A Preliminary Study of Mild Heat Stress on Inflammasome Activation in Murine Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines, Cell Culture, and Reagents
2.2. LDH Assay, ELISAs
2.3. Western Blot and Antibodies
2.4. Real-Time Cell Permeability Assay (PI Assay), Membrane Fluidity Assay
2.5. Live Cell Imaging
2.6. Statistical Analysis
3. Results
3.1. Mild and Extreme Heat Shocks Inhibit Proinflammatory Cytokines
3.2. Heat Shock after LPS Priming Differentially Inhibits Cytokines without Impacting Cell Death
3.3. Heat Shock Decreases Membrane Permeability
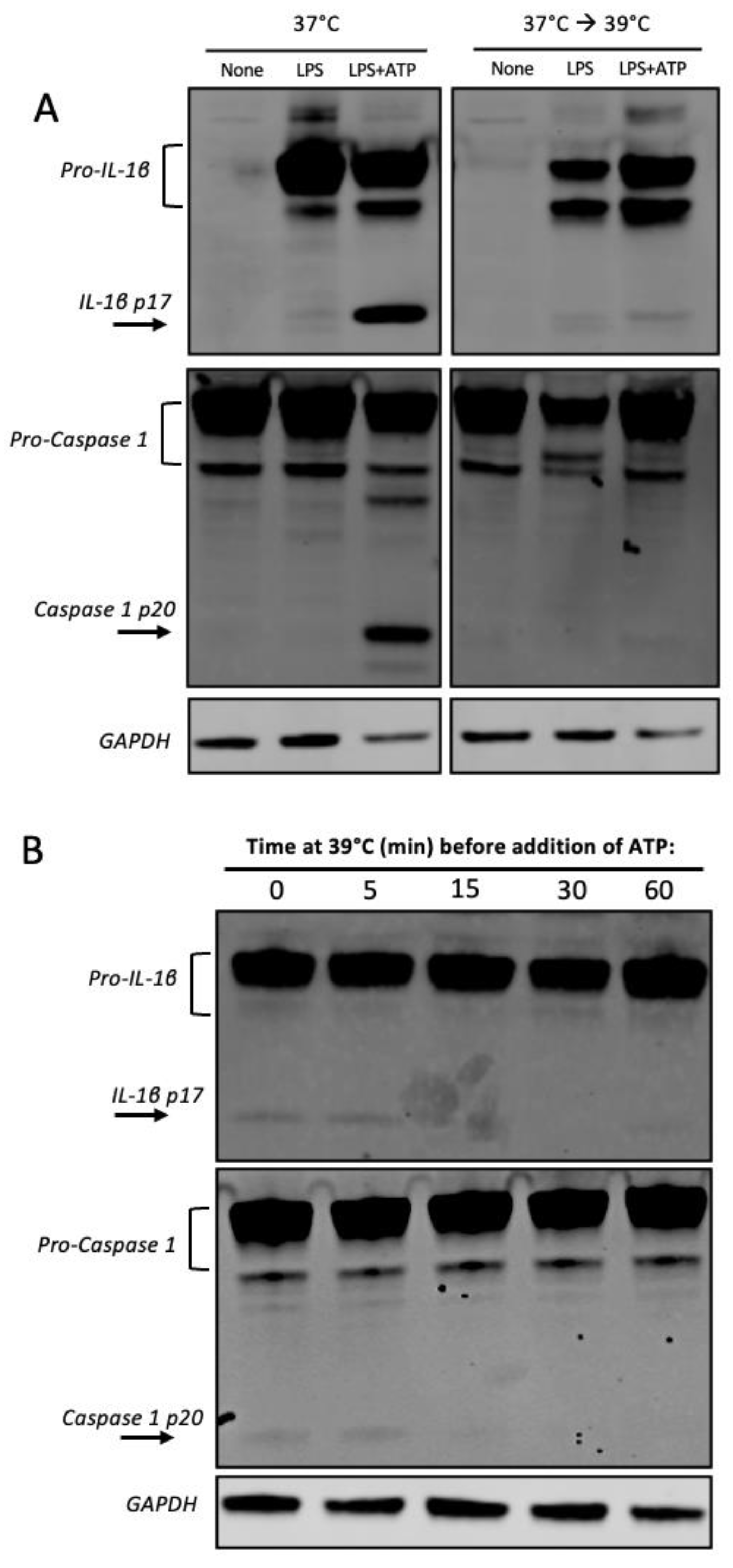
3.4. Heat Shock Inhibits Caspase 1 Processing
3.5. Mild Heat Shock Inhibits ASC Speck Formation but Not Mitochondrial Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Al-Hadrawi, D.S.; Almulla, A.F.; Maes, M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: A proof of concept and mechanism study. Mol. Psychiatry 2023, 28, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Milner, M.T.; Maddugoda, M.; Gotz, J.; Burgener, S.S.; Schroder, K. The NLRP3 inflammasome triggers sterile neuroinflammation and Alzheimer’s disease. Curr. Opin. Immunol. 2021, 68, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.W.; Lowry, C.A.; Mehl, M.R.; Allen, J.J.; Kelly, K.L.; Gartner, D.E.; Medrano, A.; Begay, T.K.; Rentscher, K.; White, J.J.; et al. Whole-Body Hyperthermia for the Treatment of Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2016, 73, 789–795. [Google Scholar] [CrossRef]
- Laukkanen, T.; Kunutsor, S.; Kauhanen, J.; Laukkanen, J.A. Sauna bathing is inversely associated with dementia and Alzheimer’s disease in middle-aged Finnish men. Age Ageing 2017, 46, 245–249. [Google Scholar] [CrossRef]
- Mallory, M.; Gogineni, E.; Jones, G.C.; Greer, L.; Simone, C.B., 2nd. Therapeutic hyperthermia: The old, the new, and the upcoming. Crit. Rev. Oncol. Hematol. 2016, 97, 56–64. [Google Scholar] [CrossRef]
- Cohen, M. Turning up the heat on COVID-19: Heat as a therapeutic intervention. F1000Res 2020, 9, 292. [Google Scholar] [CrossRef]
- Cahill, C.M.; Waterman, W.R.; Xie, Y.; Auron, P.E.; Calderwood, S.K. Transcriptional repression of the prointerleukin 1beta gene by heat shock factor 1. J. Biol. Chem. 1996, 271, 24874–24879. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, C.; Stevenson, M.A.; Auron, P.E.; Calderwood, S.K. Heat shock factor 1 represses transcription of the IL-1beta gene through physical interaction with the nuclear factor of interleukin 6. J. Biol. Chem. 2002, 277, 11802–11810. [Google Scholar] [CrossRef]
- Ensor, J.E.; Wiener, S.M.; McCrea, K.A.; Viscardi, R.M.; Crawford, E.K.; Hasday, J.D. Differential effects of hyperthermia on macrophage interleukin-6 and tumor necrosis factor-alpha expression. Am. J. Physiol. 1994, 266, C967–C974. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.; Iwasaka, H.; Matsumoto, S.; Noguchi, T. Changes in cell culture temperature alter release of inflammatory mediators in murine macrophagic RAW264.7 cells. Inflamm. Res. 2007, 56, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, F.N.; Costa, A.P.; Ghisleni, G.; Diaz, A.P.; Rodrigues, A.L.S.; Peluffo, H.; Kaster, M.P. NLRP3 inflammasome-driven pathways in depression: Clinical and preclinical findings. Brain Behav. Immun. 2017, 64, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Plassmeyer, M.; Alpan, O.; Corley, M.J.; Premeaux, T.A.; Lillard, K.; Coatney, P.; Vaziri, T.; Michalsky, S.; Pang, A.P.S.; Bukhari, Z.; et al. Caspases and therapeutic potential of caspase inhibitors in moderate-severe SARS-CoV-2 infection and long COVID. Allergy 2022, 77, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef]
- Yabal, M.; Calleja, D.J.; Simpson, D.S.; Lawlor, K.E. Stressing out the mitochondria: Mechanistic insights into NLRP3 inflammasome activation. J. Leukoc. Biol. 2019, 105, 377–399. [Google Scholar] [CrossRef]
- Erlich, Z.; Shlomovitz, I.; Edry-Botzer, L.; Cohen, H.; Frank, D.; Wang, H.; Lew, A.M.; Lawlor, K.E.; Zhan, Y.; Vince, J.E.; et al. Macrophages, rather than DCs, are responsible for inflammasome activity in the GM-CSF BMDC model. Nat. Immunol. 2019, 20, 397–406. [Google Scholar] [CrossRef]
- Evavold, C.L.; Ruan, J.; Tan, Y.; Xia, S.; Wu, H.; Kagan, J.C. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 2018, 48, 35–44.e36. [Google Scholar] [CrossRef]
- Karasawa, T.; Komada, T.; Yamada, N.; Aizawa, E.; Mizushina, Y.; Watanabe, S.; Baatarjav, C.; Matsumura, T.; Takahashi, M. Cryo-sensitive aggregation triggers NLRP3 inflammasome assembly in cryopyrin-associated periodic syndrome. eLife 2022, 11, e75166. [Google Scholar] [CrossRef]
- Tzeng, T.C.; Schattgen, S.; Monks, B.; Wang, D.; Cerny, A.; Latz, E.; Fitzgerald, K.; Golenbock, D.T. A Fluorescent Reporter Mouse for Inflammasome Assembly Demonstrates an Important Role for Cell-Bound and Free ASC Specks during In Vivo Infection. Cell. Rep. 2016, 16, 571–582. [Google Scholar] [CrossRef]
- Takii, R.; Inouye, S.; Fujimoto, M.; Nakamura, T.; Shinkawa, T.; Prakasam, R.; Tan, K.; Hayashida, N.; Ichikawa, H.; Hai, T.; et al. Heat shock transcription factor 1 inhibits expression of IL-6 through activating transcription factor 3. J. Immunol. 2010, 184, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, M.; Zhang, Y.; Miao, E.A. Detection of pyroptosis by measuring released lactate dehydrogenase activity. Methods Mol. Biol. 2013, 1040, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal. Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Balogh, G.; Horvath, I.; Nagy, E.; Hoyk, Z.; Benko, S.; Bensaude, O.; Vigh, L. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J. 2005, 272, 6077–6086. [Google Scholar] [CrossRef]
- Martine, P.; Chevriaux, A.; Derangere, V.; Apetoh, L.; Garrido, C.; Ghiringhelli, F.; Rebe, C. HSP70 is a negative regulator of NLRP3 inflammasome activation. Cell. Death Dis. 2019, 10, 256. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, D.; Hu, D.; Zhou, X.; Zhou, Y. The role of mitochondria in NLRP3 inflammasome activation. Mol. Immunol. 2018, 103, 115–124. [Google Scholar] [CrossRef]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R.; et al. NF-kappaB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef]
- Ahn, H.; Lee, G.; Lee, G.S. Lower Temperatures Exacerbate NLRP3 Inflammasome Activation by Promoting Monosodium Urate Crystallization, Causing Gout. Cells 2021, 10, 1919. [Google Scholar] [CrossRef]
- Gao, R.; Zhao, H.; Wang, X.; Tang, B.; Cai, Y.; Zhang, X.; Zong, H.; Li, Y.; Wang, Y. Mild Hypothermia Therapy Lowers the Inflammatory Level and Apoptosis Rate of Myocardial Cells of Rats with Myocardial Ischemia-Reperfusion Injury via the NLRP3 Inflammasome Pathway. Comput. Math. Methods Med. 2021, 2021, 6415275. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, N.; Zhang, B.; Lv, H.; Li, S. Cold Stress Induced Liver Injury of Mice through Activated NLRP3/Caspase-1/GSDMD Pyroptosis Signaling Pathway. Biomolecules 2022, 12, 927. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Z.; Zhang, C.; Zhang, W.; Zhang, C.; Chen, T.; Wang, Y. Mild hypothermia alleviates early brain injury after subarachnoid hemorrhage via suppressing pyroptosis through AMPK/NLRP3 inflammasome pathway in rats. Brain Res. Bull. 2023, 193, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Levin, T.C.; Wickliffe, K.E.; Leppla, S.H.; Moayeri, M. Heat shock inhibits caspase-1 activity while also preventing its inflammasome-mediated activation by anthrax lethal toxin. Cell. Microbiol. 2008, 10, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, Y.; Yu, P.; Li, Z.; Liu, Y.; Zhang, J.; Tang, X.; Yu, S. Therapeutic hypothermia alleviates myocardial ischaemia-reperfusion injury by inhibiting inflammation and fibrosis via the mediation of the SIRT3/NLRP3 signalling pathway. J. Cell. Mol. Med. 2022, 26, 4995–5007. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Regulation of the NLRP3 Inflammasome by Post-Translational Modifications and Small Molecules. Front. Immunol. 2020, 11, 618231. [Google Scholar] [CrossRef] [PubMed]
- Kassis, S.; Grondin, M.; Averill-Bates, D.A. Heat shock increases levels of reactive oxygen species, autophagy and apoptosis. Biochim. Biophys. Acta Mol. Cell. Res. 2021, 1868, 118924. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Man, S.M.; Kanneganti, T.D. Inflammasomes and Cancer. Cancer Immunol. Res. 2017, 5, 94–99. [Google Scholar] [CrossRef]
- Li, Z.; Guo, J.; Bi, L. Role of the NLRP3 inflammasome in autoimmune diseases. Biomed. Pharmacother. 2020, 130, 110542. [Google Scholar] [CrossRef]
- Vora, S.M.; Lieberman, J.; Wu, H. Inflammasome activation at the crux of severe COVID-19. Nat. Rev. Immunol. 2021, 21, 694–703. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foster, S.L.; Dutton, A.J.; Yerzhan, A.; March, L.B.; Barry, K.; Seehus, C.R.; Huang, X.; Talbot, S.; Woolf, C.J. A Preliminary Study of Mild Heat Stress on Inflammasome Activation in Murine Macrophages. Cells 2023, 12, 1189. https://doi.org/10.3390/cells12081189
Foster SL, Dutton AJ, Yerzhan A, March LB, Barry K, Seehus CR, Huang X, Talbot S, Woolf CJ. A Preliminary Study of Mild Heat Stress on Inflammasome Activation in Murine Macrophages. Cells. 2023; 12(8):1189. https://doi.org/10.3390/cells12081189
Chicago/Turabian StyleFoster, Simmie L., Abigail J. Dutton, Adina Yerzhan, Lindsay B. March, Katherine Barry, Corey R. Seehus, Xudong Huang, Sebastien Talbot, and Clifford J. Woolf. 2023. "A Preliminary Study of Mild Heat Stress on Inflammasome Activation in Murine Macrophages" Cells 12, no. 8: 1189. https://doi.org/10.3390/cells12081189
APA StyleFoster, S. L., Dutton, A. J., Yerzhan, A., March, L. B., Barry, K., Seehus, C. R., Huang, X., Talbot, S., & Woolf, C. J. (2023). A Preliminary Study of Mild Heat Stress on Inflammasome Activation in Murine Macrophages. Cells, 12(8), 1189. https://doi.org/10.3390/cells12081189







