Sperm Motility Annotated Genes: Are They Associated with Impaired Fecundity?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Sperm Purification and RNA Extraction
2.3. Selection of Target Genes
2.4. Reverse Transcription and Pre-Amplification
2.5. RT-qPCR
2.6. Protein Lysate Preparation and LC-MS/MS Analysis
2.7. Mass Spectrometry and Data Processing
2.8. Statistical and Bioinformatics Analysis
3. Results
3.1. Basic Parameters of Oligoasthenozoospermic Men and Normozoospermic Controls
3.2. Differentially Expressed Genes in Sperm (Determined by RT-qPCR Analysis)
3.3. Differentially Expressed Proteins in Sperm (Determined Using LC-MS/MS)
3.4. Significantly Shared Genes and Proteins
3.5. Correlation of Expression Levels of Significant Genes and Proteins with Basic Semen Parameters
3.6. Enrichment Analysis of the Significantly Shared Genes and Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Oud, M.S.; Houston, B.J.; Volozonoka, L.; Mastrorosa, F.K.; Holt, G.S.; Alobaidi, B.K.S.; deVries, P.F.; Astuti, G.; Ramos, L.; McLachlan, R.I.; et al. Exome sequencing reveals variants in known and novel candidate genes for severe sperm motility disorders. Hum. Reprod. 2021, 36, 2597–2611. [Google Scholar] [CrossRef]
- Oud, M.S.; Volozonoka, L.; Smits, R.M.; Vissers, L.; Ramos, L.; Veltman, J.A. A systematic review and standardized clinical validity assessment of male infertility genes. Hum. Reprod. 2019, 34, 932–941. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Shen, L.; Zheng, A.; Meng, Q.; Li, H.; Yang, S. Clinical detection, diagnosis and treatment of morphological abnormalities of sperm flagella: A review of literature. Front. Genet. 2022, 13, 1034951. [Google Scholar] [CrossRef]
- Curi, S.M.; Ariagno, J.I.; Chenlo, P.H.; Mendeluk, G.R.; Pugliese, M.N.; Sardi Segovia, L.M.; Repetto, H.E.; Blanco, A.M. Asthenozoospermia: Analysis of a large population. Arch. Androl. 2003, 49, 343–349. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Abu-Halima, M.; Becker, L.S.; Al Smadi, M.A.; Kunz, L.S.; Groger, L.; Meese, E. Expression of SPAG7 and its regulatory microRNAs in seminal plasma and seminal plasma-derived extracellular vesicles of patients with subfertility. Sci. Rep. 2023, 13, 3645. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halima, M.; Belkacemi, A.; Ayesh, B.M.; Simone Becker, L.; Sindiani, A.M.; Fischer, U.; Hammadeh, M.; Keller, A.; Meese, E. MicroRNA-targeting in spermatogenesis: Over-expressions of microRNA-23a/b-3p and its affected targeting of the genes ODF2 and UBQLN3 in spermatozoa of patients with oligoasthenozoospermia. Andrology 2021, 9, 1137–1144. [Google Scholar] [CrossRef]
- Manfrevola, F.; Ferraro, B.; Sellitto, C.; Rocco, D.; Fasano, S.; Pierantoni, R.; Chianese, R. CRISP2, CATSPER1 and PATE1 Expression in Human Asthenozoospermic Semen. Cells 2021, 10, 1956. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Viana, P.; Barros, A.; Sa, R.; Sousa, M.; Pereira, R. Further Insights on RNA Expression and Sperm Motility. Genes 2022, 13, 1291. [Google Scholar] [CrossRef]
- Abu-Halima, M.; Khaizaran, Z.A.; Ayesh, B.M.; Fischer, U.; Khaizaran, S.A.; Al-Battah, F.; Hammadeh, M.; Keller, A.; Meese, E. MicroRNAs in combined spent culture media and sperm are associated with embryo quality and pregnancy outcome. Fertil. Steril. 2020, 113, 970–980.e2. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Grow, E.J.; Mlcochova, H.; Maher, G.J.; Lindskog, C.; Nie, X.; Guo, Y.; Takei, Y.; Yun, J.; Cai, L.; et al. The adult human testis transcriptional cell atlas. Cell Res. 2018, 28, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Hermann, B.P.; Cheng, K.; Singh, A.; Roa-De La Cruz, L.; Mutoji, K.N.; Chen, I.C.; Gildersleeve, H.; Lehle, J.D.; Mayo, M.; Westernstroer, B.; et al. The Mammalian Spermatogenesis Single-Cell Transcriptome, from Spermatogonial Stem Cells to Spermatids. Cell Rep. 2018, 25, 1650–1667.e8. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halima, M.; Hammadeh, M.; Schmitt, J.; Leidinger, P.; Keller, A.; Meese, E.; Backes, C. Altered microRNA expression profiles of human spermatozoa in patients with different spermatogenic impairments. Fertil. Steril. 2013, 99, 1249–1255.e16. [Google Scholar] [CrossRef] [PubMed]
- Carbon, S.; Ireland, A.; Mungall, C.J.; Shu, S.; Marshall, B.; Lewis, S.; Ami, G.O.H.; Web Presence Working, G. AmiGO: Online access to ontology and annotation data. Bioinformatics 2009, 25, 288–289. [Google Scholar] [CrossRef]
- Chen, J.; Bardes, E.E.; Aronow, B.J.; Jegga, A.G. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009, 37, W305–W311. [Google Scholar] [CrossRef]
- Abu-Halima, M.; Becker, L.S.; Ayesh, B.M.; Meese, E. MicroRNA-targeting in male infertility: Sperm microRNA-19a/b-3p and its spermatogenesis related transcripts content in men with oligoasthenozoospermia. Front. Cell Dev. Biol. 2022, 10, 973849. [Google Scholar] [CrossRef]
- Wisniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Rappsilber, J.; Mann, M.; Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007, 2, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.S.; Al Smadi, M.A.; Raeschle, M.; Rishik, S.; Abdul-Khaliq, H.; Meese, E.; Abu-Halima, M. Proteomic Landscape of Human Sperm in Patients with Different Spermatogenic Impairments. Cells 2023, 12, 1017. [Google Scholar] [CrossRef] [PubMed]
- Gunes, S.; Esteves, S.C. Role of genetics and epigenetics in male infertility. Andrologia 2021, 53, e13586. [Google Scholar] [CrossRef] [PubMed]
- Eddy, E.M.; Toshimori, K.; O’Brien, D.A. Fibrous sheath of mammalian spermatozoa. Microsc. Res. Tech. 2003, 61, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, D.; Tu, C.; Meng, L.; Tan, Y.; Ji, Z.; Cheng, J.; Lu, G.; Lin, G.; Zhang, H.; et al. Loss-of-function missense variant of AKAP4 induced male infertility through reduced interaction with QRICH2 during sperm flagella development. Hum. Mol. Genet. 2021, 31, 219–231. [Google Scholar] [CrossRef]
- Sadakierska-Chudy, A.; Patrylak, J.; Janeczko, J.; Chudy, J. Downregulation of gene expression and the outcome of ICSI in severe oligozoospermic patients: A preliminary study. Mol. Reprod. Dev. 2020, 87, 1219–1230. [Google Scholar] [CrossRef]
- Coutton, C.; Vargas, A.S.; Amiri-Yekta, A.; Kherraf, Z.E.; Ben Mustapha, S.F.; Le Tanno, P.; Wambergue-Legrand, C.; Karaouzene, T.; Martinez, G.; Crouzy, S.; et al. Mutations in CFAP43 and CFAP44 cause male infertility and flagellum defects in Trypanosoma and human. Nat. Commun. 2018, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.N.; Amiri-Yekta, A.; Martinez, G.; Saut, A.; Tek, J.; Stouvenel, L.; Lores, P.; Karaouzene, T.; Thierry-Mieg, N.; Satre, V.; et al. Absence of CFAP69 Causes Male Infertility due to Multiple Morphological Abnormalities of the Flagella in Human and Mouse. Am. J. Hum. Genet. 2018, 102, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, F.; Li, F.; Jiang, X.; Yang, Y.; Li, X.; Li, W.; Wang, X.; Cheng, J.; Liu, M.; et al. Loss-of-function mutations in QRICH2 cause male infertility with multiple morphological abnormalities of the sperm flagella. Nat. Commun. 2019, 10, 433. [Google Scholar] [CrossRef]
- Tang, S.; Wang, X.; Li, W.; Yang, X.; Li, Z.; Liu, W.; Li, C.; Zhu, Z.; Wang, L.; Wang, J.; et al. Biallelic Mutations in CFAP43 and CFAP44 Cause Male Infertility with Multiple Morphological Abnormalities of the Sperm Flagella. Am. J. Hum. Genet. 2017, 100, 854–864. [Google Scholar] [CrossRef]
- Merveille, A.C.; Davis, E.E.; Becker-Heck, A.; Legendre, M.; Amirav, I.; Bataille, G.; Belmont, J.; Beydon, N.; Billen, F.; Clement, A.; et al. CCDC39 is required for assembly of inner dynein arms and the dynein regulatory complex and for normal ciliary motility in humans and dogs. Nat. Genet. 2011, 43, 72–78. [Google Scholar] [CrossRef]
- Wang, W.L.; Tu, C.F.; Tan, Y.Q. Insight on multiple morphological abnormalities of sperm flagella in male infertility: What is new? Asian J. Androl. 2020, 22, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Precone, V.; Cannarella, R.; Paolacci, S.; Busetto, G.M.; Beccari, T.; Stuppia, L.; Tonini, G.; Zulian, A.; Marceddu, G.; Calogero, A.E.; et al. Male Infertility Diagnosis: Improvement of Genetic Analysis Performance by the Introduction of Pre-Diagnostic Genes in a Next-Generation Sequencing Custom-Made Panel. Front. Endocrinol. 2020, 11, 605237. [Google Scholar] [CrossRef] [PubMed]
- Precone, V.; Notarangelo, A.; Marceddu, G.; D’Agruma, L.; Cannarella, R.; Calogero, A.E.; Cristofoli, F.; Guerri, G.; Paolacci, S.; Castori, M.; et al. A simultaneous next-generation sequencing approach to the diagnosis of couple infertility. Minerva Endocrinol. 2022, 47, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.; Kherraf, Z.E.; Zouari, R.; Fourati Ben Mustapha, S.; Saut, A.; Pernet-Gallay, K.; Bertrand, A.; Bidart, M.; Hograindleur, J.P.; Amiri-Yekta, A.; et al. Whole-exome sequencing identifies mutations in FSIP2 as a recurrent cause of multiple morphological abnormalities of the sperm flagella. Hum. Reprod. 2018, 33, 1973–1984. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Martinez, G.; Liu, H.; Beurois, J.; Wu, H.; Amiri-Yekta, A.; Liang, D.; Kherraf, Z.E.; Bidart, M.; Cazin, C.; et al. Bi-allelic truncating variants in CFAP206 cause male infertility in human and mouse. Hum. Genet. 2021, 140, 1367–1377. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.; Wang, X.; Yuan, G.; Zhang, C.; Yang, Y. A novel homozygous CFAP65 mutation in humans causes male infertility with multiple morphological abnormalities of the sperm flagella. Clin. Genet. 2019, 96, 541–548. [Google Scholar] [CrossRef]
- Wang, W.; Tian, S.; Nie, H.; Tu, C.; Liu, C.; Li, Y.; Li, D.; Yang, X.; Meng, L.; Hu, T.; et al. CFAP65 is required in the acrosome biogenesis and mitochondrial sheath assembly during spermiogenesis. Hum. Mol. Genet. 2021, 30, 2240–2254. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Y.; Li, Y.; Sun, J.; Yang, Y.; Shen, Y. Novel mutations in FSIP2 lead to multiple morphological abnormalities of the sperm flagella and poor ICSI prognosis. Gene 2021, 781, 145536. [Google Scholar] [CrossRef]
- Martins, A.D.; Panner Selvam, M.K.; Agarwal, A.; Alves, M.G.; Baskaran, S. Alterations in seminal plasma proteomic profile in men with primary and secondary infertility. Sci. Rep. 2020, 10, 7539. [Google Scholar] [CrossRef]
- Liu, C.; Lv, M.; He, X.; Zhu, Y.; Amiri-Yekta, A.; Li, W.; Wu, H.; Kherraf, Z.E.; Liu, W.; Zhang, J.; et al. Homozygous mutations in SPEF2 induce multiple morphological abnormalities of the sperm flagella and male infertility. J. Med. Genet. 2020, 57, 31–37. [Google Scholar] [CrossRef]
- Liu, W.; Sha, Y.; Li, Y.; Mei, L.; Lin, S.; Huang, X.; Lu, J.; Ding, L.; Kong, S.; Lu, Z. Loss-of-function mutations in SPEF2 cause multiple morphological abnormalities of the sperm flagella (MMAF). J. Med. Genet. 2019, 56, 678–684. [Google Scholar] [CrossRef]
- Tu, C.; Nie, H.; Meng, L.; Wang, W.; Li, H.; Yuan, S.; Cheng, D.; He, W.; Liu, G.; Du, J.; et al. Novel mutations in SPEF2 causing different defects between flagella and cilia bridge: The phenotypic link between MMAF and PCD. Hum. Genet. 2020, 139, 257–271. [Google Scholar] [CrossRef]
- Auguste, Y.; Delague, V.; Desvignes, J.P.; Longepied, G.; Gnisci, A.; Besnier, P.; Levy, N.; Beroud, C.; Megarbane, A.; Metzler-Guillemain, C.; et al. Loss of Calmodulin- and Radial-Spoke-Associated Complex Protein CFAP251 Leads to Immotile Spermatozoa Lacking Mitochondria and Infertility in Men. Am. J. Hum. Genet. 2018, 103, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Kherraf, Z.E.; Amiri-Yekta, A.; Dacheux, D.; Karaouzene, T.; Coutton, C.; Christou-Kent, M.; Martinez, G.; Landrein, N.; Le Tanno, P.; Fourati Ben Mustapha, S.; et al. A Homozygous Ancestral SVA-Insertion-Mediated Deletion in WDR66 Induces Multiple Morphological Abnormalities of the Sperm Flagellum and Male Infertility. Am. J. Hum. Genet. 2018, 103, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Yan, W.; Burns, K.H.; Matzuk, M.M. Tektin3 encodes an evolutionarily conserved putative testicular microtubules-related protein expressed preferentially in male germ cells. Mol. Reprod. Dev. 2004, 67, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhou, Z.; Cheng, C.; Zhao, W.; Tang, R.; Huang, Y.; Wang, W.; Xu, J.; Zeng, L.; Xie, Y.; et al. Cloning and characterization of a novel human TEKTIN1 gene. Int. J. Biochem. Cell Biol. 2001, 33, 1172–1182. [Google Scholar] [CrossRef]
- Zuccarello, D.; Ferlin, A.; Garolla, A.; Pati, M.A.; Moretti, A.; Cazzadore, C.; Francavilla, S.; Foresta, C. A possible association of a human tektin-t gene mutation (A229V) with isolated non-syndromic asthenozoospermia: Case report. Hum. Reprod. 2008, 23, 996–1001. [Google Scholar] [CrossRef]
- Alciaturi, J.; Anesetti, G.; Irigoin, F.; Skowronek, F.; Sapiro, R. Distribution of sperm antigen 6 (SPAG6) and 16 (SPAG16) in mouse ciliated and non-ciliated tissues. J. Mol. Histol. 2019, 50, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Tang, D.; Shao, Z.; Geng, H.; Gao, Y.; Li, K.; Tan, Q.; Wang, G.; Wang, C.; Wu, H.; et al. Homozygous SPAG6 variants can induce nonsyndromic asthenoteratozoospermia with severe MMAF. Reprod. Biol. Endocrinol. 2022, 20, 41. [Google Scholar] [CrossRef]
- Goldberg, E. The sperm-specific form of lactate dehydrogenase is required for fertility and is an attractive target for male contraception (a review). Biol. Reprod. 2021, 104, 521–526. [Google Scholar] [CrossRef]
- Liu, X.; Li, Q.; Wang, W.; Liu, F. Aberrant expression of spermspecific glycolytic enzymes are associated with poor sperm quality. Mol. Med. Rep. 2019, 19, 2471–2478. [Google Scholar] [CrossRef]
- Liu, X.X.; Zhang, H.; Shen, X.F.; Liu, F.J.; Liu, J.; Wang, W.J. Characteristics of testis-specific phosphoglycerate kinase 2 and its association with human sperm quality. Hum. Reprod. 2016, 31, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Dirami, T.; Rode, B.; Jollivet, M.; Da Silva, N.; Escalier, D.; Gaitch, N.; Norez, C.; Tuffery, P.; Wolf, J.P.; Becq, F.; et al. Missense mutations in SLC26A8, encoding a sperm-specific activator of CFTR, are associated with human asthenozoospermia. Am. J. Hum. Genet. 2013, 92, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Lohi, H.; Kujala, M.; Makela, S.; Lehtonen, E.; Kestila, M.; Saarialho-Kere, U.; Markovich, D.; Kere, J. Functional characterization of three novel tissue-specific anion exchangers SLC26A7, -A8, and -A9. J. Biol. Chem. 2002, 277, 14246–14254. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, H.; Xu, Y.; Shen, Q.; Xu, C.; Geng, H.; Lv, M.; Tan, Q.; Li, K.; Tang, D.; et al. Novel biallelic mutations in SLC26A8 cause severe asthenozoospermia in humans owing to midpiece defects: Insights into a putative dominant genetic disease. Hum. Mutat. 2022, 43, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Li, W.; Zhou, X.; Chen, X.; Zheng, M.; Cui, Y.; Liu, X.; Guo, X.; Zhu, H. PRSS55 plays an important role in the structural differentiation and energy metabolism of sperm and is required for male fertility in mice. J. Cell. Mol. Med. 2021, 25, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.S.; Mozdarani, H.; Ghaedi, K.; Nasr-Esfahani, M.H. Among seven testis-specific molecular markers, SPEM1 appears to have a significant clinical value for prediction of sperm retrieval in azoospermic men. Andrology 2018, 6, 890–895. [Google Scholar] [CrossRef]
- Drabovich, A.P.; Dimitromanolakis, A.; Saraon, P.; Soosaipillai, A.; Batruch, I.; Mullen, B.; Jarvi, K.; Diamandis, E.P. Differential diagnosis of azoospermia with proteomic biomarkers ECM1 and TEX101 quantified in seminal plasma. Sci. Transl. Med. 2013, 5, 212ra160. [Google Scholar] [CrossRef] [PubMed]
- Korbakis, D.; Schiza, C.; Brinc, D.; Soosaipillai, A.; Karakosta, T.D.; Legare, C.; Sullivan, R.; Mullen, B.; Jarvi, K.; Diamandis, E.P.; et al. Preclinical evaluation of a TEX101 protein ELISA test for the differential diagnosis of male infertility. BMC Med. 2017, 15, 60. [Google Scholar] [CrossRef]
- Xu, Y.; Han, Q.; Ma, C.; Wang, Y.; Zhang, P.; Li, C.; Cheng, X.; Xu, H. Comparative Proteomics and Phosphoproteomics Analysis Reveal the Possible Breed Difference in Yorkshire and Duroc Boar Spermatozoa. Front. Cell Dev. Biol. 2021, 9, 652809. [Google Scholar] [CrossRef]
- Omolaoye, T.S.; Hachim, M.Y.; du Plessis, S.S. Using publicly available transcriptomic data to identify mechanistic and diagnostic biomarkers in azoospermia and overall male infertility. Sci. Rep. 2022, 12, 2584. [Google Scholar] [CrossRef]
- Weirich, C.S.; Erzberger, J.P.; Barral, Y. The septin family of GTPases: Architecture and dynamics. Nat. Rev. Mol. Cell Biol. 2008, 9, 478–489. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Lin, Y.H.; Chen, H.I.; Wang, Y.Y.; Chiou, Y.W.; Lin, H.H.; Pan, H.A.; Wu, C.M.; Su, S.M.; Hsu, C.C.; et al. SEPT12 mutations cause male infertility with defective sperm annulus. Hum. Mutat. 2012, 33, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Wen, Y.; Zhang, T.; Wang, X.; Jiang, C.; Zheng, R.; Zhou, F.; Chen, D.; Yang, Y.; et al. Whole-exome sequencing of a cohort of infertile men reveals novel causative genes in teratozoospermia that are chiefly related to sperm head defects. Hum. Reprod. 2021, 37, 152–177. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.S.; Msaki, A.; Ghezzi, M.; Cosci, I.; Pilichou, K.; Celeghin, R.; Foresta, C.; Ferlin, A. Development of a novel next-generation sequencing panel for diagnosis of quantitative spermatogenic impairment. J. Assist. Reprod. Genet. 2020, 37, 753–762. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, Y.M.; Wang, Y.Y.; Yu, I.S.; Lin, Y.W.; Wang, Y.H.; Wu, C.M.; Pan, H.A.; Chao, S.C.; Yen, P.H.; et al. The expression level of septin12 is critical for spermiogenesis. Am. J. Pathol. 2009, 174, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.; Botana, D.; Pinero, S.; Proverbio, F.; Marin, R. Cadmium inhibits motility, activities of plasma membrane Ca(2+)-ATPase and axonemal dynein-ATPase of human spermatozoa. Andrologia 2016, 48, 464–469. [Google Scholar] [CrossRef]
- Hlivko, J.T.; Chakraborty, S.; Hlivko, T.J.; Sengupta, A.; James, P.F. The human Na,K-ATPase alpha 4 isoform is a ouabain-sensitive alpha isoform that is expressed in sperm. Mol. Reprod. Dev. 2006, 73, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.; Nguyen, A.N.; Timmerberg, B.; Tash, J.S.; Blanco, G. The Na,K-ATPase alpha4 isoform from humans has distinct enzymatic properties and is important for sperm motility. Mol. Hum. Reprod. 2006, 12, 565–576. [Google Scholar] [CrossRef]
- Dias, T.R.; Agarwal, A.; Pushparaj, P.N.; Ahmad, G.; Sharma, R. Reduced semen quality in patients with testicular cancer seminoma is associated with alterations in the expression of sperm proteins. Asian J. Androl. 2020, 22, 88–93. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Agarwal, A.; Pushparaj, P.N. A quantitative global proteomics approach to understanding the functional pathways dysregulated in the spermatozoa of asthenozoospermic testicular cancer patients. Andrology 2019, 7, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.G.; Publicover, S.J.; Barratt, C.L.R.; Martins da Silva, S.J. Human sperm ion channel (dys)function: Implications for fertilization. Hum. Reprod. Update 2019, 25, 758–776. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.R.; Fang, D.; Liu, B.H.; Cai, J.; Tang, W.H.; Jiang, H.; Xing, G.G. Roles of CatSper channels in the pathogenesis of asthenozoospermia and the therapeutic effects of acupuncture-like treatment on asthenozoospermia. Theranostics 2021, 11, 2822–2844. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Moran, M.M.; Navarro, B.; Chong, J.A.; Krapivinsky, G.; Krapivinsky, L.; Kirichok, Y.; Ramsey, I.S.; Quill, T.A.; Clapham, D.E. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl. Acad. Sci. USA 2007, 104, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Li, R.K.; Tan, J.L.; Chen, L.T.; Feng, J.S.; Liang, W.X.; Guo, X.J.; Liu, P.; Chen, Z.; Sha, J.H.; Wang, Y.F.; et al. Iqcg is essential for sperm flagellum formation in mice. PLoS ONE 2014, 9, e98053. [Google Scholar] [CrossRef]
- Yu, Y.; Oko, R.; Miranda-Vizuete, A. Developmental expression of spermatid-specific thioredoxin-1 protein: Transient association to the longitudinal columns of the fibrous sheath during sperm tail formation. Biol. Reprod. 2002, 67, 1546–1554. [Google Scholar] [CrossRef]
- Javadirad, S.M.; Mokhtari, M. TXNDC2 joint molecular marker is associated with testis pathology and is an accurate predictor of sperm retrieval. Sci. Rep. 2021, 11, 13064. [Google Scholar] [CrossRef] [PubMed]

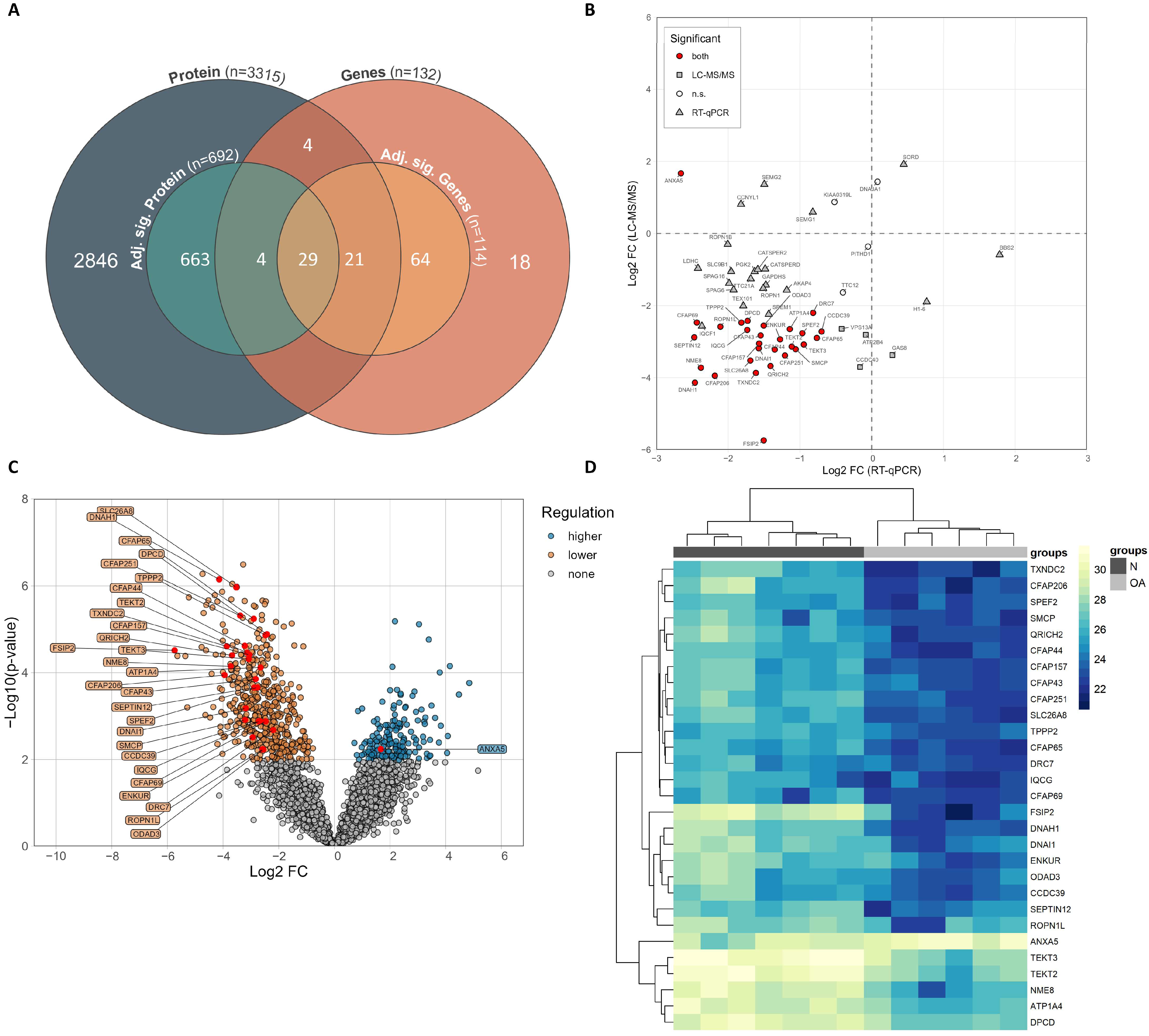
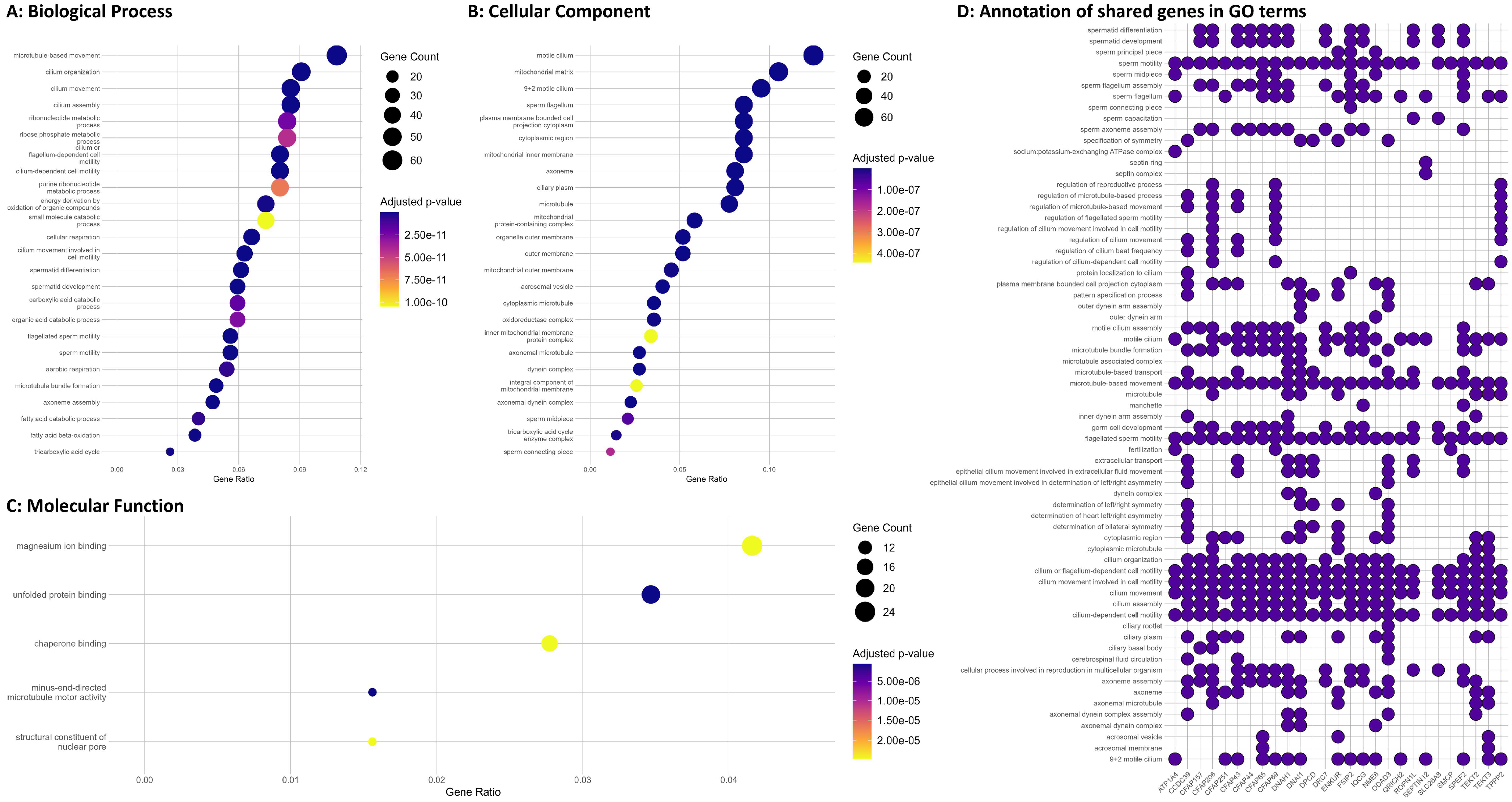
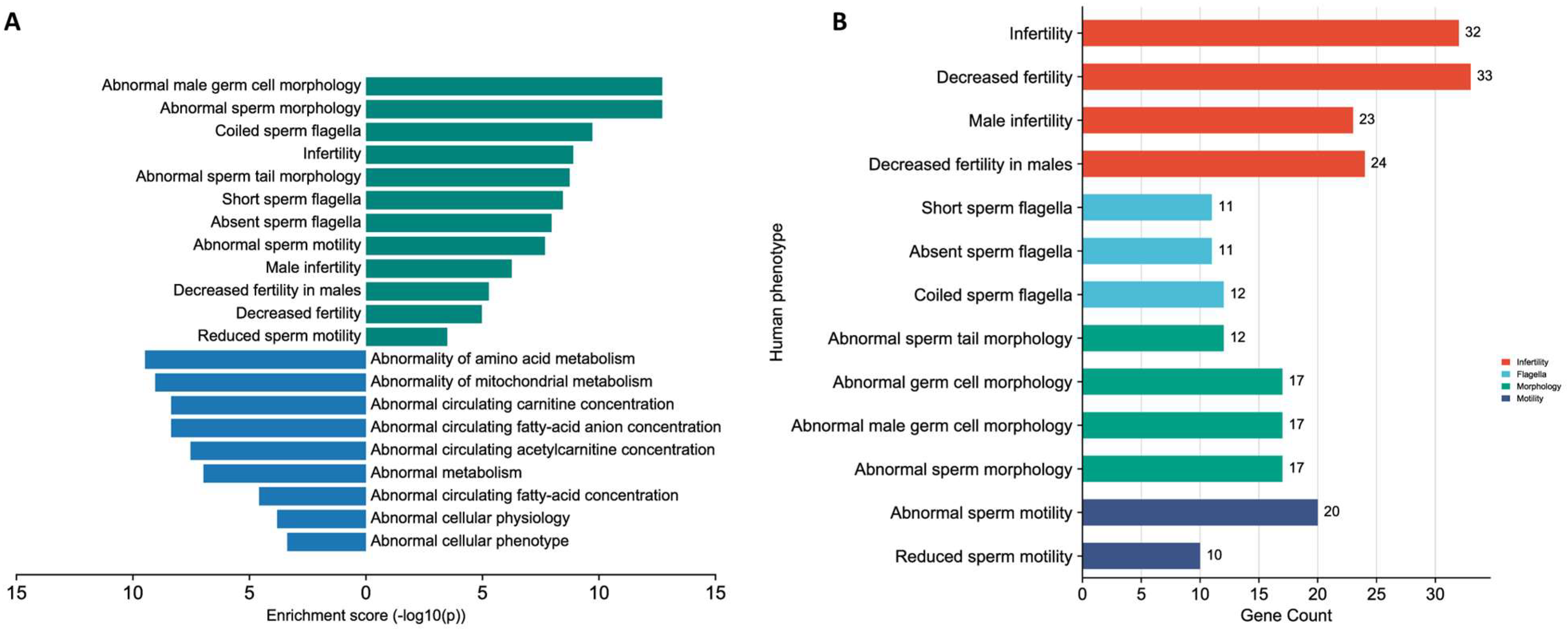
| Characteristic | Normozoospermic Men (Healthy Controls, n = 99) | Oligoasthenozoospermic Men (Subfertile Men, n = 99) | p-Value |
|---|---|---|---|
| Age (Year) | 35.49 ± 6.31 | 34.70 ± 6.73 | 3.91 × 10−1 |
| Volume (mL) | 3.45 ± 1.47 | 3.13 ± 1.34 | 1.16 × 10−1 |
| pH | 8.71 ± 0.39 | 8.71 ± 0.38 | 9.70 × 10−1 |
| Count (106/mL) | 66.51 ± 29.87 | 9.26 ± 3.56 | 3.79 × 10−46 |
| Motility (%, motile) | 56.57 ± 10.50 | 20.53 ± 4.81 | 1.26 × 10−77 |
| Morphology (%, normal) | 8.08 ± 4.62 | 5.86 ± 2.26 | 3.63 × 10−5 |
| Entrez Gene ID | Gene Symbol and Name | Log2 (Fold Change) | p-Value | Adjusted p-Value | Regulation |
|---|---|---|---|---|---|
| 100 | ADA (adenosine deaminase) | −1.24 | 4.42 × 10−4 | 6.63 × 10−4 | Lower |
| 8852 | AKAP4 (A-kinase anchoring protein 4) | −1.18 | 7.05 × 10−4 | 1.01 × 10−3 | Lower |
| 308 | ANXA5 (annexin A5) | −2.66 | 2.22 × 10−10 | 2.25 × 10−9 | Lower |
| 84071 | ARMC2 (armadillo repeat containing 2) | −1.40 | 6.31 × 10−6 | 1.41 × 10−5 | Lower |
| 55870 | ASH1L (ASH1 like histone lysine methyltransferase) | −2.06 | 2.39 × 10−10 | 2.25 × 10−9 | Lower |
| 480 | ATP1A4 (ATPase Na+/K+ transporting subunit alpha 4) | −1.14 | 7.99 × 10−4 | 1.10 × 10−3 | Lower |
| 8911 | CACNA1I (calcium voltage-gated channel subunit alpha1 I) | −1.11 | 2.07 × 10−2 | 2.51 × 10−2 | Lower |
| 117144 | CATSPER1 (cation channel sperm associated 1) | −2.01 | 1.34 × 10−7 | 4.48 × 10−7 | Lower |
| 117155 | CATSPER2 (cation channel sperm associated 2) | −1.59 | 2.47 × 10−5 | 4.66 × 10−5 | Lower |
| 347732 | CATSPER3 (cation channel sperm associated 3) | −1.67 | 2.70 × 10−7 | 8.09 × 10−7 | Lower |
| 378807 | CATSPER4 (cation channel sperm associated 4) | −1.73 | 1.87 × 10−4 | 2.98 × 10−4 | Lower |
| 257062 | CATSPERD (cation channel sperm associated auxiliary subunit delta) | −1.48 | 2.80 × 10−5 | 5.18 × 10−5 | Lower |
| 25858 | CATSPERZ (catsper channel auxiliary subunit zeta) | −2.04 | 4.33 × 10−10 | 3.57 × 10−9 | Lower |
| 339829 | CCDC39 (coiled-coil domain containing 39) | −0.70 | 3.36 × 10−2 | 3.96 × 10−2 | Lower |
| 151195 | CCNYL1 (cyclin Y like 1) | −1.82 | 1.48 × 10−10 | 1.63 × 10−9 | Lower |
| 22994 | CEP131 (centrosomal protein 131) | −1.33 | 1.86 × 10−7 | 5.85 × 10−7 | Lower |
| 286207 | CFAP157 (cilia and flagella associated protein 157) | −1.57 | 2.77 × 10−4 | 4.30 × 10−4 | Lower |
| 154313 | CFAP206 (cilia and flagella associated protein 206) | −2.19 | 7.53 × 10−10 | 5.85 × 10−9 | Lower |
| 80217 | CFAP43 (cilia and flagella associated protein 43) | −1.55 | 5.90 × 10−7 | 1.59 × 10−6 | Lower |
| 55779 | CFAP44 (cilia and flagella associated protein 44) | −1.35 | 4.11 × 10−6 | 9.86 × 10−6 | Lower |
| 255101 | CFAP65 (cilia and flagella associated protein 65) | −0.76 | 2.72 × 10−2 | 3.24 × 10−2 | Lower |
| 79846 | CFAP69 (cilia and flagella associated protein 69) | −2.44 | 4.88 × 10−8 | 1.84 × 10−7 | Lower |
| 1139 | CHRNA7 (cholinergic receptor nicotinic alpha 7 subunit) | −1.78 | 5.20 × 10−9 | 2.86 × 10−8 | Lower |
| 54514 | DDX4 (DEAD-box helicase 4) | −1.24 | 6.35 × 10−4 | 9.21 × 10−4 | Lower |
| 25981 | DNAH1 (dynein axonemal heavy chain 1) | −2.46 | 1.35 × 10−9 | 8.85 × 10−9 | Lower |
| 8701 | DNAH11 (dynein axonemal heavy chain 11) | −1.24 | 2.77 × 10−3 | 3.53 × 10−3 | Lower |
| 1767 | DNAH5 (dynein axonemal heavy chain 5) | −1.77 | 1.75 × 10−7 | 5.64 × 10−7 | Lower |
| 27019 | DNAI1 (dynein axonemal intermediate chain 1) | −1.57 | 1.08 × 10−5 | 2.30 × 10−5 | Lower |
| 25911 | DPCD (deleted in primary ciliary dyskinesia homolog (mouse)) | −1.73 | 2.37 × 10−5 | 4.60 × 10−5 | Lower |
| 84229 | DRC7 (dynein regulatory complex subunit 7) | −0.82 | 1.37 × 10−3 | 1.87 × 10−3 | Lower |
| 285588 | EFCAB9 (EF-hand calcium binding domain 9) | −2.30 | 2.10 × 10−5 | 4.15 × 10−5 | Lower |
| 219670 | ENKUR (enkurin, TRPC channel interacting protein) | −1.28 | 1.86 × 10−8 | 7.45 × 10−8 | Lower |
| 387712 | ENO4 (enolase 4) | −1.80 | 1.00 × 10−6 | 2.59 × 10−6 | Lower |
| 57119 | EPPIN (epididymal peptidase inhibitor) | −1.25 | 4.67 × 10−13 | 2.05 × 10−11 | Lower |
| 401024 | FSIP2 (fibrous sheath interacting protein 2) | −1.50 | 2.70 × 10−5 | 5.02 × 10−5 | Lower |
| 26330 | GAPDHS (glyceraldehyde-3-phosphate dehydrogenase, spermatogenic) | −1.48 | 8.77 × 10−7 | 2.32 × 10−6 | Lower |
| 8100 | IFT88 (intraflagellar transport 88) | −1.92 | 7.51 × 10−9 | 3.54 × 10−8 | Lower |
| 132141 | IQCF1 (IQ motif containing F1) | −2.37 | 2.93 × 10−7 | 8.60 × 10−7 | Lower |
| 84223 | IQCG (IQ motif containing G) | −1.73 | 2.58 × 10−8 | 1.00 × 10−7 | Lower |
| 3948 | LDHC (lactate dehydrogenase C) | −2.42 | 3.66 × 10−10 | 3.22 × 10−9 | Lower |
| 23639 | LRRC6/DNAAF11 (dynein axonemal assembly factor 11) | −1.70 | 1.36 × 10−7 | 4.48 × 10−7 | Lower |
| 644890 | MEIG1 (meiosis/spermiogenesis associated 1) | −1.60 | 1.11 × 10−7 | 3.93 × 10−7 | Lower |
| 4233 | MET (MET proto-oncogene, receptor tyrosine kinase) | −1.64 | 3.02 × 10−7 | 8.68 × 10−7 | Lower |
| 51314 | NME8 (NME/NM23 family member 8) | −2.38 | 5.98 × 10−6 | 1.36 × 10−5 | Lower |
| 79730 | NSUN7 (NOP2/Sun RNA methyltransferase family member 7) | −0.77 | 1.50 × 10−4 | 2.44 × 10−4 | Lower |
| 115948 | ODAD3/CCDC151 (outer dynein arm docking complex subunit 3) | −1.51 | 3.65 × 10−2 | 4.23 × 10−2 | Lower |
| 441531 | PGAM4 (phosphoglycerate mutase family member 4) | −1.25 | 1.45 × 10−3 | 1.96 × 10−3 | Lower |
| 5232 | PGK2 (phosphoglycerate kinase 2) | −1.63 | 4.26 × 10−3 | 5.35 × 10−3 | Lower |
| 139212 | PIH1D3/DNAAF6 (dynein axonemal assembly factor 6) | −1.13 | 1.54 × 10−2 | 1.89 × 10−2 | Lower |
| 50487 | PLA2G3 (phospholipase A2 group III) | −2.81 | 5.11 × 10−9 | 2.86 × 10−8 | Lower |
| 63978 | PRDM14 (PR/SET domain 14) | −1.07 | 1.07 × 10−2 | 1.32 × 10−2 | Lower |
| 58531 | PRM3 (protamine 3) | −2.13 | 1.67 × 10−11 | 2.46 × 10−10 | Lower |
| 203074 | PRSS55 (serine protease 55) | −1.21 | 2.46 × 10−3 | 3.19 × 10−3 | Lower |
| 84074 | QRICH2 (glutamine rich 2) | −1.41 | 1.52 × 10−5 | 3.09 × 10−5 | Lower |
| 54763 | ROPN1 (rhophilin associated tail protein 1) | −1.51 | 1.56 × 10−8 | 6.43 × 10−8 | Lower |
| 152015 | ROPN1B (rhophilin associated tail protein 1B) | −2.01 | 1.69 × 10−9 | 1.01 × 10−8 | Lower |
| 83853 | ROPN1L (rhophilin associated tail protein 1 like) | −2.11 | 1.41 × 10−8 | 6.00 × 10−8 | Lower |
| 6406 | SEMG1 (semenogelin 1) | −0.82 | 2.03 × 10−4 | 3.20 × 10−4 | Lower |
| 6407 | SEMG2 (semenogelin 2) | −1.50 | 2.86 × 10−5 | 5.18 × 10−5 | Lower |
| 124404 | SEPTIN12 (septin 12) | −2.47 | 1.17 × 10−11 | 2.20 × 10−10 | Lower |
| 9389 | SLC22A14 (solute carrier family 22 member 14) | −1.44 | 6.77 × 10−5 | 1.21 × 10−4 | Lower |
| 116369 | SLC26A8 (solute carrier family 26 member 8) | −1.69 | 1.13 × 10−7 | 3.93 × 10−7 | Lower |
| 150159 | SLC9B1 (solute carrier family 9 member B1) | −1.96 | 1.71 × 10−12 | 5.65 × 10−11 | Lower |
| 285335 | SLC9C1 (solute carrier family 9 member C1) | −1.26 | 4.19 × 10−4 | 6.37 × 10−4 | Lower |
| 81892 | SLIRP (SRA stem-loop interacting RNA binding protein) | −2.12 | 1.61 × 10−6 | 4.09 × 10−6 | Lower |
| 4184 | SMCP (sperm mitochondria associated cysteine rich protein) | −1.06 | 9.29 × 10−4 | 1.31 × 10−3 | Lower |
| 79582 | SPAG16 (sperm associated antigen 16) | −1.99 | 8.52 × 10−9 | 3.75 × 10−8 | Lower |
| 9576 | SPAG6 (sperm associated antigen 6) | −1.92 | 6.59 × 10−9 | 3.34 × 10−8 | Lower |
| 79925 | SPEF2 (sperm flagellar 2) | −0.97 | 4.20 × 10−4 | 6.37 × 10−4 | Lower |
| 374768 | SPEM1 (spermatid maturation 1) | −1.43 | 4.40 × 10−6 | 1.04 × 10−5 | Lower |
| 6863 | TAC1 (tachykinin precursor 1) | −2.25 | 2.63 × 10−6 | 6.43 × 10−6 | Lower |
| 6866 | TAC3 (tachykinin precursor 3) | −2.27 | 5.52 × 10−6 | 1.28 × 10−5 | Lower |
| 255061 | TAC4 (tachykinin precursor 4) | −1.09 | 7.27 × 10−6 | 1.57 × 10−5 | Lower |
| 6869 | TACR1 (tachykinin receptor 1) | −1.17 | 8.44 × 10−3 | 1.05 × 10−2 | Lower |
| 6865 | TACR2 (tachykinin receptor 2) | −1.73 | 6.62 × 10−6 | 1.46 × 10−5 | Lower |
| 6870 | TACR3 (tachykinin receptor 3) | −0.68 | 3.57 × 10−2 | 4.18 × 10−2 | Lower |
| 54457 | TAF7L (TATA-box binding protein associated factor 7 like) | −1.97 | 2.27 × 10−7 | 6.97 × 10−7 | Lower |
| 202500 | TCTE1 (t-complex-associated-testis-expressed 1) | −1.27 | 1.60 × 10−4 | 2.58 × 10−4 | Lower |
| 27285 | TEKT2 (tektin 2) | −1.11 | 7.57 × 10−5 | 1.33 × 10−4 | Lower |
| 64518 | TEKT3 (tektin 3) | −0.95 | 2.78 × 10−3 | 3.53 × 10−3 | Lower |
| 83639 | TEX101 (testis expressed 101) | −1.79 | 1.72 × 10−5 | 3.43 × 10−5 | Lower |
| 283471 | TMPRSS12 (transmembrane serine protease 12) | −1.39 | 9.83 × 10−5 | 1.71 × 10−4 | Lower |
| 7141 | TNP1 (transition protein 1) | −2.16 | 4.55 × 10−12 | 1.20 × 10−10 | Lower |
| 7142 | TNP2 (transition protein 2) | −0.97 | 1.80 × 10−3 | 2.40 × 10−3 | Lower |
| 122664 | TPPP2 (tubulin polymerization promoting protein family member 2) | −1.82 | 8.67 × 10−10 | 6.36 × 10−9 | Lower |
| 283629 | TSSK4 (testis specific serine kinase 4) | −1.80 | 4.93 × 10−7 | 1.36 × 10−6 | Lower |
| 199223 | TTC21A (tetratricopeptide repeat domain 21A) | −1.69 | 9.47 × 10−10 | 6.58 × 10−9 | Lower |
| 25809 | TTLL1 (TTL family tubulin polyglutamylase complex subunit L1) | −2.21 | 1.55 × 10−11 | 2.46 × 10−10 | Lower |
| 23093 | TTLL5 (tubulin tyrosine ligase like 5) | −1.58 | 7.78 × 10−9 | 3.54 × 10−8 | Lower |
| 164395 | TTLL9 (tubulin tyrosine ligase like 9) | −1.07 | 6.04 × 10−4 | 8.86 × 10−4 | Lower |
| 84203 | TXNDC2 (thioredoxin domain containing 2) | −1.61 | 3.34 × 10−7 | 9.38 × 10−7 | Lower |
| 7320 | UBE2B (ubiquitin conjugating enzyme E2 B) | −2.12 | 7.55 × 10−9 | 3.54 × 10−8 | Lower |
| 144406 | WDR66/CFAP251 (cilia and flagella associated protein 251) | −1.21 | 1.26 × 10−4 | 2.09 × 10−4 | Lower |
| 7490 | WT1 (WT1 transcription factor) | −1.33 | 1.27 × 10−4 | 2.09 × 10−4 | Lower |
| 109 | ADCY3 (adenylate cyclase 3) | 1.60 | 1.28 × 10−3 | 1.76 × 10−3 | Higher |
| 338 | APOB (apolipoprotein B) | 1.39 | 1.91 × 10−3 | 2.53 × 10−3 | Higher |
| 583 | BBS2 (Bardet-Biedl syndrome 2) | 1.78 | 5.21 × 10−8 | 1.91 × 10−7 | Higher |
| 1235 | CCR6 (C-C motif chemokine receptor 6) | 1.23 | 1.24 × 10−5 | 2.58 × 10−5 | Higher |
| 1672 | DEFB1 (defensin beta 1) | 1.30 | 1.25 × 10−5 | 2.58 × 10−5 | Higher |
| 3010 | H1-6 (H1.6 linker histone, cluster member) | 0.77 | 9.96 × 10−4 | 1.38 × 10−3 | Higher |
| 3622 | ING2 (inhibitor of growth family member 2) | 0.54 | 1.08 × 10−4 | 1.85 × 10−4 | Higher |
| 11172 | INSL6 (insulin like 6) | 0.58 | 2.30 × 10−2 | 2.76 × 10−2 | Higher |
| 55726 | INTS13 (integrator complex subunit 13) | 2.30 | 2.08 × 10−11 | 2.50 × 10−10 | Higher |
| 54742 | LY6K (lymphocyte antigen 6 family member K) | 1.57 | 6.44 × 10−9 | 3.34 × 10−8 | Higher |
| 9148 | NEURL1 (neuralized E3 ubiquitin protein ligase 1) | 0.71 | 1.95 × 10−3 | 2.55 × 10−3 | Higher |
| 261734 | NPHP4 (nephrocystin 4) | 1.49 | 1.95 × 10−6 | 4.86 × 10−6 | Higher |
| 11158 | RABL2B (RAB, member of RAS oncogene family like 2B) | 2.46 | 1.96 × 10−11 | 2.50 × 10−10 | Higher |
| 9104 | RGN (regucalcin) | 1.58 | 1.41 × 10−9 | 8.85 × 10−9 | Higher |
| 338879 | RNASE10 (ribonuclease A family member 10 (inactive)) | 2.65 | 8.57 × 10−14 | 5.66 × 10−12 | Higher |
| 390443 | RNASE9 (ribonuclease A family member 9 (inactive)) | 1.20 | 2.44 × 10−5 | 4.66 × 10−5 | Higher |
| 85413 | SLC22A16 (solute carrier family 22 member 16) | 3.18 | 7.59 × 10−12 | 1.67 × 10−10 | Higher |
| 133308 | SLC9B2 (solute carrier family 9 member B2) | 3.25 | 3.67 × 10−18 | 4.85 × 10−16 | Higher |
| 6652 | SORD (sorbitol dehydrogenase) | 0.45 | 5.32 × 10−4 | 7.89 × 10−4 | Higher |
| 91978 | TPGS1 (tubulin polyglutamylase complex subunit 1) | 0.99 | 1.25 × 10−4 | 2.09 × 10−4 | Higher |
| Entrez ID | Gene Symbol | Genes (RT-qPCR) | Proteins (LC-MS/MS) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Log2 (Fold Change) | p-Value | Adjusted p-Value | Regulation | Log2 (Fold Change) | p-Value | Adjusted p-Value | Regulation | ||
| 308 | ANXA5 | −2.66 | 2.22 × 10−10 | 2.25 × 10−9 | Lower | 1.67 | 5.79 × 10−3 | 3.39 × 10−2 | Higher |
| 480 | ATP1A4 | −1.14 | 7.99 × 10−4 | 1.13 × 10−3 | Lower | −2.65 | 7.63 × 10−5 | 2.51 × 10−3 | Lower |
| 339829 | CCDC39 | −0.70 | 3.36 × 10−2 | 3.96 × 10−2 | Lower | −2.72 | 1.33 × 10−3 | 1.31 × 10−2 | Lower |
| 286207 | CFAP157 | −1.57 | 2.77 × 10−4 | 4.30 × 10−4 | Lower | −3.05 | 3.88 × 10−5 | 1.81 × 10−3 | Lower |
| 154313 | CFAP206 | −2.19 | 7.53 × 10−10 | 5.85 × 10−9 | Lower | −3.95 | 1.14 × 10−4 | 3.14 × 10−3 | Lower |
| 144406 | CFAP251 | −1.21 | 1.26 × 10−4 | 2.09 × 10−4 | Lower | −3.38 | 4.85 × 10−6 | 6.99 × 10−4 | Lower |
| 80217 | CFAP43 | −1.55 | 5.90 × 10−7 | 1.59 × 10−6 | Lower | −2.83 | 1.41 × 10−4 | 3.62 × 10−3 | Lower |
| 55779 | CFAP44 | −1.35 | 4.11 × 10−6 | 9.86 × 10−6 | Lower | −3.22 | 2.40 × 10−5 | 1.55 × 10−3 | Lower |
| 255101 | CFAP65 | −0.76 | 2.72 × 10−2 | 3.24 × 10−2 | Lower | −2.90 | 5.73 × 10−6 | 7.60 × 10−4 | Lower |
| 79846 | CFAP69 | −2.44 | 4.88 × 10−8 | 1.84 × 10−7 | Lower | −2.47 | 1.33 × 10−3 | 1.31 × 10−2 | Lower |
| 25981 | DNAH1 | −2.46 | 1.35 × 10−9 | 8.85 × 10−9 | Lower | −4.14 | 7.19 × 10−7 | 5.05 × 10−4 | Lower |
| 27019 | DNAI1 | −1.57 | 1.08 × 10−5 | 2.30 × 10−5 | Lower | −3.18 | 6.63 × 10−4 | 8.63 × 10−3 | Lower |
| 25911 | DPCD | −1.73 | 2.37 × 10−5 | 4.60 × 10−5 | Lower | −2.42 | 1.30 × 10−5 | 1.16 × 10−3 | Lower |
| 84229 | DRC7 | −0.82 | 1.37 × 10−3 | 1.87 × 10−3 | Lower | −2.20 | 2.11 × 10−3 | 1.76 × 10−2 | Lower |
| 219670 | ENKUR | −1.28 | 1.86 × 10−8 | 7.45 × 10−8 | Lower | −2.94 | 3.09 × 10−3 | 2.28 × 10−2 | Lower |
| 401024 | FSIP2 | −1.50 | 2.70 × 10−5 | 5.02 × 10−5 | Lower | −5.74 | 3.06 × 10−5 | 1.69 × 10−3 | Lower |
| 84223 | IQCG | −1.73 | 2.58 × 10−8 | 1.00 × 10−7 | Lower | −2.68 | 1.31 × 10−3 | 1.31 × 10−2 | Lower |
| 51314 | NME8 | −2.38 | 5.98 × 10−6 | 1.36 × 10−5 | Lower | −3.72 | 7.13 × 10−5 | 2.42 × 10−3 | Lower |
| 115948 | ODAD3 | −1.51 | 3.65 × 10−2 | 4.23 × 10−2 | Lower | −2.56 | 5.91 × 10−3 | 3.40 × 10−2 | Lower |
| 84074 | QRICH2 | −1.41 | 1.52 × 10−5 | 3.09 × 10−5 | Lower | −3.68 | 3.99 × 10−5 | 1.81 × 10−3 | Lower |
| 83853 | ROPN1L | −2.11 | 1.41 × 10−8 | 6.00 × 10−8 | Lower | −2.59 | 5.87 × 10−3 | 3.39 × 10−2 | Lower |
| 124404 | SEPTIN12 | −2.47 | 1.17 × 10−11 | 2.20 × 10−10 | Lower | −2.88 | 2.21 × 10−4 | 4.66× 10−3 | Lower |
| 116369 | SLC26A8 | −1.69 | 1.13 × 10−7 | 3.93 × 10−7 | Lower | −3.53 | 1.08 × 10−6 | 5.05 × 10−4 | Lower |
| 4184 | SMCP | −1.06 | 9.29 × 10−4 | 1.31 × 10−3 | Lower | −3.21 | 1.23 × 10−3 | 1.27 × 10−2 | Lower |
| 79925 | SPEF2 | −0.97 | 4.20 × 10−4 | 6.37 × 10−4 | Lower | −2.77 | 2.19 × 10−4 | 4.66 × 10−3 | Lower |
| 27285 | TEKT2 | −1.11 | 7.57 × 10−5 | 1.33 × 10−4 | Lower | −3.14 | 3.49 × 10−5 | 1.78 × 10−3 | Lower |
| 64518 | TEKT3 | −0.95 | 2.78 × 10−3 | 3.53 × 10−3 | Lower | −3.08 | 4.86 × 10−5 | 2.01 × 10−3 | Lower |
| 122664 | TPPP2 | −1.82 | 8.67 × 10−10 | 6.36 × 10−9 | Lower | −2.47 | 1.37 × 10−5 | 1.19 × 10−3 | Lower |
| 84203 | TXNDC2 | −1.61 | 3.34 × 10−7 | 9.38 × 10−7 | Lower | −3.87 | 2.47 × 10−5 | 1.55 × 10−3 | Lower |
| Genes/Proteins | Motility (% Motile) (Genes Determined by RT-qPCR) | Motility (% Motile) (Proteins Determined by LC-MS/MS) | ||
|---|---|---|---|---|
| Correlation Coefficient (r) | p-Value | Correlation Coefficient (r) | p-Value | |
| ATP1A4 | 0.18 | 1.69 × 10−2 | 0.61 | 2.69 × 10−2 |
| CFAP157 | 0.21 | 1.29 × 10−2 | 0.66 | 1.42 × 10−2 |
| CFAP206 | 0.32 | 1.56 × 10−5 | 0.61 | 2.69 × 10−2 |
| CFAP251 | 0.18 | 1.46 × 10−2 | 0.66 | 1.42 × 10−2 |
| CFAP43 | 0.26 | 4.42 × 10−4 | 0.59 | 3.25 × 10−2 |
| CFAP44 | 0.16 | 3.10 × 10−2 | 0.62 | 2.52 × 10−2 |
| CFAP65 | 0.22 | 1.32 × 10−2 | 0.65 | 1.53 × 10−2 |
| DNAH1 | 0.35 | 1.79 × 10−6 | 0.62 | 2.52 × 10−2 |
| DNAI1 | 0.26 | 5.46 × 10−4 | 0.60 | 2.87 × 10−2 |
| DPCD | 0.16 | 4.67 × 10−2 | 0.65 | 1.53 × 10−2 |
| FSIP2 | 0.22 | 3.07 × 10−3 | 0.62 | 2.52 × 10−2 |
| QRICH2 | 0.26 | 4.05 × 10−4 | 0.63 | 2.05 × 10−2 |
| ROPN1L | 0.31 | 1.71 × 10−5 | 0.74 | 4.11 × 10−3 |
| SEPTIN12 | 0.38 | 9.71 × 10−8 | 0.80 | 9.69 × 10−4 |
| SLC26A8 | 0.31 | 2.77 × 10−5 | 0.59 | 3.25 × 10−2 |
| SPEF2 | 0.15 | 4.16 × 10−2 | 0.66 | 1.42 × 10−2 |
| TEKT2 | 0.18 | 1.74 × 10−2 | 0.61 | 2.69 × 10−2 |
| TPPP2 | 0.38 | 1.40 × 10−7 | 0.63 | 2.20 × 10−2 |
| TXNDC2 | 0.26 | 4.83 × 10−4 | 0.71 | 6.68 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Halima, M.; Becker, L.S.; Al Smadi, M.A.; Abdul-Khaliq, H.; Raeschle, M.; Meese, E. Sperm Motility Annotated Genes: Are They Associated with Impaired Fecundity? Cells 2023, 12, 1239. https://doi.org/10.3390/cells12091239
Abu-Halima M, Becker LS, Al Smadi MA, Abdul-Khaliq H, Raeschle M, Meese E. Sperm Motility Annotated Genes: Are They Associated with Impaired Fecundity? Cells. 2023; 12(9):1239. https://doi.org/10.3390/cells12091239
Chicago/Turabian StyleAbu-Halima, Masood, Lea Simone Becker, Mohammad A. Al Smadi, Hashim Abdul-Khaliq, Markus Raeschle, and Eckart Meese. 2023. "Sperm Motility Annotated Genes: Are They Associated with Impaired Fecundity?" Cells 12, no. 9: 1239. https://doi.org/10.3390/cells12091239
APA StyleAbu-Halima, M., Becker, L. S., Al Smadi, M. A., Abdul-Khaliq, H., Raeschle, M., & Meese, E. (2023). Sperm Motility Annotated Genes: Are They Associated with Impaired Fecundity? Cells, 12(9), 1239. https://doi.org/10.3390/cells12091239








