The Glymphatic System May Play a Vital Role in the Pathogenesis of Hepatic Encephalopathy: A Narrative Review
Abstract
1. Introduction
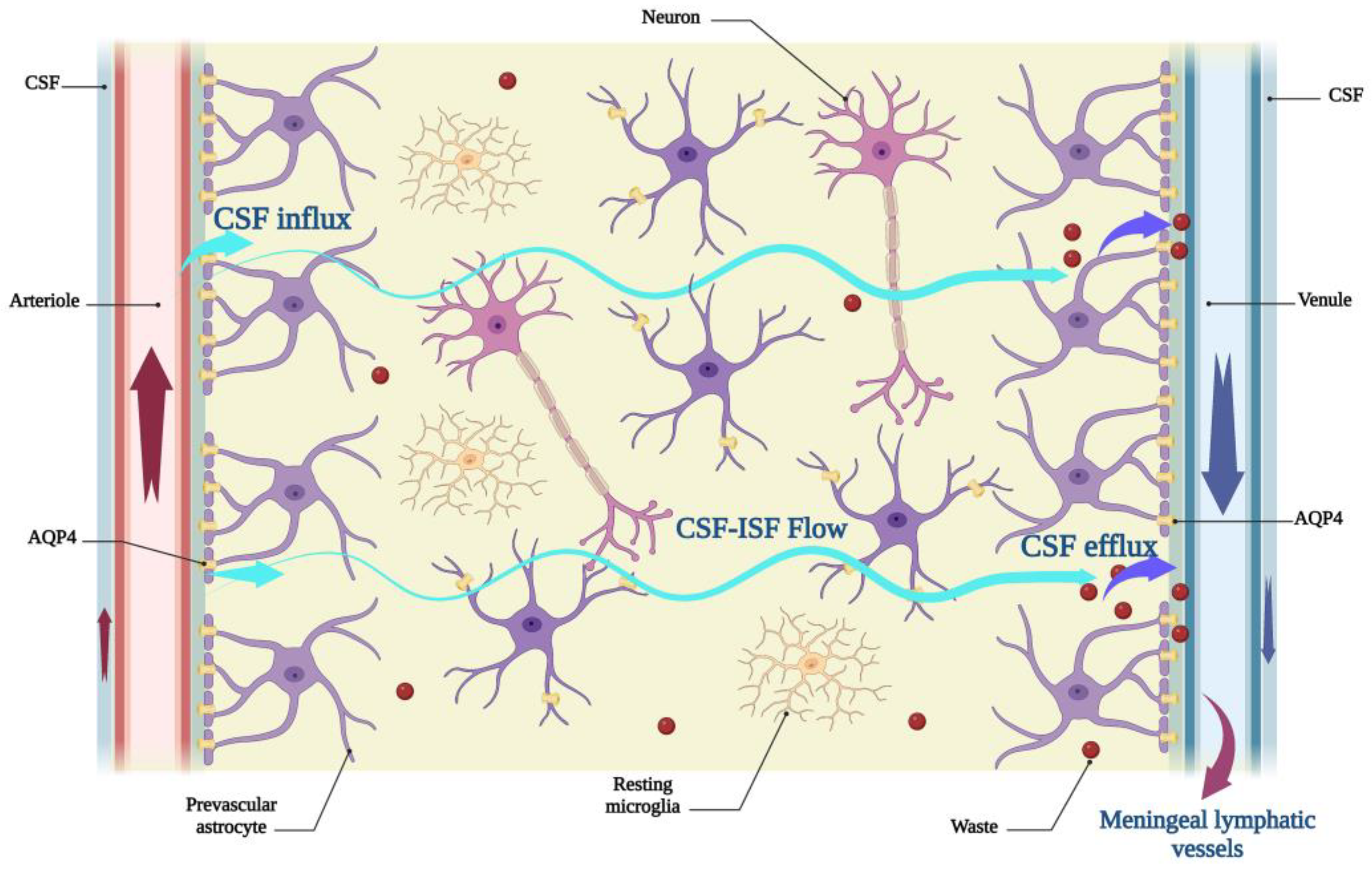
2. An Overview
3. Factors Affecting the Function of the Glymphatic System
4. Direct Evidence of Glymphatic System Impairment in HE
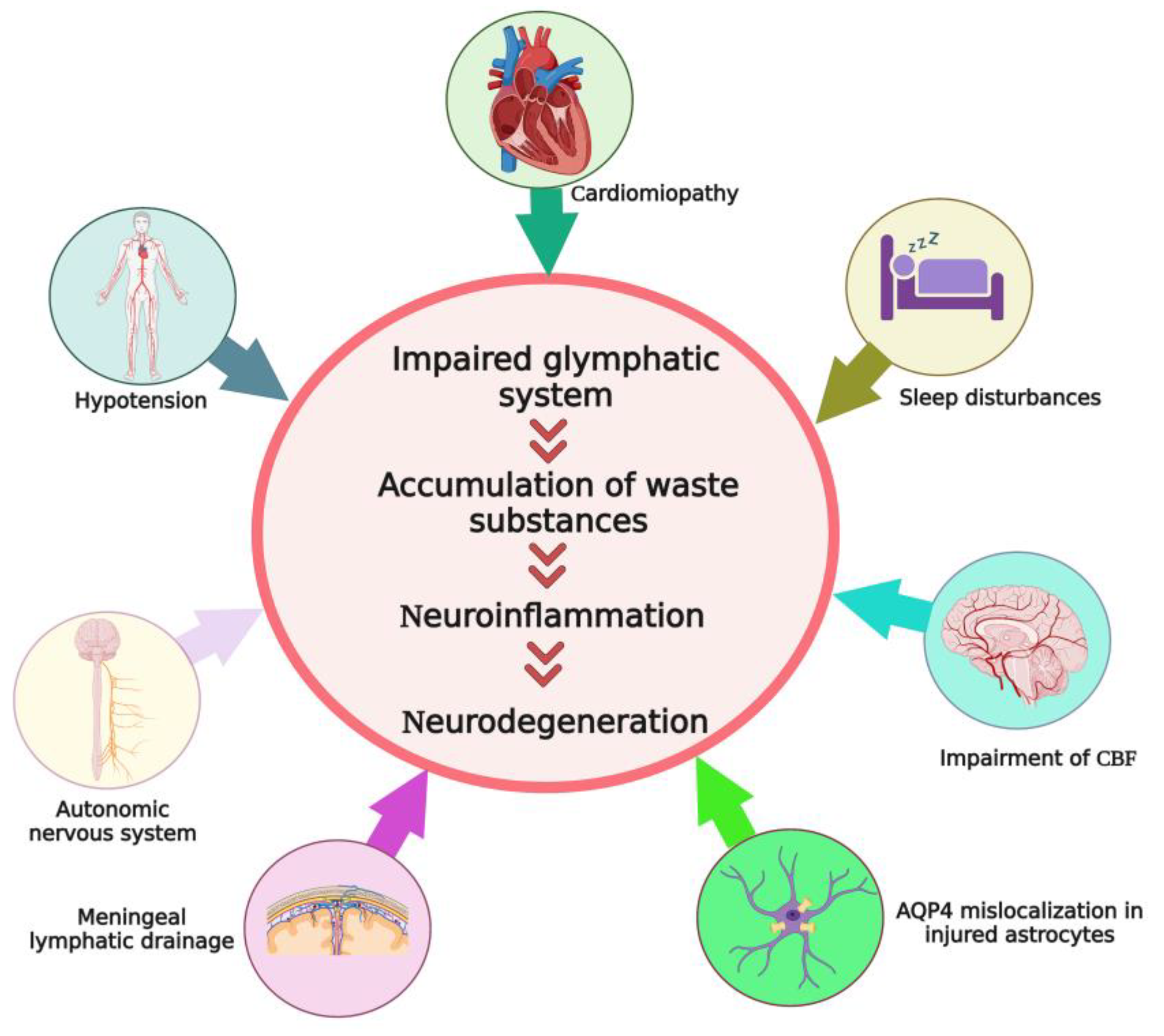
5. Factors That May Indirectly Affect the Efficacy of Glymphatic System in Hepatic Encephalopathy
5.1. Sleep Disturbances in HE
5.2. Cardiomyopathy, Arterial Hypotension, and Impaired Cerebral Blood Flow in Cirrhosis and HE
5.3. Alteration in the Function of Autonomic Nervous System in HE
5.4. Astrocyte Dysfunction and AQP4 Mislocalization in HE
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wijdicks, E.F.M. Hepatic Encephalopathy. N. Engl. J. Med. 2016, 375, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Malinchoc, M.; Kim, W.R.; Kamath, P.S. Hepatic encephalopathy as a predictor of survival in patients with end-stage liver disease. Liver Transplant. 2007, 13, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Bloom, P.P.; Tapper, E.B.; Young, V.B.; Lok, A.S. Microbiome therapeutics for hepatic encephalopathy. J. Hepatol. 2021, 75, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Vigo, M.B.; Pérez, M.J.; De Fino, F.; Gómez, G.; Martínez, S.A.; Bisagno, V.; Di Carlo, M.B.; Scazziota, A.; Manautou, J.E.; Ghanem, C.I. Acute acetaminophen intoxication induces direct neurotoxicity in rats manifested as astrogliosis and decreased dopaminergic markers in brain areas associated with locomotor regulation. Biochem. Pharmacol. 2019, 170, 113662. [Google Scholar] [CrossRef]
- Tevethia, H.V.; Choudhury, A.; Maiwall, R.; Mitra, L.; Saluja, V.; Singh, P.; Trigatia, A.; Kumar, G.; Thapar, S.; Sarin, S.K. Noninvasive diagnosis of cerebral edema in patients of acute liver failure. Hepatol. Int. 2018, 12, S614. [Google Scholar] [CrossRef]
- Tamnanloo, F.; Ochoa-Sanchez, R.; Tremblay, M.; Rose, C.F. Repeated ammonia-induced episodes of hepatic encephalopathy leads to neuronal cell loss in rats with chronic liver disease. Hepatology 2022, 76, S1168–S1169. [Google Scholar] [CrossRef]
- Claeys, W.; Van Hoecke, L.; Geerts, A.; Van Vlierberghe, H.; Lefere, S.; Van Imschoot, G.; Van Wonterghem, E.; Ghesquière, B.; Vandenbroucke, R.E.; Van Steenkiste, C. A mouse model of hepatic encephalopathy: Bile duct ligation induces brain ammonia overload, glial cell activation and neuroinflammation. Sci. Rep. 2022, 12, 17558. [Google Scholar] [CrossRef] [PubMed]
- Balzano, T.; Forteza, J.; Molina, P.; Giner, J.; Monzó, A.; Sancho-Jiménez, J.; Urios, A.; Montoliu, C.; Felipo, V. Lymphocyte infiltration, glial activation and neuronal loss in cerebellum of patients with different stages of chronic liver disease. Glia 2019, 67, E518–E519. [Google Scholar] [CrossRef]
- Agarwal, A.N.; Mais, D.D. Sensitivity and Specificity of Alzheimer Type II Astrocytes in Hepatic Encephalopathy. Arch. Pathol. Lab. Med. 2019, 143, 1256–1258. [Google Scholar] [CrossRef]
- Sepehrinezhad, A.; Zarifkar, A.; Namvar, G.; Shahbazi, A.; Williams, R. Astrocyte swelling in hepatic encephalopathy: Molecular perspective of cytotoxic edema. Metab. Brain Dis. 2020, 35, 559–578. [Google Scholar] [CrossRef]
- Weiss, N.; Barbier Saint Hilaire, P.; Colsch, B.; Isnard, F.; Attala, S.; Schaefer, A.; Amador, M.D.; Rudler, M.; Lamari, F.; Sedel, F.; et al. Cerebrospinal fluid metabolomics highlights dysregulation of energy metabolism in overt hepatic encephalopathy. J. Hepatol 2016, 65, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- Bjerring, P.N.; Gluud, L.L.; Larsen, F.S. Cerebral blood flow and metabolism in hepatic encephalopathy—A meta-analysis. J. Clin. Exp. Hepatol. 2018, 8, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Nedelsky, N.B.; Todd, P.K.; Taylor, J.P. Autophagy and the ubiquitin-proteasome system: Collaborators in neuroprotection. Biochim. Et Biophys. Acta 2008, 1782, 691–699. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, J.; Wei, F.; Song, J.; Chen, W.; Shan, L.; Xue, R.; Wang, G.; Tao, J.; Zhang, G.; et al. Characterizing the glymphatic influx by utilizing intracisternal infusion of fluorescently conjugated cadaverine. Life Sci. 2018, 201, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The role of brain barriers in fluid movement in the CNS: Is there a ‘glymphatic’ system? Acta Neuropathol. 2018, 135, 387–407. [Google Scholar] [CrossRef]
- Reeves, B.C.; Karimy, J.K.; Kundishora, A.J.; Mestre, H.; Cerci, H.M.; Matouk, C.; Alper, S.L.; Lundgaard, I.; Nedergaard, M.; Kahle, K.T. Glymphatic System Impairment in Alzheimer’s Disease and Idiopathic Normal Pressure Hydrocephalus. Trends Mol. Med. 2020, 26, 285–295. [Google Scholar] [CrossRef]
- Silva, I.; Silva, J.; Ferreira, R.; Trigo, D. Glymphatic system, AQP4, and their implications in Alzheimer’s disease. Neurol. Res. Pract. 2021, 3, 5. [Google Scholar] [CrossRef]
- Harrison, I.F.; Ismail, O.; Machhada, A.; Colgan, N.; Ohene, Y.; Nahavandi, P.; Ahmed, Z.; Fisher, A.; Meftah, S.; Murray, T.K.; et al. Impaired glymphatic function and clearance of tau in an Alzheimer’s disease model. Brain 2020, 143, 2576–2593. [Google Scholar] [CrossRef]
- Goulay, R.; Flament, J.; Gauberti, M.; Naveau, M.; Pasquet, N.; Gakuba, C.; Emery, E.; Hantraye, P.; Vivien, D.; Aron-Badin, R.; et al. Subarachnoid Hemorrhage Severely Impairs Brain Parenchymal Cerebrospinal Fluid Circulation in Nonhuman Primate. Stroke 2017, 48, 2301–2305. [Google Scholar] [CrossRef]
- Plog, B.A.; Dashnaw, M.L.; Hitomi, E.; Peng, W.; Liao, Y.; Lou, N.; Deane, R.; Nedergaard, M. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J. Neurosci. 2015, 35, 518–526. [Google Scholar] [CrossRef]
- Iliff, J.J.; Chen, M.J.; Plog, B.A.; Zeppenfeld, D.M.; Soltero, M.; Yang, L.; Singh, I.; Deane, R.; Nedergaard, M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014, 34, 16180–16193. [Google Scholar] [CrossRef] [PubMed]
- Hadjihambi, A.; Harrison, I.F.; Costas-Rodríguez, M.; Vanhaecke, F.; Arias, N.; Gallego-Durán, R.; Mastitskaya, S.; Hosford, P.S.; Olde Damink, S.W.M.; Davies, N.; et al. Impaired brain glymphatic flow in experimental hepatic encephalopathy. J. Hepatol. 2019, 70, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Gallina, P.; Gallo, O.; Nicoletti, C.; Romanelli, R.G. A hydrodynamic hypothesis for the pathogenesis of glymphatic system impairment in hepatic encephalopathy. J. Hepatol. 2019, 71, 228–229. [Google Scholar] [CrossRef] [PubMed]
- Valenza, M.; Facchinetti, R.; Steardo, L.; Scuderi, C. Altered waste disposal system in aging and Alzheimer’s disease: Focus on astrocytic aquaporin-4. Front. Pharmacol. 2020, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Plá, V.; Giannetto, M.; Vinitsky, H.S.; Stæger, F.F.; Metcalfe, T.; Nguyen, R.; Benrais, A.; Nedergaard, M. Circadian control of brain glymphatic and lymphatic fluid flow. Nat. Commun. 2020, 11, 4411. [Google Scholar] [CrossRef]
- Camassa, L.M.A.; Lunde, L.K.; Hoddevik, E.H.; Stensland, M.; Boldt, H.B.; De Souza, G.A.; Ottersen, O.P.; Amiry-Moghaddam, M. Mechanisms underlying AQP4 accumulation in astrocyte endfeet. Glia 2015, 63, 2073–2091. [Google Scholar] [CrossRef]
- De Bellis, M.; Pisani, F.; Mola, M.G.; Rosito, S.; Simone, L.; Buccoliero, C.; Trojano, M.; Nicchia, G.P.; Svelto, M.; Frigeri, A. Translational readthrough generates new astrocyte AQP4 isoforms that modulate supramolecular clustering, glial endfeet localization, and water transport. Glia 2017, 65, 790–803. [Google Scholar] [CrossRef]
- Mestre, H.; Hablitz, L.M.; Xavier, A.L.; Feng, W.; Zou, W.; Pu, T.; Monai, H.; Murlidharan, G.; Rivera, R.M.C.; Simon, M.J. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 2018, 7, e40070. [Google Scholar] [CrossRef]
- Eide, P.K.; Ringstad, G. Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: A glymphatic magnetic resonance imaging study. J. Cereb. Blood Flow Metab. 2019, 39, 1355–1368. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Vinitsky, H.S.; Sun, Q.; Stæger, F.F.; Sigurdsson, B.; Mortensen, K.N.; Lilius, T.O.; Nedergaard, M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 2019, 5, eaav5447. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, R.J.; Steffey, E.P.; LeCouteur, R.A.; Imai, A.; Farver, T.B.; Kortz, G.D. Effects of body position on intracranial and cerebral perfusion pressures in isoflurane-anesthetized horses. J. Appl. Physiol. 2002, 92, 2542–2546. [Google Scholar] [CrossRef]
- Kose, G.; Hatipoglu, S. Effect of head and body positioning on cerebral blood flow velocity in patients who underwent cranial surgery. J. Clin. Nurs. 2012, 21, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Xie, L.; Yu, M.; Kang, H.; Feng, T.; Deane, R.; Logan, J.; Nedergaard, M.; Benveniste, H. The Effect of Body Posture on Brain Glymphatic Transport. J. Neurosci. 2015, 35, 11034–11044. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.K.; Li, Y.-H.; Pham, T.T.; Ochoa, L.N.; Treviño, M.T.; Hartley, C.J.; Michael, L.H.; Entman, M.L.; Taffet, G.E. Measurement of aortic input impedance in mice: Effects of age on aortic stiffness. Am. J. Physiol.-Heart Circ. Physiol. 2003, 285, H1464–H1470. [Google Scholar] [CrossRef]
- Avolio, A.P.; Kuznetsova, T.; Heyndrickx, G.R.; Kerkhof, P.L.M.; Li, J.K. Arterial Flow, Pulse Pressure and Pulse Wave Velocity in Men and Women at Various Ages. Adv. Exp. Med. Biol. 2018, 1065, 153–168. [Google Scholar] [CrossRef]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef]
- Ma, Q.; Ineichen, B.V.; Detmar, M.; Proulx, S.T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 2017, 8, 1434. [Google Scholar] [CrossRef]
- Chandra, P.K.; Cikic, S.; Rutkai, I.; Guidry, J.J.; Katakam, P.V.G.; Mostany, R.; Busija, D.W. Effects of aging on protein expression in mice brain microvessels: ROS scavengers, mRNA/protein stability, glycolytic enzymes, mitochondrial complexes, and basement membrane components. GeroScience 2022, 44, 371–388. [Google Scholar] [CrossRef]
- Bronzuoli, M.R.; Facchinetti, R.; Valenza, M.; Cassano, T.; Steardo, L.; Scuderi, C. Astrocyte Function Is Affected by Aging and Not Alzheimer’s Disease: A Preliminary Investigation in Hippocampi of 3xTg-AD Mice. Front. Pharmacol. 2019, 10, 644. [Google Scholar] [CrossRef]
- Palmer, A.L.; Ousman, S.S. Astrocytes and Aging. Front. Aging Neurosci. 2018, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Q.; Miyajima, M.; Ogino, I.; Arai, H. Expression of the water-channel protein aquaporin 4 in the H-Tx rat: Possible compensatory role in spontaneously arrested hydrocephalus. J. Neurosurg. 2006, 105, 459–464. [Google Scholar] [CrossRef]
- Owasil, R.; O’Neill, R.; Keable, A.; Nimmo, J.; MacGregor Sharp, M.; Kelly, L.; Saito, S.; Simpson, J.E.; Weller, R.O.; Smith, C.; et al. The Pattern of AQP4 Expression in the Ageing Human Brain and in Cerebral Amyloid Angiopathy. Int. J. Mol. Sci. 2020, 21, 1225. [Google Scholar] [CrossRef]
- Cheng, K.P.; Brodnick, S.K.; Blanz, S.L.; Zeng, W.; Kegel, J.; Pisaniello, J.A.; Ness, J.P.; Ross, E.; Nicolai, E.N.; Settell, M.L.; et al. Clinically-derived vagus nerve stimulation enhances cerebrospinal fluid penetrance. Brain Stimul. 2020, 13, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, H.; Lee, H.; Ding, F.; Sun, Q.; Al-Bizri, E.; Makaryus, R.; Probst, S.; Nedergaard, M.; Stein, E.A.; Lu, H. Anesthesia with Dexmedetomidine and Low-dose Isoflurane Increases Solute Transport via the Glymphatic Pathway in Rat Brain When Compared with High-dose Isoflurane. Anesthesiology 2017, 127, 976–988. [Google Scholar] [CrossRef]
- Hsu, S.J.; Zhang, C.; Jeong, J.; Lee, S.I.; McConnell, M.; Utsumi, T.; Iwakiri, Y. Enhanced Meningeal Lymphatic Drainage Ameliorates Neuroinflammation and Hepatic Encephalopathy in Cirrhotic Rats. Gastroenterology 2021, 160, 1315–1329.e13. [Google Scholar] [CrossRef] [PubMed]
- Claeys, W.; Van Hoecke, L.; Lefere, S.; Geerts, A.; Verhelst, X.; Van Vlierberghe, H.; Degroote, H.; Devisscher, L.; Vandenbroucke, R.E.; Van Steenkiste, C. The neurogliovascular unit in hepatic encephalopathy. JHEP Rep. 2021, 3, 100352. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, B.C.; Puri, V.; Sachdeva, S.; Srivastava, S. Sleep disturbances in patients of liver cirrhosis with minimal hepatic encephalopathy before and after lactulose therapy. Metab. Brain Dis. 2017, 32, 595–605. [Google Scholar] [CrossRef]
- Agrawal, S.; Umapathy, S.; Dhiman, R.K. Minimal Hepatic Encephalopathy Impairs Quality of Life. J. Clin. Exp. Hepatol. 2015, 5, S42–S48. [Google Scholar] [CrossRef]
- Velissaris, D.; Solomou, E.; Kalogeropoulos, A.; Georgiopoulou, V.; Thomopoulos, C.; Karatza, C. Sleep disorders and brain MRI as early indicators of subclinical hepatic encephalopathy. Hepatogastroenterology 2006, 53, 51–54. [Google Scholar]
- Labenz, C.; Baron, J.S.; Toenges, G.; Schattenberg, J.M.; Nagel, M.; Sprinzl, M.F.; Nguyen-Tat, M.; Zimmermann, T.; Huber, Y.; Marquardt, J.U.; et al. Prospective evaluation of the impact of covert hepatic encephalopathy on quality of life and sleep in cirrhotic patients. Aliment. Pharmacol. Ther. 2018, 48, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Bruyneel, M.; Sersté, T.; Libert, W.; van den Broecke, S.; Ameye, L.; Dachy, B.; Mulkay, J.P.; Moreno, C.; Gustot, T. Improvement of sleep architecture parameters in cirrhotic patients with recurrent hepatic encephalopathy with the use of rifaximin. Eur. J. Gastroenterol. Hepatol. 2017, 29, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Spahr, L.; Coeytaux, A.; Giostra, E.; Hadengue, A.; Annoni, J.M. Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: A randomized controlled pilot trial. Am. J. Gastroenterol. 2007, 102, 744–753. [Google Scholar] [CrossRef]
- Llansola, M.; Cantero, J.L.; Hita-Yañez, E.; Mirones-Maldonado, M.J.; Piedrafita, B.; Ahabrach, H.; Errami, M.; Agusti, A.; Felipo, V. Progressive reduction of sleep time and quality in rats with hepatic encephalopathy caused by portacaval shunts. Neuroscience 2012, 201, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Beaubernard, C.; Salomon, F.; Bismuth, H. Experimental hepatic encephalopathy. Study of the organization of a diurnal sleep pattern in rats with portocaval anastomosis. Biomedicine 1980, 32, 76–80. [Google Scholar] [PubMed]
- Larsen, F.S. Cerebral blood flow in hyperammonemia: Heterogeneity and starling forces in capillaries. Metab. Brain Dis. 2002, 17, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.S.; Wendon, J. Brain edema in liver failure: Basic physiologic principles and management. Liver Transplant. 2002, 8, 983–989. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Zeppenfeld, D.M.; Venkataraman, A.; Plog, B.A.; Liao, Y.; Deane, R.; Nedergaard, M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 2013, 33, 18190–18199. [Google Scholar] [CrossRef]
- Liu, H.; Gaskari, S.A.; Lee, S.S. Cardiac and vascular changes in cirrhosis: Pathogenic mechanisms. World J. Gastroenterol. 2006, 12, 837–842. [Google Scholar] [CrossRef]
- Pudil, R.; Pelouch, R.; Praus, R.; Vašatová, M.; Hůlek, P. Heart failure in patients with liver cirrhosis. Cor Et Vasa 2013, 55, e391–e396. [Google Scholar] [CrossRef]
- Berzigotti, A.; Bosch, J. Editorial: Increased cardiac output in cirrhosis—Non-invasive assessment of regional blood flow by magnetic resonance angiography. Aliment. Pharmacol. Ther. 2016, 43, 1340–1342. [Google Scholar] [CrossRef]
- Li, X.; Yu, S.; Li, L.; Han, D.; Dai, S.; Gao, Y. Cirrhosis-related changes in left ventricular function and correlation with the model for end-stage liver disease score. Int. J. Clin. Exp. Med. 2014, 7, 5751–5757. [Google Scholar]
- Sampaio, F.; Pimenta, J.; Bettencourt, N.; Fontes-Carvalho, R.; Silva, A.P.; Valente, J.; Bettencourt, P.; Fraga, J.; Gama, V. Systolic and diastolic dysfunction in cirrhosis: A tissue-Doppler and speckle tracking echocardiography study. Liver Int. 2013, 33, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, M.; Salerno, F.; Pagnozzi, G.; Dionigi, E.; Visentin, S.; Cirello, I.; Meregaglia, D.; Nicolini, A. Diastolic dysfunction is associated with poor survival in patients with cirrhosis with transjugular intrahepatic portosystemic shunt. Gut 2007, 56, 869–875. [Google Scholar] [CrossRef]
- Srinivasamurthy, B.C.; Saravanan, S.P.; Marak, F.K.; Manivel, P.; Bhat, R.V.; Mathiyazhagan, D. Morphological Cardiac Alterations in Liver Cirrhosis: An Autopsy Study. Heart Views Off. J. Gulf Heart Assoc. 2021, 22, 96–101. [Google Scholar] [CrossRef]
- Gregolin, C.S.; do Nascimento, M.; Borges de Souza, S.L.; Ferreira Mota, G.A.; Bomfim, G.F.; de Azevedo Melo Luvizotto, R.; Sugizaki, M.M.; Zanati Bazan, S.G.; Salomé de Campos, D.H.; Dias, M.C.; et al. Myocardial Dysfunction in Cirrhotic Cardiomyopathy is Associated with Alterations of Phospholamban Phosphorylation and IL-6 Levels. Arch. Med. Res. 2021, 52, 284–293. [Google Scholar] [CrossRef]
- Yu, S.; Sun, L.; Wang, H.; Jiang, J.; Zhou, Q. Autonomic regulation of imbalance-induced myocardial fibrosis and its mechanism in rats with cirrhosis. Exp. Ther. Med. 2021, 22, 1040. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Ting, W.J.; Day, C.H.; Ju, D.T.; Yeh, Y.L.; Chung, L.C.; Tsai, F.J.; Tsai, C.H.; Tsai, Y.; Huang, C.Y. SHSST cyclodextrin complex prevents the fibrosis effect on CCl₄-induced cirrhotic cardiomyopathy in rats through TGF-β pathway inhibition effects. Int. J. Mol. Sci. 2014, 15, 8037–8048. [Google Scholar] [CrossRef]
- Farahnaz, J.; Razieh, A.; Alireza, A.; Giti, G.; Ahmad Reza, D. Evaluation of Chronic Losartan Treatment Effect on Cardiac Chronotropic Dysfunction in Biliary Cirrhotic Rats. Acta Med. Iran. 2018, 56, 4–13. [Google Scholar]
- Wiest, R.; Groszmann, R.J. The paradox of nitric oxide in cirrhosis and portal hypertension: Too much, not enough. Hepatology 2002, 35, 478–491. [Google Scholar] [CrossRef]
- Zardi, E.M.; Abbate, A.; Zardi, D.M.; Dobrina, A.; Margiotta, D.; Tassel, B.W.V.; Afeltra, A.; Sanyal, A.J. Cirrhotic Cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ma, Z.; Lee, S.S. Contribution of nitric oxide to the pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Gastroenterology 2000, 118, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Bátkai, S.; Járai, Z.; Wagner, J.A.; Goparaju, S.K.; Varga, K.; Liu, J.; Wang, L.; Mirshahi, F.; Khanolkar, A.D.; Makriyannis, A.; et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat. Med. 2001, 7, 827–832. [Google Scholar] [CrossRef]
- Trewby, P.N.; Williams, R. Pathophysiology of hypotension in patients with fulminant hepatic failure. Gut 1977, 18, 1021–1026. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Su, C.-H.; Chan, J.Y.H.; Chan, S.H.H. Nitrosative Stress-Induced Disruption of Baroreflex Neural Circuits in a Rat Model of Hepatic Encephalopathy: A DTI Study. Sci. Rep. 2017, 7, 40111. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, Y.; Johnny, E.; Brian, J.; Todd, M.M. Plasma viscosity and cerebral blood flow. Am. J. Physiol.-Heart Circ. Physiol. 2000, 279, H1949–H1954. [Google Scholar] [CrossRef]
- Odunayo, A. Chapter 80–Traumatic Brain Injury. In August’s Consultations in Feline Internal Medicine; Little, S.E., Ed.; W.B. Saunders: St. Louis, PA, USA, 2016; Volume 7, pp. 799–802. [Google Scholar] [CrossRef]
- Bjerring, P.N.; Bjerrum, E.J.; Larsen, F.S. Impaired cerebral microcirculation induced by ammonium chloride in rats is due to cortical adenosine release. J. Hepatol. 2018, 68, 1137–1143. [Google Scholar] [CrossRef]
- Dam, G.; Keiding, S.; Munk, O.L.; Ott, P.; Vilstrup, H.; Bak, L.K.; Waagepetersen, H.S.; Schousboe, A.; Sørensen, M. Hepatic encephalopathy is associated with decreased cerebral oxygen metabolism and blood flow, not increased ammonia uptake. Hepatology 2013, 57, 258–265. [Google Scholar] [CrossRef]
- Zheng, G.; Zhang, L.J.; Cao, Y.; Lu, G.M. Venous blood ammonia can be associated with cerebral blood flow in hepatic encephalopathy. Hepatology 2013, 58, 832–833. [Google Scholar] [CrossRef]
- Strauss, G.; Hansen, B.A.; Kirkegaard, P.; Rasmussen, A.; Hjortrup, A.; Larsen, F.S. Liver function, cerebral blood flow autoregulation, and hepatic encephalopathy in fulminant hepatic failure. Hepatology 1997, 25, 837–839. [Google Scholar] [CrossRef]
- Larsen, F.S.; Knudsen, G.M.; Paulson, O.B.; Vilstrup, H. Cerebral blood flow autoregulation is absent in rats with thioacetamide-induced hepatic failure. J. Hepatol. 1994, 21, 491–495. [Google Scholar] [CrossRef]
- Donovan, J.P.; Schafer, D.F.; Shaw, B.W., Jr.; Sorrell, M.F. Cerebral oedema and increased intracranial pressure in chronic liver disease. Lancet 1998, 351, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Crippin, J.S.; Gross, J.B., Jr.; Lindor, K.D. Increased intracranial pressure and hepatic encephalopathy in chronic liver disease. Am. J. Gastroenterol. 1992, 87, 879–882. [Google Scholar] [PubMed]
- Detry, O.; De Roover, A.; Honoré, P.; Meurisse, M. Brain edema and intracranial hypertension in fulminant hepatic failure: Pathophysiology and management. WJG 2006, 12, 7405. [Google Scholar] [CrossRef]
- Bernal, W.; Murphy, N.; Brown, S.; Whitehouse, T.; Bjerring, P.N.; Hauerberg, J.; Frederiksen, H.J.; Auzinger, G.; Wendon, J.; Larsen, F.S. A multicentre randomized controlled trial of moderate hypothermia to prevent intracranial hypertension in acute liver failure. J. Hepatol. 2016, 65, 273–279. [Google Scholar] [CrossRef]
- Bjerring, P.N.; Eefsen, M.; Hansen, B.A.; Larsen, F.S. The brain in acute liver failure. A tortuous path from hyperammonemia to cerebral edema. Metab. Brain Dis. 2009, 24, 5–14. [Google Scholar] [CrossRef]
- Blei, A.T.; Larsen, F.S. Pathophysiology of cerebral edema in fulminant hepatic failure. J. Hepatol. 1999, 31, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Jalan, R.; Damink, S.W.O.; Deutz, N.E.; Hayes, P.C.; Lee, A. Moderate hypothermia in patients with acute liver failure and uncontrolled intracranial hypertension. Gastroenterology 2004, 127, 1338–1346. [Google Scholar] [CrossRef]
- Tofteng, F.; Larsen, F.S. The Effect of Indomethacin on Intracranial Pressure, Cerebral Perfusion and Extracellular Lactate and Glutamate Concentrations in Patients with Fulminant Hepatic Failure. J. Cereb. Blood Flow Metab. 2004, 24, 798–804. [Google Scholar] [CrossRef]
- Tofteng, F.; Hauerberg, J.; Hansen, B.A.; Pedersen, C.B.; Jørgensen, L.; Larsen, F.S. Persistent Arterial Hyperammonemia Increases the Concentration of Glutamine and Alanine in the Brain and Correlates with Intracranial Pressure in Patients with Fulminant Hepatic Failure. J. Cereb. Blood Flow Metab. 2006, 26, 21–27. [Google Scholar] [CrossRef]
- Ranjan, P.; Mishra, A.M.; Kale, R.; Saraswat, V.A.; Gupta, R.K. Cytotoxic Edema Is Responsible for Raised Intracranial Pressure in Fulminant Hepatic Failure: In Vivo Demonstration Using Diffusion-Weighted MRI in Human Subjects. Metab. Brain Dis. 2005, 20, 181–192. [Google Scholar] [CrossRef]
- Bjerring, P.N.; Hauerberg, J.; Jørgensen, L.; Frederiksen, H.-J.; Tofteng, F.; Hansen, B.A.; Larsen, F.S. Brain hypoxanthine concentration correlates to lactate/pyruvate ratio but not intracranial pressure in patients with acute liver failure. J. Hepatol. 2010, 53, 1054–1058. [Google Scholar] [CrossRef]
- Davenport, A.; Will, E.J.; Davison, A.M. Early Changes in Intracranial Pressure During Haemofiltration Treatment in Patients with Grade 4 Hepatic Encephalopathy and Acute Oliguric Renal Failure. Nephrol. Dial. Transplant. 1990, 5, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.E.; Tofteng, F.; Strauss, G.I.; Larsen, F.S. Effect of treatment with the Molecular Adsorbents Recirculating System on arterial amino acid levels and cerebral amino acid metabolism in patients with hepatic encephalopathy. Scand. J. Gastroenterol. 2004, 39, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Bjerring, P.N.; Dale, N.; Larsen, F.S. Acute hyperammonemia and systemic inflammation is associated with increased extracellular brain adenosine in rats: A biosensor study. Neurochem. Res. 2015, 40, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Bjerring, P.N.; Hauerberg, J.; Frederiksen, H.J.; Nielsen, H.B.; Clemmesen, J.O.; Larsen, F.S. The effect of fractionated plasma separation and adsorption on cerebral amino acid metabolism and oxidative metabolism during acute liver failure. J. Hepatol. 2012, 57, 774–779. [Google Scholar] [CrossRef]
- Lenaerts, A.; Codden, T.; Meunier, J.-C.; Henry, J.-P.; Ligny, G. Effects of clonidine on diuretic response in ascitic patients with cirrhosis and activation of sympathetic nervous system. Hepatology 2006, 44, 844–849. [Google Scholar] [CrossRef]
- Miyajima, H.; Nomura, M.; Muguruma, N.; Okahisa, T.; Shibata, H.; Okamura, S.; Honda, H.; Shimizu, I.; Harada, M.; Saito, K.; et al. Relationship among gastric motility, autonomic activity, and portal hemodynamics in patients with liver cirrhosis. J. Gastroenterol. Hepatol. 2001, 16, 647–659. [Google Scholar] [CrossRef]
- Henriksen, J.H.; Ring-Larsen, H.; Christensen, N.J. Sympathetic nervous activity in cirrhosis: A survey of plasma catecholamine studies. J. Hepatol. 1985, 1, 55–65. [Google Scholar] [CrossRef]
- Barron, H.V.; Alam, I.; Lesh, M.D.; Strunk, A.; Bass, N.M. Autonomic nervous system tone measured by baroreflex sensitivity is depressed in patients with end-stage liver disease. Am. J. Gastroenterol. 1999, 94, 986–989. [Google Scholar] [CrossRef]
- Lara, C.; Gajardo, A.; Giubergia, F.; Bustos, N.; Roblero, J.P.; Urzúa, A.; Poniachik, J.; Cattaneo, M. P-38 Utility of pupillary reactivity in the functional assessment of the autonomous nervous system in patients with chronic liver disease: Preliminary results. Ann. Hepatol. 2021, 24, 100402. [Google Scholar] [CrossRef]
- Esler, M.; Dudley, F.; Jennings, G.; Debinski, H.; Lambert, G.; Jones, P.; Crotty, B.; Colman, J.; Willett, I. Increased Sympathetic Nervous Activity and the Effects of Its Inhibition with Clonidine in Alcoholic Cirrhosis. Ann. Intern. Med. 1992, 116, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Lenz, K.; Hörtnagl, H.; Magometschnigg, D.; Kleinberger, G.; Druml, W.; Laggner, A. Function of the autonomic nervous system in patients with hepatic encephalopathy. Hepatology 1985, 5, 831–836. [Google Scholar] [CrossRef]
- Rega, D.; Aiko, M.; Peñaranda, N.; Urios, A.; Gallego, J.J.; Giménez-Garzó, C.; Casanova, F.; Fiorillo, A.; Cabrera-Pastor, A.; San-Miguel, T.; et al. Patients with Minimal Hepatic Encephalopathy Show Altered Thermal Sensitivity and Autonomic Function. J. Clin. Med. 2021, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, S.-L.; Du, J.; Chen, Y.; Jia, J.; Feng, J.-G.; Liu, K.-X.; Zhou, J. Dexmedetomidine alleviates neuroinflammation, restores sleep disorders and neurobehavioral abnormalities in rats with minimal hepatic encephalopathy. Int. Immunopharmacol. 2021, 96, 107795. [Google Scholar] [CrossRef]
- Kirstetter, P.; Moreau, R.; Soupison, T.; Cailmail, S.; Hartleb, M.; Lebrec, D. Role of sympathetic cardiovascular tone in control of arterial pressure in rats with cirrhosis. Liver 1996, 16, 263–266. [Google Scholar] [CrossRef]
- Dietrich, P.; Moleda, L.; Kees, F.; Müller, M.; Straub, R.H.; Hellerbrand, C.; Wiest, R. Dysbalance in sympathetic neurotransmitter release and action in cirrhotic rats: Impact of exogenous neuropeptide Y. J. Hepatol. 2013, 58, 254–261. [Google Scholar] [CrossRef]
- Jaeger, V.; DeMorrow, S.; McMillin, M. The direct contribution of astrocytes and microglia to the pathogenesis of hepatic encephalopathy. J. Clin. Transl. Hepatol. 2019, 7, 352. [Google Scholar] [CrossRef]
- Ott, P.; Larsen, F.S. Blood-brain barrier permeability to ammonia in liver failure: A critical reappraisal. Neurochem. Int. 2004, 44, 185–198. [Google Scholar] [CrossRef]
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and Functions in Brain Pathologies. Front. Pharmacol. 2019, 10, 1114. [Google Scholar] [CrossRef]
- Broadhead, M.J.; Miles, G.B. Bi-Directional Communication Between Neurons and Astrocytes Modulates Spinal Motor Circuits. Front. Cell. Neurosci. 2020, 14, 30. [Google Scholar] [CrossRef]
- Hol, E.M.; Pekny, M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef]
- Kretzschmar, H.A.; DeArmond, S.J.; Forno, L.S. Measurement of GFAP in Hepatic Encephalopathy by ELISA and Transblots. J. Neuropathol. Exp. Neurol. 1985, 44, 459–471. [Google Scholar] [CrossRef]
- Hiba, O.E.; Elgot, A.; Ahboucha, S.; Gamrani, H. Differential regional responsiveness of astroglia in mild hepatic encephalopathy: An Immunohistochemical approach in bile duct ligated rat. Acta Histochem. 2016, 118, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Shuliatnikova, T. Region-dependent heterogeneity in GFAP expression in acute hepatic encephalopathy. Interdiscip.Res. Sci. Horiz. Perspect. 2021, 2, 56–57. [Google Scholar]
- Chileski, G.S.; García, E.N.; Lértora, J.W.; Mussart, N.; Hernández, D.R.; Cholich, L.A. Hepatic encephalopathy in swine experimentally poisoned with Senna occidentalis seeds: Effects on astrocytes. Toxicon 2021, 201, 86–91. [Google Scholar] [CrossRef]
- Bélanger, M.; Desjardins, P.; Chatauret, N.; Butterworth, R.F. Loss of expression of glial fibrillary acidic protein in acute hyperammonemia. Neurochem. Int. 2002, 41, 155–160. [Google Scholar] [CrossRef]
- Jayakumar, A.; Rao, K.R.; Murthy, C.R.; Norenberg, M. Glutamine in the mechanism of ammonia-induced astrocyte swelling. Neurochem. Int. 2006, 48, 623–628. [Google Scholar] [CrossRef]
- Strauss, G.I.; Knudsen, G.M.; Kondrup, J.; Møller, K.; Larsen, F.S. Cerebral metabolism of ammonia and amino acids in patients with fulminant hepatic failure. Gastroenterology 2001, 121, 1109–1119. [Google Scholar] [CrossRef]
- Albrecht, J.; Norenberg, M.D. Glutamine: A Trojan horse in ammonia neurotoxicity. Hepatology 2006, 44, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Tanigami, H.; Rebel, A.; Martin, L.J.; Chen, T.Y.; Brusilow, S.W.; Traystman, R.J.; Koehler, R.C. Effect of glutamine synthetase inhibition on astrocyte swelling and altered astroglial protein expression during hyperammonemia in rats. Neuroscience 2005, 131, 437–449. [Google Scholar] [CrossRef]
- Zemtsova, I.; Görg, B.; Keitel, V.; Bidmon, H.J.; Schrör, K.; Häussinger, D. Microglia activation in hepatic encephalopathy in rats and humans. Hepatology 2011, 54, 204–215. [Google Scholar] [CrossRef] [PubMed]
- McMillin, M.; Frampton, G.; Thompson, M.; Galindo, C.; Standeford, H.; Whittington, E.; Alpini, G.; DeMorrow, S. Neuronal CCL2 is upregulated during hepatic encephalopathy and contributes to microglia activation and neurological decline. J. Neuroinflammation 2014, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V.R.; Brahmbhatt, M.; Norenberg, M.D. Microglia contribute to ammonia-induced astrocyte swelling in culture. Metab. Brain Dis. 2013, 28, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.; Miller, F.; Cazaubon, S.; Couraud, P.O. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim. Et Biophys. Acta 2009, 1788, 842–857. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Hajal, C.; Le Roi, B.; Kamm, R.D.; Maoz, B.M. Biology and Models of the Blood–brain Barrier. Annu. Rev. Biomed. Eng. 2021, 23, 359–384. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood–brain barrier disruption: Effects and mechanisms. CNS Neurosci. Ther. 2021, 27, 36–47. [Google Scholar] [CrossRef]
- Galea, I. The blood–brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The blood–brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 2020, 217, e20190062. [Google Scholar] [CrossRef]
- Song, K.; Li, Y.; Zhang, H.; An, N.; Wei, Y.; Wang, L.; Tian, C.; Yuan, M.; Sun, Y.; Xing, Y. Oxidative stress-mediated blood-brain barrier (BBB) disruption in neurological diseases. Oxidative Med. Cell. Longev. 2020, 2020, 4356386. [Google Scholar] [CrossRef]
- Marchetti, L.; Engelhardt, B. Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc. Biol. 2020, 2, H1–H18. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.R.; Rama Rao, K.V.; Norenberg, M.D. Neuroinflammation in hepatic encephalopathy: Mechanistic aspects. J. Clin. Exp. Hepatol. 2015, 5, S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ruan, J.; Li, D.; Wang, M.; Han, Z.; Qiu, W.; Wu, G. The Role of Intestinal Bacteria and Gut–Brain Axis in Hepatic Encephalopathy. Front. Cell. Infect. Microbiol. 2021, 10, 595759. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.; Journaux, M.; Mourabit, H.E.; Mouri, S.; Wendum, D.; Lasnier, E.; Couraud, P.-O.; Housset, C.; Thabut, D.; Rudler, M.; et al. A systemic mechanism of increased transendothelial migration of leukocytes through the blood-brain barrier in hepatic encephalopathy. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101801. [Google Scholar] [CrossRef]
- Masago, K.; Kihara, Y.; Yanagida, K.; Hamano, F.; Nakagawa, S.; Niwa, M.; Shimizu, T. Lysophosphatidic acid receptor, LPA6, regulates endothelial blood-brain barrier function: Implication for hepatic encephalopathy. Biochem. Biophys. Res. Commun. 2018, 501, 1048–1054. [Google Scholar] [CrossRef]
- McMillin, M.A.; Frampton, G.A.; Seiwell, A.P.; Patel, N.S.; Jacobs, A.N.; DeMorrow, S. TGFβ1 exacerbates blood–brain barrier permeability in a mouse model of hepatic encephalopathy via upregulation of MMP9 and downregulation of claudin-5. Lab. Investig. 2015, 95, 903–913. [Google Scholar] [CrossRef]
- Kato, M.; Sugihara, J.; Nakamura, T.; Muto, Y. Electron microscopic study of the blood-brain barrier in rats with brain edema and encephalopathy due to acute hepatic failure. Gastroenterol. Jpn. 1989, 24, 135–142. [Google Scholar] [CrossRef]
- Wiltfang, J.; Nolte, W.; Otto, M.; Wildberg, J.; Bahn, E.; Figulla, H.R.; Pralle, L.; Hartmann, H.; Rüther, E.; Ramadori, G. Elevated serum levels of astroglial S100beta in patients with liver cirrhosis indicate early and subclinical portal-systemic encephalopathy. Metab. Brain Dis. 1999, 14, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Kamel, L.; Ghali, A.; Ismail, A.; El Khayat, H. Serum levels of astroglial S100-beta and neuron-specific enolase in hepatic encephalopathy patients. EMHJ-East. Mediterr. Health J. 2007, 13, 1114–1123. [Google Scholar] [CrossRef]
- Wright, G.; Davies, N.A.; Shawcross, D.L.; Hodges, S.J.; Zwingmann, C.; Brooks, H.F.; Mani, A.R.; Harry, D.; Stadlbauer, V.; Zou, Z.; et al. Endotoxemia produces coma and brain swelling in bile duct ligated rats. Hepatology 2007, 45, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Cauli, O.; Llansola, M.; Agustí, A.; Rodrigo, R.; Hernández-Rabaza, V.; Rodrigues, T.B.; López-Larrubia, P.; Cerdán, S.; Felipo, V. Cerebral oedema is not responsible for motor or cognitive deficits in rats with hepatic encephalopathy. Liver Int. 2014, 34, 379–387. [Google Scholar] [CrossRef]
- Kale, R.A.; Gupta, R.K.; Saraswat, V.A.; Hasan, K.M.; Trivedi, R.; Mishra, A.M.; Ranjan, P.; Pandey, C.M.; Narayana, P.A. Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology 2006, 43, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, J.; Sanpedro, F.; Alonso, J.; Rovira, A. 1H Magnetic Resonance in the Study of Hepatic Encephalopathy in Humans. Metab. Brain Dis. 2002, 17, 415–429. [Google Scholar] [CrossRef]
- Rai, R.; Ahuja, C.K.; Agrawal, S.; Kalra, N.; Duseja, A.; Khandelwal, N.; Chawla, Y.; Dhiman, R.K. Reversal of low-grade cerebral edema after lactulose/rifaximin therapy in patients with cirrhosis and minimal hepatic encephalopathy. Clin. Transl. Gastroenterol. 2015, 6, e111. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.; McMillin, M.; Frampton, G.; Petrescu, A.D.; Williams, E.; Jaeger, V.; Kain, J.; DeMorrow, S. Direct Comparison of the Thioacetamide and Azoxymethane Models of Type A Hepatic Encephalopathy in Mice. Gene Expr. 2018, 18, 171–185. [Google Scholar] [CrossRef]
- Mardini, H.; Smith, F.E.; Record, C.O.; Blamire, A.M. Magnetic resonance quantification of water and metabolites in the brain of cirrhotics following induced hyperammonaemia. J. Hepatol. 2011, 54, 1154–1160. [Google Scholar] [CrossRef]
- Häussinger, D.; Kircheis, G.; Fischer, R.; Schliess, F.; vom Dahl, S. Hepatic encephalopathy in chronic liver disease: A clinical manifestation of astrocyte swelling and low-grade cerebral edema? J. Hepatol. 2000, 32, 1035–1038. [Google Scholar] [CrossRef]
- Martinez, A. Electron microscopy in human hepatic encephalopathy. Acta Neuropathol. 1968, 11, 82–86. [Google Scholar] [CrossRef]
- Jiang, W.; Desjardins, P.; Butterworth, R.F. Hypothermia attenuates oxidative/nitrosative stress, encephalopathy and brain edema in acute (ischemic) liver failure. Neurochem. Int. 2009, 55, 124–128. [Google Scholar] [CrossRef]
- Jayakumar, A.R.; Tong, X.Y.; Curtis, K.M.; Ruiz-Cordero, R.; Abreu, M.T.; Norenberg, M.D. Increased toll-like receptor 4 in cerebral endothelial cells contributes to the astrocyte swelling and brain edema in acute hepatic encephalopathy. J Neurochem. 2014, 128, 890–903. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Hughes, R.D.; Keays, R.T.; Williams, R. Electron microscopic study of brain capillaries in cerebral edema from fulminant hepatic failure. Hepatology 1992, 15, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Sepehrinezhad, A.; Shahbazi, A.; Sahab Negah, S.; Joghataei, M.T.; Larsen, F.S. Drug-induced-acute liver failure: A critical appraisal of the thioacetamide model for the study of hepatic encephalopathy. Toxicol. Rep. 2021, 8, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.P.Q.; Perreau, V.M.; Shultz, S.R.; Brady, R.D.; Lei, E.; Dixit, S.; Taylor, J.M.; Beart, P.M.; Boon, W.C. Inflammation in Traumatic Brain Injury: Roles for Toxic A1 Astrocytes and Microglial–Astrocytic Crosstalk. Neurochem. Res. 2019, 44, 1410–1424. [Google Scholar] [CrossRef]
- Zha, Z.; Liu, Y.-J.; Liu, S.-S.; Zhang, N.; Li, J.-L.; Qi, F.; Jin, L.-Y.; Xue, B.; Yang, T.; Fan, Y.-P.; et al. Bu Shen Yi Sui capsule promotes myelin repair by modulating the transformation of A1/A2 reactive astrocytes In Vivo and In Vitro. Oxidative Med. Cell. Longev. 2022, 2022, 3800004. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, C.; Shi, R.; Zhou, S.; Shan, H.; Deng, L.; Chen, T.; Guo, Y.; Zhang, Z.; Yang, G.Y.; et al. Blocking C3d(+)/GFAP(+) A1 Astrocyte Conversion with Semaglutide Attenuates Blood-Brain Barrier Disruption in Mice after Ischemic Stroke. Aging Dis. 2022, 13, 943–959. [Google Scholar] [CrossRef]
- Song, N.; Zhu, H.; Xu, R.; Liu, J.; Fang, Y.; Zhang, J.; Ding, J.; Hu, G.; Lu, M. Induced Expression of kir6.2 in A1 Astrocytes Propagates Inflammatory Neurodegeneration via Drp1-dependent Mitochondrial Fission. Front. Pharmacol. 2021, 11, 618992. [Google Scholar] [CrossRef]
- Manley, G.T.; Binder, D.K.; Papadopoulos, M.C.; Verkman, A.S. New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice. Neuroscience 2004, 129, 983–991. [Google Scholar] [CrossRef]
- Yi, M.-H.; Lee, Y.S.; Kang, J.W.; Kim, S.J.; Oh, S.-H.; Kim, Y.M.; Lee, Y.H.; Lee, S.D.; Kim, D.W. NFAT5-Dependent Expression of AQP4 in Astrocytes. Cell. Mol. Neurobiol. 2013, 33, 223–232. [Google Scholar] [CrossRef]
- Stokum, J.A.; Mehta, R.I.; Ivanova, S.; Yu, E.; Gerzanich, V.; Simard, J.M. Heterogeneity of aquaporin-4 localization and expression after focal cerebral ischemia underlies differences in white versus grey matter swelling. Acta Neuropathol. Commun. 2015, 3, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-A.; Li, P.; Ye, S.-Y.; Ning, Y.-L.; Wang, H.; Peng, Y.; Yang, N.; Zhao, Y.; Zhang, Z.-H.; Chen, J.-F.; et al. Perivascular AQP4 dysregulation in the hippocampal CA1 area after traumatic brain injury is alleviated by adenosine A2A receptor inactivation. Sci. Rep. 2017, 7, 2254. [Google Scholar] [CrossRef] [PubMed]
- Binder, D.K. Astrocytes: Stars of the Sacred Disease. Epilepsy Curr. 2018, 18, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Potokar, M.; Stenovec, M.; Jorgačevski, J.; Holen, T.; Kreft, M.; Ottersen, O.P.; Zorec, R. Regulation of AQP4 surface expression via vesicle mobility in astrocytes. Glia 2013, 61, 917–928. [Google Scholar] [CrossRef]
- Xu, Z.; Xiao, N.; Chen, Y.; Huang, H.; Marshall, C.; Gao, J.; Cai, Z.; Wu, T.; Hu, G.; Xiao, M. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol. Neurodegener. 2015, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Wang, M.X.; Ismail, O.; Braun, M.; Schindler, A.G.; Reemmer, J.; Wang, Z.; Haveliwala, M.A.; O’Boyle, R.P.; Han, W.Y.; et al. Loss of perivascular aquaporin-4 localization impairs glymphatic exchange and promotes amyloid β plaque formation in mice. Alzheimer’s Res. Ther. 2022, 14, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Sun, L.; Zhang, Y.; Wang, A.; Yan, J. Aquaporin4 Knockout Aggravates Early Brain Injury Following Subarachnoid Hemorrhage Through Impairment of the Glymphatic System in Rat Brain. Acta Neurochir. Suppl. 2020, 127, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, Y.; Zou, Y.; Pang, Y.; He, X.; Chen, Y.; Liu, Y.; Feng, W.; Zhang, Y.; Li, Q.; et al. Locomotor Hyperactivity in the Early-Stage Alzheimer’s Disease-like Pathology of APP/PS1 Mice: Associated with Impaired Polarization of Astrocyte Aquaporin 4. Aging Dis. 2022, 13, 1504–1522. [Google Scholar] [CrossRef]
- Kamagata, K.; Andica, C.; Takabayashi, K.; Saito, Y.; Taoka, T.; Nozaki, H.; Kikuta, J.; Fujita, S.; Hagiwara, A.; Kamiya, K. Association of MRI indices of glymphatic system with amyloid deposition and cognition in mild cognitive impairment and Alzheimer disease. Neurology 2022, 99, e2648–e2660. [Google Scholar] [CrossRef]
- Taoka, T.; Masutani, Y.; Kawai, H.; Nakane, T.; Matsuoka, K.; Yasuno, F.; Kishimoto, T.; Naganawa, S. Evaluation of glymphatic system activity with the diffusion MR technique: Diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn. J. Radiol. 2017, 35, 172–178. [Google Scholar] [CrossRef]
- Zeppenfeld, D.M.; Simon, M.; Haswell, J.D.; D’Abreo, D.; Murchison, C.; Quinn, J.F.; Grafe, M.R.; Woltjer, R.L.; Kaye, J.; Iliff, J.J. Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol. 2017, 74, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Iliff, J.J.; Yang, L.; Yang, J.; Chen, X.; Chen, M.J.; Giese, R.N.; Wang, B.; Shi, X.; Nedergaard, M. ‘Hit & Run’ model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J. Cereb. Blood Flow Metab. 2013, 33, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhan, Y.; Ai, L.; Chen, H.; Chen, J. AQP4-siRNA alleviates traumatic brain edema by altering post-traumatic AQP4 polarity reversal in TBI rats. J. Clin. Neurosci. 2020, 81, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; Wright, D.K.; Yamakawa, G.R.; Shultz, S.R.; Mychasiuk, R. Repetitive Mild Traumatic Brain Injury Alters Glymphatic Clearance Rates in Limbic Structures of Adolescent Female Rats. Sci. Rep. 2020, 10, 6254. [Google Scholar] [CrossRef] [PubMed]
- Banitalebi, S.; Skauli, N.; Geiseler, S.; Ottersen, O.P.; Amiry-Moghaddam, M. Disassembly and Mislocalization of AQP4 in Incipient Scar Formation after Experimental Stroke. Int. J. Mol. Sci. 2022, 23, 1117. [Google Scholar] [CrossRef] [PubMed]
- Migliati, E.R.; Amiry-Moghaddam, M.; Froehner, S.C.; Adams, M.E.; Ottersen, O.P.; Bhardwaj, A. Na+-K+-2Cl− cotransport inhibitor attenuates cerebral edema following experimental stroke via the perivascular pool of aquaporin-4. Neurocritical Care 2010, 13, 123–131. [Google Scholar] [CrossRef]
- Steiner, E.; Enzmann, G.U.; Lin, S.; Ghavampour, S.; Hannocks, M.J.; Zuber, B.; Rüegg, M.A.; Sorokin, L.; Engelhardt, B. Loss of astrocyte polarization upon transient focal brain ischemia as a possible mechanism to counteract early edema formation. Glia 2012, 60, 1646–1659. [Google Scholar] [CrossRef]
- Gaberel, T.; Gakuba, C.; Goulay, R.; Martinez De Lizarrondo, S.; Hanouz, J.L.; Emery, E.; Touze, E.; Vivien, D.; Gauberti, M. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: A new target for fibrinolysis? Stroke 2014, 45, 3092–3096. [Google Scholar] [CrossRef]
- García-Lezana, T.; Oria, M.; Romero-Giménez, J.; Bové, J.; Vila, M.; Genescà, J.; Chavarria, L.; Cordoba, J. Cerebellar neurodegeneration in a new rat model of episodic hepatic encephalopathy. J. Cereb. Blood Flow Metab. 2017, 37, 927–937. [Google Scholar] [CrossRef]
- Gelpi, E.; Rahimi, J.; Klotz, S.; Schmid, S.; Ricken, G.; Forcen-Vega, S.; Budka, H.; Kovacs, G.G. The autophagic marker p62 highlights Alzheimer type II astrocytes in metabolic/hepatic encephalopathy. Neuropathology 2020, 40, 358–366. [Google Scholar] [CrossRef]
- Shulyatnikova, T.; Shavrin, V. Mobilisation and redistribution of multivesicular bodies to the endfeet of reactive astrocytes in acute endogenous toxic encephalopathies. Brain Res. 2021, 1751, 147174. [Google Scholar] [CrossRef] [PubMed]
- Raviz, E.K.; Noormand, F.; Kermani, A.S.; Galedari, A.; Esmaeilpour, K.; Maneshian, M.; Kalantaripour, T.P.; Dabiri, S.; Asadi-Shekaari, M. Promising Effects of Naringenin and Melatonin against Hepatic Encephalopathy Impairments Induced by Bile Duct Ligation in Male Rats. Cent. Nerv. Syst. Agents Med. Chem. 2022, 22, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Shuliatnikova, T.; Tumanskyi, V. Expression of the water channel protein aquaporin-4 in the brain during human liver cirrhosis. Grail Sci. 2022, 22, 287–288. [Google Scholar] [CrossRef]
- Eslimi Esfahani, D.; Oryan, S.; Nabiuni, M.; Hosseinynia, T.S. The effects of bile duct ligation on motor cortex region morphology and aquaporin 4 protein concentration in male Wistar rats. Nova Biol. Reper. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- Bodega, G.; Suárez, I.; López-Fernández, L.A.; García, M.I.; Köber, M.; Penedo, M.; Luna, M.; Juárez, S.; Ciordia, S.; Oria, M.; et al. Ammonia induces aquaporin-4 rearrangement in the plasma membrane of cultured astrocytes. Neurochem. Int. 2012, 61, 1314–1324. [Google Scholar] [CrossRef]
- Lichter-Konecki, U.; Mangin, J.M.; Gordish-Dressman, H.; Hoffman, E.P.; Gallo, V. Gene expression profiling of astrocytes from hyperammonemic mice reveals altered pathways for water and potassium homeostasis in vivo. Glia 2008, 56, 365–377. [Google Scholar] [CrossRef]
- De Rui, M.; Schiff, S.; Aprile, D.; Angeli, P.; Bombonato, G.; Bolognesi, M.; Sacerdoti, D.; Gatta, A.; Merkel, C.; Amodio, P.; et al. Excessive daytime sleepiness and hepatic encephalopathy: It is worth asking. Metab. Brain Dis. 2013, 28, 245–248. [Google Scholar] [CrossRef]
- Velissaris, D.; Karamouzos, V.; Polychronopoulos, P.; Karanikolas, M. Chronotypology and melatonin alterations in minimal hepatic encephalopathy. J. Circadian Rhythm. 2009, 7, 6. [Google Scholar] [CrossRef]
- Lozeva, V.; Tuomisto, L.; Sola, D.; Plumed, C.; Hippeläinen, M.; Butterworth, R. Increased density of brain histamine H1 receptors in rats with portacaval anastomosis and in cirrhotic patients with chronic hepatic encephalopathy. Hepatology 2001, 33, 1370–1376. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Saeian, K.; Schubert, C.M.; Franco, R.; Franco, J.; Heuman, D.M. Disruption of sleep architecture in minimal hepatic encephalopathy and ghrelin secretion. Aliment Pharm. 2011, 34, 103–105. [Google Scholar] [CrossRef]
- Vora, R.S.; Subramanian, R.M. Hypotension in Cirrhosis. Clin. Liver Dis. 2019, 13, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Choi, D.; Lim, H.K.; Lim, J.H. Multiple infarcted regenerative nodules in liver cirrhosis after systemic hypotension due to septic shock: Radiologic findings. Abdom. Imaging 2004, 29, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Sourianarayanane, A.; Barnes, D.S.; McCullough, A.J. Beneficial effect of midodrine in hypotensive cirrhotic patients with refractory ascites. Gastroenterol Hepatol 2011, 7, 132–134. [Google Scholar]
- Lozeva, V.; Valjakka, A.; Lecklin, A.; Olkkonen, H.; Hippeläinen, M.; Itkonen, M.; Plumed, C.; Tuomisto, L. Effects of the histamine H(1) receptor blocker, pyrilamine, on spontaneous locomotor activity of rats with long-term portacaval anastomosis. Hepatology 2000, 31, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Ahabrach, H.; Piedrafita, B.; Ayad, A.; El Mlili, N.; Errami, M.; Felipo, V.; Llansola, M. Chronic hyperammonemia alters the circadian rhythms of corticosteroid hormone levels and of motor activity in rats. J. Neurosci. Res. 2010, 88, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Bomzon, A.; Binh, O.; Blendis, L.M. Hypotension in experimental cirrhosis: Is loss of vascular responsiveness to norepinephrine the cause of hypotension in chronic bile-duct-ligated dogs? J. Hepatol. 1993, 17, 116–123. [Google Scholar] [CrossRef]
- Bomzon, A.; Rosenberg, M.; Gali, D.; Binah, O.; Mordechovitz, D.; Better, O.S.; Greig, P.D.; Blendis, L.M. Systemic hypotension and decreased pressor response in dogs with chronic bile duct ligation. Hepatology 1986, 6, 595–600. [Google Scholar] [CrossRef]
- Clària, J.; Jiménez, W.; Ros, J.; Asbert, M.; Castro, A.; Arroyo, V.; Rivera, F.; Rodés, J. Pathogenesis of arterial hypotension in cirrhotic rats with ascites: Role of endogenous nitric oxide. Hepatology 1992, 15, 343–349. [Google Scholar] [CrossRef]
- Caracuel, L.; Sastre, E.; Callejo, M.; Rodrigues-Díez, R.; García-Redondo, A.B.; Prieto, I.; Nieto, C.; Salaices, M.; Aller, M.Á.; Arias, J.; et al. Hepatic Encephalopathy-Associated Cerebral Vasculopathy in Acute-on-Chronic Liver Failure: Alterations on Endothelial Factor Release and Influence on Cerebrovascular Function. Front. Physiol. 2020, 11, 593371. [Google Scholar] [CrossRef] [PubMed]
- Trewby, P.N.; Hanid, M.A.; Mackenzie, R.L.; Mellon, P.J.; Williams, R. Effects of cerebral oedema and arterial hypotension on cerebral blood flow in an animal model of hepatic failure. Gut 1978, 19, 999–1005. [Google Scholar] [CrossRef]
- Estrela, H.F.G.; Damásio, E.S.; Fonseca, E.K.U.N.; Bergamaschi, C.T.; Campos, R.R. Differential Sympathetic Vasomotor Activation Induced by Liver Cirrhosis in Rats. PLoS ONE 2016, 11, e0152512. [Google Scholar] [CrossRef] [PubMed]
- Rohde, J.; Pedersen, H.R.; Bjerring, P.N.; Larsen, F.S. Effects of Dexamethasone and Cox Inhibitors on Intracranial Pressure and Cerebral Perfusion in the Lipopolysaccharide Treated Rats with Hyperammonemia. PLoS ONE 2015, 10, e0117416. [Google Scholar] [CrossRef]
- Larsen, R.H.; Kjær, M.S.; Eefsen, M.; Larsen, F.S.; Bjerring, P.N. Ciclosporin does not attenuate intracranial hypertension in rats with acute hyperammonaemia. World J. Hepatol. 2013, 5, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Master, S.; Gottstein, J.; Blei, A.T. Cerebral blood flow and the development of ammonia-induced brain edema in rats after portacaval anastomosis. Hepatology 1999, 30, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Cauli, O.; López–Larrubia, P.; Rodrigo, R.; Agusti, A.; Boix, J.; Nieto–Charques, L.; Cerdán, S.; Felipo, V. Brain Region-Selective Mechanisms Contribute to the Progression of Cerebral Alterations in Acute Liver Failure in Rats. Gastroenterology 2011, 140, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Wang, Y.-Y.; Chuang, Y.-H.; Chen, C.-J. Skeletal muscle proteolysis is associated with sympathetic activation and TNF-α-ubiquitin-proteasome pathway in liver cirrhotic rats. J. Gastroenterol. Hepatol. 2016, 31, 890–896. [Google Scholar] [CrossRef]
- Jia, W.; Liu, J.; Hu, R.; Hu, A.; Tang, W.; Li, L.; Li, J. Xiaochaihutang improves the cortical astrocyte edema in thioacetamide-induced rat acute hepatic encephalopathy by activating NRF2 pathway. Front. Pharmacol. 2020, 11, 382. [Google Scholar] [CrossRef]
- Chen, J.-R.; Wang, B.-N.; Tseng, G.-F.; Wang, Y.-J.; Huang, Y.-S.; Wang, T.-J. Morphological changes of cortical pyramidal neurons in hepatic encephalopathy. BMC Neurosci. 2014, 15, 1–12. [Google Scholar] [CrossRef]
- Ismail, F.S.; Faustmann, T.J.; Corvace, F.; Tsvetanova, A.; Moinfar, Z.; Faustmann, P.M. Ammonia induced microglia activation was associated with limited effects on connexin 43 and aquaporin 4 expression in an astrocyte-microglia co-culture model. BMC Neurosci. 2021, 22, 21. [Google Scholar] [CrossRef]
- Rama Rao, K.V.; Chen, M.; Simard, J.M.; Norenberg, M.D. Increased aquaporin-4 expression in ammonia-treated cultured astrocytes. Neuroreport 2003, 14, 2379–2382. [Google Scholar] [CrossRef]
- Pan, C.-F. Ammonia induces upregulation of aquaporin-4 in neocortical astrocytes of rats through the p38 mitogen-activated protein kinase pathway. Chin. Med. J. 2010, 123, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Stokum, J.A.; Kwon, M.S.; Woo, S.K.; Tsymbalyuk, O.; Vennekens, R.; Gerzanich, V.; Simard, J.M. SUR1-TRPM4 and AQP4 form a heteromultimeric complex that amplifies ion/water osmotic coupling and drives astrocyte swelling. Glia 2018, 66, 108–125. [Google Scholar] [CrossRef]
- Sinke, A.P.; Jayakumar, A.R.; Panickar, K.S.; Moriyama, M.; Reddy, P.V.B.; Norenberg, M.D. NFκB in the mechanism of ammonia-induced astrocyte swelling in culture. J. Neurochem. 2008, 106, 2302–2311. [Google Scholar] [CrossRef]
- Jayakumar, A.R.; Rama Rao, K.V.; Tong, X.Y.; Norenberg, M.D. Calcium in the mechanism of ammonia-induced astrocyte swelling. J. Neurochem. 2009, 109, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, R.; Görg, B.; Becker, S.; Qvartskhava, N.; Bidmon, H.J.; Selbach, O.; Haas, H.L.; Schliess, F.; Häussinger, D. Hypoosmotic swelling and ammonia increase oxidative stress by NADPH oxidase in cultured astrocytes and vital brain slices. Glia 2007, 55, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Felipo, V.; Urios, A.; Montesinos, E.; Molina, I.; Garcia-Torres, M.L.; Civera, M.; Olmo, J.A.; Ortega, J.; Martinez-Valls, J.; Serra, M.A.; et al. Contribution of hyperammonemia and inflammatory factors to cognitive impairment in minimal hepatic encephalopathy. Metab. Brain Dis. 2012, 27, 51–58. [Google Scholar] [CrossRef] [PubMed]

| Studies | Affected Factor | Key Results | References |
|---|---|---|---|
| Clinical human cases | Sleep disorders | Sleep disturbances are defined as common features of liver diseases and indicators of subclinical HE in cirrhosis, evaluated by Pittsburgh Sleep Quality Index, Epworth Sleepiness Scale, and polysomnography. | [48,50,53,188,189,190,191] |
| Arterial hypotension | Portosystemic shunt and accumulation of vasodilator mediators in systemic circulation, shock, decrease systemic vascular resistance, and blood pressure in HE and cirrhosis. | [70,71,73,74,192,193,194] | |
| Impaired cerebral blood flow | Decrease in the cerebral perfusion pressure, intracranial hypertension, and impairment of CBF following liver failure and HE. | [83,84,85,86,87,88,89,90,91,92,93,94] | |
| Altered autonomic nervous system | General imbalance in ANS tone, sympathetic tone hyperactivity, increase in plasma levels of norepinephrine and inhibition of vagal tone in cirrhosis, chronic liver disease and HE. | [98,99,100,101,102,103,104,105] | |
| Astrocyte dysfunction | Decrease in GFAP expression in the frontal cortex and basal ganglia in postmortem analysis of HE patients, solutes accumulation in astrocytes, brain edema, and astrocyte swelling in patients with fulminant hepatic failure. | [114,144,150,153] | |
| Dysregulation of AQP4 | Postmortem brain analysis of cirrhosis patients indicated an increase in the expression of AQP4 in cell body. | [184] | |
| In Vivo | Sleep disorders | Paradoxical sleep, disruption of sleep patterns, suppression in the duration of rapid eye movement sleep, non-rapid eye movement sleep, and alternation of circadian rhythms in rat models of HE. | [54,55,190,195,196] |
| Cardiomyopathy | Cardiac hypertrophy, myocyte swelling, gross abnormalities in cardiomyocytes in histological examinations, increase plasma levels of myocardial enzymes, systolic and diastolic abnormalities, inotropic and chronotropic dysfunction, and QT interval prolongation in rat models of HE and cirrhosis. | [66,67,68,69] | |
| Arterial hypotension | Decrease in the systemic arterial blood pressure in animal models of HE and liver failure. | [75,197,198,199,200,201,202] | |
| Impaired cerebral blood flow | Raising intracranial pressure, cerebral hyperemia, vasogenic edema, and impairment of CBF in hyperammonemic HE rats. | [82,201,203,204,205,206] | |
| Altered autonomic nervous system | Sympathetic tone hyperactivity and increase in circulatory norepinephrine in rats model of cirrhosis and HE. | [106,107,108,202,207] | |
| Astrocyte dysfunction | Decrease in GFAP expression in substantia nigra, ventral tegmental area, hippocampus, sensorimotor cortex, thalamus, cerebral edema, astrogliosis, and astrocyte swelling in animal models of HE. | [115,116,117,118,142,208,209] | |
| Dysregulation of AQP4 | Decreased expression of AQP4 in olfactory bulb and prefrontal cortex accompanied with glymphatic impairment in BDL rats. | [22] | |
| Meningeal lymphatic drainage | Improvement in the severity of HE in an experimental rat model following enhanced meningeal lymphatic drainage (requires further experiments) | [46] | |
| In Vitro | Astrocyte dysfunction | Cell swelling and AQP4 rearrangement in ammonia-exposed astrocytes. | [186,210,211,212,213,214,215,216] |
| Dysregulation of AQP4 | Decreased AQP4 expression in astrocytes isolated from hyperammonemic mice and AQP4 rearrangement in ammonia-exposed astrocytes. | [187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepehrinezhad, A.; Stolze Larsen, F.; Ashayeri Ahmadabad, R.; Shahbazi, A.; Sahab Negah, S. The Glymphatic System May Play a Vital Role in the Pathogenesis of Hepatic Encephalopathy: A Narrative Review. Cells 2023, 12, 979. https://doi.org/10.3390/cells12070979
Sepehrinezhad A, Stolze Larsen F, Ashayeri Ahmadabad R, Shahbazi A, Sahab Negah S. The Glymphatic System May Play a Vital Role in the Pathogenesis of Hepatic Encephalopathy: A Narrative Review. Cells. 2023; 12(7):979. https://doi.org/10.3390/cells12070979
Chicago/Turabian StyleSepehrinezhad, Ali, Fin Stolze Larsen, Rezan Ashayeri Ahmadabad, Ali Shahbazi, and Sajad Sahab Negah. 2023. "The Glymphatic System May Play a Vital Role in the Pathogenesis of Hepatic Encephalopathy: A Narrative Review" Cells 12, no. 7: 979. https://doi.org/10.3390/cells12070979
APA StyleSepehrinezhad, A., Stolze Larsen, F., Ashayeri Ahmadabad, R., Shahbazi, A., & Sahab Negah, S. (2023). The Glymphatic System May Play a Vital Role in the Pathogenesis of Hepatic Encephalopathy: A Narrative Review. Cells, 12(7), 979. https://doi.org/10.3390/cells12070979







