High-Fat Diet Modulates the Excitability of Neurons within the Brain–Liver Pathway
Abstract
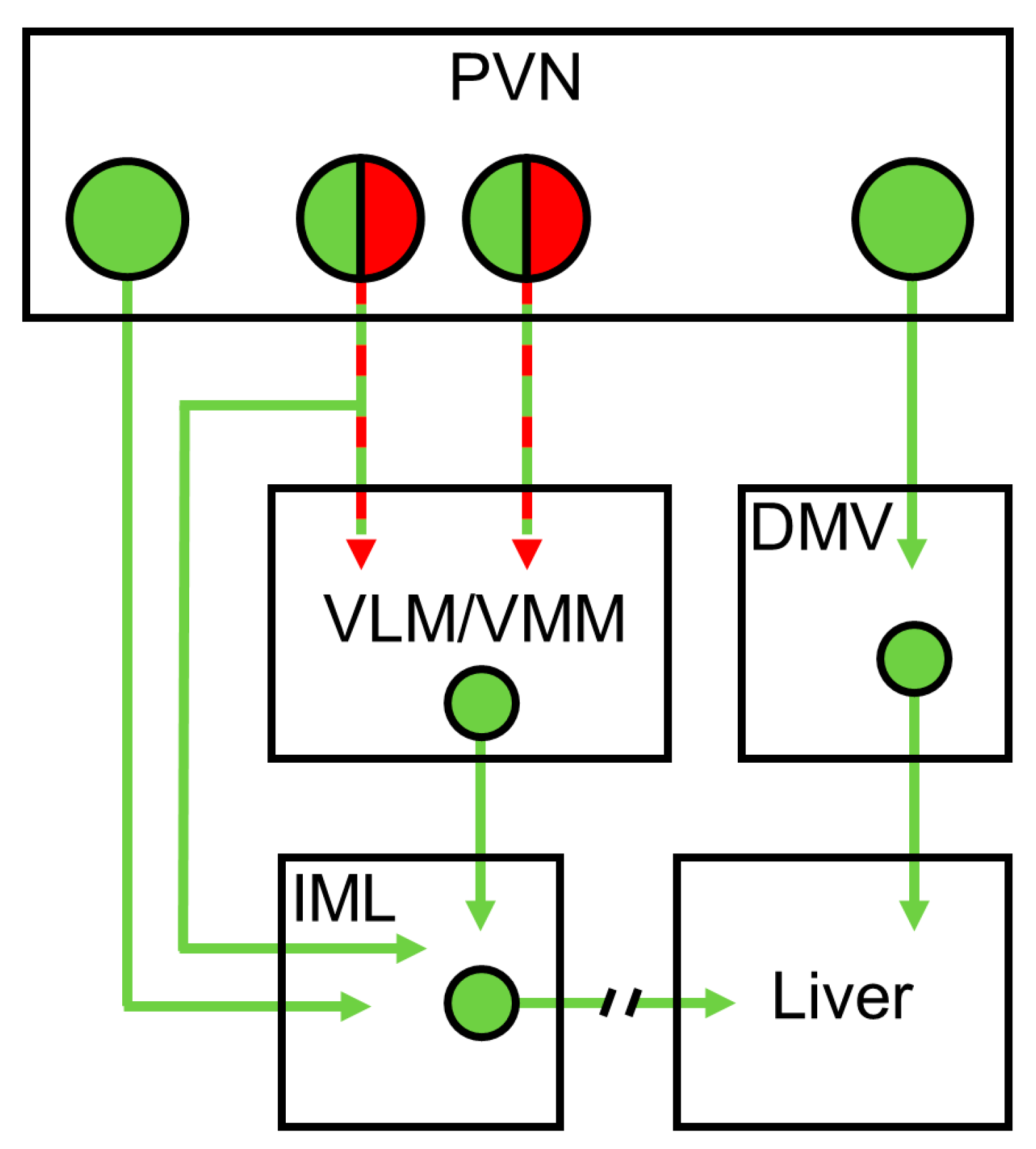
:1. Introduction
2. Materials and Methods
2.1. Animals
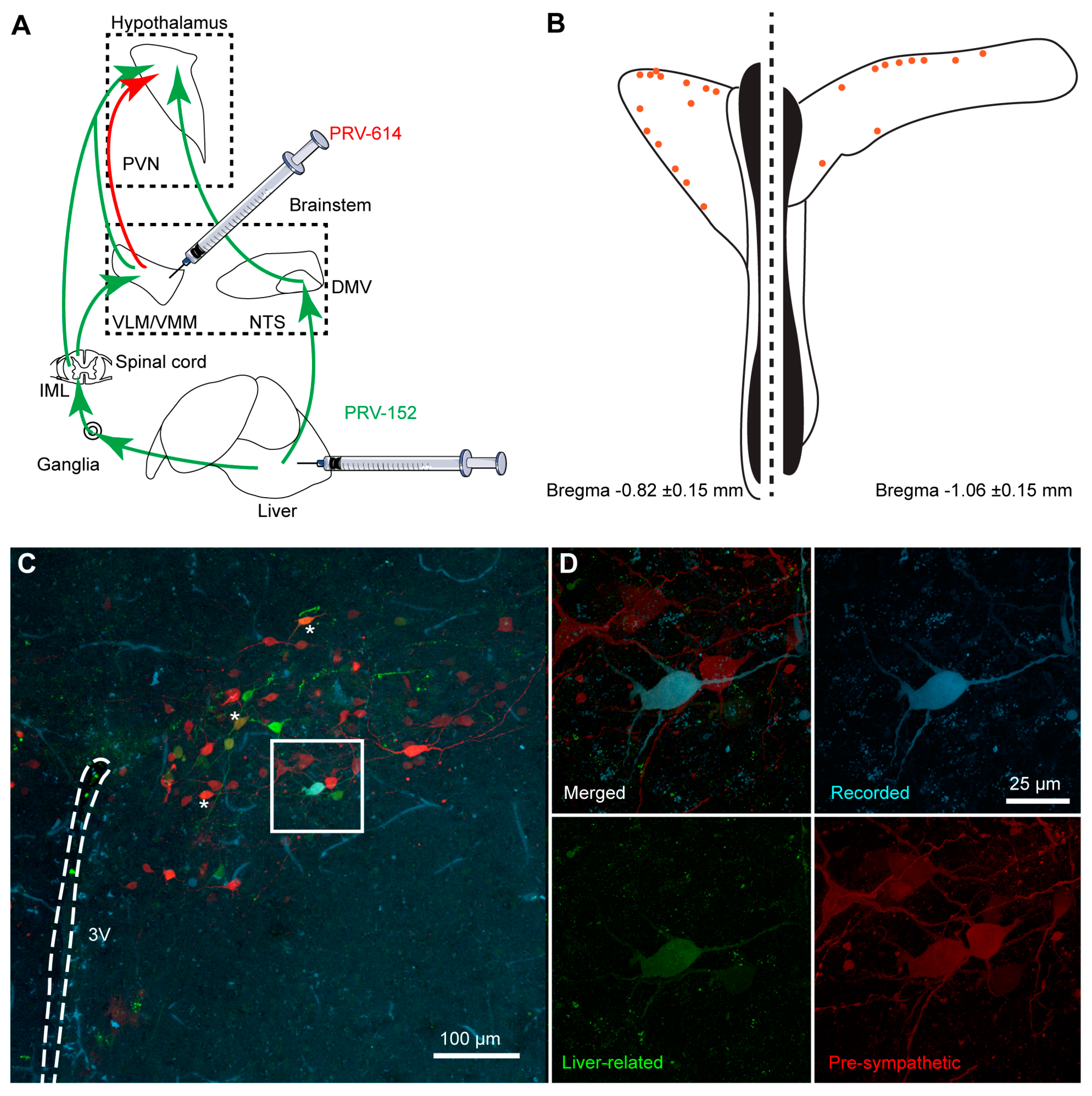
2.2. Pseudorabies Virus Inoculation
2.3. Brain Injections
2.4. Brain Slices Preparation
2.5. Whole-Cell Patch-Clamp Recordings
2.6. Visualization of the Recorded Cells
2.7. Gene Expression with Droplet Digital PCR
2.8. Statistical Analysis
3. Results
3.1. Increased Excitability of Liver-Related PVN Neurons in HFD-Fed Mice
3.2. The Excitability of Liver-Related PVN Neurons Is Decreased in Response to Insulin in Mice Fed with HFD
3.3. HFD and Insulin Do Not Alter the Excitability of VLM-Projecting Liver-Related PVN Neurons
3.4. Insulin Decreases the Excitability of Liver-Related Neurons in the VLM/VMM in HFD-Fed Mice
3.5. Insulin Receptor Expression in Liver-Related Neurons
4. Discussion
Plasticity of Liver-Related Neurons in HFD-Fed Mice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jensen, K.J.; Alpini, G.; Glaser, S. Hepatic nervous system and neurobiology of the liver. Compr. Physiol. 2013, 3, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Biffi, A.; Seravalle, G.; Trevano, F.Q.; Dell’Oro, R.; Corrao, G.; Mancia, G. Sympathetic Neural Overdrive in the Obese and Overweight State. Hypertension 2019, 74, 349–358. [Google Scholar] [CrossRef]
- Schlaich, M.; Straznicky, N.; Lambert, E.; Lambert, G. Metabolic syndrome: A sympathetic disease? Lancet Diabetes Endocrinol. 2015, 3, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Thorp, A.A.; Schlaich, M.P. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J. Diabetes Res. 2015, 2015, 341583. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Gang, G.T.; Ryu, D.; Koh, M.; Kim, Y.N.; Kim, S.S.; Park, J.; Kim, Y.H.; Sim, T.; Lee, I.K.; et al. Inverse agonist of nuclear receptor ERRγ mediates antidiabetic effect through inhibition of hepatic gluconeogenesis. Diabetes 2013, 62, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.T.; Frederico, M.J.; da Luz, G.; Cintra, D.E.; Ropelle, E.R.; Pauli, J.R.; Velloso, L.A. Acute exercise reduces hepatic glucose production through inhibition of the Foxo1/HNF-4alpha pathway in insulin resistant mice. J. Physiol. 2010, 588, 2239–2253. [Google Scholar] [CrossRef]
- Enriori, P.J.; Sinnayah, P.; Simonds, S.E.; Garcia Rudaz, C.; Cowley, M.A. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J. Neurosci. 2011, 31, 12189–12197. [Google Scholar] [CrossRef]
- Uyama, N.; Geerts, A.; Reynaert, H. Neural connections between the hypothalamus and the liver. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2004, 280, 808–820. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Bruinstroop, E.; Yi, C.X.; Klieverik, L.P.; La Fleur, S.E.; Fliers, E. Hypothalamic control of energy metabolism via the autonomic nervous system. Ann. N. Y. Acad. Sci. 2010, 1212, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.X.; la Fleur, S.E.; Fliers, E.; Kalsbeek, A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochim. Biophys. Acta 2010, 1802, 416–431. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, J.D.; Zsombok, A. Brain-liver connections: Role of the preautonomic PVN neurons. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E183–E189. [Google Scholar] [CrossRef]
- Kalsbeek, A.; La Fleur, S.; Van Heijningen, C.; Buijs, R.M. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J. Neurosci. 2004, 24, 7604–7613. [Google Scholar] [CrossRef] [PubMed]
- Bisschop, P.H.; Fliers, E.; Kalsbeek, A. Autonomic regulation of hepatic glucose production. Compr. Physiol. 2015, 5, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Pyner, S.; Coote, J.H. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 2000, 100, 549–556. [Google Scholar] [CrossRef]
- Badoer, E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin. Exp. Pharmacol. Physiol. 2001, 28, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Zsombok, A.; Gao, H.; Miyata, K.; Issa, A.; Derbenev, A.V. Immunohistochemical localization of transient receptor potential vanilloid type 1 and insulin receptor substrate 2 and their co-localization with liver-related neurons in the hypothalamus and brainstem. Brain Res. 2011, 1398, 30–39. [Google Scholar] [CrossRef]
- Stanley, S.; Pinto, S.; Segal, J.; Perez, C.A.; Viale, A.; DeFalco, J.; Cai, X.; Heisler, L.K.; Friedman, J.M. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc. Natl. Acad. Sci. USA 2010, 107, 7024–7029. [Google Scholar] [CrossRef] [PubMed]
- La Fleur, S.E.; Kalsbeek, A.; Wortel, J.; Buijs, R.M. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res. 2000, 871, 50–56. [Google Scholar] [CrossRef]
- Gao, H.; Miyata, K.; Bhaskaran, M.D.; Derbenev, A.V.; Zsombok, A. Transient receptor potential vanilloid type 1-dependent regulation of liver-related neurons in the paraventricular nucleus of the hypothalamus diminished in the type 1 diabetic mouse. Diabetes 2012, 61, 1381–1390. [Google Scholar] [CrossRef]
- Gao, H.; Molinas, A.J.R.; Miyata, K.; Qiao, X.; Zsombok, A. Overactivity of Liver-Related Neurons in the Paraventricular Nucleus of the Hypothalamus: Electrophysiological Findings in db/db Mice. J. Neurosci. 2017, 37, 11140–11150. [Google Scholar] [CrossRef]
- Card, J.P. Practical considerations for the use of pseudorabies virus in transneuronal studies of neural circuitry. Neurosci. Biobehav. Rev. 1998, 22, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Cano, G.; Card, J.P.; Sved, A.F. Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J. Comp. Neurol. 2004, 471, 462–481. [Google Scholar] [CrossRef]
- Franklin, K.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA , 2007. [Google Scholar]
- Jiang, Y.; Gao, H.; Krantz, A.M.; Derbenev, A.V.; Zsombok, A. Reduced GABAergic inhibition of kidney-related PVN neurons in streptozotocin-treated type 1 diabetic mouse. J. Neurophysiol. 2013, 110, 2192–2202. [Google Scholar] [CrossRef]
- Stern, J.E. Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J. Physiol. 2001, 537, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Rezai-Zadeh, K.; Desmoulins, L.D.; Muenzberg, H.; Derbenev, A.V.; Zsombok, A. GABAergic leptin receptor-expressing neurons in the dorsomedial hypothalamus project to brown adipose tissue-related neurons in the paraventricular nucleus of mice. Auton. Neurosci. 2022, 245, 103058. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.A.; Tasker, J.G. Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. J. Physiol. 2000, 523 Pt 1, 193–209. [Google Scholar] [CrossRef]
- Molinas, A.J.R.; Desmoulins, L.D.; Hamling, B.V.; Butcher, S.M.; Anwar, I.J.; Miyata, K.; Enix, C.L.; Dugas, C.M.; Satou, R.; Derbenev, A.V.; et al. Interaction between TRPV1-expressing neurons in the hypothalamus. J. Neurophysiol. 2019, 121, 140–151. [Google Scholar] [CrossRef]
- Bruinstroop, E.; Fliers, E.; Kalsbeek, A. Hypothalamic control of hepatic lipid metabolism via the autonomic nervous system. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 673–684. [Google Scholar] [CrossRef]
- Torres, H.; Huesing, C.; Burk, D.H.; Molinas, A.J.R.; Neuhuber, W.L.; Berthoud, H.-R.; Münzberg, H.; Derbenev, A.V.; Zsombok, A. Sympathetic innervation of the mouse kidney and liver arising from prevertebral ganglia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R328–R337. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, L.; Gao, W.; Hu, F.; Zhang, J.; Ren, Y.; Lin, R.; Feng, Q.; Cheng, M.; Ju, D.; et al. A Central Catecholaminergic Circuit Controls Blood Glucose Levels during Stress. Neuron 2017, 95, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Wang, Q.; Ritter, S. Selective Pharmacogenetic Activation of Catecholamine Subgroups in the Ventrolateral Medulla Elicits Key Glucoregulatory Responses. Endocrinology 2018, 159, 341–355. [Google Scholar] [CrossRef] [PubMed]
- The Jackson Laboratory. Phenotype Information for Diet-Induced Obese C57BL/6J (#380050). Available online: https://www.jax.org/jax-mice-and-services/strain-data-sheet-pages/phenotype-information-380050 (accessed on 15 March 2023).
- He, M.Q.; Wang, J.Y.; Wang, Y.; Sui, J.; Zhang, M.; Ding, X.; Zhao, Y.; Chen, Z.Y.; Ren, X.X.; Shi, B.Y. High-fat diet-induced adipose tissue expansion occurs prior to insulin resistance in C57BL/6J mice. Chronic Dis. Transl. Med. 2020, 6, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.J.; Yuan, F.; Zhang, Y.; Li, D.P. Upregulation of orexin receptor in paraventricular nucleus promotes sympathetic outflow in obese Zucker rats. Neuropharmacology 2015, 99, 481–490. [Google Scholar] [CrossRef]
- Buijs, R.M.; la Fleur, S.E.; Wortel, J.; Van Heyningen, C.; Zuiddam, L.; Mettenleiter, T.C.; Kalsbeek, A.; Nagai, K.; Niijima, A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J. Comp. Neurol. 2003, 464, 36–48. [Google Scholar] [CrossRef]
- Williams, K.W.; Margatho, L.O.; Lee, C.E.; Choi, M.; Lee, S.; Scott, M.M.; Elias, C.F.; Elmquist, J.K. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J. Neurosci. 2010, 30, 2472–2479. [Google Scholar] [CrossRef] [PubMed]
- Spanswick, D.; Smith, M.A.; Mirshamsi, S.; Routh, V.H.; Ashford, M.L. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat. Neurosci. 2000, 3, 757–758. [Google Scholar] [CrossRef]
- Zhang, B.; Nakata, M.; Nakae, J.; Ogawa, W.; Yada, T. Central insulin action induces activation of paraventricular oxytocin neurons to release oxytocin into circulation. Sci. Rep. 2018, 8, 10415. [Google Scholar] [CrossRef]
- Garcia-Caceres, C.; Quarta, C.; Varela, L.; Gao, Y.; Gruber, T.; Legutko, B.; Jastroch, M.; Johansson, P.; Ninkovic, J.; Yi, C.X.; et al. Astrocytic Insulin Signaling Couples Brain Glucose Uptake with Nutrient Availability. Cell 2016, 166, 867–880. [Google Scholar] [CrossRef]
- Herrera Moro Chao, D.; Kirchner, M.K.; Pham, C.; Foppen, E.; Denis, R.G.P.; Castel, J.; Morel, C.; Montalban, E.; Hassouna, R.; Bui, L.C.; et al. Hypothalamic astrocytes control systemic glucose metabolism and energy balance. Cell Metab. 2022, 34, 1532–1547.e6. [Google Scholar] [CrossRef]
- Inoue, H.; Ogawa, W.; Asakawa, A.; Okamoto, Y.; Nishizawa, A.; Matsumoto, M.; Teshigawara, K.; Matsuki, Y.; Watanabe, E.; Hiramatsu, R.; et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab. 2006, 3, 267–275. [Google Scholar] [CrossRef]
- Pocai, A.; Obici, S.; Schwartz, G.J.; Rossetti, L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005, 1, 53–61. [Google Scholar] [CrossRef]
- Pagotto, U. Where does insulin resistance start? The brain. Diabetes Care 2009, 32 (Suppl. 2), S174–S177. [Google Scholar] [CrossRef] [PubMed]
- Klöckener, T.; Hess, S.; Belgardt, B.F.; Paeger, L.; Verhagen, L.A.; Husch, A.; Sohn, J.W.; Hampel, B.; Dhillon, H.; Zigman, J.M.; et al. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat. Neurosci. 2011, 14, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Suyama, S.; Ralevski, A.; Liu, Z.W.; Dietrich, M.O.; Yada, T.; Simonds, S.E.; Cowley, M.A.; Gao, X.B.; Diano, S.; Horvath, T.L. Plasticity of calcium-permeable AMPA glutamate receptors in Pro-opiomelanocortin neurons. Elife 2017, 6, e25755. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H. Chronic high-fat diet induces overeating and impairs synaptic transmission in feeding-related brain regions. Front. Mol. Neurosci. 2022, 15, 1019446. [Google Scholar] [CrossRef]
- Liu, J.; Dimitrov, S.; Sawangjit, A.; Born, J.; Ehrlich, I.; Hallschmid, M. Short-term high-fat feeding induces a reversible net decrease in synaptic AMPA receptors in the hypothalamus. J. Nutr. Biochem. 2021, 87, 108516. [Google Scholar] [CrossRef]
- Linehan, V.; Fang, L.Z.; Parsons, M.P.; Hirasawa, M. High-fat diet induces time-dependent synaptic plasticity of the lateral hypothalamus. Mol. Metab. 2020, 36, 100977. [Google Scholar] [CrossRef]
- de Noronha, S.R.; Campos, G.V.; Abreu, A.R.; de Souza, A.A.; Chianca, D.A., Jr.; de Menezes, R.C. High fat diet induced-obesity facilitates anxiety-like behaviors due to GABAergic impairment within the dorsomedial hypothalamus in rats. Behav. Brain Res. 2017, 316, 38–46. [Google Scholar] [CrossRef]
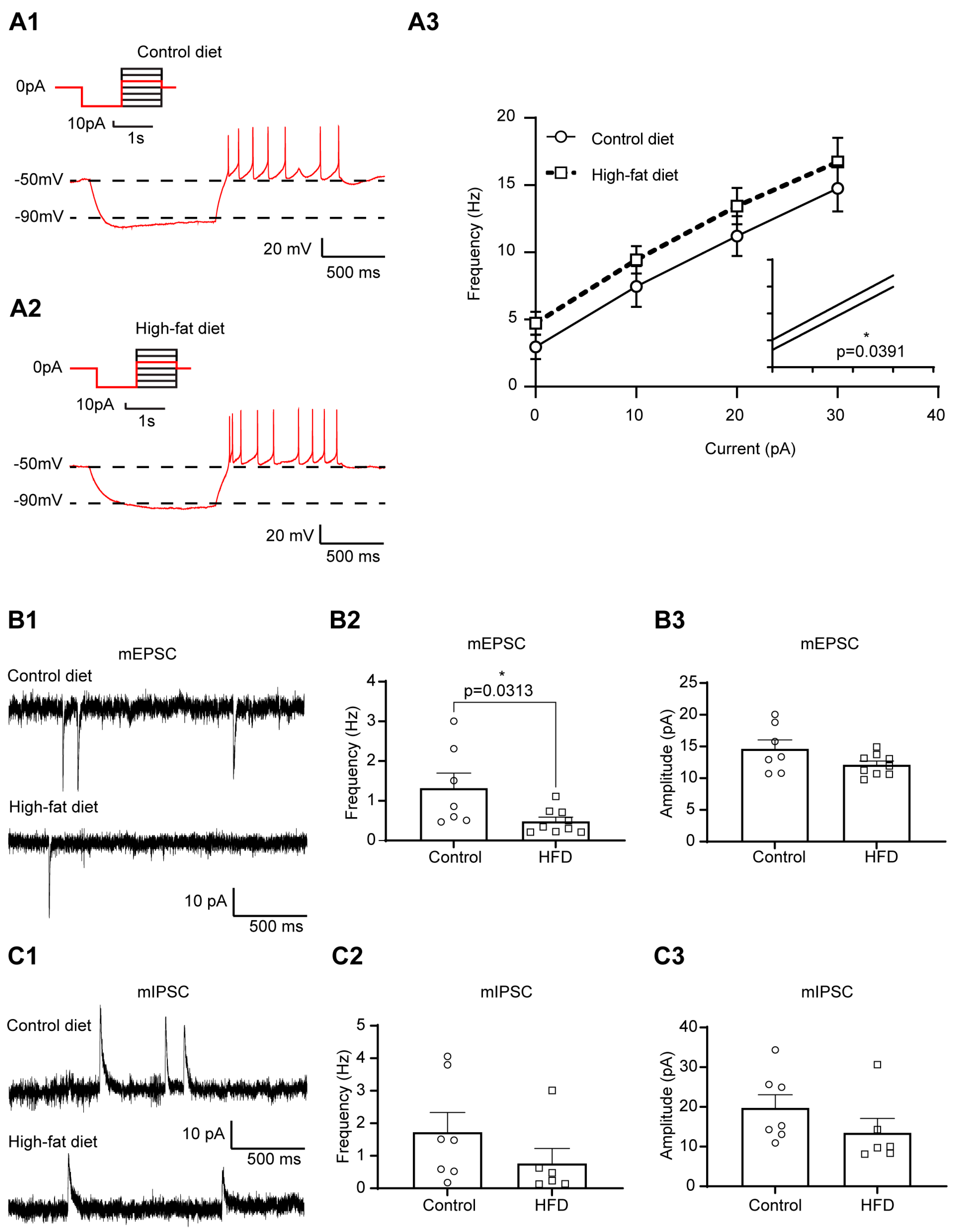

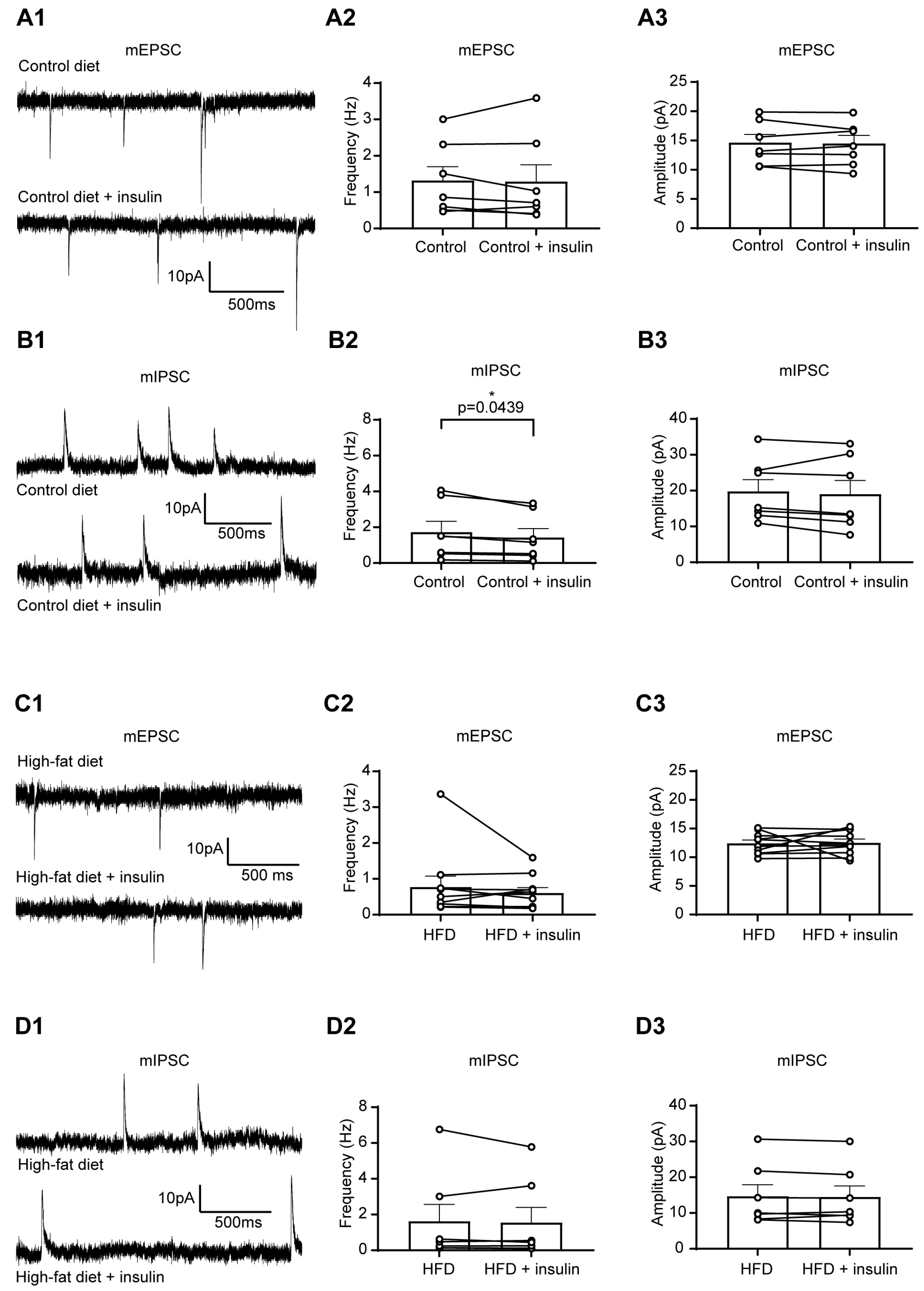
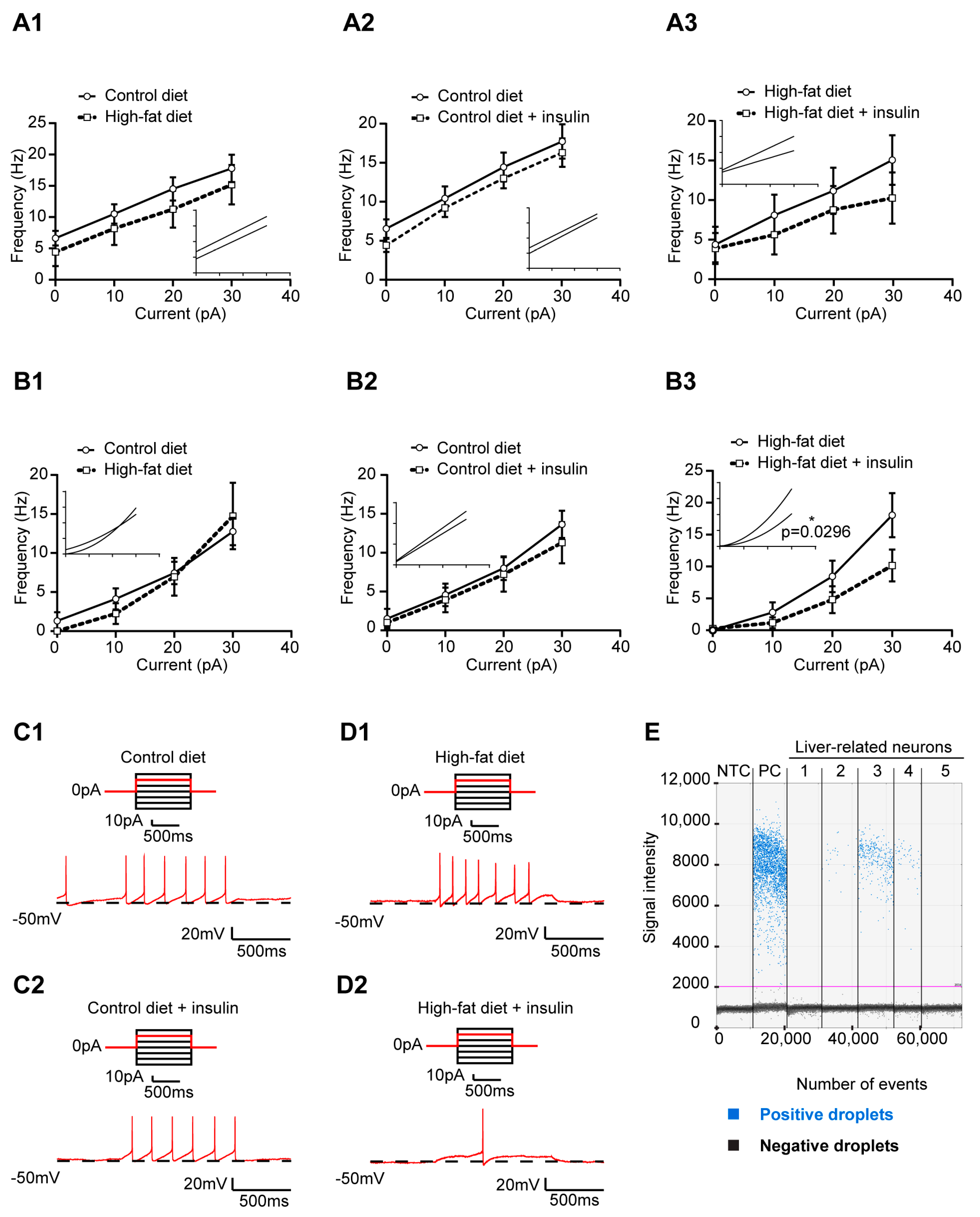
| Membrane Properties | mEPSC | mIPSC | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RMP (mV) | AP Frequency (Hz) | Rin (GΩ) | Frequency (Hz) | Amplitude (pA) | Frequency (Hz) | Amplitude (pA) | ||||||||||||||||||||||
| Mean | SEM | n | p | Mean | SEM | n | p | Mean | SEM | n | p | Mean | SEM | n | p | Mean | SEM | n | p | Mean | SEM | n | p | Mean | SEM | n | p | |
| Control diet | −50.71 | 2.17 | 10 | 0.69 | 0.79 | 0.40 | 6 | 0.61 | 1.00 | 0.09 | 10 | 0.38 | 1.17 | 0.17 | 9 | 0.29 | 14.47 | 1.19 | 9 | 0.78 | 0.81 | 0.16 | 11 | 0.14 | 13.43 | 0.70 | 11 | 0.07 |
| High-fat diet | −49.43 | 2.30 | 8 | 1.20 | 0.52 | 4 | 1.15 | 0.16 | 8 | 1.50 | 0.22 | 14 | 14.90 | 0.99 | 14 | 1.21 | 0.19 | 15 | 17.10 | 1.55 | 15 | |||||||
| Control diet | −50.71 | 2.17 | 10 | 0.27 | 0.59 | 0.32 | 8 | 0.25 | 1.00 | 0.09 | 10 | 0.21 | 1.62 | 0.40 | 8 | 0.06 | 13.77 | 1.50 | 8 | 0.66 | 0.79 | 0.18 | 10 | 0.60 | 13.17 | 0.72 | 10 | 0.20 |
| Control diet + Insulin | −49.35 | 1.81 | 10 | 0.25 | 0.06 | 8 | 1.07 | 0.11 | 10 | 1.40 | 0.37 | 8 | 14.06 | 1.74 | 8 | 0.86 | 0.15 | 10 | 12.08 | 0.74 | 10 | |||||||
| High-fat diet | −49.43 | 2.30 | 8 | 0.68 | 1.20 | 0.52 | 4 | >0.99 | 1.151 | 0.16 | 8 | 0.54 | 1.54 | 0.24 | 12 | 0.30 | 14.59 | 0.87 | 12 | 0.08 | 1.27 | 0.23 | 11 | 0.08 | 18.02 | 1.93 | 11 | 0.01 |
| High-fat diet + Insulin | −48.87 | 2.91 | 8 | 1.09 | 0.49 | 4 | 1.07 | 0.16 | 8 | 1.50 | 0.44 | 12 | 13.32 | 1.12 | 12 | 1.13 | 0.22 | 11 | 16.17 | 1.56 | 11 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinas, A.J.R.; Desmoulins, L.D.; Davis, R.K.; Gao, H.; Satou, R.; Derbenev, A.V.; Zsombok, A. High-Fat Diet Modulates the Excitability of Neurons within the Brain–Liver Pathway. Cells 2023, 12, 1194. https://doi.org/10.3390/cells12081194
Molinas AJR, Desmoulins LD, Davis RK, Gao H, Satou R, Derbenev AV, Zsombok A. High-Fat Diet Modulates the Excitability of Neurons within the Brain–Liver Pathway. Cells. 2023; 12(8):1194. https://doi.org/10.3390/cells12081194
Chicago/Turabian StyleMolinas, Adrien J. R., Lucie D. Desmoulins, Roslyn K. Davis, Hong Gao, Ryousuke Satou, Andrei V. Derbenev, and Andrea Zsombok. 2023. "High-Fat Diet Modulates the Excitability of Neurons within the Brain–Liver Pathway" Cells 12, no. 8: 1194. https://doi.org/10.3390/cells12081194
APA StyleMolinas, A. J. R., Desmoulins, L. D., Davis, R. K., Gao, H., Satou, R., Derbenev, A. V., & Zsombok, A. (2023). High-Fat Diet Modulates the Excitability of Neurons within the Brain–Liver Pathway. Cells, 12(8), 1194. https://doi.org/10.3390/cells12081194






