Advanced 3D Models of Human Brain Tissue Using Neural Cell Lines: State-of-the-Art and Future Prospects
Abstract
:1. Introduction
2. Search Methodology
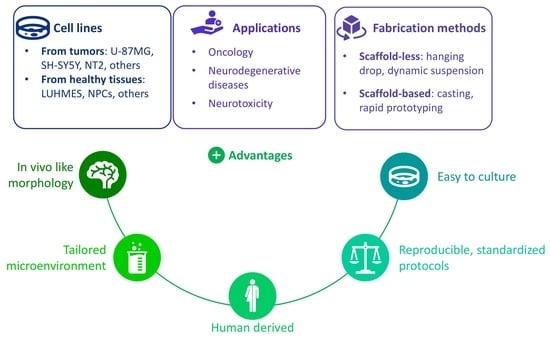
3. Cell Lines in 3D Culture
3.1. Cancer Cell Lines
3.1.1. Glioblastoma Cell Lines
3.1.2. Neuroblastoma Cell Lines
3.1.3. Other Cancer Cell Lines
3.2. Cell Lines Derived from Healthy Tissues
4. Discussion
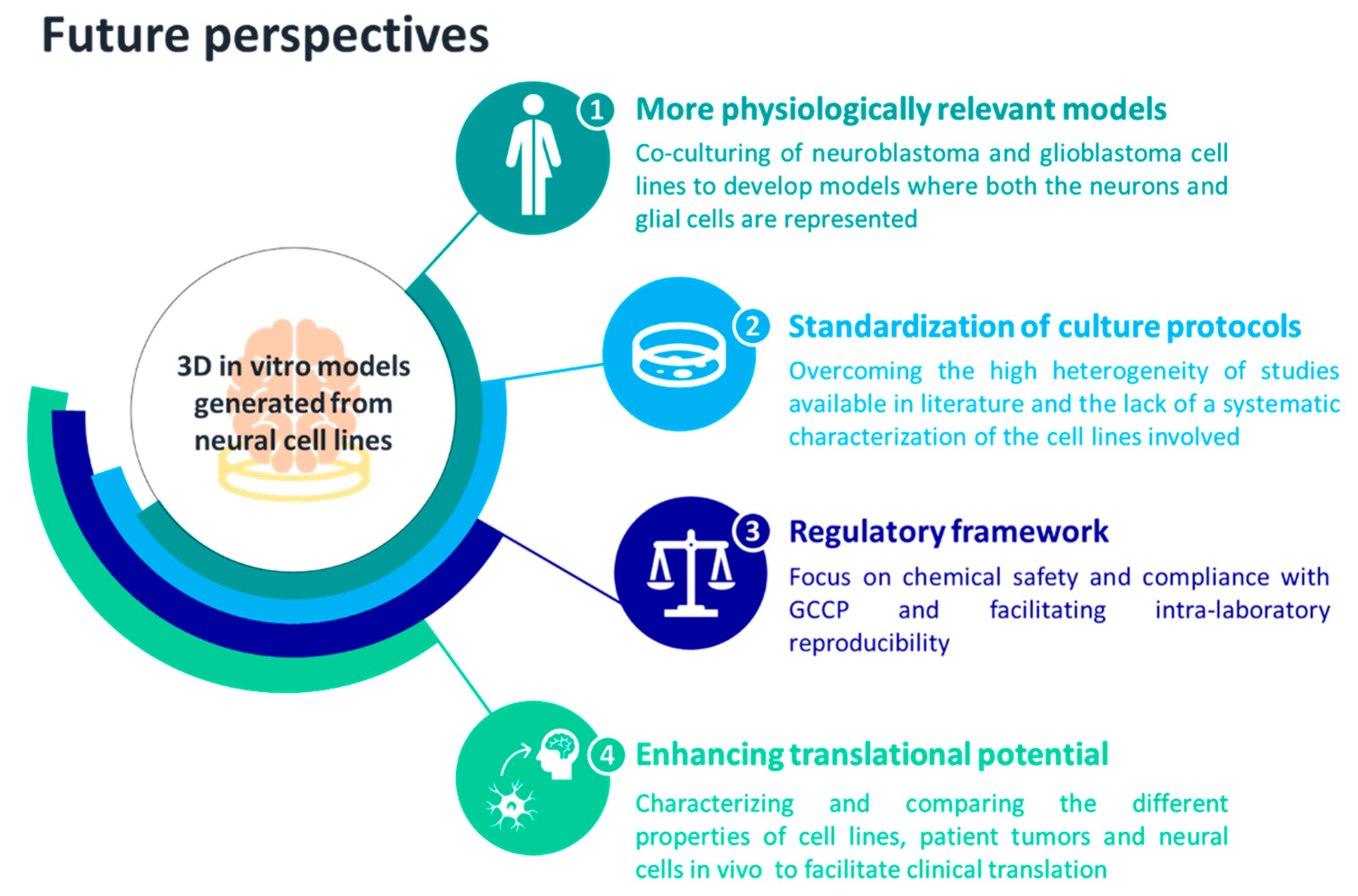
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Tsilidis, K.K.; Panagiotou, O.A.; Sena, E.S.; Aretouli, E.; Evangelou, E. Evaluation of Excess Significance Bias in Animal Studies of Neurological Diseases. PLoS Biol. 2013, 11, 1001609. [Google Scholar] [CrossRef] [PubMed]
- Premack, D. Human and Animal Cognition: Continuity and Discontinuity. Proc. Natl. Acad. Sci. USA 2007, 104, 13861–13867. [Google Scholar] [CrossRef] [PubMed]
- Courtine, G.; Bunge, M.B.; Fawcett, J.W.; Grossman, R.G.; Kaas, J.H.; Lemon, R.; Maier, I.; Martin, J.; Nudo, R.J.; Ramon-Cueto, A.; et al. Can Experiments in Nonhuman Primates Expedite the Translation of Treatments for Spinal Cord Injury in Humans? Nat. Med. 2007, 13, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Lemon, R.N. Descending Pathways in Motor Control. Annu. Rev. Neurosci. 2008, 31, 195–218. [Google Scholar] [CrossRef]
- Mattes, W.B. In Vitro to in Vivo Translation. Curr. Opin. Toxicol. 2020, 23–24, 114–118. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Koh, I.; Kim, P. In Vitro Reconstruction of Brain Tumor Microenvironment. Rev. Artic. BioChip J. 2019, 13, 1–7. [Google Scholar] [CrossRef]
- Bal-Price, A.K.; Hogberg, H.T.; Buzanska, L.; Coecke, S. Relevance of in Vitro Neurotoxicity Testing for Regulatory Requirements: Challenges to Be Considered. Neurotoxicol Teratol. 2008, 32, 36–41. [Google Scholar] [CrossRef]
- Slanzi, A.; Iannoto, G.; Rossi, B.; Zenaro, E.; Constantin, G. In Vitro Models of Neurodegenerative Diseases. Front. Cell. Dev. Biol. 2020, 8, 328. [Google Scholar] [CrossRef]
- da Silva Siqueira, L.; Majolo, F.; da Silva, A.P.B.; da Costa, J.C.; Marinowic, D.R. Neurospheres: A Potential in Vitro Model for the Study of Central Nervous System Disorders. Mol. Biol. Rep. 2021, 48, 3649–3663. [Google Scholar] [CrossRef]
- Calissano, P.; Matrone, C.; Amadoro, G. Apoptosis and in Vitro Alzheimer Disease Neuronal Models. Commun. Integr. Biol. 2009, 2, 163–169. [Google Scholar] [CrossRef]
- Nikolakopoulou, P.; Rauti, R.; Voulgaris, D.; Shlomy, I.; Maoz, B.M.; Herland, A. Recent Progress in Translational Engineered in Vitro Models of the Central Nervous System. Brain 2020, 143, 3181–3213. [Google Scholar] [CrossRef]
- van Veen, E.; van der Jagt, M.; Cnossen, M.C.; Maas, A.I.R.; de Beaufort, I.D.; Menon, D.K.; Citerio, G.; Stocchetti, N.; Rietdijk, W.J.R.; van Dijck, J.T.J.M.; et al. Brain Death and Postmortem Organ Donation: Report of a Questionnaire from the CENTER-TBI Study. Crit. Care 2018, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.; Amini, S. General Overview of Neuronal Cell Culture. Methods Mol. Biol. 2021, 2311, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zahumenska, R.; Nosal, V.; Smolar, M.; Okajcekova, T.; Skovierova, H.; Strnadel, J.; Halasova, E. Induced Pluripotency: A Powerful Tool for In Vitro Modeling. Int. J. Mol. Sci. 2020, 21, 8910. [Google Scholar] [CrossRef] [PubMed]
- Farahany, N.A.; Greely, H.T.; Hyman, S.; Koch, C.; Grady, C.; Pasca, S.P.; Sestan, N.; Arlotta, P.; Bernat, J.L.; Ting, J.; et al. The Ethics of Experimenting with Human Brain Tissue. Nature 2018, 556, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Dunnett, S.B.; Rosser, A.E. Challenges for Taking Primary and Stem Cells into Clinical Neurotransplantation Trials for Neurodegenerative Disease. Neurobiol. Dis. 2014, 61, 79–89. [Google Scholar] [CrossRef]
- Cullen, D.K.; Pfister, B. State of the Art and Future Challenges in Neural Engineering: Neural Interfaces: Foreword/Editors’ Commentary (Volume 1). Crit. Rev. Biomed. Eng. 2011, 39, 1–3. [Google Scholar] [CrossRef]
- Wagenaar, D.A.; Madhavan, R.; Pine, J.; Potter, S.M. Controlling Bursting in Cortical Cultures with Closed-Loop Multi-Electrode Stimulation. J. Neurosci. 2005, 25, 680–688. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesisin a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Monzel, A.S.; Smits, L.M.; Hemmer, K.; Hachi, S.; Moreno, E.L.; van Wuellen, T.; Jarazo, J.; Walter, J.; Brüggemann, I.; Boussaad, I.; et al. Derivation of Human Midbrain-Specific Organoids from Neuroepithelial Stem Cells. Stem Cell. Rep. 2017, 8, 1144–1154. [Google Scholar] [CrossRef]
- Quadrato, G.; Nguyen, T.; Macosko, E.Z.; Sherwood, J.L.; Yang, S.M.; Berger, D.R.; Maria, N.; Scholvin, J.; Goldman, M.; Kinney, J.P.; et al. Cell Diversity and Network Dynamics in Photosensitive Human Brain Organoids. Nature 2017, 545, 48–53. [Google Scholar] [CrossRef]
- Poli, D.; Magliaro, C.; Ahluwalia, A. Experimental and Computational Methods for the Study of Cerebral Organoids: A Review. Front. Neurosci. 2019, 13, 162. [Google Scholar] [CrossRef]
- Berger, E.; Magliaro, C.; Paczia, N.; Monzel, A.S.; Antony, P.; Linster, C.L.; Bolognin, S.; Ahluwalia, A.; Schwamborn, J.C. Millifluidic Culture Improves Human Midbrain Organoid Vitality and Differentiation. Lab. Chip 2018, 18, 3172–3183. [Google Scholar] [CrossRef]
- McMurtrey, R.J. Analytic Models of Oxygen and Nutrient Diffusion, Metabolism Dynamics, and Architecture Optimization in Three-Dimensional Tissue Constructs with Applications and Insights in Cerebral Organoids. Tissue Eng. Part. C Methods 2016, 22, 221–249. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Alifieris, C.; Trafalis, D.T. Glioblastoma Multiforme: Pathogenesis and Treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef]
- Pontén, J.; Macintyre, E.H. Long Term Culture of Normal and Neoplastic Human Glia. Acta Pathol. Microbiol. Scand. 1968, 74, 465–486. [Google Scholar] [CrossRef]
- Bigner, D.D.; Bigner, S.H.; Pontén, J.; Westermark, B.; Mahaley, M.S.; Ruoslahti, E.; Herschman, H.; Eng, L.F.; Wlkstrand, C.J. Heterogeneity of Genotypic and Phenotypic Characteristics of Fifteen Permanent Cell Lines Derived from Human Gliomas. J. Neuropathol. Exp. Neurol. 1981, 40, 201–229. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Kum, W.; Cockram, C.S.; Teoh, R.; Young, J.D. Functional Substance P Receptors on a Human Astrocytoma Cell Line (U-373 MG). Brain Res. 1989, 488, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.H. T98G: An Anchorage-Independent Human Tumor Cell Line That Exhibits Stationary Phase G1 Arrest in Vitro. J. Cell. Physiol. 1979, 99, 43–54. [Google Scholar] [CrossRef]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In Vitro Cultivation of Human Tumors: Establishment of Cell Lines Derived From a Series of Solid Tumors. JNCI J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Yahyanejad, S.; Van Hoof, S.J.; Theys, J.; Barbeau, L.M.O.; Granton, P.V.; Paesmans, K.; Verhaegen, F.; Vooijs, M. An Image Guided Small Animal Radiation Therapy Platform (SmART) to Monitor Glioblastoma Progression and Therapy Response. Radiother. Oncol. 2015, 116, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Yahyanejad, S.; King, H.; Iglesias, V.S.; Granton, P.V.; Barbeau, L.M.O.; van Hoof, S.J.; Groot, A.J.; Habets, R.; Prickaerts, J.; Chalmers, A.J.; et al. NOTCH Blockade Combined with Radiation Therapy and Temozolomide Prolongs Survival of Orthotopic Glioblastoma. Oncotarget 2016, 7, 41251. [Google Scholar] [CrossRef]
- Morimoto, T.; Nakazawa, T.; Matsuda, R.; Nishimura, F.; Nakamura, M.; Yamada, S.; Nakagawa, I.; Park, Y.S.; Tsujimura, T.; Nakase, H. Evaluation of Comprehensive Gene Expression and Nk Cellmediated Killing in Glioblastoma Cell Line-Derived Spheroids. Cancers 2021, 13, 4896. [Google Scholar] [CrossRef]
- Stein, A.M.; Demuth, T.; Mobley, D.; Berens, M.; Sander, L.M. A Mathematical Model of Glioblastoma Tumor Spheroid Invasion in a Three-Dimensional In Vitro Experiment. Biophys. J. 2007, 92, 356–365. [Google Scholar] [CrossRef]
- Wang, C.; Tong, X.; Yang, F. Bioengineered 3D Brain Tumor Model to Elucidate the Effects of Matrix Stiffness on Glioblastoma Cell Behavior Using Peg-Based Hydrogels. Mol. Pharm. 2014, 11, 2115–2125. [Google Scholar] [CrossRef]
- Hermida, M.A.; Kumar, J.D.; Schwarz, D.; Laverty, K.G.; Di Bartolo, A.; Ardron, M.; Bogomolnijs, M.; Clavreul, A.; Brennan, P.M.; Wiegand, U.K.; et al. Three Dimensional in Vitro Models of Cancer: Bioprinting Multilineage Glioblastoma Models. Adv. Biol. Regul. 2020, 75, 100658. [Google Scholar] [CrossRef]
- Marino, A.; Tricinci, O.; Battaglini, M.; Filippeschi, C.; Mattoli, V.; Sinibaldi, E.; Ciofani, G.; Marino, B.A.; Battaglini, M.; Ciofani, G.; et al. A 3D Real-Scale, Biomimetic, and Biohybrid Model of the Blood-Brain Barrier Fabricated through Two-Photon Lithography. Small 2018, 14, 1702959. [Google Scholar] [CrossRef]
- Lee, C.; Abelseth, E.; de la Vega, L.; Willerth, S.M. Bioprinting a Novel Glioblastoma Tumor Model Using a Fibrin-Based Bioink for Drug Screening. Mater. Today Chem. 2019, 12, 78–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.X.; Ma, J.W.; Li, H.Y.; Ye, J.C.; Xie, S.M.; Du, B.; Zhong, X.Y. EGCG Inhibits Properties of Glioma Stem-like Cells and Synergizes with Temozolomide through Downregulation of P-Glycoprotein Inhibition. J. Neurooncol. 2015, 121, 41–52. [Google Scholar] [CrossRef]
- Calori, I.R.; Alves, S.R.; Bi, H.; Tedesco, A.C. Type-I Collagen/Collagenase Modulates the 3D Structure and Behavior of Glioblastoma Spheroid Models. ACS Appl. Bio. Mater. 2022, 5, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, K.; Hara, J.; Ito, C.E.; Asuri, P. Role of Three-Dimensional Matrix Stiffness in Regulating the Response of Human Neural Cells to Toxins. Cell. Mol. Bioeng. 2014, 7, 278–284. [Google Scholar] [CrossRef]
- Chung, C.; Boterberg, T.; Lucas, J.; Panoff, J.; Valteau-Couanet, D.; Hero, B.; Bagatell, R.; Hill-Kayser, C.E. Neuroblastoma. Pediatr. Blood Cancer 2021, 68, e28473. [Google Scholar] [CrossRef]
- Maris, J.M. Recent Advances in Neuroblastoma. NIH Public Acess 2012, 23, 1–7. [Google Scholar] [CrossRef]
- Biedler, J.L.; Helson, L.; Spengler, B.A. Morphology and Growth, Tumorigenicity, and Cytogenetics of Human Neuroblastoma Cells in Continuous Culture. Cancer Res. 1973, 33, 2643–2652. [Google Scholar]
- Tumilowicz, J.J.; Nichols, W.W.; Cholon, J.J.; Greene Departments, A.E.; Biology, C. Definition of a Continuous Human Cell Line Derived from Neuroblastoma1. Cancer Res. 1970, 30, 2110–2118. [Google Scholar]
- Matsushima, H.; Bogenmann, E. BI-Modal Differentiation Pattern in a New Human Neuroblastoma Cell Line in Vitro. Int. J. Cancer 1992, 51, 250–258. [Google Scholar] [CrossRef]
- Gilbert, F.; Balaban, G.; Moorhead, P.; Bianchi, D.; Schlesinger, H. Abnormalities of Chromosome 1p in Human Neuroblastoma Tumors and Cell Lines. Cancer Genet. Cytogenet. 1982, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.F.; Cui, L.; Jin, M.M.; Hu, D.Y.; Hou, X.G.; Liu, S.S.; Zhang, X.; Zhu, J.H. A Matrigel-Based 3D Construct of SH-SY5Y Cells Models the α-Synuclein Pathologies of Parkinson’s Disease. DMM Dis. Model. Mech. 2022, 15. [Google Scholar] [CrossRef]
- Taylor-Whiteley, T.R.; Le Maitre, C.L.; Duce, J.A.; Dalton, C.F.; Smith, D.P. Recapitulating Parkinson’s Disease Pathology in a Three-Dimensional Human Neural Cell Culture Model. DMM Dis. Model. Mech. 2019, 12, dmm038042. [Google Scholar] [CrossRef]
- Domert, J.; Sackmann, C.; Agholme, L.; Bergström, J.; Ingelsson, M.; Hallbeck, M.; Severinsson, E. Aggregated Alpha-Synuclein Transfer Efficiently between Cultured Human Neuron- like Cells and Localize to Lysosomes. PLoS ONE 2016, 11, e0168700. [Google Scholar] [CrossRef] [PubMed]
- Fiore, N.J.; Tamer-Mahoney, J.D.; Beheshti, A.; Nieland, T.J.F.; Kaplan, D.L. 3D Biocomposite Culture Enhances Differentiation of Dopamine-like Neurons from SH-SY5Y Cells: A Model for Studying Parkinson’s Disease Phenotypes. Biomaterials 2022, 290, 121858. [Google Scholar] [CrossRef] [PubMed]
- Chemmarappally, J.M.; Pegram, H.C.N.; Abeywickrama, N.; Fornari, E.; Hargreaves, A.J.; De Girolamo, L.A.; Stevens, B. A Co-Culture Nanofibre Scaffold Model of Neural Cell Degeneration in Relevance to Parkinson’s Disease. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Krinke, D.; Jahnke, H.G.; Mack, T.G.A.; Hirche, A.; Striggow, F.; Robitzki, A.A. A Novel Organotypic Tauopathy Model on a New Microcavity Chip for Bioelectronic Label-Free and Real Time Monitoring. Biosens. Bioelectron. 2010, 26, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Seidel, D.; Krinke, D.; Jahnke, H.G.; Hirche, A.; Kloß, D.; Mack, T.G.A.; Striggow, F.; Robitzki, A. Induced Tauopathy in a Novel 3D-Culture Model Mediates Neurodegenerative Processes: A Real-Time Study on Biochips. PLoS ONE 2012, 7, e49150. [Google Scholar] [CrossRef] [PubMed]
- Fantini, V.; Bordoni, M.; Scocozza, F.; Conti, M.; Scarian, E.; Carelli, S.; Di Giulio, A.M.; Marconi, S.; Pansarasa, O.; Auricchio, F.; et al. Bioink Composition and Printing Parameters for 3D Modeling Neural Tissue. Cells 2019, 8, 830. [Google Scholar] [CrossRef]
- Dominguez-Alfaro, A.; Alegret, N.; Arnaiz, B.; Salsamendi, M.; Mecerreyes, D.; Prato, M. Toward Spontaneous Neuronal Differentiation of SH-SY5Y Cells Using Novel Three-Dimensional Electropolymerized Conductive Scaffolds. ACS Appl. Mater. Interfaces 2020, 12, 57330–57342. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Nan, F.; Xiao, J.; Lian, J.C.; He, X.; Guo, X.; Sun, G.W.; Ma, X.J. Microcapsule Co-Culture System Enhances Neural Differentiation of Mesenchymal Stem Cells. J. Hard Tissue Biol. 2013, 22, 241–248. [Google Scholar] [CrossRef]
- Marrazzo, P.; Angeloni, C.; Hrelia, S. Combined Treatment with Three Natural Antioxidants Enhances Neuroprotection in a SH-SY5Y 3D Culture Model. Antioxidants 2019, 8, 420. [Google Scholar] [CrossRef]
- Eleftheriadou, D.; Evans, R.E.; Atkinson, E.; Abdalla, A.; Gavins, F.K.H.; Boyd, A.S.; Williams, G.R.; Knowles, J.C.; Roberton, V.H.; Phillips, J.B. An Alginate-Based Encapsulation System for Delivery of Therapeutic Cells to the CNS. RSC Adv. 2022, 12, 4005–4015. [Google Scholar] [CrossRef]
- Bordoni, M.; Karabulut, E.; Kuzmenko, V.; Fantini, V.; Pansarasa, O.; Cereda, C.; Gatenholm, P. 3D Printed Conductive Nanocellulose Scaffolds for the Differentiation of Human Neuroblastoma Cells. Cells 2020, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Wang, X.; Chen, X.Z.; Mushtaq, F.; Deng, S.; Zhu, C.; Torlakcik, H.; Terzopoulou, A.; Qin, X.H.; Xiao, X.; et al. 3D-Printed Soft Magnetoelectric Microswimmers for Delivery and Differentiation of Neuron-Like Cells. Adv. Funct. Mater. 2020, 30, 1910323. [Google Scholar] [CrossRef]
- Liaudanskaya, V.; Migliaresi, C.; Motta, A. Homeostasis Maintenance of Encapsulated Cells. J. Tissue Eng. Regen. Med. 2018, 12, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Geetha Marapureddy, S.; Hivare, P.; Sharma, A.; Chakraborty, J.; Ghosh, S.; Gupta, S.; Thareja, P. Rheology and Direct Write Printing of Chitosan-Graphene Oxide Nanocomposite Hydrogels for Differentiation of Neuroblastoma Cells. Carbohydr. Polym. 2021, 269, 118254. [Google Scholar] [CrossRef]
- De Conto, V.; Cheung, V.; Maubon, G.; Souguir, Z.; Maubon, N.; Vandenhaute, E.; Bérézowski, V. In Vitro Differentiation Modifies the Neurotoxic Response of SH-SY5Y Cells. Toxicol. Vitr. 2021, 77, 105235. [Google Scholar] [CrossRef]
- Ko, K.R.; Tam, N.W.; Teixeira, A.G.; Frampton, J.P. SH-SY5Y and LUHMES Cells Display Differential Sensitivity to MPP+, Tunicamycin, and Epoxomicin in 2D and 3D Cell Culture. Biotechnol. Prog. 2020, 36, e2942. [Google Scholar] [CrossRef]
- Desai, A.; Kisaalita, W.S.; Keith, C.; Wu, Z.Z. Human Neuroblastoma (SH-SY5Y) Cell Culture and Differentiation in 3-D Collagen Hydrogels for Cell-Based Biosensing. Biosens. Bioelectron. 2006, 21, 1483–1492. [Google Scholar] [CrossRef]
- Gallagher, C.; Murphy, C.; Kelly, G.; O’brien, F.J.; Piskareva, O. Three-Dimensional In Vitro Biomimetic Model of Neuroblastoma Using Collagen-Based Scaffolds. J. Vis. Exp. 2021, 173, e62627. [Google Scholar] [CrossRef]
- Marrella, A.; Dondero, A.; Aiello, M.; Casu, B.; Olive, D.; Regis, S.; Bottino, C.; Pende, D.; Meazza, R.; Caluori, G.; et al. Cell-Laden Hydrogel as a Clinical-Relevant 3D Model for Analyzing Neuroblastoma Growth, Immunophenotype, and Susceptibility to Therapies. Front. Immunol. 2019, 10, 1876. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y Cell Line in Parkinson’s Disease Research: A Systematic Review. Mol. Neurodegener. 2017, 12, 1–11. [Google Scholar] [CrossRef]
- Whitworth, C.L.; Redfern, C.P.F.; Cheek, T.R. Differentiation-Induced Remodelling of Store-Operated Calcium Entry Is Independent of Neuronal or Glial Phenotype but Modulated by Cellular Context. Mol. Neurobiol. 2019, 56, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Patte-Mensah, C.; Meyer, L.; Schaeffer, V.; Eckert, A.; Mensah-Nyagan, A.G. Transfection of Human Neuroblastoma Cells with Alzheimer’s Disease Brain Hallmarks as a Promising Strategy to Investigate the Role of Neurosteroidogenesis in Neuroprotection. Genet. Modif. Org. Genet. Eng. Res. Ther. 2012, 3, 50–59. [Google Scholar] [CrossRef]
- Martin, E.R.; Gandawijaya, J.; Oguro-Ando, A. A Novel Method for Generating Glutamatergic SH-SY5Y Neuron-like Cells Utilizing B-27 Supplement. Front. Pharm. 2022, 13, 4042. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Suarez, L.; Awabdh, S.A.; Coumoul, X.; Chauvet, C. The SH-SY5Y Human Neuroblastoma Cell Line, a Relevant in Vitro Cell Model for Investigating Neurotoxicology in Human: Focus on Organic Pollutants. Neurotoxicology 2022, 92, 131–155. [Google Scholar] [CrossRef]
- Datta, P.K. Neuronal Cell Culture. Neuronal Cell. Cult. Methods Protoc. 2013, 1078, 35–44. [Google Scholar] [CrossRef]
- Riegerová, P.; Brejcha, J.; Bezděková, D.; Chum, T.; Mašínová, E.; Čermáková, N.; Ovsepian, S.V.; Cebecauer, M.; Štefl, M. Expression and Localization of AβPP in SH-SY5Y Cells Depends on Differentiation State. J. Alzheimer’s Dis. 2021, 82, 485. [Google Scholar] [CrossRef]
- Abolpour Mofrad, S.; Kuenzel, K.; Friedrich, O.; Gilbert, D.F. Optimizing Neuronal Differentiation of Human Pluripotent NT2 Stem Cells in Monolayer Cultures. Dev. Growth Differ. 2016, 58, 664–676. [Google Scholar] [CrossRef]
- Pleasure, S.J.; Lee, V.Y. NTera 2 Cells: A Human Cell Line Which Displays Characteristics Expected of a Human Committed Neuronal Progenitor Cell. J. Neurosci. Res. 1993, 35, 585–602. [Google Scholar] [CrossRef]
- Terrasso, A.P.; Pinto, C.; Serra, M.; Filipe, A.; Almeida, S.; Ferreira, A.L.; Pedroso, P.; Brito, C.; Alves, P.M. Novel Scalable 3D Cell Based Model for in Vitro Neurotoxicity Testing: Combining Human Differentiated Neurospheres with Gene Expression and Functional Endpoints. J. Biotechnol. 2015, 205, 82–92. [Google Scholar] [CrossRef]
- Xu, T.; Gregory, C.A.; Molnar, P.; Cui, X.; Jalota, S.; Bhaduri, S.B.; Boland, T. Viability and Electrophysiology of Neural Cell Structures Generated by the Inkjet Printing Method. Biomaterials 2006, 27, 3580–3588. [Google Scholar] [CrossRef]
- Keles, G.E.; Berger, M.S.; Srinivasan, J.; Kolstoe, D.D.; Bobola, M.S.; Silber, J.R. Establishment and Characterization of Four Human Medulloblastoma-Derived Cell Lines. Oncol. Res. 1995, 7, 493–503. [Google Scholar] [PubMed]
- Zhuang, P.; Sun, A.X.; An, J.; Chua, C.K.; Chew, S.Y. 3D Neural Tissue Models: From Spheroids to Bioprinting. Biomaterials 2018, 154, 113–133. [Google Scholar] [CrossRef]
- Ivanov, D.P.; Parker, T.L.; Walker, D.A.; Alexander, C.; Ashford, M.B.; Gellert, P.R.; Garnett, M.C. In Vitro Co-Culture Model of Medulloblastoma and Human Neural Stem Cells for Drug Delivery Assessment. J. Biotechnol. 2015, 205, 3–13. [Google Scholar] [CrossRef]
- Sanchez-Diaz, P.C.; Burton, T.L.; Burns, S.C.; Hung, J.Y.; Penalva, L.O.F. Musashi 1 Modulates Cell Proliferation Genes in the Medulloblastoma Cell Line Daoy. BMC Cancer 2008, 8, 1–12. [Google Scholar] [CrossRef]
- Lotharius, J.; Falsig, J.; Van Beek, J.; Payne, S.; Dringen, R.; Brundin, P.; Leist, M. Progressive Degeneration of Human Mesencephalic Neuron-Derived Cells Triggered by Dopamine-Dependent Oxidative Stress Is Dependent on the Mixed-Lineage Kinase Pathway. J. Neurosci. 2005, 25, 6329–6342. [Google Scholar] [CrossRef] [PubMed]
- Scholz, D.; Pöltl, D.; Genewsky, A.; Weng, M.; Waldmann, T.; Schildknecht, S.; Leist, M. Rapid, Complete and Large-Scale Generation of Post-Mitotic Neurons from the Human LUHMES Cell Line. J. Neurochem. 2011, 119, 957–971. [Google Scholar] [CrossRef]
- Martínez-Cerdeño, V.; Noctor, S.C. Neural Progenitor Cell Terminology. Front. Neuroanat. 2018, 12, 104. [Google Scholar] [CrossRef]
- Cheng, L.; Hu, W.; Qiu, B.; Zhao, J.; Yu, Y.; Guan, W.; Wang, M.; Yang, W.; Pei, G. Generation of Neural Progenitor Cells by Chemical Cocktails and Hypoxia. Cell. Res. 2014, 24, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, L.; Harris, G.; Delp, J.; Valadares, M.; Pamies, D.; Hogberg, H.T.; Waldmann, T.; Leist, M.; Hartung, T. A LUHMES 3D Dopaminergic Neuronal Model for Neurotoxicity Testing Allowing Long-Term Exposure and Cellular Resilience Analysis. Arch. Toxicol. 2016, 3, 2725–2743. [Google Scholar] [CrossRef]
- Leite, P.E.C.; Pereira, M.R.; Harris, G.; Pamies, D.; Dos Santos, L.M.G.; Granjeiro, J.M.; Hogberg, H.T.; Hartung, T.; Smirnova, L. Suitability of 3D Human Brain Spheroid Models to Distinguish Toxic Effects of Gold and Poly-Lactic Acid Nanoparticles to Assess Biocompatibility for Brain Drug Delivery. Part. Fibre Toxicol. 2019, 16, 1–20. [Google Scholar] [CrossRef]
- Harris, G.; Eschment, M.; Orozco, S.P.; Mccaffery, J.M.; Maclennan, R.; Severin, D.; Leist, M.; Kleensang, A.; Pamies, D.; Maertens, A.; et al. Toxicity, Recovery, and Resilience in a 3D Dopaminergic Neuronal in Vitro Model Exposed to Rotenone. Arch. Toxicol. 2018, 92, 2587–2606. [Google Scholar] [CrossRef]
- Joshi, P.; Yu, K.N.; Kang, S.Y.; Kwon, S.J.; Kwon, P.S.; Dordick, J.S.; Kothapalli, C.R.; Lee, M.Y. 3D-Cultured Neural Stem Cell Microarrays on a Micropillar Chip for High-Throughput Developmental Neurotoxicology. Exp. Cell. Res. 2018, 370, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Nierode, G.J.; Gopal, S.; Kwon, P.; Clark, D.S.; Schaffer, D.V.; Dordick, J.S. High-Throughput Identification of Factors Promoting Neuronal Differentiation of Human Neural Progenitor Cells in Microscale 3D Cell Culture. Biotechnol. Bioeng. 2019, 116, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Tomaskovic-Crook, E.; Zhang, P.; Ahtiainen, A.; Kaisvuo, H.; Lee, C.-Y.; Beirne, S.; Aqrawe, Z.; Svirskis, D.; Hyttinen, J.; Wallace, G.G.; et al. Human Neural Tissues from Neural Stem Cells Using Conductive Biogel and Printed Polymer Microelectrode Arrays for 3D Electrical Stimulation. Adv. Heal. Mater. 2019, 8, 1900425. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells. Adv. Heal. Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef]
- Agholme, L.; Lindström, T.; Kgedal, K.; Marcusson, J.; Hallbeck, M. An In Vitro Model for Neuroscience: Differentiation of SH-SY5Y Cells into Cells with Morphological and Biochemical Characteristics of Mature Neurons. J. Alzheimer’s Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Cacopardo, L.; Guazzelli, N.; Ahluwalia, A. Characterizing and Engineering Biomimetic Materials for Viscoelastic Mechanotransduction Studies. Tissue Eng. Part. B Rev. 2022, 28, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Mattei, G.; Magliaro, C.; Giusti, S.; Ramachandran, S.D.; Heinz, S.; Braspenning, J.; Ahluwalia, A. On the Adhesion-Cohesion Balance and Oxygen Consumption Characteristics of Liver Organoids. PLoS ONE 2017, 12, e0173206. [Google Scholar] [CrossRef]
- Serpeloni, M.; Ilce Mara, C.; Gania, Z.; Tiara Noorintan, S.; Putu Diah Pradnya Septiari, N.; Sandra Fitriany, D.; Gandhi Torizal, F. Strategies for Generating Human Pluripotent Stem Cell-Derived-Organoid Culture for Disease Modeling, Drug Screening, and Regenerative Therapy. Future Pharmacol. 2022, 2, 360–376. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-Induced Gelation of Alginate: Mechanisms and Applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Mastrorocco, A.; Cacopardo, L.; Lamanna, D.; Temerario, L.; Brunetti, G.; Carluccio, A.; Robbe, D.; Dell’aquila, M.E. Bioengineering Approaches to Improve In Vitro Performance of Prepubertal Lamb Oocytes. Cells 2021, 10, 1458. [Google Scholar] [CrossRef] [PubMed]
- Tirella, A.; Magliaro, C.; Penta, M.; Troncone, M.; Pimentel, R.; Ahluwalia, A. Sphyga: A Multiparameter Open Source Tool for Fabricating Smart and Tunable Hydrogel Microbeads. Biofabrication 2014, 6, 025009. [Google Scholar] [CrossRef] [PubMed]
- Innala, M.; Riebe, I.; Kuzmenko, V.; Sundberg, J.; Gatenholm, P.; Hanse, E.; Johannesson, S. 3D Culturing and Differentiation of SH-SY5Y Neuroblastoma Cells on Bacterial Nanocellulose Scaffolds 3D Culturing and Differentiation of SH-SY5Y Neuroblastoma Cells on Bacterial Nanocellulose Scaffolds. Nanomed. Biotechnol. 2014, 42, 302–308. [Google Scholar] [CrossRef]
- Frimat, J.-P.; Xie, S.; Bastiaens, A.; Schurink, B.; Wolbers, F.; Toonder, J.D.; Luttge, R. Advances in 3D Neuronal Cell Culture. J. Vac. Sci. Technology. 2015, 33, 06F902. [Google Scholar] [CrossRef]
- Hopkins, A.M.; DeSimone, E.; Chwalek, K.; Kaplan, D.L. 3D in Vitro Modeling of the Central Nervous System. Prog. Neurobiol. 2015, 125, 1–25. [Google Scholar] [CrossRef]
- Budday, S.; Nay, R.; de Rooij, R.; Steinmann, P.; Wyrobek, T.; Ovaert, T.C.; Kuhl, E. Mechanical Properties of Gray and White Matter Brain Tissue by Indentation. J. Mech. Behav. Biomed. Mater. 2015, 46, 318. [Google Scholar] [CrossRef]
- Park, K.; Lonsberry, G.E.; Gearing, M.; Levey, A.I.; Desai, J.P. Viscoelastic Properties of Human Autopsy Brain Tissues as Biomarkers for Alzheimer’s Diseases. IEEE Trans. Biomed. Eng. 2019, 66, 1705–1713. [Google Scholar] [CrossRef]
- Trédan, O.; Galmarini, C.M.; Patel, K.; Tannock, I.F. Drug Resistance and the Solid Tumor Microenvironment. J. Natl. Cancer Inst. 2007, 99, 1441–1454. [Google Scholar] [CrossRef]
- Bal-Price, A.K.; Hogberg, H.T.; Buzanska, L.; Lenas, P.; van Vliet, E.; Hartung, T. In Vitro Developmental Neurotoxicity (DNT) Testing: Relevant Models and Endpoints. Neurotoxicology 2010, 31, 545–554. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef]
- Alexander, H.P.; Ironside, J.W. Molecular Pathology in Neurodegenerative Diseases. Curr. Drug. Targets 2012, 13, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- McComish, S.F.; MacMahon Copas, A.N.; Caldwell, M.A. Human Brain-Based Models Provide a Powerful Tool for the Advancement of Parkinson’s Disease Research and Therapeutic Development. Front. Neurosci. 2022, 16, 851058. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.; Marshall, D.; Reid, Y.; Parkes, H.; Gelber, C. The Costs of Using Unauthenticated, over-Passaged Cell Lines: How Much More Data Do We Need? Biotechniques 2007, 43, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Moors, M.; Rockel, T.D.; Abel, J.; Cline, J.E.; Gassmann, K.; Schreiber, T.; Shuwald, J.; Weinmann, N.; Fritsche, E. Human Neurospheres as Three-Dimensional Cellular Systems for Developmental Neurotoxicity Testing. Env. Health Perspect. 2009, 117, 1131–1138. [Google Scholar] [CrossRef]
- Coecke, S.; Balls, M.; Bowe, G.; Davis, J.; Gstraunthaler, G.; Hartung, T.; Hay, R.; Merten, O.W.; Price, A.; Schechtman, L.; et al. Guidance on Good Cell Culture Practice: A Report of the Second ECVAM Task Force on Good Cell Culture Practice. ATLA Altern. Lab. Anim. 2005, 33, 261–287. [Google Scholar] [CrossRef]
- Ball, N.; Bars, R.; Botham, P.A.; Cuciureanu, A.; Cronin, M.T.D.; Doe, J.E.; Dudzina, T.; Gant, T.W.; Leist, M.; van Ravenzwaay, B. A Framework for Chemical Safety Assessment Incorporating New Approach Methodologies within REACH. Arch. Toxicol. 2022, 96, 743–766. [Google Scholar] [CrossRef]
- Rasband, M.N. Glial Contributions to Neural Function and Disease. Mol. Cell. Proteom. 2016, 15, 355. [Google Scholar] [CrossRef]
- Hirsch, C.; Schildknecht, S. In Vitro Research Reproducibility: Keeping up High Standards. Front. Pharm. 2019, 10, 1484. [Google Scholar] [CrossRef]
- Zingales, V.; Torriero, N.; Zanella, L.; Fernández-Franzón, M.; Ruiz, M.J.; Esposito, M.R.; Cimetta, E. Development of an in Vitro Neuroblastoma 3D Model and Its Application for Sterigmatocystin-Induced Cytotoxicity Testing. Food Chem. Toxicol. 2021, 157, 112605. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nicol, C.J.B.; Lo, S.S.; Hung, S.W.; Wang, C.J.; Lin, C.H. Resveratrol Mitigates Oxygen and Glucose Deprivation-Induced Inflammation, NLRP3 Inflammasome, and Oxidative Stress in 3D Neuronal Culture. Int. J. Mol. Sci. 2022, 23, 11678. [Google Scholar] [CrossRef]
- Gomez-Lechon, M.; Donato, M.; Lahoz, A.; Castell, J. Cell Lines: A Tool for In Vitro Drug Metabolism Studies. Curr. Drug. Metab. 2008, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]

| Cell Line | Origin | Gender and Age | Morphology | [Ref.], Year |
|---|---|---|---|---|
| U-87MG | Malignant glioma (likely glioblastoma) | Male, unspecified | Epithelial | [28], 1968 |
| U-251MG | Glioblastoma-astrocytoma | Male, 75 years old | Pleomorphic/ astrocytoid | [29], 1984 |
| U-373MG | Glioblastoma-astrocytoma | Male, 75 years old | Pleomorphic/astrocytoid | [30], 1989 |
| T-98G | Glioblastoma multiforme | Male, 61 years old | Fibroblast | [31], 1979 |
| A-172 | Glioblastoma | Male, 53 years old | Fibroblast | [32], 1973 |
| Cell lines | Application | Materials and Methods | Main Findings | [Ref.], Year |
|---|---|---|---|---|
| U-87MG | Oncology | Self-assembled spheroids in agarose-coated 96-well plates treated with increasing concentrations of temozolomide | Spheroid growth influenced by administered dose | [33], 2015 |
| Self-assembled spheroids in agarose-coated 96-well plates treated with an inhibitor of the NOTCH signaling pathway | Reduced resistance of treated cells within spheroids to chemotherapeutic agents | [34], 2016 | ||
| Gene expression of spheroids obtained in low attachment wells compared with 2D controls | Upregulated gene expression of the inspected molecular characteristics in the 3D spheroid models compared with the 2D model | [35], 2021 | ||
| Self-assembled spheroids laden with wild-type and cells with increased malignancy implanted in collagen-I gels. | Differences in the cell proliferation between the wild-type and the more malignant ones due to lower cell adhesion | [36] 2007 | ||
| Spheroids with PEG-based hydrogel matrix with characteristics mimicking the physiological and glioblastoma-altered properties of in vivo ECM | Reduced cell proliferation and spreading on stiffer matrices | [37] 2014 | ||
| Bio-printed 3D constructs laden with glioblastoma and monocytic cells compared to 2D controls for cancer drug sensitivity | Optimization of the bio-printing procedure to promote a tumor microenvironment; 3D showed higher drug resistance than 2D | [38] 2020 | ||
| Co-culture of glioblastoma and endothelial-like cells in scaffolds fabricated with two-photon lithography, with microtubes resembling capillaries | Development of a realistic and 1:1 scale system mimicking the blood–brain barrier with good adhesion and covering by both cell types | [39] 2018 | ||
| Bioprinting of cell-laden 3D structures with a bioink made of fibrin, alginate and genipin | Good viability and tendency to form spheroids resulting in a more physiologically relevant glioblastoma model | [40] 2019 | ||
| U-87, SHG-44 and U-251 | Multicellular spheroids supplemented with B27, human basic fibroblast and epidermal growth factors, treated with EGCG for evaluating inhibition of cell stemness | Efficacy of the EGCG treatment in inhibiting cell viability and migration and inducing cell apoptosis, hence of potential in assessing glioblastoma therapy | [41] 2015 | |
| U-87MG, T-98G, A-172 and UW473 | Compact multicellular spheroids formed with type-I collagen colloidal solutions (with increasing collagen concentration from 0 to 80 mg mL−1) | Development of a cheap and accessible method for building multicellular spheroids, usable for drug screening and glioblastoma cell infiltration | [42] 2022 | |
| U-87MG, U-251MG and IMR-32 | Neurotoxicity | Spheroids obtained encapsulating cells in alginate, with concentration of 0.25 or 1% weight/volume and exposed to different toxins for 24 hr for testing cell viability | Higher sensitiveness to the toxins of the cells within the soft matrices than those in the stiffer ones, suggesting a role of matrix stiffness in neurotoxicity regulation | [43] 2014 |
| Cell Line | Origin | Gender and Age | Morphology | [Ref.], Year |
|---|---|---|---|---|
| SH-SY5Y | Thrice cloned subline of the neuroblastoma cell line SK-N-SH | Female, 4 years old | Neuroblast | [46], 1973 |
| IMR-32 | Neuroblastoma | Male, 13 months old | Neuroblast; fibroblast | [47], 1970 |
| HTLA-230 | Neuroblastoma | Male, 11 months old | Round to bi-polar morphology | [48], 1992 |
| Kelly | Neuroblastoma | Female, 1 year old | Round to fusiform with polar neurite processes | [49], 1982 |
| Cell lines | Application | Materials and Methods | Main Findings | [Ref.], Year |
|---|---|---|---|---|
| SH-SY5Y | Neurodegenerative diseases | Cells grown either on Matrigel or ECM scaffolds, differentiated with retinoic acid | 3D models of the alpha-synuclein pathology associated with PD | [50,51,52], 2016, 2019, 2022 |
| RA-differentiated SH-SY5Y cells grown in silk-hydrogel or Matrigel, exposed to neurotoxicants | Model exploitable for studying the pathogenesis of PD | [53], 2022 | ||
| Cells grown on 3D nanoscaffold fabricated with polyacrylonitrile and Jeffamine® doped polyacrylonitrile | Improved survival, growth and sensitivity to treatments mimicking PD features | [54], 2020 | ||
| Wild type and tau-mutated cells seeded on well plates, placed on a shaker to generate spheroids | Salient features of AD at the microscale recapitulated better by the spheroid model than 2D cultures | [55,56], 2010, 2012 | ||
| 3D printed structures laden with cells in alginate and gelatin, using commercial printer | Good cell viability, maintenance of the 3D structure and spatial organization | [57], 2019 | ||
| Conductive and porous scaffolds fabricated by electro-polymerization using carbon nanotubes and PEDOT | Good biocompatibility shown by the improved tubulin expression on conductive scaffolds | [58], 2020 | ||
| Bacterial nanocellulose scaffolds coated with collagen I for promoting cell adhesion and differentiation | Functional action potentials were observed thanks to electrophysiological recordings | [59], 2013 | ||
| Collagen sponges (BIOPAD™) seeded with cells for investigating the neuroprotective effect of phytochemicals | Improved cell viability, upregulated antioxidant and insulin-degrading enzymes and reduced glutathione levels | [60], 2019 | ||
| 0.3 % w/v alginate beads, obtained via syringe-pump-controlled extrusion from 15 to 27G needles, coated with 0.1% w/v poly-L-ornithine or 0.3% w/v hyaluronic acid | Suitability for CNS implantation and delivery of therapeutic cells for the treatment of neurodegenerative disorders | [61], 2022 | ||
| 3D bioprinting of cells with bioinks composed of nanofibrils alginate and single-walled carbon nanotubes | Conductive scaffold-promoted cell differentiation (TUBB3 and NESTIN expression) | [62], 2020 | ||
| Cells seeded on scaffold generated by two-photon lithography of gelatin–methacryloyl and impregnated with magnetoelectric NPs | Electrostimulation allowed cell differentiation in the absence of chemical factors (neurite outgrowth with multipolar shape) | [63], 2020 | ||
| Oncology | Cells encapsulated in 2% w/v alginate thanks to electrohydrodynamic jetting and cultured for 4 weeks | Tissue maturation and higher cell viability, metabolic activity and proliferation level than cells cultured on TCP | [64], 2018 | |
| Generation of chitosan (CH)–graphene oxide (GO) nanocomposite hydrogels seeded with cells | Cell differentiation (extensive neurite outgrowth) promoted by the CH–GO hydrogels | [65], 2021 | ||
| Neurotoxicity | 3D hyaluronic acid-based hydro-scaffold (BIOMIMESYS®) seeded with cells | Higher neuronal differentiation and lower sensitivity to neurotoxic compounds with respect to 2D cultures | [66], 2021 | |
| Microporous silk scaffolds coated with poly-L-ornithine and laminin, seeded with cells, encapsulated with collagen or Matrigel, and exposed to 1-methyl-4-phenylpyridinium | During differentiation, reduced proliferation and higher sensitivity to neurotoxins in comparison with 2D cultures | [67], 2020 | ||
| Cells encapsulated in 1 mg/mL collagen gels obtained by casting in Petri dishes and differentiated | Lower responsiveness of cells in 3D to potassium-induced cell depolarization with respect to 2D | [68], 2006 | ||
| IMR-32, Kelly and SH-SY5Y | Oncology | Cells in collagen-based porous scaffold. Assessment of cell proliferation, viability and spatial within the scaffolds | Precise manipulation of cells and ECM components allowed by the 3D culture system; environment more physiologically similar to tumor tissue | [69], 2021 |
| HTLA-230 and SH-SY5Y | Oncology | Cells suspended in alginate and manually extruded for mimicking the extracellular microenvironment experienced by tumor cells in in vivo settings | Reduced sensitivity to imatinib mesylate—a cytotoxic drug—with respect to cells cultured in monolayer and characteristics similar to the in vivo immunophenotype of tumor cells | [70], 2019 |
| Cell line | Application | Materials and Methods | Main Findings | [Ref.], Year |
|---|---|---|---|---|
| LUHMES | Neurodegenerative | 3D constructs obtained by shaking (80 rpm) of cells seeded in 6-well plates with differentiation medium | Optimization of the differentiation protocol for a 3D construct with the formation of a pronounced neuronal network | [90], 2016 |
| Neurotoxicity | Spheroid formation by differentiation with neurotrophic factor and shaking (80 rpm). Treatment with different NP concentrations | Alteration of cell physiology and morphology of the spheroid surface provoked by the NPs, with induction of neurotoxic effects at the highest concentrations | [91], 2019 | |
| 3D constructs obtained by shaking (80 rpm) followed by 24 h exposure to rotenone | Recovery of ATP levels, mitochondria functions and neurite outgrowth after rotenone wash out showing good functional recovery | [92], 2018 | ||
| ReNcell VM | Neurotoxicity | Cells encapsulated in alginate and Matrigel and bioprinted on microarray chip platforms | Successful establishment of miniaturized 3D culture of cells in alginate–Matrigel matrices useful for assessing toxicity | [93], 2018 |
| Microarray chip-based platform for the screening of the effect of 12 toxicants on neuronal differentiation | Enhanced neurogenesis and decreased astrocyte differentiation with the combined treatment of RA and CHIR | [94], 2019 | ||
| Neurodegenerative diseases, neurotoxicity | Direct write printing of a conductive polymer for the development of a 3D electrical stimulation tool of cells encapsulated within a conductive biogel | In situ differentiation of the NPCs into neurons and neuroglial cells and formation of tissue with high density and mature neurons | [95], 2019 | |
| ReNcell CX | Neurodegenerative diseases, neurotoxicity | Direct write printing of cells over a supporting polysaccharide (alginate, carboxymethyl-chitosan, and agarose) | In situ differentiation of NPCs to neurons with synaptic connections and spontaneous electrical activity | [96], 2016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabbri, R.; Cacopardo, L.; Ahluwalia, A.; Magliaro, C. Advanced 3D Models of Human Brain Tissue Using Neural Cell Lines: State-of-the-Art and Future Prospects. Cells 2023, 12, 1181. https://doi.org/10.3390/cells12081181
Fabbri R, Cacopardo L, Ahluwalia A, Magliaro C. Advanced 3D Models of Human Brain Tissue Using Neural Cell Lines: State-of-the-Art and Future Prospects. Cells. 2023; 12(8):1181. https://doi.org/10.3390/cells12081181
Chicago/Turabian StyleFabbri, Rachele, Ludovica Cacopardo, Arti Ahluwalia, and Chiara Magliaro. 2023. "Advanced 3D Models of Human Brain Tissue Using Neural Cell Lines: State-of-the-Art and Future Prospects" Cells 12, no. 8: 1181. https://doi.org/10.3390/cells12081181
APA StyleFabbri, R., Cacopardo, L., Ahluwalia, A., & Magliaro, C. (2023). Advanced 3D Models of Human Brain Tissue Using Neural Cell Lines: State-of-the-Art and Future Prospects. Cells, 12(8), 1181. https://doi.org/10.3390/cells12081181