumatoid Arthritis. Sci. Rep. 2021, 11, 22168. [Google Scholar] [CrossRef]
- Orange, D.E.; Yao, V.; Sawicka, K.; Fak, J.; Frank, M.O.; Parveen, S.; Blachere, N.E.; Hale, C.; Zhang, F.; Raychaudhuri, S.; et al. RNA Identification of PRIME Cells Predicting Rheumatoid Arthritis Flares. N. Engl. J. Med. 2020, 383, 218–228. [Google Scholar] [CrossRef]
- Sfikakis, P.P.; Vlachogiannis, N.I.; Christopoulos, P.F. Cadherin-11 as a Therapeutic Target in Chronic, Inflammatory Rheumatic Diseases. Clin. Immunol. 2017, 176, 107–113. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, H.; Yu, S.; Lu, Y.; Wu, T. Research Progress in the Role and Mechanism of Cadherin-11 in Different Diseases. J. Cancer 2021, 12, 1190–1199. [Google Scholar] [CrossRef]
- Cao, C.; Wu, F.; Niu, X.; Hu, X.; Cheng, J.; Zhang, Y.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; et al. Cadherin-11 Cooperates with Inflammatory Factors to Promote the Migration and Invasion of Fibroblast-like Synoviocytes in Pigmented Villonodular Synovitis. Theranostics 2020, 10, 10573–10588. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, D.; Römer-Hillmann, A.; Krause, A.; Hansen, U.; Wehmeyer, C.; Intemann, J.; de Gorter, D.J.J.; Dankbar, B.; Hillen, J.; Heitzmann, M.; et al. Lasp1 Regulates Adherens Junction Dynamics and Fibroblast Transformation in Destructive Arthritis. Nat. Commun. 2021, 12, 3624. [Google Scholar] [CrossRef] [PubMed]
- Passanha, F.R.; Geuens, T.; LaPointe, V.L.S. Cadherin-11 Influences Differentiation in Human Mesenchymal Stem Cells by Regulating the Extracellular Matrix Via the TGFβ1 Pathway. Stem Cells 2022, 40, 669–677. [Google Scholar] [CrossRef]
- Chang, S.K.; Kohlgruber, A.C.; Mizoguchi, F.; Michelet, X.; Wolf, B.J.; Wei, K.; Lee, P.Y.; Lynch, L.; Duquette, D.; Ceperuelo-Mallafré, V.; et al. Stromal Cell Cadherin-11 Regulates Adipose Tissue Inflammation and Diabetes. J. Clin. Investig. 2017, 127, 3300–3312. [Google Scholar] [CrossRef]
- Sun, S.; Karsdal, M.A. Type VI Collagen. In Biochemistry of Collagens, Laminins and Elastin; Elsevier: Amsterdam, The Netherlands, 2016; pp. 49–55. ISBN 978-0-12-809847-9. [Google Scholar]
- Gilbert, S.J.; Bonnet, C.S.; Blain, E.J. Mechanical Cues: Bidirectional Reciprocity in the Extracellular Matrix Drives Mechano-Signalling in Articular Cartilage. Int. J. Mol. Sci. 2021, 22, 13595. [Google Scholar] [CrossRef]
- Wolf, J.; Carsons, S.E. Distribution of Type VI Collagen Expression in Synovial Tissue and Cultured Synoviocytes: Relation to Fibronectin Expression. Ann. Rheum. Dis. 1991, 50, 493–496. [Google Scholar] [CrossRef]
- Okawa, S.; Unuma, K.; Yamada, A.; Aki, T.; Uemura, K. Lipopolysaccharide Induces Expression of Collagen VI in the Rat Lung. J. Toxicol. Pathol. 2015, 28, 37–41. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Chávez, S.A.; Chaparro-Barrera, E.; Alvarado-Jáquez, M.F.; Cuevas-Martínez, R.; Ochoa-Albíztegui, R.E.; Pacheco-Tena, C. Complete Freund’s Adjuvant Induces a Fibroblast-like Synoviocytes (FLS) Metabolic and Migratory Phenotype in Resident Fibroblasts of the Inoculated Footpad at the Earliest Stage of Adjuvant-Induced Arthritis. Cells 2023, 12, 842. https://doi.org/10.3390/cells12060842
González-Chávez SA, Chaparro-Barrera E, Alvarado-Jáquez MF, Cuevas-Martínez R, Ochoa-Albíztegui RE, Pacheco-Tena C. Complete Freund’s Adjuvant Induces a Fibroblast-like Synoviocytes (FLS) Metabolic and Migratory Phenotype in Resident Fibroblasts of the Inoculated Footpad at the Earliest Stage of Adjuvant-Induced Arthritis. Cells. 2023; 12(6):842. https://doi.org/10.3390/cells12060842
Chicago/Turabian StyleGonzález-Chávez, Susana Aideé, Eduardo Chaparro-Barrera, María Fernanda Alvarado-Jáquez, Rubén Cuevas-Martínez, Rosa Elena Ochoa-Albíztegui, and César Pacheco-Tena. 2023. "Complete Freund’s Adjuvant Induces a Fibroblast-like Synoviocytes (FLS) Metabolic and Migratory Phenotype in Resident Fibroblasts of the Inoculated Footpad at the Earliest Stage of Adjuvant-Induced Arthritis" Cells 12, no. 6: 842. https://doi.org/10.3390/cells12060842
APA StyleGonzález-Chávez, S. A., Chaparro-Barrera, E., Alvarado-Jáquez, M. F., Cuevas-Martínez, R., Ochoa-Albíztegui, R. E., & Pacheco-Tena, C. (2023). Complete Freund’s Adjuvant Induces a Fibroblast-like Synoviocytes (FLS) Metabolic and Migratory Phenotype in Resident Fibroblasts of the Inoculated Footpad at the Earliest Stage of Adjuvant-Induced Arthritis. Cells, 12(6), 842. https://doi.org/10.3390/cells12060842






