Derivation and Characterization of Novel Cytocompatible Decellularized Tissue Scaffold for Myoblast Growth and Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Decellularization of Mushroom Tissue
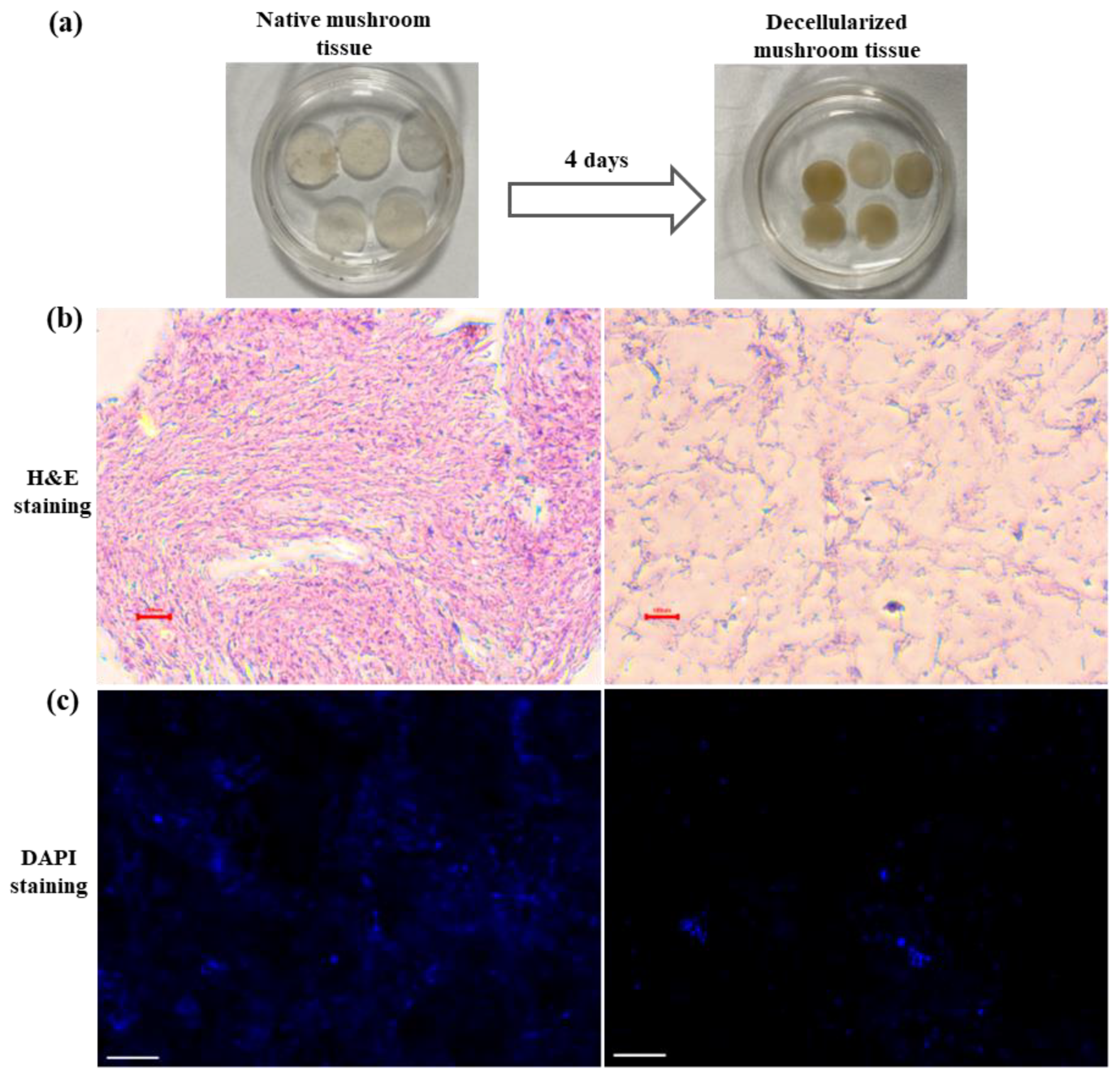
2.4. Characterization of Decellularization in Mushroom Tissue
2.5. DNA Isolation and Quantification
2.6. Biodegradation Analysis of DMS
2.7. Cytocompatibility of DMS
2.8. In Vitro Myogenesis on DMS
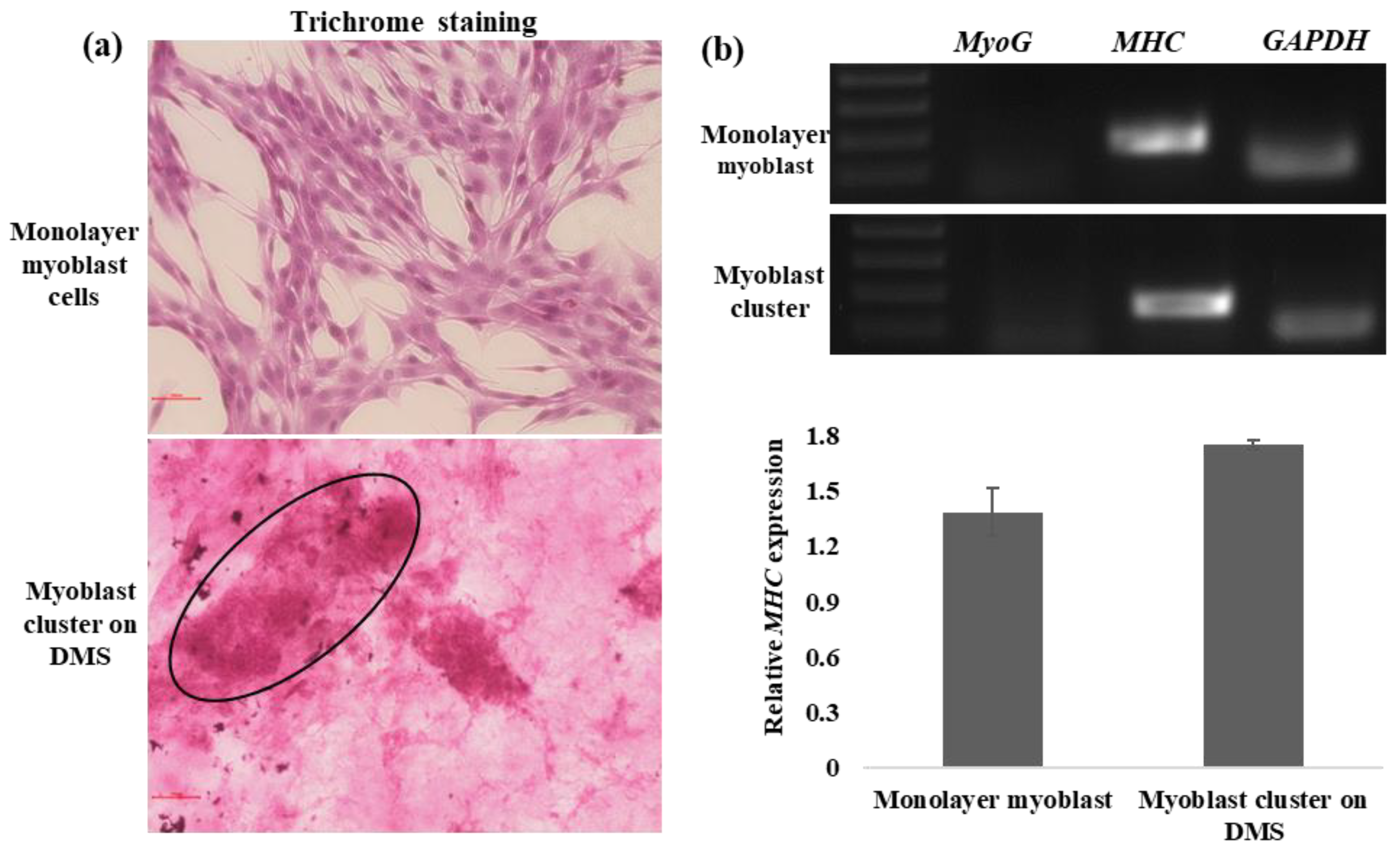
2.9. Gene Expression Analysis of Differentiated Myoblast Clusters on DMS
2.10. Statistical Analysis
3. Results
3.1. Generation and Characterization of Decellularized Mushroom Tissue
3.2. Evaluation of Scaffold Properties of Decellularized Mushroom Tissue: Biodegradation and Cytocompatibility
3.3. In Vitro Myogenesis on DMS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekmekcioglu, C.; Wallner, P.; Kundi, M.; Weisz, U.; Haas, W.; Hutter, H.P. Red meat, diseases, and healthy alternatives: A critical review. Crit. Rev. Food Sci. Nutr. 2018, 58, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, R.; Tago, D.; Treich, N. Infectious diseases and meat production. Environ. Resour. Econ. 2020, 76, 1019–1044. [Google Scholar] [CrossRef] [PubMed]
- Papier, K.; Knuppel, A.; Syam, N.; Jebb, S.A.; Key, T.J. Meat consumption and risk of ischemic heart disease: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 63, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Sobur, M.; Islam, M.; Ievy, S.; Hossain, M.; El Zowalaty, M.E.; Rahman, A.M.M.; Ashour, H.M. Zoonotic diseases: Etiology, impact, and control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Verma, V.; Kumar, M.; Kumar, A.; Sarma, D.K.; Singh, B.; Jha, R. Stem cells-derived in vitro meat: From petri dish to dinner plate. Crit. Rev. Food Sci. Nutr. 2022, 62, 2641–2654. [Google Scholar] [CrossRef] [PubMed]
- Soice, E.; Johnston, J. How cellular agriculture systems can promote food security. Front. Sustain. Food Syst. 2021, 5, 753996. [Google Scholar] [CrossRef]
- Munteanu, C.; Mireşan, V.; Răducu, C.; Ihuţ, A.; Uiuiu, P.; Pop, D.; Neacşu, A.; Cenariu, M.; Groza, I. Can cultured meat be an alternative to farm animal production for a sustainable and healthier lifestyle? Front. Nutr. 2021, 8, 749298. [Google Scholar] [CrossRef]
- Ben-Arye, T.; Levenberg, S. Tissue Engineering for Clean Meat Production. Front. Sustain. Food Syst. 2019, 3, 46. [Google Scholar] [CrossRef]
- McLeod, A. World Livestock 2011—Livestock in Food Security; Food and Agriculture Organization of the United Nations FAO: Rome, Italy, 2011. [Google Scholar]
- Chriki, S.; Hocquette, J.F. The myth of cultured meat: A review. Front. Nutr. 2020, 7, 7. [Google Scholar] [CrossRef]
- Ben-Arye, T.; Shandalov, Y.; Ben-Shaul, S.; Landau, S.; Zagury, Y.; Ianovici, I.; Lavon, N.; Levenberg, S. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat. Food 2020, 1, 210–220. [Google Scholar] [CrossRef]
- Seah, J.S.H.; Singh, S.; Tan, L.P.; Choudhury, D. Scaffolds for the manufacture of cultured meat. Crit. Rev. Biotechnol. 2022, 42, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.K.; Shin, D.M.; Choi, J.; Do, J.T.; Han, S.G. Current issues and technical advances in cultured meat production: A review. Food Sci. Anim. Resour. 2021, 41, 355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, H.; Zhang, Z.; Lu, Y.; Huang, X.; Yang, L.; Xu, J.; Yang, W.; Fan, X.; Du, B.; et al. A nerve graft constructed with xenogeneic acellular nerve matrix and autologous adipose-derived mesenchymal stem cells. Biomaterials 2010, 31, 5312–5324. [Google Scholar] [CrossRef] [PubMed]
- Modulevsky, D.J.; Lefebvre, C.; Haase, K.; Al-Rekabi, Z.; Pelling, A.E. Apple derived cellulose scaffolds for 3D mammalian cell culture. PLoS ONE 2014, 9, e97835. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, V.; Singh, S.K.; Gupta, J.; Kumar, M.; Sarma, D.K.; Verma, V. Recent advances in bioengineered scaffold for in vitro meat production. Cell Tissue Res. 2022, 391, 235–247. [Google Scholar] [CrossRef]
- Urciuolo, A.; De Coppi, P. Decellularized tissue for muscle regeneration. Int. J. Mol. Sci. 2018, 19, 2392. [Google Scholar] [CrossRef]
- Lu, H.; Ying, K.; Shi, Y.; Liu, D.; Chen, Q. Bioprocessing by Decellularized Scaffold Biomaterials in Cultured Meat: A Review. Bioengineering 2022, 9, 787. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Shiwarski, D.J.; Ball, R.L.; Whitehead, K.A.; Feinberg, A.W. Engineering aligned skeletal muscle tissue using decellularized plant-derived scaffolds. ACS Biomater. Sci. Eng. 2020, 6, 3046–3054. [Google Scholar] [CrossRef]
- Thyden, R.; Perreault, L.R.; Jones, J.D.; Notman, H.; Varieur, B.M.; Patmanidis, A.A.; Dominko, T.; Gaudette, G.R. An edible, decellularized plant derived cell carrier for lab grown meat. Appl. Sci. 2022, 12, 5155. [Google Scholar] [CrossRef]
- Asgar, M.A.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A.A. Nonmeat protein alternatives as meat extenders and meat analogs. Compr. Rev. Food Sci. Food Saf. 2010, 9, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Petroski, W.; Minich, D.M. Is there such a thing as “anti-nutrients”? A narrative review of perceived problematic plant compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Shahzadi, S.; Ransom, R.F.; Kloczkowski, A. Nature’s Own Pharmacy: Mushroom-Based Chemical Scaffolds and Their Therapeutic Implications. Int. J. Mol. Sci. 2023, 24, 15596. [Google Scholar] [CrossRef] [PubMed]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Zo, S.M.; Han, S.S. Novel biomimetic chitin-glucan polysaccharide nano/microfibrous fungal-scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2020, 149, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Balasundari, R.; Bishi, D.K.; Mathapati, S.; Naser, S.B.; Cherian, K.M.; Guhathakurta, S. Nanocoated botanical scaffold in salvage for human tissue regeneration. J. Biomater. Tissue Eng. 2012, 2, 330–335. [Google Scholar] [CrossRef]
- Shahini, A.; Vydiam, K.; Choudhury, D.; Rajabian, N.; Nguyen, T.; Lei, P.; Andreadis, S.T. Efficient and high yield isolation of myoblasts from skeletal muscle. Stem Cell Res. 2018, 30, 122–129. [Google Scholar] [CrossRef]
- Valderrama, K.; Oliva, M.; Campos, B.; Brown, D. Parasitic castration of Eurhomalea lenticularis Bivalvia: Veneridae by a digenetic trematode: Quantitative histological analysis. Dis. Aquat. Org. 2004, 59, 151–158. [Google Scholar] [CrossRef]
- Kim, Y.S.; Majid, M.; Melchiorri, A.J.; Mikos, A.G. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng. Transl. Med. 2019, 4, 83–95. [Google Scholar] [CrossRef]
- Yu, Y.; Alkhawaji, A.; Ding, Y.; Mei, J. Decellularized scaffolds in regenerative medicine. Oncotarget 2016, 7, 58671. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef] [PubMed]
- Enrione, J.; Blaker, J.J.; Brown, D.I.; Weinstein-Oppenheimer, C.R.; Pepczynska, M.; Olguín, Y.; Sánchez, E.; Acevedo, C.A. Edible scaffolds based on non-mammalian biopolymers for myoblast growth. Materials 2017, 10, 1404. [Google Scholar] [CrossRef] [PubMed]
- Cascella, R.; Chen, S.W.; Bigi, A.; Camino, J.D.; Xu, C.K.; Dobson, C.M.; Chiti, F.; Cremades, N.; Cecchi, C. The release of toxic oligomers from α-synuclein fibrils induces dysfunction in neuronal cells. Nat. Commun. 2021, 12, 1814. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, I.; Szeliga, A.; Moraczewski, J.; Czaplicka, I.; Brzóska, E. Comparison of satellite cell-derived myoblasts and C2C12 differentiation in two-and three-dimensional cultures: Changes in adhesion protein expression. Cell Biol. Int. 2011, 35, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Lanzoni, D.; Bracco, F.; Cheli, F.; Colosimo, B.M.; Moscatelli, D.; Baldi, A.; Rebucci, R.; Giromini, C. Biotechnological and Technical Challenges Related to Cultured Meat Production. Appl. Sci. 2022, 12, 6771. [Google Scholar] [CrossRef]
- Wollschlaeger, J.O.; Maatz, R.; Albrecht, F.B.; Klatt, A.; Heine, S.; Blaeser, A.; Kluger, P.J. Scaffolds for cultured meat on the basis of polysaccharide hydrogels enriched with plant-based proteins. Gels 2022, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Rebello, A.S.; Gaudette, G.R. Decellularized spinach: An edible scaffold for laboratory-grown meat. Food Biosci. 2021, 41, 100986. [Google Scholar] [CrossRef]
- Salehi, A.; Mobarhan, M.A.; Mohammadi, J.; Shahsavarani, H.; Shokrgozar, M.A.; Alipour, A. Efficient mineralization and osteogenic gene overexpression of mesenchymal stem cells on decellularized spinach leaf scaffold. Gene 2020, 757, 144852. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Q.; Wang, S.; Zhang, J.; Fan, S.; Lin, X. Current advances in the development of decellularized plant extracellular matrix. Front. Bioeng. Biotechnol. 2021, 9, 712262. [Google Scholar] [CrossRef]
- Hsieh, D.J.; Srinivasan, P.; Yen, K.C.; Yeh, Y.C.; Chen, Y.J.; Wang, H.C.; Tarng, Y.W. Protocols for the preparation and characterization of decellularized tissue and organ scaffolds for tissue engineering. BioTechniques 2021, 70, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Basiri, A.; Farokhi, M.; Azami, M.; Ebrahimi-Barough, S.; Mohamadnia, A.; Rashtbar, M.; Hasanzadeh, E.; Mahmoodi, N.; Baghaban, E.M.; Ai, J. A silk fibroin/decellularized extract of Wharton’s jelly hydrogel intended for cartilage tissue engineering. Prog. Biomater. 2019, 8, 31–42. [Google Scholar] [CrossRef] [PubMed]
- McCrary, M.W.; Vaughn, N.E.; Hlavac, N.; Song, Y.H.; Wachs, R.A.; Schmidt, C.E. Novel sodium deoxycholate-based chemical decellularization method for peripheral nerve. Tissue Eng. Part C Methods 2020, 26, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Griffin, M.; Naik, A.; Szarko, M.; Butler, P.E. Optimising the decellularization of human elastic cartilage with trypsin for future use in ear reconstruction. Sci. Rep. 2018, 8, 3097. [Google Scholar] [CrossRef] [PubMed]
- Simsa, R.; Padma, A.M.; Heher, P.; Hellström, M.; Teuschl, A.; Jenndahl, L.; Bergh, N.; Fogelstrand, P. Systematic in vitro comparison of decellularization protocols for blood vessels. PLoS ONE 2018, 13, e0209269. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, A.; Talaei-Khozani, T.; Kargar-Abarghouei, E.; Razban, V.; Vojdani, Z. Decellularized human ovarian scaffold based on a sodium lauryl ester sulfate SLES-treated protocol, as a natural three-dimensional scaffold for construction of bioengineered ovaries. Stem Cell Res. Ther. 2018, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Fontana, G.; Gershlak, J.; Adamski, M.; Lee, J.S.; Matsumoto, S.; Le, H.D.; Binder, B.; Wirth, J.; Gaudette, G.; Murphy, W.L. Biofunctionalized plants as diverse biomaterials for human cell culture. Adv. Healthc. Mater. 2017, 6, 1601225. [Google Scholar] [CrossRef]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.; Dolivo, D.M.; Yang, T.; Dominko, T.; et al. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef]
- Chang, J.Y.F.; Kessler, H.P. Masson trichrome stain helps differentiate myofibroma from smooth muscle lesions in the head and neck region. J. Formos. Med. Assoc. 2008, 107, 767–773. [Google Scholar] [CrossRef]
- Lee, J.; Jung, H.; Park, N.; Park, S.H.; Ju, J.H. Induced osteogenesis in plants decellularized scaffolds. Sci. Rep. 2019, 9, 20194. [Google Scholar] [CrossRef]



| Sr. | Gene | Primer Sequence |
|---|---|---|
| 1. | MyoG F MyoG R | 5′GCTCAAGAAAGTGAATGAGGC 3′ 5′ CTGGTAGACTCCTTCCTGCAG 3′ |
| 2. | MHC F MHC R | 5′ GGCCAAATCAAAGAGGTGA 3′ 5′ CGTGCTTCTCCTTCTCAACC 3′ |
| 3. | GAPDH F GAPDH R | 5′ GAAGGTCGGTGTGAACGGAT 3′ 5′ ATGAAGGGGTCGTTGATGGC 3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Singh, S.K.; Kumar, V.; Gupta, J.; Kumar, M.; Sarma, D.K.; Singh, S.; Kumawat, M.; Verma, V. Derivation and Characterization of Novel Cytocompatible Decellularized Tissue Scaffold for Myoblast Growth and Differentiation. Cells 2024, 13, 41. https://doi.org/10.3390/cells13010041
Singh A, Singh SK, Kumar V, Gupta J, Kumar M, Sarma DK, Singh S, Kumawat M, Verma V. Derivation and Characterization of Novel Cytocompatible Decellularized Tissue Scaffold for Myoblast Growth and Differentiation. Cells. 2024; 13(1):41. https://doi.org/10.3390/cells13010041
Chicago/Turabian StyleSingh, Anshuman, Suraj Kumar Singh, Vinod Kumar, Jalaj Gupta, Manoj Kumar, Devojit Kumar Sarma, Samradhi Singh, Manoj Kumawat, and Vinod Verma. 2024. "Derivation and Characterization of Novel Cytocompatible Decellularized Tissue Scaffold for Myoblast Growth and Differentiation" Cells 13, no. 1: 41. https://doi.org/10.3390/cells13010041
APA StyleSingh, A., Singh, S. K., Kumar, V., Gupta, J., Kumar, M., Sarma, D. K., Singh, S., Kumawat, M., & Verma, V. (2024). Derivation and Characterization of Novel Cytocompatible Decellularized Tissue Scaffold for Myoblast Growth and Differentiation. Cells, 13(1), 41. https://doi.org/10.3390/cells13010041








