Contribution of Axon Initial Segment Structure and Channels to Brain Pathology
Abstract
1. Introduction
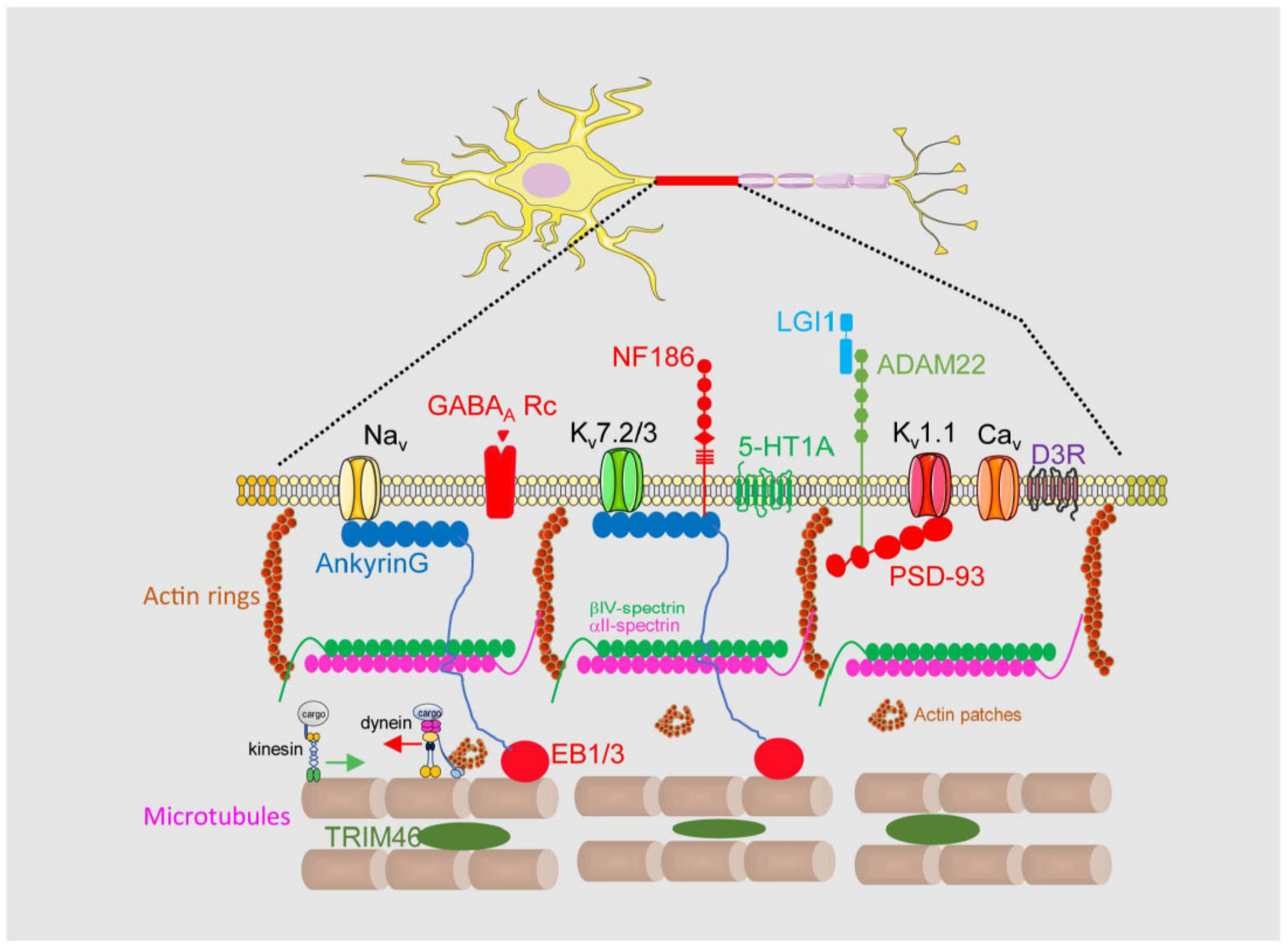
1.1. Axon Initial Segment Structure and Physiology
1.2. Axon Initial Segment Composition and Structure
1.3. Voltage-Gated Ion Channels in the AIS
1.4. Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels
1.5. Neurotransmitter Receptors at the AIS
1.6. Axon Initial Segment Plasticity and Modulation
2. Intrinsic and Extrinsic Axon Initial Segment Factors Contributing to Channelopathies
2.1. AIS Intrinsic Factors
2.1.1. AIS Voltage-Gated Ion Channels Mutations
2.1.2. Voltage-Gated Ion Channels Scaffold Proteins at the AIS
2.2. AIS Extrinsic Factors
3. Axon Initial Segment, Mental Disorders, and Neurodegenerative Diseases
3.1. Mental Disorders
3.2. Neurodevelopmental Disorders
3.3. Neuroinflammatory Diseases and Glial Cells
3.4. Autoimmune Diseases
3.5. Alzheimer’s Disease
Funding
Conflicts of Interest
References
- Imbrici, P.; Liantonio, A.; Camerino, G.M.; De Bellis, M.; Camerino, C.; Mele, A.; Giustino, A.; Pierno, S.; De Luca, A.; Tricarico, D.; et al. Therapeutic Approaches to Genetic Ion Channelopathies and Perspectives in Drug Discovery. Front. Pharmacol. 2016, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.C.; Jan, L.Y. The distribution and targeting of neuronal voltage-gated ion channels. Nat. Rev. Neurosci. 2006, 7, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, D.M. The neuronal channelopathies. Brain 2002, 125, 1177–1195. [Google Scholar] [CrossRef]
- Debanne, D.; Campanac, E.; Bialowas, A.; Carlier, E.; Alcaraz, G. Axon physiology. Physiol. Rev. 2011, 91, 555–602. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.; Del Puerto, A.; Puime, A.; Sanchez-Ponce, D.; Fronzaroli-Molinieres, L.; Pallas-Bazarra, N.; Carlier, E.; Giraud, P.; Debanne, D.; Wandosell, F.; et al. GSK3 and beta-catenin determines functional expression of sodium channels at the axon initial segment. Cell. Mol. Life Sci. 2013, 70, 105–120. [Google Scholar] [CrossRef]
- Tapia, M.; Wandosell, F.; Garrido, J.J. Impaired function of HDAC6 slows down axonal growth and interferes with axon initial segment development. PLoS ONE 2010, 5, e12908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ciorraga, M.; Mendez, P.; Retana, D.; Boumedine-Guignon, N.; Achon, B.; Russier, M.; Debanne, D.; Garrido, J.J. Formin Activity and mDia1 Contribute to Maintain Axon Initial Segment Composition and Structure. Mol. Neurobiol. 2021, 58, 6153–6169. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.W.; Rasband, M.N.; Meseguer, V.; Kramer, R.H.; Golding, N.L. Serotonin modulates spike probability in the axon initial segment through HCN channels. Nat. Neurosci. 2016, 19, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ben-Shalom, R.; Ahn, M.; Liptak, A.T.; van Rijn, R.M.; Whistler, J.L.; Bender, K.J. beta-Arrestin-Dependent Dopaminergic Regulation of Calcium Channel Activity in the Axon Initial Segment. Cell Rep. 2016, 16, 1518–1526. [Google Scholar] [CrossRef]
- Bender, K.J.; Trussell, L.O. The physiology of the axon initial segment. Annu. Rev. Neurosci. 2012, 35, 249–265. [Google Scholar] [CrossRef]
- Kole, M.H.; Ilschner, S.U.; Kampa, B.M.; Williams, S.R.; Ruben, P.C.; Stuart, G.J. Action potential generation requires a high sodium channel density in the axon initial segment. Nat. Neurosci. 2008, 11, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Del Puerto, A.; Fronzaroli-Molinieres, L.; Perez-Alvarez, M.J.; Giraud, P.; Carlier, E.; Wandosell, F.; Debanne, D.; Garrido, J.J. ATP-P2X7 Receptor Modulates Axon Initial Segment Composition and Function in Physiological Conditions and Brain Injury. Cereb. Cortex 2015, 25, 2282–2294. [Google Scholar] [CrossRef] [PubMed]
- Grubb, M.S.; Burrone, J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 2010, 465, 1070–1074. [Google Scholar] [CrossRef]
- Kuba, H. Plasticity at the axon initial segment. Commun. Integr. Biol. 2010, 3, 597–598. [Google Scholar] [CrossRef]
- Rasband, M.N. The axon initial segment and the maintenance of neuronal polarity. Nat. Rev. Neurosci. 2010, 11, 552–562. [Google Scholar] [CrossRef]
- Schafer, D.P.; Jha, S.; Liu, F.; Akella, T.; McCullough, L.D.; Rasband, M.N. Disruption of the axon initial segment cytoskeleton is a new mechanism for neuronal injury. J. Neurosci. 2009, 29, 13242–13254. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.M.; Bennett, V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J. Cell Biol. 2001, 155, 739–746. [Google Scholar] [CrossRef]
- Ogawa, Y.; Horresh, I.; Trimmer, J.S.; Bredt, D.S.; Peles, E.; Rasband, M.N. Postsynaptic density-93 clusters Kv1 channels at axon initial segments independently of Caspr2. J. Neurosci. 2008, 28, 5731–5739. [Google Scholar] [CrossRef] [PubMed]
- Nakada, C.; Ritchie, K.; Oba, Y.; Nakamura, M.; Hotta, Y.; Iino, R.; Kasai, R.S.; Yamaguchi, K.; Fujiwara, T.; Kusumi, A. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat. Cell Biol. 2003, 5, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Leterrier, C.; Vacher, H.; Fache, M.P.; d’Ortoli, S.A.; Castets, F.; Autillo-Touati, A.; Dargent, B. End-binding proteins EB3 and EB1 link microtubules to ankyrin G in the axon initial segment. Proc. Natl. Acad. Sci. USA 2011, 108, 8826–8831. [Google Scholar] [CrossRef]
- Leterrier, C. The Axon Initial Segment: An Updated Viewpoint. J. Neurosci. 2018, 38, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ponce, D.; Munoz, A.; Garrido, J.J. Casein kinase 2 and microtubules control axon initial segment formation. Mol. Cell. Neurosci. 2011, 46, 222–234. [Google Scholar] [CrossRef]
- Konishi, Y.; Setou, M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat. Neurosci. 2009, 12, 559–567. [Google Scholar] [CrossRef]
- Harterink, M.; Vocking, K.; Pan, X.; Soriano Jerez, E.M.; Slenders, L.; Freal, A.; Tas, R.P.; van de Wetering, W.J.; Timmer, K.; Motshagen, J.; et al. TRIM46 Organizes Microtubule Fasciculation in the Axon Initial Segment. J. Neurosci. 2019, 39, 4864–4873. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, M.J.; Leterrier, C. The functional architecture of axonal actin. Mol. Cell. Neurosci. 2018, 91, 151–159. [Google Scholar] [CrossRef]
- Eichel, K.; Shen, K. The function of the axon initial segment in neuronal polarity. Dev. Biol. 2022, 489, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, M.; van de Willige, D.; Freal, A.; Chazeau, A.; Franker, M.A.; Hofenk, J.; Rodrigues, R.J.; Kapitein, L.C.; Akhmanova, A.; Jaarsma, D.; et al. Dynein Regulator NDEL1 Controls Polarized Cargo Transport at the Axon Initial Segment. Neuron 2016, 89, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Benedeczky, I.; Molnar, E.; Somogyi, P. The cisternal organelle as a Ca(2+)-storing compartment associated with GABAergic synapses in the axon initial segment of hippocampal pyramidal neurones. Exp. Brain Res. 1994, 101, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Schluter, A.; Del Turco, D.; Deller, T.; Gutzmann, A.; Schultz, C.; Engelhardt, M. Structural Plasticity of Synaptopodin in the Axon Initial Segment during Visual Cortex Development. Cereb. Cortex 2017, 27, 4662–4675. [Google Scholar] [CrossRef] [PubMed]
- Hedstrom, K.L.; Ogawa, Y.; Rasband, M.N. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J. Cell Biol. 2008, 183, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Sobotzik, J.M.; Sie, J.M.; Politi, C.; Del Turco, D.; Bennett, V.; Deller, T.; Schultz, C. AnkyrinG is required to maintain axo-dendritic polarity in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 17564–17569. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.J.; Giraud, P.; Carlier, E.; Fernandes, F.; Moussif, A.; Fache, M.P.; Debanne, D.; Dargent, B. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science 2003, 300, 2091–2094. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Kao, T.; Horvath, Z.; Lemos, J.; Sul, J.Y.; Cranstoun, S.D.; Bennett, V.; Scherer, S.S.; Cooper, E.C. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J. Neurosci. 2006, 26, 2599–2613. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Lambert, S.; Malen, P.L.; Carpenter, S.; Boland, L.M.; Bennett, V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 1998, 143, 1295–1304. [Google Scholar] [CrossRef]
- Child, N.D.; Benarroch, E.E. Differential distribution of voltage-gated ion channels in cortical neurons: Implications for epilepsy. Neurology 2014, 82, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Boiko, T.; Van Wart, A.; Caldwell, J.H.; Levinson, S.R.; Trimmer, J.S.; Matthews, G. Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J. Neurosci. 2003, 23, 2306–2313. [Google Scholar] [CrossRef] [PubMed]
- Ogiwara, I.; Miyamoto, H.; Morita, N.; Atapour, N.; Mazaki, E.; Inoue, I.; Takeuchi, T.; Itohara, S.; Yanagawa, Y.; Obata, K.; et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: A circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J. Neurosci. 2007, 27, 5903–5914. [Google Scholar] [CrossRef]
- Hu, W.; Tian, C.; Li, T.; Yang, M.; Hou, H.; Shu, Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat. Neurosci. 2009, 12, 996–1002. [Google Scholar] [CrossRef]
- Rama, S.; Zbili, M.; Fekete, A.; Tapia, M.; Benitez, M.J.; Boumedine, N.; Garrido, J.J.; Debanne, D. The role of axonal Kv1 channels in CA3 pyramidal cell excitability. Sci. Rep. 2017, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Maureira, C.; Liu, X.; McCormick, D. P/Q and N channels control baseline and spike-triggered calcium levels in neocortical axons and synaptic boutons. J. Neurosci. 2010, 30, 11858–11869. [Google Scholar] [CrossRef] [PubMed]
- Bender, K.J.; Trussell, L.O. Axon initial segment Ca2+ channels influence action potential generation and timing. Neuron 2009, 61, 259–271. [Google Scholar] [CrossRef]
- Combe, C.L.; Gasparini, S. I(h) from synapses to networks: HCN channel functions and modulation in neurons. Prog. Biophys. Mol. Biol. 2021, 166, 119–132. [Google Scholar] [CrossRef]
- Bender, R.A.; Baram, T.Z. Hyperpolarization activated cyclic-nucleotide gated (HCN) channels in developing neuronal networks. Prog. Neurobiol. 2008, 86, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Baruscotti, M.; Bottelli, G.; Milanesi, R.; DiFrancesco, J.C.; DiFrancesco, D. HCN-related channelopathies. Pflug. Arch. 2010, 460, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Cruz, D.A.; Eggan, S.M.; Azmitia, E.C.; Lewis, D.A. Serotonin1A receptors at the axon initial segment of prefrontal pyramidal neurons in schizophrenia. Am. J. Psychiatry 2004, 161, 739–742. [Google Scholar] [CrossRef]
- Bender, K.J.; Ford, C.P.; Trussell, L.O. Dopaminergic modulation of axon initial segment calcium channels regulates action potential initiation. Neuron 2010, 68, 500–511. [Google Scholar] [CrossRef]
- Polter, A.M.; Li, X. 5-HT1A receptor-regulated signal transduction pathways in brain. Cell. Signal. 2010, 22, 1406–1412. [Google Scholar] [CrossRef]
- D’Amico, J.M.; Butler, A.A.; Heroux, M.E.; Cotel, F.; Perrier, J.M.; Butler, J.E.; Gandevia, S.C.; Taylor, J.L. Human motoneurone excitability is depressed by activation of serotonin 1A receptors with buspirone. J. Physiol. 2017, 595, 1763–1773. [Google Scholar] [CrossRef]
- Kuba, H.; Oichi, Y.; Ohmori, H. Presynaptic activity regulates Na(+) channel distribution at the axon initial segment. Nature 2010, 465, 1075–1078. [Google Scholar] [CrossRef]
- Kuba, H.; Yamada, R.; Ishiguro, G.; Adachi, R. Redistribution of Kv1 and Kv7 enhances neuronal excitability during structural axon initial segment plasticity. Nat. Commun. 2015, 6, 8815. [Google Scholar] [CrossRef] [PubMed]
- Chand, A.N.; Galliano, E.; Chesters, R.A.; Grubb, M.S. A distinct subtype of dopaminergic interneuron displays inverted structural plasticity at the axon initial segment. J. Neurosci. 2015, 35, 1573–1590. [Google Scholar] [CrossRef]
- von Reyn, C.R.; Spaethling, J.M.; Mesfin, M.N.; Ma, M.; Neumar, R.W.; Smith, D.H.; Siman, R.; Meaney, D.F. Calpain mediates proteolysis of the voltage-gated sodium channel alpha-subunit. J. Neurosci. 2009, 29, 10350–10356. [Google Scholar] [CrossRef] [PubMed]
- Reeves, T.M.; Greer, J.E.; Vanderveer, A.S.; Phillips, L.L. Proteolysis of submembrane cytoskeletal proteins ankyrin-G and alphaII-spectrin following diffuse brain injury: A role in white matter vulnerability at Nodes of Ranvier. Brain Pathol. 2010, 20, 1055–1068. [Google Scholar] [CrossRef]
- King, A.N.; Manning, C.F.; Trimmer, J.S. A unique ion channel clustering domain on the axon initial segment of mammalian neurons. J. Comp. Neurol. 2014, 522, 2594–2608. [Google Scholar] [CrossRef] [PubMed]
- Lipkin, A.M.; Cunniff, M.M.; Spratt, P.W.E.; Lemke, S.M.; Bender, K.J. Functional Microstructure of Ca(V)-Mediated Calcium Signaling in the Axon Initial Segment. J. Neurosci. 2021, 41, 3764–3776. [Google Scholar] [CrossRef]
- Jamann, N.; Dannehl, D.; Lehmann, N.; Wagener, R.; Thielemann, C.; Schultz, C.; Staiger, J.; Kole, M.H.P.; Engelhardt, M. Sensory input drives rapid homeostatic scaling of the axon initial segment in mouse barrel cortex. Nat. Commun. 2021, 12, 23. [Google Scholar] [CrossRef]
- Akter, N.; Fukaya, R.; Adachi, R.; Kawabe, H.; Kuba, H. Structural and Functional Refinement of the Axon Initial Segment in Avian Cochlear Nucleus during Development. J. Neurosci. 2020, 40, 6709–6721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bonadiman, A.; Ciorraga, M.; Benitez, M.J.; Garrido, J.J. P2Y1 Purinergic Receptor Modulate Axon Initial Segment Initial Development. Front. Cell. Neurosci. 2019, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.L.; Leo-Macias, A.; Yuen, S.; Khatri, L.; Pfennig, S.; Zhang, Y.; Agullo-Pascual, E.; Caillol, G.; Zhu, M.S.; Rothenberg, E.; et al. Localized Myosin II Activity Regulates Assembly and Plasticity of the Axon Initial Segment. Neuron 2018, 97, 555–570.e6. [Google Scholar] [CrossRef]
- Jahan, I.; Adachi, R.; Egawa, R.; Nomura, H.; Kuba, H. CDK5/p35-Dependent Microtubule Reorganization Contributes to Homeostatic Shortening of the Axon Initial Segment. J. Neurosci. 2023, 43, 359–372. [Google Scholar] [CrossRef]
- Spillane, J.; Kullmann, D.M.; Hanna, M.G. Genetic neurological channelopathies: Molecular genetics and clinical phenotypes. J. Neurol. Neurosurg. Psychiatry 2016, 87, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Mantegazza, M.; Cestele, S.; Catterall, W.A. Sodium channelopathies of skeletal muscle and brain. Physiol. Rev. 2021, 101, 1633–1689. [Google Scholar] [CrossRef]
- Escayg, A.; Goldin, A.L. Sodium channel SCN1A and epilepsy: Mutations and mechanisms. Epilepsia 2010, 51, 1650–1658. [Google Scholar] [CrossRef]
- Nappi, P.; Miceli, F.; Soldovieri, M.V.; Ambrosino, P.; Barrese, V.; Taglialatela, M. Epileptic channelopathies caused by neuronal Kv7 (KCNQ) channel dysfunction. Pflug. Arch. 2020, 472, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shapiro, M.S. Activity-dependent transcriptional regulation of M-Type (Kv7) K(+) channels by AKAP79/150-mediated NFAT actions. Neuron 2012, 76, 1133–1146. [Google Scholar] [CrossRef]
- Geiger, J.; Weber, Y.G.; Landwehrmeyer, B.; Sommer, C.; Lerche, H. Immunohistochemical analysis of KCNQ3 potassium channels in mouse brain. Neurosci. Lett. 2006, 400, 101–104. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, M.C.; Hasan, S.; Guglielmi, L.; Servettini, I.; Cenciarini, M.; Catacuzzeno, L.; Franciolini, F. New insights into the pathogenesis and therapeutics of episodic ataxia type 1. Front. Cell. Neurosci. 2015, 9, 317. [Google Scholar] [CrossRef] [PubMed]
- Zbili, M.; Rama, S.; Benitez, M.J.; Fronzaroli-Molinieres, L.; Bialowas, A.; Boumedine-Guignon, N.; Garrido, J.J.; Debanne, D. Homeostatic regulation of axonal Kv1.1 channels accounts for both synaptic and intrinsic modifications in the hippocampal CA3 circuit. Proc. Natl. Acad. Sci. USA 2021, 118, e2110601118. [Google Scholar] [CrossRef] [PubMed]
- Simms, B.A.; Zamponi, G.W. Neuronal voltage-gated calcium channels: Structure, function, and dysfunction. Neuron 2014, 82, 24–45. [Google Scholar] [CrossRef] [PubMed]
- Pietrobon, D. CaV2.1 channelopathies. Pflug. Arch. 2010, 460, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Klassen, T.; Davis, C.; Goldman, A.; Burgess, D.; Chen, T.; Wheeler, D.; McPherson, J.; Bourquin, T.; Lewis, L.; Villasana, D.; et al. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell 2011, 145, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Splawski, I.; Yoo, D.S.; Stotz, S.C.; Cherry, A.; Clapham, D.E.; Keating, M.T. CACNA1H mutations in autism spectrum disorders. J. Biol. Chem. 2006, 281, 22085–22091. [Google Scholar] [CrossRef] [PubMed]
- Stringer, R.N.; Jurkovicova-Tarabova, B.; Huang, S.; Haji-Ghassemi, O.; Idoux, R.; Liashenko, A.; Souza, I.A.; Rzhepetskyy, Y.; Lacinova, L.; Van Petegem, F.; et al. A rare CACNA1H variant associated with amyotrophic lateral sclerosis causes complete loss of Ca(v)3.2 T-type channel activity. Mol. Brain 2020, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Baez-Nieto, D.; Allen, A.; Akers-Campbell, S.; Yang, L.; Budnik, N.; Pupo, A.; Shin, Y.C.; Genovese, G.; Liao, M.; Perez-Palma, E.; et al. Analysing an allelic series of rare missense variants of CACNA1I in a Swedish schizophrenia cohort. Brain 2022, 145, 1839–1853. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.R.; Rasband, M.N. Ankyrins and neurological disease. Curr. Opin. Neurobiol. 2021, 69, 51–57. [Google Scholar] [CrossRef]
- Rueckert, E.H.; Barker, D.; Ruderfer, D.; Bergen, S.E.; O’Dushlaine, C.; Luce, C.J.; Sheridan, S.D.; Theriault, K.M.; Chambert, K.; Moran, J.; et al. Cis-acting regulation of brain-specific ANK3 gene expression by a genetic variant associated with bipolar disorder. Mol. Psychiatry 2013, 18, 922–929. [Google Scholar] [CrossRef]
- Roussos, P.; Katsel, P.; Davis, K.L.; Bitsios, P.; Giakoumaki, S.G.; Jogia, J.; Rozsnyai, K.; Collier, D.; Frangou, S.; Siever, L.J.; et al. Molecular and genetic evidence for abnormalities in the nodes of Ranvier in schizophrenia. Arch. Gen. Psychiatry 2012, 69, 7–15. [Google Scholar] [CrossRef]
- Logue, M.W.; Solovieff, N.; Leussis, M.P.; Wolf, E.J.; Melista, E.; Baldwin, C.; Koenen, K.C.; Petryshen, T.L.; Miller, M.W. The ankyrin-3 gene is associated with posttraumatic stress disorder and externalizing comorbidity. Psychoneuroendocrinology 2013, 38, 2249–2257. [Google Scholar] [CrossRef]
- Bi, C.; Wu, J.; Jiang, T.; Liu, Q.; Cai, W.; Yu, P.; Cai, T.; Zhao, M.; Jiang, Y.H.; Sun, Z.S. Mutations of ANK3 identified by exome sequencing are associated with autism susceptibility. Hum. Mutat. 2012, 33, 1635–1638. [Google Scholar] [CrossRef]
- Iqbal, Z.; Vandeweyer, G.; van der Voet, M.; Waryah, A.M.; Zahoor, M.Y.; Besseling, J.A.; Roca, L.T.; Vulto-van Silfhout, A.T.; Nijhof, B.; Kramer, J.M.; et al. Homozygous and heterozygous disruptions of ANK3: At the crossroads of neurodevelopmental and psychiatric disorders. Hum. Mol. Genet. 2013, 22, 1960–1970. [Google Scholar] [CrossRef]
- Liu, C.H.; Rasband, M.N. Axonal Spectrins: Nanoscale Organization, Functional Domains and Spectrinopathies. Front. Cell. Neurosci. 2019, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Saitsu, H.; Tohyama, J.; Kumada, T.; Egawa, K.; Hamada, K.; Okada, I.; Mizuguchi, T.; Osaka, H.; Miyata, R.; Furukawa, T.; et al. Dominant-negative mutations in alpha-II spectrin cause West syndrome with severe cerebral hypomyelination, spastic quadriplegia, and developmental delay. Am. J. Hum. Genet. 2010, 86, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, T.; Nelson, A.D.; Glanowska, K.; Murphy, G.G.; Jenkins, P.M.; Parent, J.M. Critical roles of alphaII spectrin in brain development and epileptic encephalopathy. J. Clin. Investig. 2018, 128, 760–773. [Google Scholar] [CrossRef]
- Knierim, E.; Gill, E.; Seifert, F.; Morales-Gonzalez, S.; Unudurthi, S.D.; Hund, T.J.; Stenzel, W.; Schuelke, M. A recessive mutation in beta-IV-spectrin (SPTBN4) associates with congenital myopathy, neuropathy, and central deafness. Hum. Genet. 2017, 136, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Ortiz-Gonzalez, X.R.; Yum, S.W.; Gill, S.M.; White, A.; Kelter, E.; Seaver, L.H.; Lee, S.; Wiley, G.; Gaffney, P.M.; et al. βIV Spectrinopathies Cause Profound Intellectual Disability, Congenital Hypotonia, and Motor Axonal Neuropathy. Am. J. Hum. Genet. 2018, 102, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- del Puerto, A.; Diaz-Hernandez, J.I.; Tapia, M.; Gomez-Villafuertes, R.; Benitez, M.J.; Zhang, J.; Miras-Portugal, M.T.; Wandosell, F.; Diaz-Hernandez, M.; Garrido, J.J. Adenylate cyclase 5 coordinates the action of ADP, P2Y1, P2Y13 and ATP-gated P2X7 receptors on axonal elongation. J. Cell Sci. 2012, 125, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Andrejew, R.; Oliveira-Giacomelli, A.; Ribeiro, D.E.; Glaser, T.; Arnaud-Sampaio, V.F.; Lameu, C.; Ulrich, H. The P2X7 Receptor: Central Hub of Brain Diseases. Front. Mol. Neurosci. 2020, 13, 124. [Google Scholar] [CrossRef]
- Kaphzan, H.; Buffington, S.A.; Jung, J.I.; Rasband, M.N.; Klann, E. Alterations in intrinsic membrane properties and the axon initial segment in a mouse model of Angelman syndrome. J. Neurosci. 2011, 31, 17637–17648. [Google Scholar] [CrossRef] [PubMed]
- Lossie, A.C.; Whitney, M.M.; Amidon, D.; Dong, H.J.; Chen, P.; Theriaque, D.; Hutson, A.; Nicholls, R.D.; Zori, R.T.; Williams, C.A.; et al. Distinct phenotypes distinguish the molecular classes of Angelman syndrome. J. Med. Genet. 2001, 38, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Rayi, P.R.; Bagrov, A.Y.; Kaphzan, H. Chronic alpha1-Na/K-ATPase inhibition reverses the elongation of the axon initial segment of the hippocampal CA1 pyramidal neurons in Angelman syndrome model mice. Neuropsychopharmacology 2021, 46, 654–664. [Google Scholar] [CrossRef]
- Gulledge, A.T.; Bravo, J.J. Neuron Morphology Influences Axon Initial Segment Plasticity. eNeuro 2016, 3, ENEURO.0085-15.2016. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.; Dominguez, A.; Zhang, W.; Del Puerto, A.; Ciorraga, M.; Benitez, M.J.; Guaza, C.; Garrido, J.J. Cannabinoid Receptors Modulate Neuronal Morphology and AnkyrinG Density at the Axon Initial Segment. Front. Cell. Neurosci. 2017, 11, 5. [Google Scholar] [CrossRef]
- Hamada, M.S.; Goethals, S.; de Vries, S.I.; Brette, R.; Kole, M.H. Covariation of axon initial segment location and dendritic tree normalizes the somatic action potential. Proc. Natl. Acad. Sci. USA 2016, 113, 14841–14846. [Google Scholar] [CrossRef]
- Benned-Jensen, T.; Christensen, R.K.; Denti, F.; Perrier, J.F.; Rasmussen, H.B.; Olesen, S.P. Live Imaging of Kv7.2/7.3 Cell Surface Dynamics at the Axon Initial Segment: High Steady-State Stability and Calpain-Dependent Excitotoxic Downregulation Revealed. J. Neurosci. 2016, 36, 2261–2266. [Google Scholar] [CrossRef] [PubMed]
- Atapour, N.; Rosa, M.G.P. Age-related plasticity of the axon initial segment of cortical pyramidal cells in marmoset monkeys. Neurobiol. Aging 2017, 57, 95–103. [Google Scholar] [CrossRef]
- Nascimento, A.I.; Da Silva, T.F.; Fernandes, E.C.; Luz, L.L.; Mar, F.M.; Safronov, B.V.; Sousa, M.M. Sensory neurons have an axon initial segment that initiates spontaneous activity in neuropathic pain. Brain 2022, 145, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Yermakov, L.M.; Drouet, D.E.; Griggs, R.B.; Elased, K.M.; Susuki, K. Type 2 Diabetes Leads to Axon Initial Segment Shortening in db/db Mice. Front. Cell. Neurosci. 2018, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Smolin, B.; Karry, R.; Gal-Ben-Ari, S.; Ben-Shachar, D. Differential expression of genes encoding neuronal ion-channel subunits in major depression, bipolar disorder and schizophrenia: Implications for pathophysiology. Int. J. Neuropsychopharmacol. 2012, 15, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Imbrici, P.; Camerino, D.C.; Tricarico, D. Major channels involved in neuropsychiatric disorders and therapeutic perspectives. Front. Genet. 2013, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Bavamian, S.; Mellios, N.; Lalonde, J.; Fass, D.M.; Wang, J.; Sheridan, S.D.; Madison, J.M.; Zhou, F.; Rueckert, E.H.; Barker, D.; et al. Dysregulation of miR-34a links neuronal development to genetic risk factors for bipolar disorder. Mol. Psychiatry 2015, 20, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Walder-Christensen, K.K.; Lalani, S.; Yan, H.; Garcia-Prieto, I.D.; Alvarez, S.; Fernandez-Jaen, A.; Speltz, L.; Jiang, Y.H.; Bennett, V. Neurodevelopmental mutation of giant ankyrin-G disrupts a core mechanism for axon initial segment assembly. Proc. Natl. Acad. Sci. USA 2019, 116, 19717–19726. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Cordner, Z.A.; Xiong, J.; Chiu, C.T.; Artola, A.; Zuo, Y.; Nelson, A.D.; Kim, T.Y.; Zaika, N.; Woolums, B.M.; et al. Genetic disruption of ankyrin-G in adult mouse forebrain causes cortical synapse alteration and behavior reminiscent of bipolar disorder. Proc. Natl. Acad. Sci. USA 2017, 114, 10479–10484. [Google Scholar] [CrossRef] [PubMed]
- Rees, E.; Carrera, N.; Morgan, J.; Hambridge, K.; Escott-Price, V.; Pocklington, A.J.; Richards, A.L.; Pardinas, A.F.; Investigators, G.; McDonald, C.; et al. Targeted Sequencing of 10,198 Samples Confirms Abnormalities in Neuronal Activity and Implicates Voltage-Gated Sodium Channels in Schizophrenia Pathogenesis. Biol. Psychiatry 2019, 85, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Mi, Z.; Yang, J.; He, Q.; Zhang, X.; Xiao, Y.; Shu, Y. Alterations of Electrophysiological Properties and Ion Channel Expression in Prefrontal Cortex of a Mouse Model of Schizophrenia. Front. Cell. Neurosci. 2019, 13, 554. [Google Scholar] [CrossRef]
- Page, S.C.; Sripathy, S.R.; Farinelli, F.; Ye, Z.; Wang, Y.; Hiler, D.J.; Pattie, E.A.; Nguyen, C.V.; Tippani, M.; Moses, R.L.; et al. Electrophysiological measures from human iPSC-derived neurons are associated with schizophrenia clinical status and predict individual cognitive performance. Proc. Natl. Acad. Sci. USA 2022, 119, e2109395119. [Google Scholar] [CrossRef]
- Lam, M.; Chen, C.Y.; Li, Z.; Martin, A.R.; Bryois, J.; Ma, X.; Gaspar, H.; Ikeda, M.; Benyamin, B.; Brown, B.C.; et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat. Genet. 2019, 51, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Booker, S.A.; Simoes de Oliveira, L.; Anstey, N.J.; Kozic, Z.; Dando, O.R.; Jackson, A.D.; Baxter, P.S.; Isom, L.L.; Sherman, D.L.; Hardingham, G.E.; et al. Input-Output Relationship of CA1 Pyramidal Neurons Reveals Intact Homeostatic Mechanisms in a Mouse Model of Fragile X Syndrome. Cell Rep. 2020, 32, 107988. [Google Scholar] [CrossRef]
- Tian, T.; Quintana-Urzainqui, I.; Kozic, Z.; Pratt, T.; Price, D.J. Pax6 loss alters the morphological and electrophysiological development of mouse prethalamic neurons. Development 2022, 149, dev200052. [Google Scholar] [CrossRef]
- Jacko, M.; Weyn-Vanhentenryck, S.M.; Smerdon, J.W.; Yan, R.; Feng, H.; Williams, D.J.; Pai, J.; Xu, K.; Wichterle, H.; Zhang, C. Rbfox Splicing Factors Promote Neuronal Maturation and Axon Initial Segment Assembly. Neuron 2018, 97, 853–868.e6. [Google Scholar] [CrossRef]
- Hong, T.; Falcone, C.; Dufour, B.; Amina, S.; Castro, R.P.; Regalado, J.; Pearson, W.; Noctor, S.C.; Martinez-Cerdeno, V. GABA(A)Ralpha2 is Decreased in the Axon Initial Segment of Pyramidal Cells in Specific Areas of the Prefrontal Cortex in Autism. Neuroscience 2020, 437, 76–86. [Google Scholar] [CrossRef]
- Usui, N.; Tian, X.; Harigai, W.; Togawa, S.; Utsunomiya, R.; Doi, T.; Miyoshi, K.; Shinoda, K.; Tanaka, J.; Shimada, S.; et al. Length impairments of the axon initial segment in rodent models of attention-deficit hyperactivity disorder and autism spectrum disorder. Neurochem. Int. 2022, 153, 105273. [Google Scholar] [CrossRef]
- Baalman, K.L.; Cotton, R.J.; Rasband, S.N.; Rasband, M.N. Blast wave exposure impairs memory and decreases axon initial segment length. J. Neurotrauma 2013, 30, 741–751. [Google Scholar] [CrossRef]
- Craner, M.J.; Newcombe, J.; Black, J.A.; Hartle, C.; Cuzner, M.L.; Waxman, S.G. Molecular changes in neurons in multiple sclerosis: Altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger. Proc. Natl. Acad. Sci. USA 2004, 101, 8168–8173. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, H.S.; Jensen, D.B.; Dimintiyanova, K.P.; Bonnevie, V.S.; Hedegaard, A.; Lehnhoff, J.; Moldovan, M.; Grondahl, L.; Meehan, C.F. Increased Axon Initial Segment Length Results in Increased Na(+) Currents in Spinal Motoneurones at Symptom Onset in the G127X SOD1 Mouse Model of Amyotrophic Lateral Sclerosis. Neuroscience 2021, 468, 247–264. [Google Scholar] [CrossRef]
- Sasaki, S.; Warita, H.; Abe, K.; Iwata, M. Impairment of axonal transport in the axon hillock and the initial segment of anterior horn neurons in transgenic mice with a G93A mutant SOD1 gene. Acta Neuropathol. 2005, 110, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Senol, A.D.; Pinto, G.; Beau, M.; Guillemot, V.; Dupree, J.L.; Stadelmann, C.; Ranft, J.; Lubetzki, C.; Davenne, M. Alterations of the axon initial segment in multiple sclerosis grey matter. Brain Commun. 2022, 4, fcac284. [Google Scholar] [CrossRef] [PubMed]
- Baalman, K.; Marin, M.A.; Ho, T.S.; Godoy, M.; Cherian, L.; Robertson, C.; Rasband, M.N. Axon initial segment-associated microglia. J. Neurosci. 2015, 35, 2283–2292. [Google Scholar] [CrossRef]
- Gallo, N.B.; Berisha, A.; Van Aelst, L. Microglia regulate chandelier cell axo-axonic synaptogenesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2114476119. [Google Scholar] [CrossRef]
- Schmaul, S.; Hanuscheck, N.; Bittner, S. Astrocytic potassium and calcium channels as integrators of the inflammatory and ischemic CNS microenvironment. Biol. Chem. 2021, 402, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Lezmy, J.; Arancibia-Carcamo, I.L.; Quintela-Lopez, T.; Sherman, D.L.; Brophy, P.J.; Attwell, D. Astrocyte Ca(2+)-evoked ATP release regulates myelinated axon excitability and conduction speed. Science 2021, 374, eabh2858. [Google Scholar] [CrossRef] [PubMed]
- Bartley, C.M.; Ngo, T.T.; Alvarenga, B.D.; Kung, A.F.; Teliska, L.H.; Sy, M.; DeRisi, J.L.; Rasband, M.N.; Pittock, S.J.; Dubey, D.; et al. betaIV-Spectrin Autoantibodies in 2 Individuals With Neuropathy of Possible Paraneoplastic Origin: A Case Series. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1188. [Google Scholar] [CrossRef] [PubMed]
- Berghs, S.; Ferracci, F.; Maksimova, E.; Gleason, S.; Leszczynski, N.; Butler, M.; De Camilli, P.; Solimena, M. Autoimmunity to beta IV spectrin in paraneoplastic lower motor neuron syndrome. Proc. Natl. Acad. Sci. USA 2001, 98, 6945–6950. [Google Scholar] [CrossRef]
- Valencia-Sanchez, C.; Knight, A.M.; Hammami, M.B.; Guo, Y.; Mills, J.R.; Kryzer, T.J.; Piquet, A.L.; Amin, A.; Heinzelmann, M.; Lucchinetti, C.F.; et al. Characterisation of TRIM46 autoantibody-associated paraneoplastic neurological syndrome. J. Neurol. Neurosurg. Psychiatry 2022, 93, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Bartley, C.M.; Ngo, T.T.; Cadwell, C.R.; Harroud, A.; Schubert, R.D.; Alvarenga, B.D.; Hawes, I.A.; Zorn, K.C.; Hunyh, T.; Teliska, L.H.; et al. Dual ankyrinG and subpial autoantibodies in a man with well-controlled HIV infection with steroid-responsive meningoencephalitis: A case report. Front. Neurol. 2022, 13, 1102484. [Google Scholar] [CrossRef]
- Ohkawa, T.; Fukata, Y.; Yamasaki, M.; Miyazaki, T.; Yokoi, N.; Takashima, H.; Watanabe, M.; Watanabe, O.; Fukata, M. Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. J. Neurosci. 2013, 33, 18161–18174. [Google Scholar] [CrossRef]
- Seagar, M.; Russier, M.; Caillard, O.; Maulet, Y.; Fronzaroli-Molinieres, L.; De San Feliciano, M.; Boumedine-Guignon, N.; Rodriguez, L.; Zbili, M.; Usseglio, F.; et al. LGI1 tunes intrinsic excitability by regulating the density of axonal Kv1 channels. Proc. Natl. Acad. Sci. USA 2017, 114, 7719–7724. [Google Scholar] [CrossRef] [PubMed]
- Pinatel, D.; Hivert, B.; Boucraut, J.; Saint-Martin, M.; Rogemond, V.; Zoupi, L.; Karagogeos, D.; Honnorat, J.; Faivre-Sarrailh, C. Inhibitory axons are targeted in hippocampal cell culture by anti-Caspr2 autoantibodies associated with limbic encephalitis. Front. Cell. Neurosci. 2015, 9, 265. [Google Scholar] [CrossRef]
- Zandi, M.S.; Irani, S.R.; Lang, B.; Waters, P.; Jones, P.B.; McKenna, P.; Coles, A.J.; Vincent, A.; Lennox, B.R. Disease-relevant autoantibodies in first episode schizophrenia. J. Neurol. 2011, 258, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wu, Y.; Gu, M.; Liu, Z.; Ma, Y.; Li, J.; Zhang, Y. Selective filtering defect at the axon initial segment in Alzheimer’s disease mouse models. Proc. Natl. Acad. Sci. USA 2014, 111, 14271–14276. [Google Scholar] [CrossRef]
- Vitale, P.; Salgueiro-Pereira, A.R.; Lupascu, C.A.; Willem, M.; Migliore, R.; Migliore, M.; Marie, H. Analysis of Age-Dependent Alterations in Excitability Properties of CA1 Pyramidal Neurons in an APPPS1 Model of Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 668948. [Google Scholar] [CrossRef]
- Liu, C.; Tan, F.C.; Xiao, Z.C.; Dawe, G.S. Amyloid precursor protein enhances Nav1.6 sodium channel cell surface expression. J. Biol. Chem. 2015, 290, 12048–12057. [Google Scholar] [CrossRef]
- Yuan, D.J.; Yang, G.; Wu, W.; Li, Q.F.; Xu, D.E.; Ntim, M.; Jiang, C.Y.; Liu, J.C.; Zhang, Y.; Wang, Y.Z.; et al. Reducing Nav1.6 expression attenuates the pathogenesis of Alzheimer’s disease by suppressing BACE1 transcription. Aging Cell 2022, 21, e13593. [Google Scholar] [CrossRef]
- Hessler, S.; Zheng, F.; Hartmann, S.; Rittger, A.; Lehnert, S.; Volkel, M.; Nissen, M.; Edelmann, E.; Saftig, P.; Schwake, M.; et al. beta-Secretase BACE1 regulates hippocampal and reconstituted M-currents in a beta-subunit-like fashion. J. Neurosci. 2015, 35, 3298–3311. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, G.; Burgaletto, C.; Bellanca, C.M.; Munafo, A.; Bernardini, R.; Cantarella, G. Role of Microglia and Astrocytes in Alzheimer’s Disease: From Neuroinflammation to Ca(2+) Homeostasis Dysregulation. Cells 2022, 11, 2728. [Google Scholar] [CrossRef] [PubMed]
- Anton-Fernandez, A.; Leon-Espinosa, G.; DeFelipe, J.; Munoz, A. Pyramidal cell axon initial segment in Alzheimer s disease. Sci. Rep. 2022, 12, 8722. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Akolkar, H.; Xu, J.; Liu, Y.; Popova, D.; Xie, J.; Youssef, M.M.; Benosman, R.; Hart, R.P.; Herrup, K. The Amyloid Precursor Protein Modulates the Position and Length of the Axon Initial Segment. J. Neurosci. 2023, 43, 1830–1844. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.A.; Ziburkus, J.; Jankowsky, J.; Rasband, M.N. Amyloid-beta plaques disrupt axon initial segments. Exp. Neurol. 2016, 281, 93–98. [Google Scholar] [CrossRef]
- Sohn, P.D.; Huang, C.T.; Yan, R.; Fan, L.; Tracy, T.E.; Camargo, C.M.; Montgomery, K.M.; Arhar, T.; Mok, S.A.; Freilich, R.; et al. Pathogenic Tau Impairs Axon Initial Segment Plasticity and Excitability Homeostasis. Neuron 2019, 104, 458–470.e5. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido, J.J. Contribution of Axon Initial Segment Structure and Channels to Brain Pathology. Cells 2023, 12, 1210. https://doi.org/10.3390/cells12081210
Garrido JJ. Contribution of Axon Initial Segment Structure and Channels to Brain Pathology. Cells. 2023; 12(8):1210. https://doi.org/10.3390/cells12081210
Chicago/Turabian StyleGarrido, Juan José. 2023. "Contribution of Axon Initial Segment Structure and Channels to Brain Pathology" Cells 12, no. 8: 1210. https://doi.org/10.3390/cells12081210
APA StyleGarrido, J. J. (2023). Contribution of Axon Initial Segment Structure and Channels to Brain Pathology. Cells, 12(8), 1210. https://doi.org/10.3390/cells12081210




