High-Fat Diet Modulates the Excitability of Neurons within the Brain–Liver Pathway
Abstract
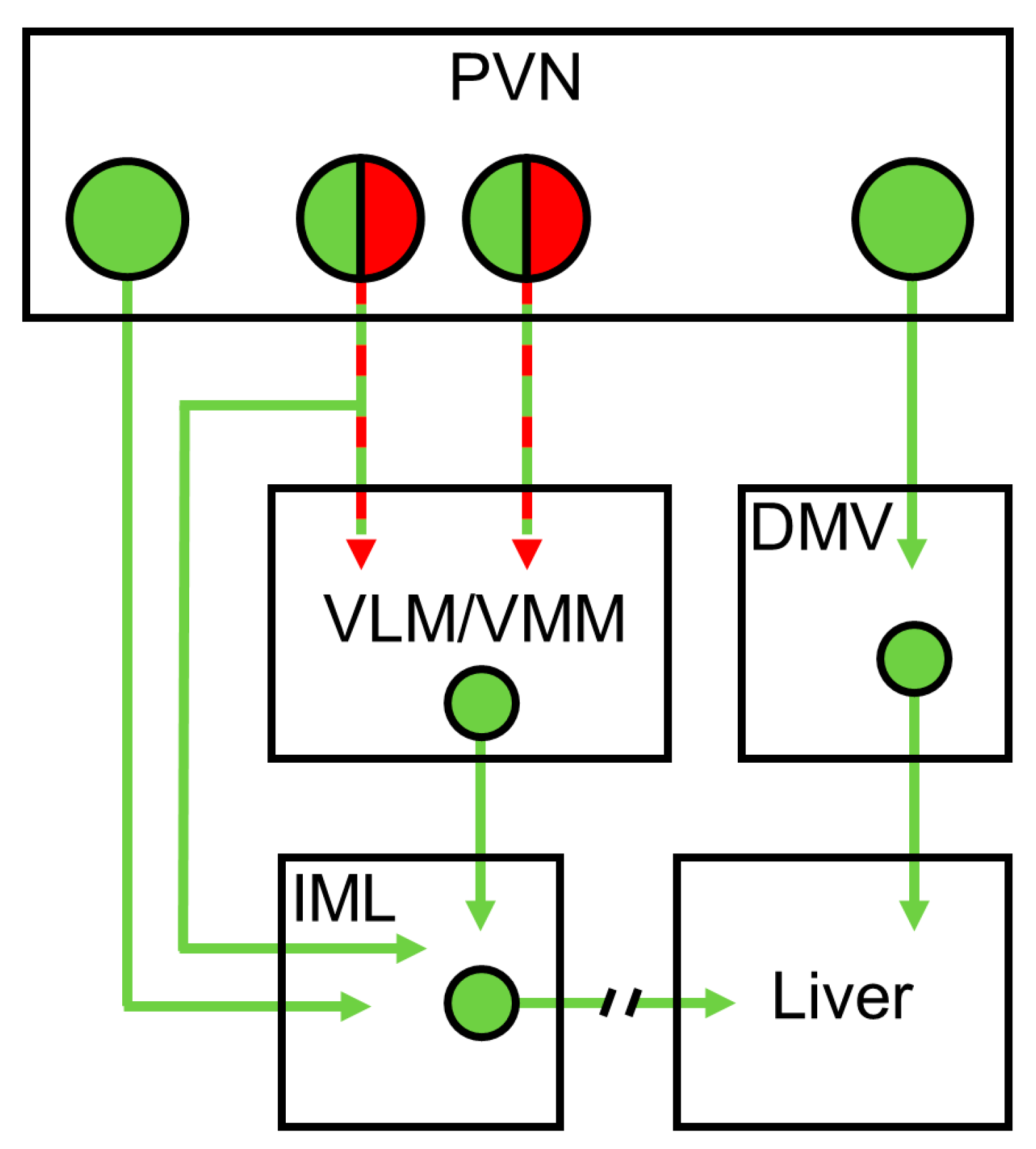
1. Introduction
2. Materials and Methods
2.1. Animals
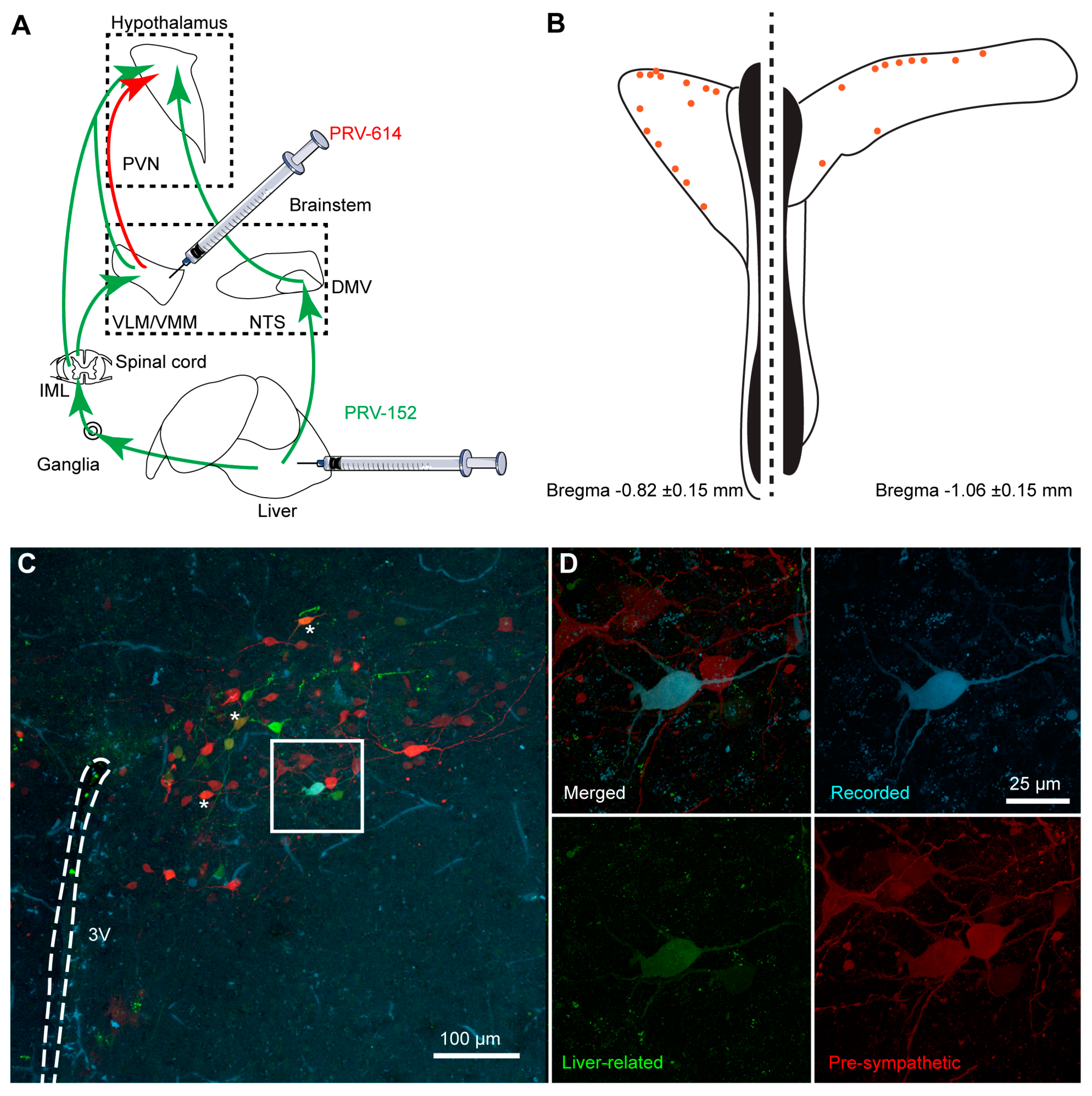
2.2. Pseudorabies Virus Inoculation
2.3. Brain Injections
2.4. Brain Slices Preparation
2.5. Whole-Cell Patch-Clamp Recordings
2.6. Visualization of the Recorded Cells
2.7. Gene Expression with Droplet Digital PCR
2.8. Statistical Analysis
3. Results
3.1. Increased Excitability of Liver-Related PVN Neurons in HFD-Fed Mice
3.2. The Excitability of Liver-Related PVN Neurons Is Decreased in Response to Insulin in Mice Fed with HFD
3.3. HFD and Insulin Do Not Alter the Excitability of VLM-Projecting Liver-Related PVN Neurons
3.4. Insulin Decreases the Excitability of Liver-Related Neurons in the VLM/VMM in HFD-Fed Mice
3.5. Insulin Receptor Expression in Liver-Related Neurons
4. Discussion
Plasticity of Liver-Related Neurons in HFD-Fed Mice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jensen, K.J.; Alpini, G.; Glaser, S. Hepatic nervous system and neurobiology of the liver. Compr. Physiol. 2013, 3, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Biffi, A.; Seravalle, G.; Trevano, F.Q.; Dell’Oro, R.; Corrao, G.; Mancia, G. Sympathetic Neural Overdrive in the Obese and Overweight State. Hypertension 2019, 74, 349–358. [Google Scholar] [CrossRef]
- Schlaich, M.; Straznicky, N.; Lambert, E.; Lambert, G. Metabolic syndrome: A sympathetic disease? Lancet Diabetes Endocrinol. 2015, 3, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Thorp, A.A.; Schlaich, M.P. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J. Diabetes Res. 2015, 2015, 341583. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Gang, G.T.; Ryu, D.; Koh, M.; Kim, Y.N.; Kim, S.S.; Park, J.; Kim, Y.H.; Sim, T.; Lee, I.K.; et al. Inverse agonist of nuclear receptor ERRγ mediates antidiabetic effect through inhibition of hepatic gluconeogenesis. Diabetes 2013, 62, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.T.; Frederico, M.J.; da Luz, G.; Cintra, D.E.; Ropelle, E.R.; Pauli, J.R.; Velloso, L.A. Acute exercise reduces hepatic glucose production through inhibition of the Foxo1/HNF-4alpha pathway in insulin resistant mice. J. Physiol. 2010, 588, 2239–2253. [Google Scholar] [CrossRef]
- Enriori, P.J.; Sinnayah, P.; Simonds, S.E.; Garcia Rudaz, C.; Cowley, M.A. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J. Neurosci. 2011, 31, 12189–12197. [Google Scholar] [CrossRef]
- Uyama, N.; Geerts, A.; Reynaert, H. Neural connections between the hypothalamus and the liver. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2004, 280, 808–820. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Bruinstroop, E.; Yi, C.X.; Klieverik, L.P.; La Fleur, S.E.; Fliers, E. Hypothalamic control of energy metabolism via the autonomic nervous system. Ann. N. Y. Acad. Sci. 2010, 1212, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.X.; la Fleur, S.E.; Fliers, E.; Kalsbeek, A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim. Biophys. Acta 2010, 1802, 416–431. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, J.D.; Zsombok, A. Brain-liver connections: Role of the preautonomic PVN neurons. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E183–E189. [Google Scholar] [CrossRef]
- Kalsbeek, A.; La Fleur, S.; Van Heijningen, C.; Buijs, R.M. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J. Neurosci. 2004, 24, 7604–7613. [Google Scholar] [CrossRef] [PubMed]
- Bisschop, P.H.; Fliers, E.; Kalsbeek, A. Autonomic regulation of hepatic glucose production. Compr. Physiol. 2015, 5, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Pyner, S.; Coote, J.H. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 2000, 100, 549–556. [Google Scholar] [CrossRef]
- Badoer, E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin. Exp. Pharmacol. Physiol. 2001, 28, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Zsombok, A.; Gao, H.; Miyata, K.; Issa, A.; Derbenev, A.V. Immunohistochemical localization of transient receptor potential vanilloid type 1 and insulin receptor substrate 2 and their co-localization with liver-related neurons in the hypothalamus and brainstem. Brain Res. 2011, 1398, 30–39. [Google Scholar] [CrossRef]
- Stanley, S.; Pinto, S.; Segal, J.; Perez, C.A.; Viale, A.; DeFalco, J.; Cai, X.; Heisler, L.K.; Friedman, J.M. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc. Natl. Acad. Sci. USA 2010, 107, 7024–7029. [Google Scholar] [CrossRef] [PubMed]
- La Fleur, S.E.; Kalsbeek, A.; Wortel, J.; Buijs, R.M. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res. 2000, 871, 50–56. [Google Scholar] [CrossRef]
- Gao, H.; Miyata, K.; Bhaskaran, M.D.; Derbenev, A.V.; Zsombok, A. Transient receptor potential vanilloid type 1-dependent regulation of liver-related neurons in the paraventricular nucleus of the hypothalamus diminished in the type 1 diabetic mouse. Diabetes 2012, 61, 1381–1390. [Google Scholar] [CrossRef]
- Gao, H.; Molinas, A.J.R.; Miyata, K.; Qiao, X.; Zsombok, A. Overactivity of Liver-Related Neurons in the Paraventricular Nucleus of the Hypothalamus: Electrophysiological Findings in db/db Mice. J. Neurosci. 2017, 37, 11140–11150. [Google Scholar] [CrossRef]
- Card, J.P. Practical considerations for the use of pseudorabies virus in transneuronal studies of neural circuitry. Neurosci. Biobehav. Rev. 1998, 22, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Cano, G.; Card, J.P.; Sved, A.F. Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J. Comp. Neurol. 2004, 471, 462–481. [Google Scholar] [CrossRef]
- Franklin, K.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA , 2007. [Google Scholar]
- Jiang, Y.; Gao, H.; Krantz, A.M.; Derbenev, A.V.; Zsombok, A. Reduced GABAergic inhibition of kidney-related PVN neurons in streptozotocin-treated type 1 diabetic mouse. J. Neurophysiol. 2013, 110, 2192–2202. [Google Scholar] [CrossRef]
- Stern, J.E. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J. Physiol. 2001, 537, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Rezai-Zadeh, K.; Desmoulins, L.D.; Muenzberg, H.; Derbenev, A.V.; Zsombok, A. GABAergic leptin receptor-expressing neurons in the dorsomedial hypothalamus project to brown adipose tissue-related neurons in the paraventricular nucleus of mice. Auton. Neurosci. 2022, 245, 103058. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.A.; Tasker, J.G. Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. J. Physiol. 2000, 523 Pt 1, 193–209. [Google Scholar] [CrossRef]
- Molinas, A.J.R.; Desmoulins, L.D.; Hamling, B.V.; Butcher, S.M.; Anwar, I.J.; Miyata, K.; Enix, C.L.; Dugas, C.M.; Satou, R.; Derbenev, A.V.; et al. Interaction between TRPV1-expressing neurons in the hypothalamus. J. Neurophysiol. 2019, 121, 140–151. [Google Scholar] [CrossRef]
- Bruinstroop, E.; Fliers, E.; Kalsbeek, A. Hypothalamic control of hepatic lipid metabolism via the autonomic nervous system. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 673–684. [Google Scholar] [CrossRef]
- Torres, H.; Huesing, C.; Burk, D.H.; Molinas, A.J.R.; Neuhuber, W.L.; Berthoud, H.-R.; Münzberg, H.; Derbenev, A.V.; Zsombok, A. Sympathetic innervation of the mouse kidney and liver arising from prevertebral ganglia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R328–R337. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, L.; Gao, W.; Hu, F.; Zhang, J.; Ren, Y.; Lin, R.; Feng, Q.; Cheng, M.; Ju, D.; et al. A Central Catecholaminergic Circuit Controls Blood Glucose Levels during Stress. Neuron 2017, 95, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Wang, Q.; Ritter, S. Selective Pharmacogenetic Activation of Catecholamine Subgroups in the Ventrolateral Medulla Elicits Key Glucoregulatory Responses. Endocrinology 2018, 159, 341–355. [Google Scholar] [CrossRef] [PubMed]
- The Jackson Laboratory. Phenotype Information for Diet-Induced Obese C57BL/6J (#380050). Available online: https://www.jax.org/jax-mice-and-services/strain-data-sheet-pages/phenotype-information-380050 (accessed on 15 March 2023).
- He, M.Q.; Wang, J.Y.; Wang, Y.; Sui, J.; Zhang, M.; Ding, X.; Zhao, Y.; Chen, Z.Y.; Ren, X.X.; Shi, B.Y. High-fat diet-induced adipose tissue expansion occurs prior to insulin resistance in C57BL/6J mice. Chronic Dis. Transl. Med. 2020, 6, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.J.; Yuan, F.; Zhang, Y.; Li, D.P. Upregulation of orexin receptor in paraventricular nucleus promotes sympathetic outflow in obese Zucker rats. Neuropharmacology 2015, 99, 481–490. [Google Scholar] [CrossRef]
- Buijs, R.M.; la Fleur, S.E.; Wortel, J.; Van Heyningen, C.; Zuiddam, L.; Mettenleiter, T.C.; Kalsbeek, A.; Nagai, K.; Niijima, A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J. Comp. Neurol. 2003, 464, 36–48. [Google Scholar] [CrossRef]
- Williams, K.W.; Margatho, L.O.; Lee, C.E.; Choi, M.; Lee, S.; Scott, M.M.; Elias, C.F.; Elmquist, J.K. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J. Neurosci. 2010, 30, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Spanswick, D.; Smith, M.A.; Mirshamsi, S.; Routh, V.H.; Ashford, M.L. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat. Neurosci. 2000, 3, 757–758. [Google Scholar] [CrossRef]
- Zhang, B.; Nakata, M.; Nakae, J.; Ogawa, W.; Yada, T. Central insulin action induces activation of paraventricular oxytocin neurons to release oxytocin into circulation. Sci. Rep. 2018, 8, 10415. [Google Scholar] [CrossRef]
- Garcia-Caceres, C.; Quarta, C.; Varela, L.; Gao, Y.; Gruber, T.; Legutko, B.; Jastroch, M.; Johansson, P.; Ninkovic, J.; Yi, C.X.; et al. Astrocytic Insulin Signaling Couples Brain Glucose Uptake with Nutrient Availability. Cell 2016, 166, 867–880. [Google Scholar] [CrossRef]
- Herrera Moro Chao, D.; Kirchner, M.K.; Pham, C.; Foppen, E.; Denis, R.G.P.; Castel, J.; Morel, C.; Montalban, E.; Hassouna, R.; Bui, L.C.; et al. Hypothalamic astrocytes control systemic glucose metabolism and energy balance. Cell Metab. 2022, 34, 1532–1547.e6. [Google Scholar] [CrossRef]
- Inoue, H.; Ogawa, W.; Asakawa, A.; Okamoto, Y.; Nishizawa, A.; Matsumoto, M.; Teshigawara, K.; Matsuki, Y.; Watanabe, E.; Hiramatsu, R.; et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab. 2006, 3, 267–275. [Google Scholar] [CrossRef]
- Pocai, A.; Obici, S.; Schwartz, G.J.; Rossetti, L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005, 1, 53–61. [Google Scholar] [CrossRef]
- Pagotto, U. Where does insulin resistance start? The brain. Diabetes Care 2009, 32 (Suppl. 2), S174–S177. [Google Scholar] [CrossRef] [PubMed]
- Klöckener, T.; Hess, S.; Belgardt, B.F.; Paeger, L.; Verhagen, L.A.; Husch, A.; Sohn, J.W.; Hampel, B.; Dhillon, H.; Zigman, J.M.; et al. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat. Neurosci. 2011, 14, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Suyama, S.; Ralevski, A.; Liu, Z.W.; Dietrich, M.O.; Yada, T.; Simonds, S.E.; Cowley, M.A.; Gao, X.B.; Diano, S.; Horvath, T.L. Plasticity of calcium-permeable AMPA glutamate receptors in Pro-opiomelanocortin neurons. Elife 2017, 6, e25755. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H. Chronic high-fat diet induces overeating and impairs synaptic transmission in feeding-related brain regions. Front. Mol. Neurosci. 2022, 15, 1019446. [Google Scholar] [CrossRef]
- Liu, J.; Dimitrov, S.; Sawangjit, A.; Born, J.; Ehrlich, I.; Hallschmid, M. Short-term high-fat feeding induces a reversible net decrease in synaptic AMPA receptors in the hypothalamus. J. Nutr. Biochem. 2021, 87, 108516. [Google Scholar] [CrossRef]
- Linehan, V.; Fang, L.Z.; Parsons, M.P.; Hirasawa, M. High-fat diet induces time-dependent synaptic plasticity of the lateral hypothalamus. Mol. Metab. 2020, 36, 100977. [Google Scholar] [CrossRef]
- de Noronha, S.R.; Campos, G.V.; Abreu, A.R.; de Souza, A.A.; Chianca, D.A., Jr.; de Menezes, R.C. High fat diet induced-obesity facilitates anxiety-like behaviors due to GABAergic impairment within the dorsomedial hypothalamus in rats. Behav. Brain Res. 2017, 316, 38–46. [Google Scholar] [CrossRef]
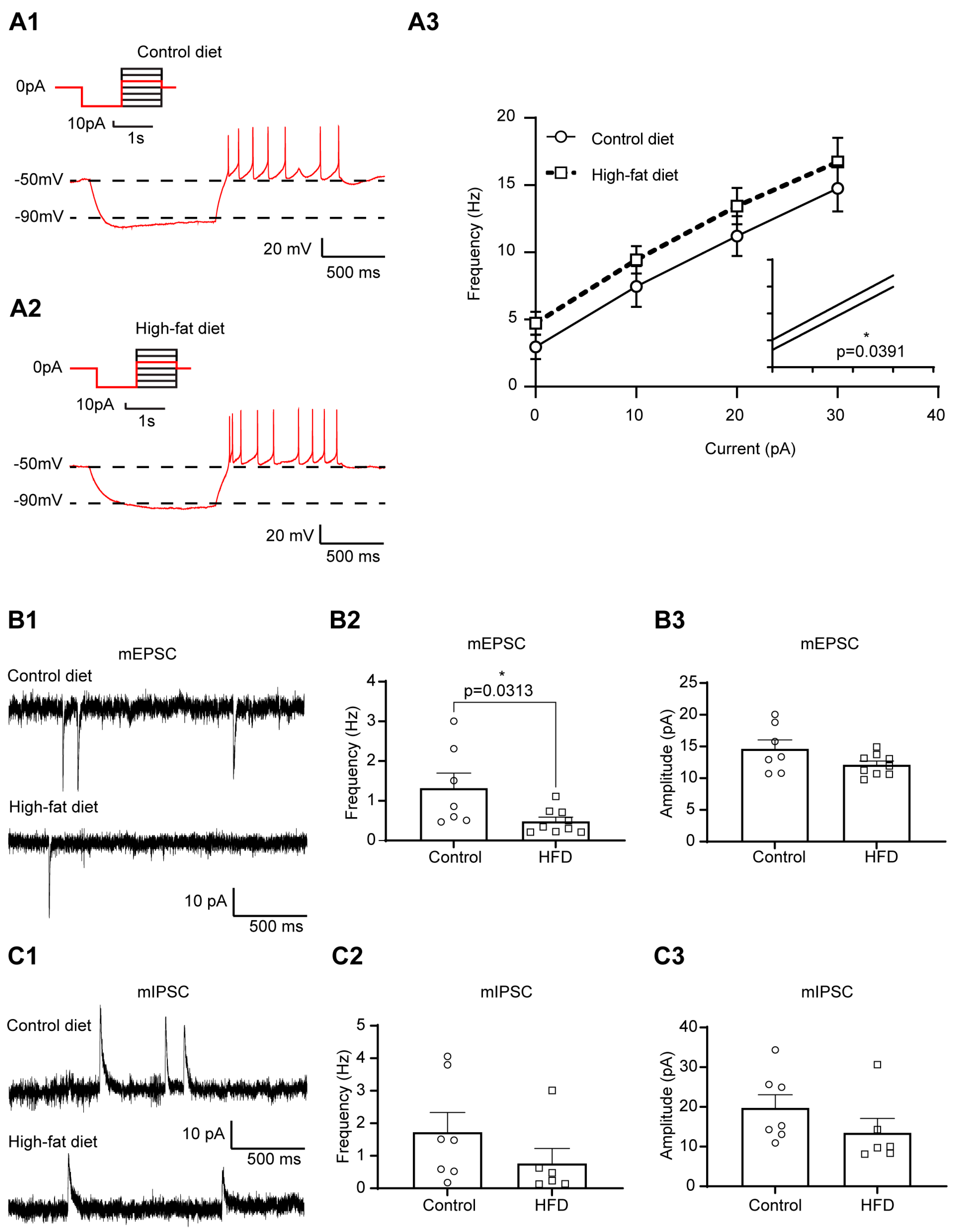

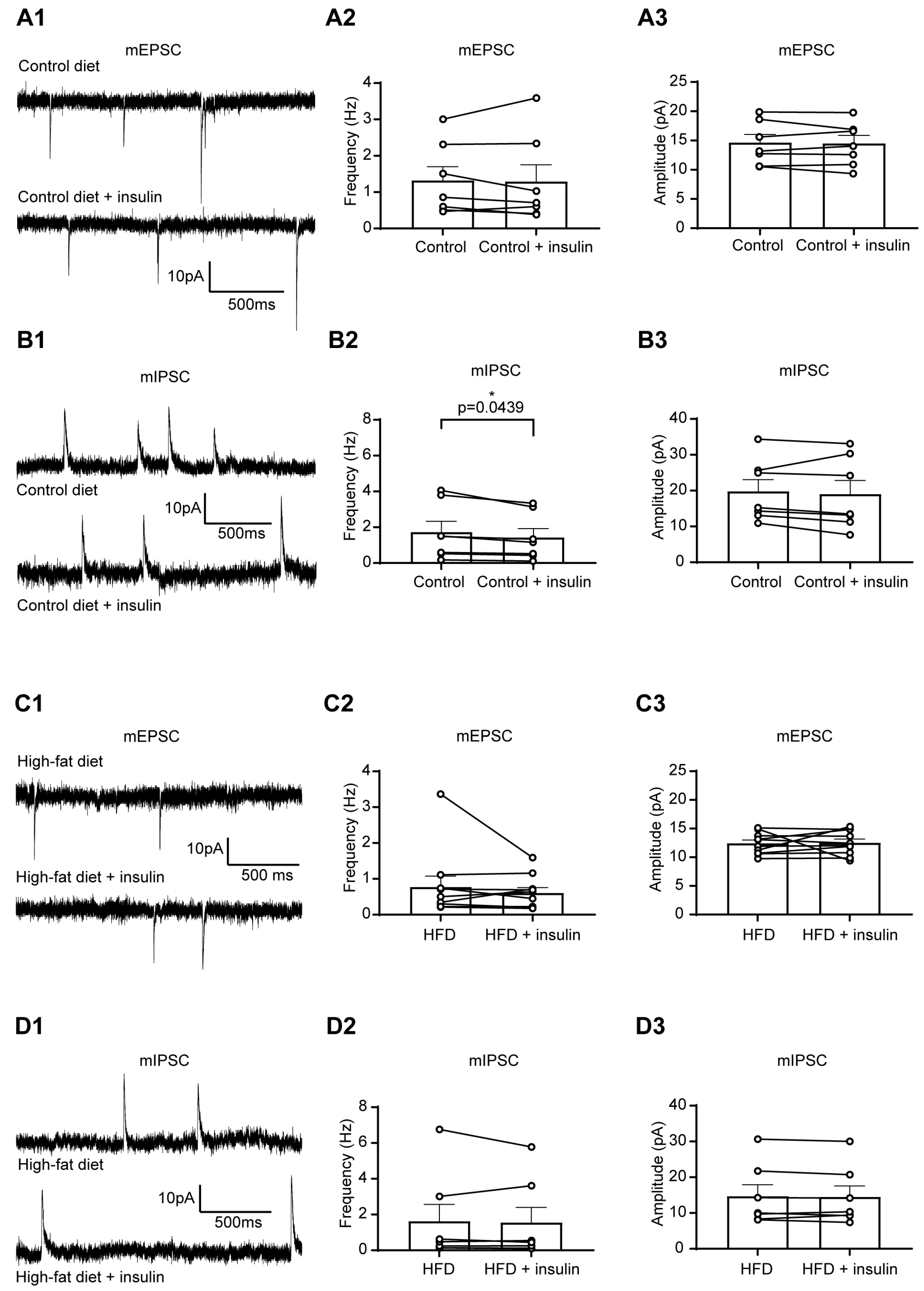
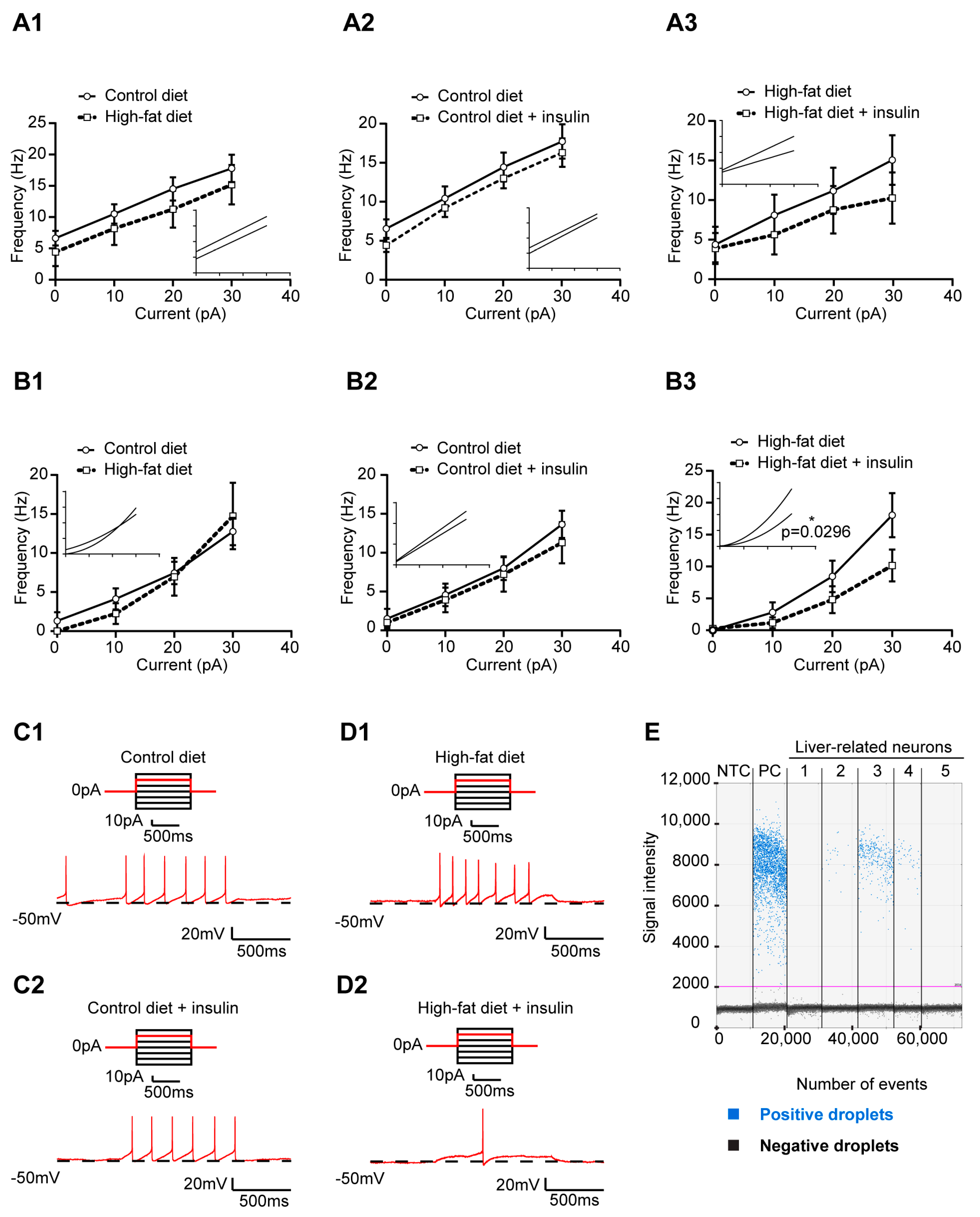
| Membrane Properties | mEPSC | mIPSC | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RMP (mV) | AP Frequency (Hz) | Rin (GΩ) | Frequency (Hz) | Amplitude (pA) | Frequency (Hz) | Amplitude (pA) | ||||||||||||||||||||||
| Mean | SEM | n | p | Mean | SEM | n | p | Mean | SEM | n | p | Mean | SEM | n | p | Mean | SEM | n | p | Mean | SEM | n | p | Mean | SEM | n | p | |
| Control diet | −50.71 | 2.17 | 10 | 0.69 | 0.79 | 0.40 | 6 | 0.61 | 1.00 | 0.09 | 10 | 0.38 | 1.17 | 0.17 | 9 | 0.29 | 14.47 | 1.19 | 9 | 0.78 | 0.81 | 0.16 | 11 | 0.14 | 13.43 | 0.70 | 11 | 0.07 |
| High-fat diet | −49.43 | 2.30 | 8 | 1.20 | 0.52 | 4 | 1.15 | 0.16 | 8 | 1.50 | 0.22 | 14 | 14.90 | 0.99 | 14 | 1.21 | 0.19 | 15 | 17.10 | 1.55 | 15 | |||||||
| Control diet | −50.71 | 2.17 | 10 | 0.27 | 0.59 | 0.32 | 8 | 0.25 | 1.00 | 0.09 | 10 | 0.21 | 1.62 | 0.40 | 8 | 0.06 | 13.77 | 1.50 | 8 | 0.66 | 0.79 | 0.18 | 10 | 0.60 | 13.17 | 0.72 | 10 | 0.20 |
| Control diet + Insulin | −49.35 | 1.81 | 10 | 0.25 | 0.06 | 8 | 1.07 | 0.11 | 10 | 1.40 | 0.37 | 8 | 14.06 | 1.74 | 8 | 0.86 | 0.15 | 10 | 12.08 | 0.74 | 10 | |||||||
| High-fat diet | −49.43 | 2.30 | 8 | 0.68 | 1.20 | 0.52 | 4 | >0.99 | 1.151 | 0.16 | 8 | 0.54 | 1.54 | 0.24 | 12 | 0.30 | 14.59 | 0.87 | 12 | 0.08 | 1.27 | 0.23 | 11 | 0.08 | 18.02 | 1.93 | 11 | 0.01 |
| High-fat diet + Insulin | −48.87 | 2.91 | 8 | 1.09 | 0.49 | 4 | 1.07 | 0.16 | 8 | 1.50 | 0.44 | 12 | 13.32 | 1.12 | 12 | 1.13 | 0.22 | 11 | 16.17 | 1.56 | 11 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinas, A.J.R.; Desmoulins, L.D.; Davis, R.K.; Gao, H.; Satou, R.; Derbenev, A.V.; Zsombok, A. High-Fat Diet Modulates the Excitability of Neurons within the Brain–Liver Pathway. Cells 2023, 12, 1194. https://doi.org/10.3390/cells12081194
Molinas AJR, Desmoulins LD, Davis RK, Gao H, Satou R, Derbenev AV, Zsombok A. High-Fat Diet Modulates the Excitability of Neurons within the Brain–Liver Pathway. Cells. 2023; 12(8):1194. https://doi.org/10.3390/cells12081194
Chicago/Turabian StyleMolinas, Adrien J. R., Lucie D. Desmoulins, Roslyn K. Davis, Hong Gao, Ryousuke Satou, Andrei V. Derbenev, and Andrea Zsombok. 2023. "High-Fat Diet Modulates the Excitability of Neurons within the Brain–Liver Pathway" Cells 12, no. 8: 1194. https://doi.org/10.3390/cells12081194
APA StyleMolinas, A. J. R., Desmoulins, L. D., Davis, R. K., Gao, H., Satou, R., Derbenev, A. V., & Zsombok, A. (2023). High-Fat Diet Modulates the Excitability of Neurons within the Brain–Liver Pathway. Cells, 12(8), 1194. https://doi.org/10.3390/cells12081194






