Improving Schwann Cell Differentiation from Human Adipose Stem Cells with Metabolic Glycoengineering
Abstract
1. Introduction
2. Materials and Methods
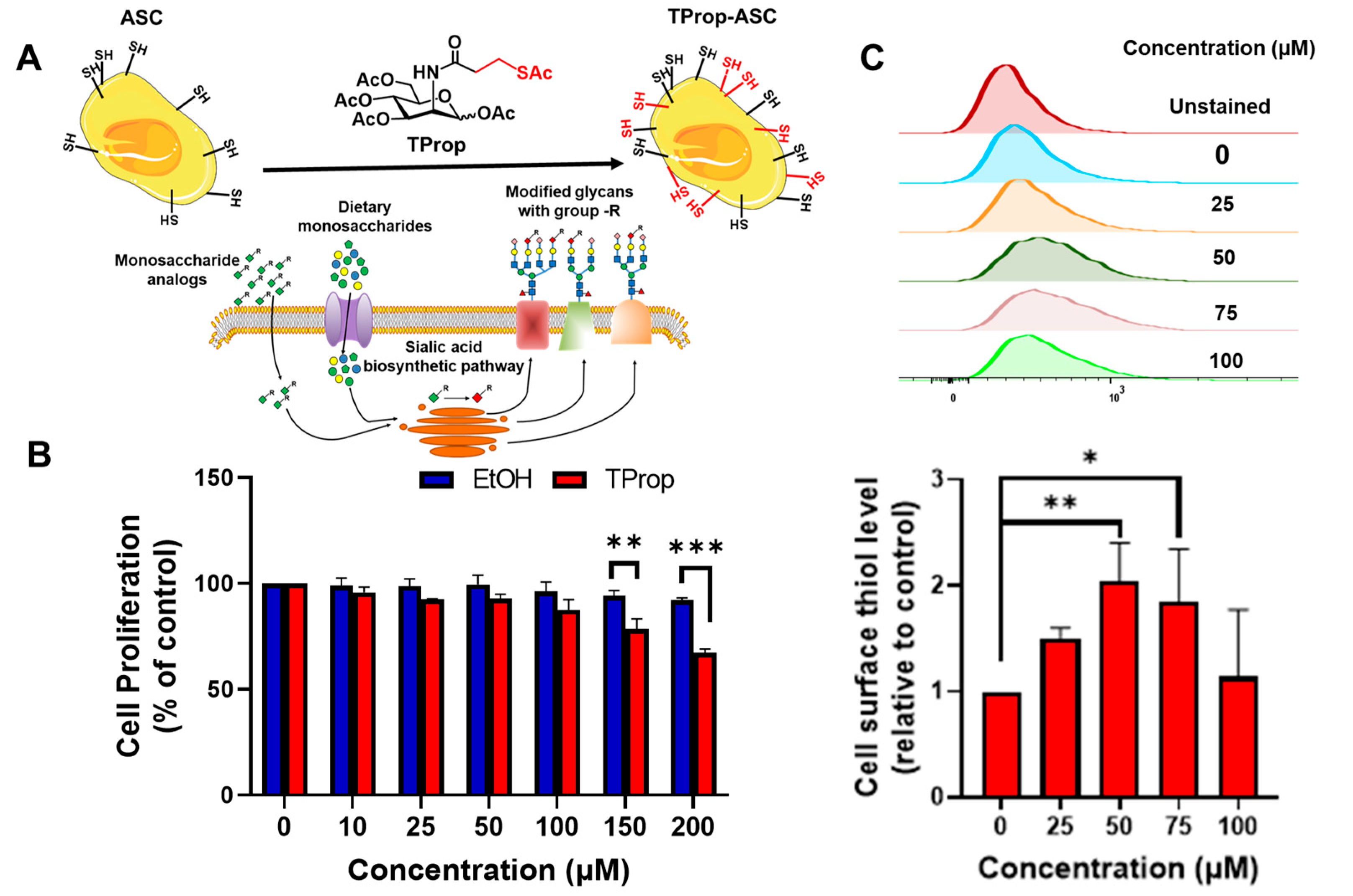
2.1. Metabolic Activity
2.2. Flow Cytometry
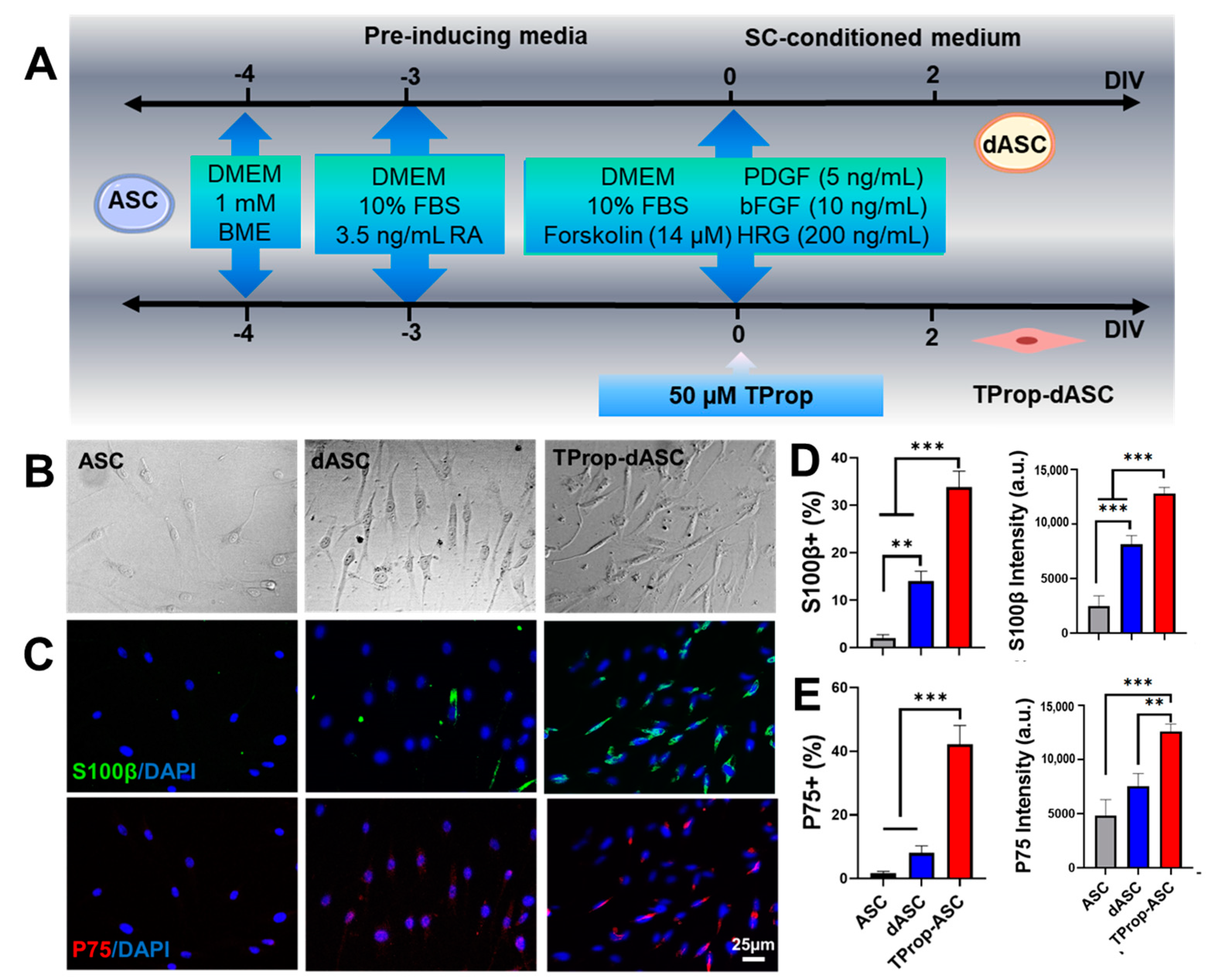
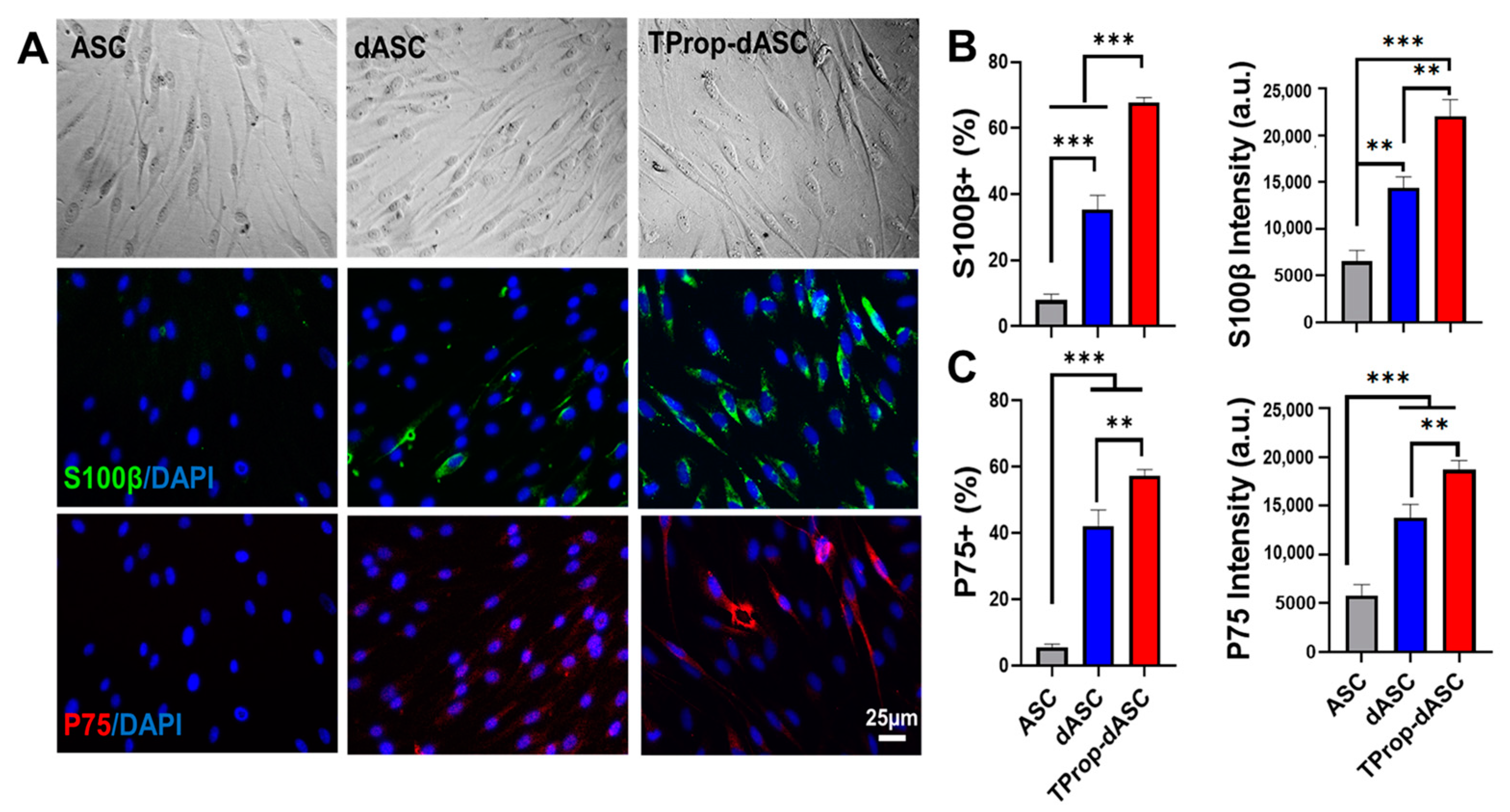
2.3. ASC Schwann Cell Differentiation
2.4. Identification of Schwann-Like Cells by Immunofluorescence
2.5. Detection of Secreted Nerve Growth Factor Beta (NGFβ) and Glial Cell Line-Derived Neurotrophic Factor (GDNF) by Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. mRNA Extraction and RT-qPCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Metabolic Impact of Thiol-Modified ManNAc Analogs in ASCs
3.2. Differentiation of ASCs to a Schwann Cell Phenotype
3.3. RT-PCR Results
3.4. Secretion of Neurotrophins
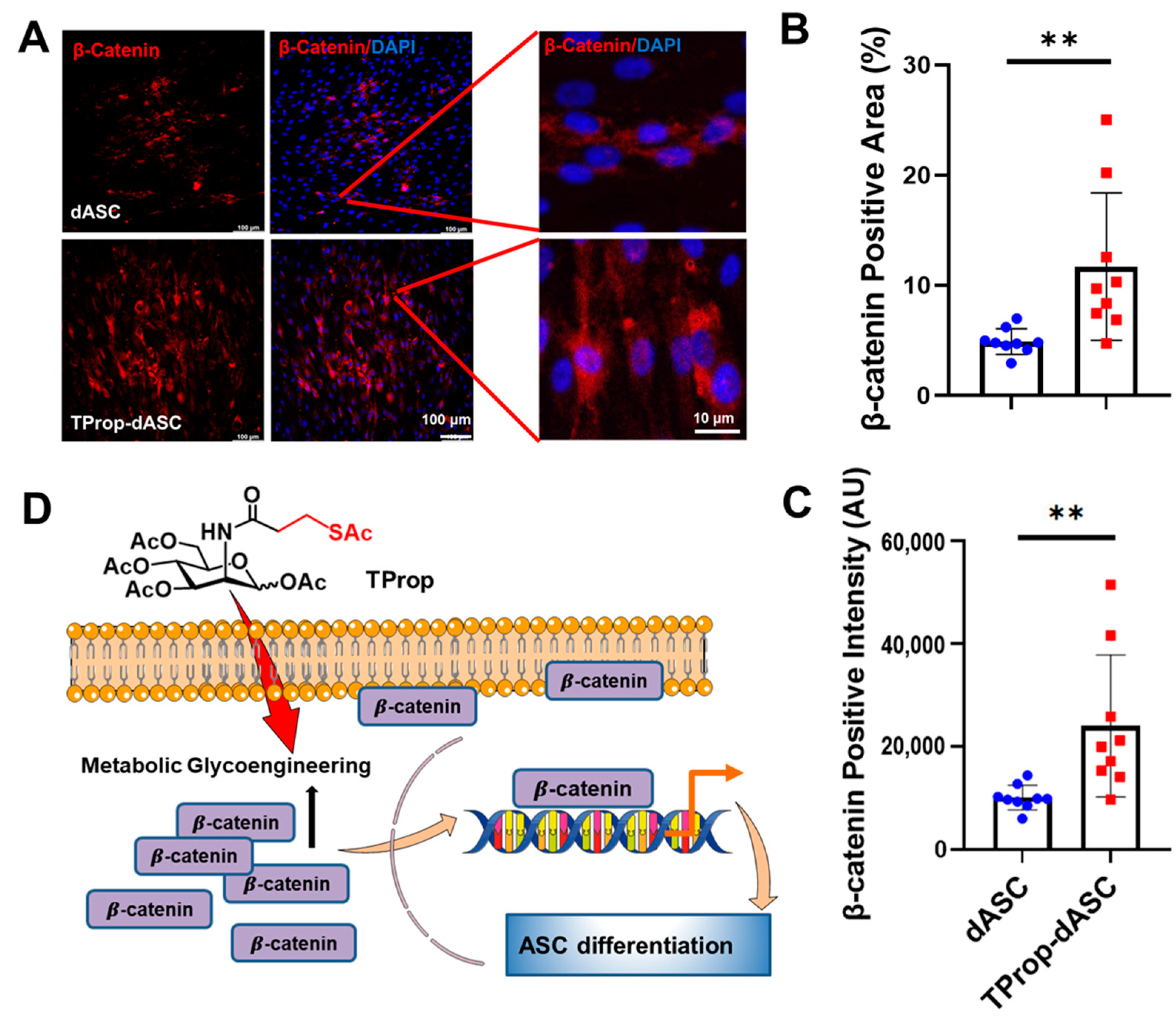
3.5. Wnt Signaling Activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Du, J.; Zhen, G.; Chen, H.; Zhang, S.; Qing, L.; Yang, X.; Lee, G.; Mao, H.Q.; Jia, X. Optimal electrical stimulation boosts stem cell therapy in nerve regeneration. Biomaterials 2018, 181, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Chen, H.; Zhou, K.; Jia, X. Quantitative multimodal evaluation of passaging human neural crest stem cells for peripheral nerve regeneration. Stem Cell Rev. Rep. 2018, 14, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.N.; Lancaster, K.Z.; Zhen, G.; He, J.; Gupta, M.K.; Kong, Y.L.; Engel, E.A.; Krick, K.D.; Ju, A.; Meng, F.; et al. 3D Printed Anatomical nerve regeneration pathways. Adv. Funct. Mater. 2015, 25, 6205–6217. [Google Scholar] [CrossRef] [PubMed]
- Boucanova, F.; Chrast, R. Metabolic interaction between Schwann cells and axons under physiological and disease conditions. Front. Cell. Neurosci. 2020, 14, 148. [Google Scholar] [CrossRef]
- Liu, Y.P.; Tian, M.Y.; Yang, Y.D.; Li, H.; Zhao, T.T.; Zhu, J.; Mou, F.F.; Cui, G.H.; Guo, H.D.; Shao, S.J. Schwann cells-derived exosomal miR-21 participates in high glucose regulation of neurite outgrowth. iScience 2022, 25, 105141. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Belfiore, L.; Chu, T.H.; Fleming, T.; Midha, R.; Biernaskie, J.; Schuurmans, C. Insights into the role and potential of Schwann cells for peripheral nerve repair from studies of development and injury. Front. Mol. Neurosci. 2020, 13, 608442. [Google Scholar] [CrossRef]
- Vallejo, F.A.; Diaz, A.; Errante, E.L.; Smartz, T.; Khan, A.; Silvera, R.; Brooks, A.E.; Lee, Y.S.; Burks, S.S.; Levi, A.D. Systematic review of the therapeutic use of Schwann cells in the repair of peripheral nerve injuries: Advancements from animal studies to clinical trials. Front. Cell. Neurosci. 2022, 16, 929593. [Google Scholar] [CrossRef]
- Wilson, E.R.; Della-Flora Nunes, G.; Weaver, M.R.; Frick, L.R.; Feltri, M.L. Schwann cell interactions during the development of the peripheral nervous system. Dev. Neurobiol. 2021, 81, 464–489. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Q.; Parkinson, D.B.; Dun, X.P. Analysis of Schwann cell Mmigration and axon Rregeneration following nerve injury in the sciatic nerve bridge. Front. Mol. Neurosci. 2019, 12, 308. [Google Scholar] [CrossRef]
- ElAbd, R.; Alabdulkarim, A.; AlSabah, S.; Hazan, J.; Alhalabi, B.; Thibaudeau, S. Role of electrical Stimulation in peripheral nerve regeneration: A systematic review. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4115. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, F.Y.; Ling, Z.M.; Su, W.F.; Zhao, Y.Y.; Chen, G.; Wei, Z.Y. The Effect of Schwann cells/Schwann cell-like cells on cell therapy for peripheral neuropathy. Front. Cell. Neurosci. 2022, 16, 836931. [Google Scholar] [CrossRef]
- Hopf, A.; Schaefer, D.J.; Kalbermatten, D.F.; Guzman, R.; Madduri, S. Schwann cell-like clls: Origin and usability for repair and regeneration of the peripheral and central nervous system. Cells 2020, 9, 1990. [Google Scholar] [CrossRef]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef]
- Ching, R.C.; Wiberg, M.; Kingham, P.J. Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cell Res. Ther. 2018, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Piovesana, R.; Faroni, A.; Tata, A.M.; Reid, A.J. Schwann-like adipose-derived stem cells as a promising therapeutic tool for peripheral nerve regeneration: Effects of cholinergic stimulation. Neural Regen. Res. 2021, 16, 1218–1220. [Google Scholar] [CrossRef] [PubMed]
- Sumarwoto, T.; Suroto, H.; Mahyudin, F.; Utomo, D.N.; Romaniyanto; Tinduh, D.; Notobroto, H.B.; Sigit Prakoeswa, C.R.; Rantam, F.A.; Rhatomy, S. Role of adipose mesenchymal stem cells and secretome in peripheral nerve regeneration. Ann. Med. Surg. 2021, 67, 102482. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, C.A.; Grochmal, J.; Kung, T.A.; Cederna, P.S.; Midha, R.; Kemp, S.W.P. Stem-cell-based therapies to enhance peripheral nerve regeneration. Muscle Nerve 2020, 61, 449–459. [Google Scholar] [CrossRef]
- Jiang, L.; Mee, T.; Zhou, X.; Jia, X. Augmenting peripheral nerve regeneration with adipose-derived stem cells. Stem Cell Rev. Rep. 2022, 18, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Jones, S.; Jia, X. Stem cell transplantation for peripheral nerve regeneration: Current options and opportunities. Int. J. Mol. Sci. 2017, 18, 94. [Google Scholar] [CrossRef]
- Su, Q.; Nasser, M.I.; He, J.; Deng, G.; Ouyang, Q.; Zhuang, D.; Deng, Y.; Hu, H.; Liu, N.; Li, Z.; et al. Engineered Schwann cell-based therapies for injury peripheral nerve reconstruction. Front. Cell. Neurosci. 2022, 16, 865266. [Google Scholar] [CrossRef]
- Zhou, N.; Xu, Z.; Li, X.; Ren, S.; Chen, J.; Xiong, H.; Wang, C.; Guo, J.; Kang, Y.; Chen, Z.; et al. Schwann cell-derived exosomes induce the differentiation of human adipose-derived stem cells Into Schwann cells. Front. Mol. Biosci. 2021, 8, 835135. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.T.; Fang, J.T.; Li, L.; Chen, G.; Qin, B.G.; Gu, L.Q. Contralateral C7 transfer combined with acellular nerve allografts seeded with differentiated adipose stem cells for repairing upper brachial plexus injury in rats. Neural Regen. Res. 2019, 14, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhu, Y.; Yin, H.Y.; Guo, Z.Y.; Xu, F.; Xiao, B.; Jiang, W.L.; Guo, W.M.; Meng, H.Y.; Lu, S.B.; et al. Differentiation of adipose-derived stem cells into Schwann cell-like cells through intermittent induction: Potential advantage of cellular transient memory function. Stem Cell Res. Ther. 2018, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Siegmund, A.; Eigenberger, A.; Hartmann, V.; Langewost, F.; Hammer, N.; Anker, A.; Klein, K.; Morsczeck, C.; Prantl, L.; et al. Peripheral nerve regeneration-adipose-tissue-derived stem cells differentiated by a three-step protocol promote neurite elongation via NGF secretion. Cells 2022, 11, 2887. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cao, L.; Wang, Y.; Hua, Y.; Cai, Z.; Chen, J.; Chen, L.; Jin, Y.; Niu, L.; Shen, H.; et al. Human eyelid adipose tissue-derived Schwann cells promote regeneration of a transected sciatic nerve. Sci. Rep. 2017, 7, 43248. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Xu, Y.; Liu, X.; Xu, S.; Zhang, Y.; Zhu, Z.; He, B. Effect of induction time on the proliferation and differentiation of induced Schwann-like cells from adipose-derived stem cells. Cell. Mol. Neurobiol. 2020, 40, 1105–1116. [Google Scholar] [CrossRef]
- Sampathkumar, S.G.; Li, A.V.; Jones, M.B.; Sun, Z.; Yarema, K.J. Metabolic installation of thiols into sialic acid modulates adhesion and stem cell biology. Nat. Chem. Biol. 2006, 2, 149–152. [Google Scholar] [CrossRef]
- Du, J.; Meledeo, M.A.; Wang, Z.; Khanna, H.S.; Paruchuri, V.D.; Yarema, K.J. Metabolic glycoengineering: Sialic acid and beyond. Glycobiology 2009, 19, 1382–1401. [Google Scholar] [CrossRef]
- Du, J.; Yarema, K.J. Carbohydrate engineered cells for regenerative medicine. Adv. Drug Deliv. Rev. 2010, 62, 671–682. [Google Scholar] [CrossRef]
- Du, J.; Agatemor, C.; Saeui, C.T.; Bhattacharya, R.; Jia, X.; Yarema, K.J. Glycoengineering human neural and adipose stem cells with novel thiol-modified N-acetylmannosamine (ManNAc) analogs. Cells 2021, 10, 377. [Google Scholar] [CrossRef]
- Du, J.; Liu, X.; Yarema, K.J.; Jia, X. Glycoengineering human neural stem cells (hNSCs) for adhesion improvement using a novel thiol-modified N-acetylmannosamine (ManNAc) analog. Biomater. Adv. 2022, 134, 112675. [Google Scholar] [CrossRef]
- Hutton, D.L.; Grayson, W.L. Hypoxia inhibits de novo Vvascular assembly of adipose-derived stromal/stem cell populations, but promotes growth of preformed vessels. Tissue Eng. Part A 2016, 22, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Est-Witte, S.E.; Farris, A.L.; Tzeng, S.Y.; Hutton, D.L.; Gong, D.H.; Calabresi, K.G.; Grayson, W.L.; Green, J.J. Non-viral gene delivery of HIF-1α promotes angiogenesis in human adipose-derived stem cells. Acta. Biomater. 2020, 113, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, E.; Farris, A.; O’Sullivan, A.; Rodriguez, R.; Grayson, W. Comparison of stromal vascular vraction and passaged adipose-derived stromal/stem cells as point-of-care agents for bone regeneration. Tissue Eng. Part A 2019, 25, 1459–1469. [Google Scholar] [CrossRef]
- Temple, J.P.; Hutton, D.L.; Hung, B.P.; Huri, P.Y.; Cook, C.A.; Kondragunta, R.; Jia, X.; Grayson, W.L. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J. Biomed. Mater. Res. Part A 2014, 102, 4317–4325. [Google Scholar] [CrossRef]
- Hutton, D.L.; Kondragunta, R.; Moore, E.M.; Hung, B.P.; Jia, X.; Grayson, W.L. Tumor necrosis factor improves vascularization in osteogenic grafts engineered with human adipose-derived stem/stromal cells. PLoS ONE 2014, 9, e107199. [Google Scholar] [CrossRef]
- Sampathkumar, S.G.; Jones, M.B.; Yarema, K.J. Metabolic expression of thiol-derivatized sialic acids on the cell surface and their quantitative estimation by flow cytometry. Nat. Protoc. 2006, 1, 1840–1851. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Che, P.L.; Aich, U.; Tan, E.; Kim, H.J.; Sampathkumar, S.G.; Yarema, K.J. Deciphering glycan linkages involved in Jurkat cell interactions with gold-coated nanofibers via sugar-displayed thiols. Bioorg. Med. Chem. Lett. 2011, 21, 4980–4984. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Che, P.L.; Wang, Z.Y.; Aich, U.; Yarema, K.J. Designing a binding interface for control of cancer cell adhesion via 3D topography and metabolic oligosaccharide engineering. Biomaterials 2011, 32, 5427–5437. [Google Scholar] [CrossRef] [PubMed]
- Di Summa, P.G.; Schiraldi, L.; Cherubino, M.; Oranges, C.M.; Kalbermatten, D.F.; Raffoul, W.; Madduri, S. Adipose derived stem cells reduce fibrosis and promote nerve regeneration in rats. Anat. Rec. 2018, 301, 1714–1721. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.M.; Wang, Y.; Fu, W.L.; Liu, D.H.; Zhang, C.Y.; Wang, Q.L.; Tong, X.J. The combination of adipose-derived Schwann-like cells and acellular nerve allografts promotes sciatic nerve regeneration and repair through the JAK2/STAT3 signaling pathway in rats. Neuroscience 2019, 422, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mooney, D.J. Metabolic glycan labelling for cancer-targeted therapy. Nat. Chem. 2020, 12, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Agatemor, C.; Buettner, M.J.; Ariss, R.; Muthiah, K.; Saeui, C.T.; Yarema, K.J. Exploiting metabolic glycoengineering to advance healthcare. Nat. Rev. Chem. 2019, 3, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Horstkorte, R.; Rau, K.; Laabs, S.; Danker, K.; Reutter, W. Biochemical engineering of the N-acyl side chain of sialic acid leads to increased calcium influx from intracellular compartments and promotes differentiation of HL60 cells. FEBS Lett. 2004, 571, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Templeton, K.; Ramos, M.; Rose, J.; Le, B.; Zhou, Q.; Cressman, A.; Ferreyra, S.; Lebrilla, C.B.; Fierro, F.A. Mesenchymal stromal cells regulate sialylations of N-glycans, affecting cell migration and survival. Int. J. Mol. Sci. 2021, 22, 6868. [Google Scholar] [CrossRef]
- Prautsch, K.M.; Schmidt, A.; Paradiso, V.; Schaefer, D.J.; Guzman, R.; Kalbermatten, D.F.; Madduri, S. Modulation of human adipose stem cells’ neurotrophic capacity Uusing a variety of growth factors for neural tissue engineering applications: Zxonal growth, transcriptional, and phosphoproteomic analyses in vitro. Cells 2020, 9, 1939. [Google Scholar] [CrossRef]
- Trevor, L.V.; Riches-Suman, K.; Mahajan, A.L.; Thornton, M.J. Adipose tissue: A source of stem cells with potential for regenerative therapies for wound healing. J. Clin. Med. 2020, 9, 2161. [Google Scholar] [CrossRef]
- Fornaro, M.; Giovannelli, A.; Foggetti, A.; Muratori, L.; Geuna, S.; Novajra, G.; Perroteau, I. Role of neurotrophic factors in enhancing linear axonal growth of ganglionic sensory neurons in vitro. Neural Regen. Res. 2020, 15, 1732–1739. [Google Scholar] [CrossRef]
- Zarinfard, G.; Aliakbari, M.; Asgari, V.; Razavi, S. Upregulation of neurotrophic factors and myelin basic protein in Schwann-like cells by T3 hormone following transdifferentiation of human adipose-derived stem cells. Int. J. Mol. Cell. Med. 2022, 11, 41–54. [Google Scholar] [CrossRef]
- Piovesana, R.; Faroni, A.; Taggi, M.; Matera, A.; Soligo, M.; Canipari, R.; Manni, L.; Reid, A.J.; Tata, A.M. Muscarinic receptors modulate Nerve Growth Factor production in rat Schwann-like adipose-derived stem cells and in Schwann cells. Sci. Rep. 2020, 10, 7159. [Google Scholar] [CrossRef]
- Onesto, M.M.; Short, C.A.; Rempel, S.K.; Catlett, T.S.; Gomez, T.M. Growth factors as axon guidance molecules: Lessons from in vitro studies. Front. Neurosci. 2021, 15, 678454. [Google Scholar] [CrossRef] [PubMed]
- Rhode, S.C.; Beier, J.P.; Ruhl, T. Adipose tissue stem cells in peripheral nerve regeneration—In vitro and in vivo. J. Neurosci. Res. 2021, 99, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Eggers, R.; de Winter, F.; Hoyng, S.A.; Hoeben, R.C.; Malessy, M.J.A.; Tannemaat, M.R.; Verhaagen, J. Timed GDNF gene therapy using an immune-evasive gene switch promotes long distance axon regeneration. Brain 2019, 142, 295–311. [Google Scholar] [CrossRef] [PubMed]
- de León, A.; Gibon, J.; Barker, P.A. NGF-Dependent and BDNF-dependent DRG sensory neurons deploy distinct degenerative signaling mechanisms. eNeuro 2021, 8, ENEURO.0277-20.2020. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D. Nerve growth factor: A neuroimmune crosstalk mediator for all seasons. Immunology 2017, 151, 1–15. [Google Scholar] [CrossRef]
- Apfel, S.C.; Kessler, J.A.; Adornato, B.T.; Litchy, W.J.; Sanders, C.; Rask, C.A. Recombinant human nerve growth factor in the treatment of diabetic polyneuropathy. NGF Study Group. Neurology 1998, 51, 695–702. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Wang, Z.; Liu, X.; Hu, C.; Yarema, K.J.; Jia, X. Improving Schwann Cell Differentiation from Human Adipose Stem Cells with Metabolic Glycoengineering. Cells 2023, 12, 1190. https://doi.org/10.3390/cells12081190
Du J, Wang Z, Liu X, Hu C, Yarema KJ, Jia X. Improving Schwann Cell Differentiation from Human Adipose Stem Cells with Metabolic Glycoengineering. Cells. 2023; 12(8):1190. https://doi.org/10.3390/cells12081190
Chicago/Turabian StyleDu, Jian, Zihui Wang, Xiao Liu, Cecilia Hu, Kevin J. Yarema, and Xiaofeng Jia. 2023. "Improving Schwann Cell Differentiation from Human Adipose Stem Cells with Metabolic Glycoengineering" Cells 12, no. 8: 1190. https://doi.org/10.3390/cells12081190
APA StyleDu, J., Wang, Z., Liu, X., Hu, C., Yarema, K. J., & Jia, X. (2023). Improving Schwann Cell Differentiation from Human Adipose Stem Cells with Metabolic Glycoengineering. Cells, 12(8), 1190. https://doi.org/10.3390/cells12081190






