LGR5+ Intestinal Stem Cells Display Sex-Dependent Radiosensitivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. In Vitro Culture of Intestinal Crypt Organoids
2.3. Irradiation Procedure
2.4. Histology
2.5. Crypt Proliferation Rate
2.6. Determination of Villous Length and Crypt Depth
2.7. qPCR Analysis
2.8. Western Blot Analysis
2.9. In Vivo Lineage Tracing Assay
2.10. LGR5+ Cell Isolation
2.11. RNAseq Analysis
2.12. Serum Exosomal miRNA Analysis
3. Results
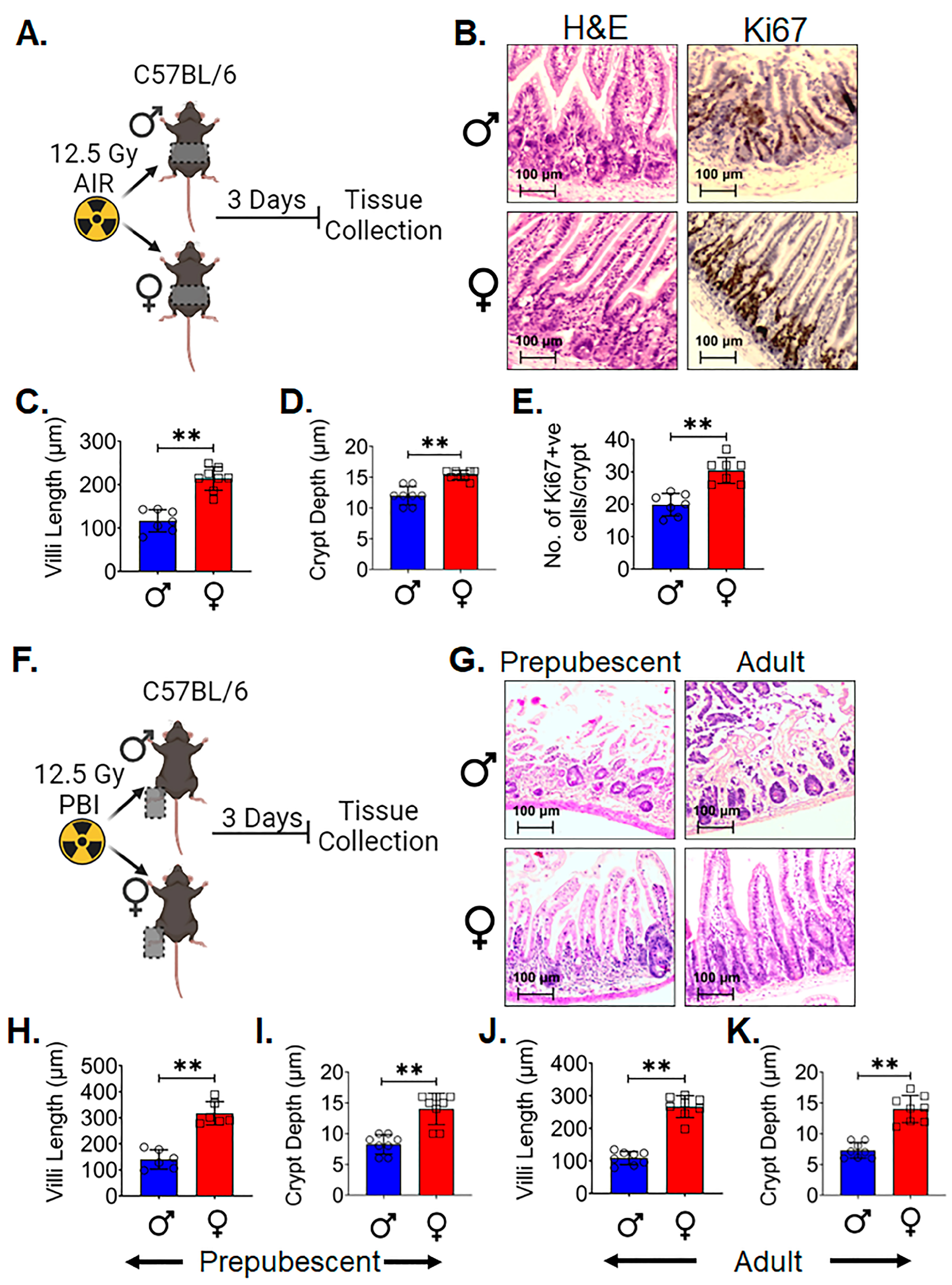
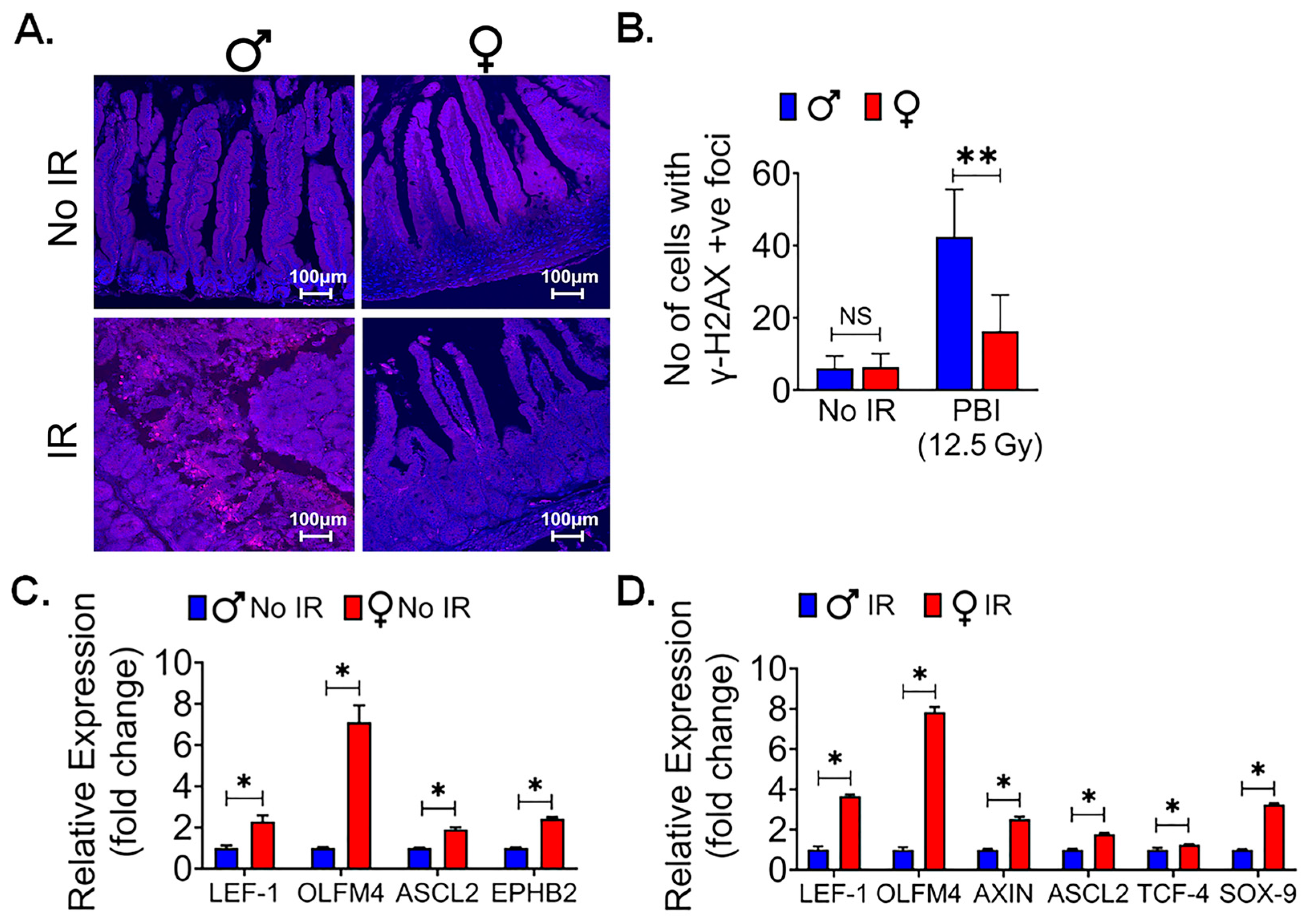
3.1. Female Intestinal Epithelium Is Resistant to Radiation-Induced Toxicity
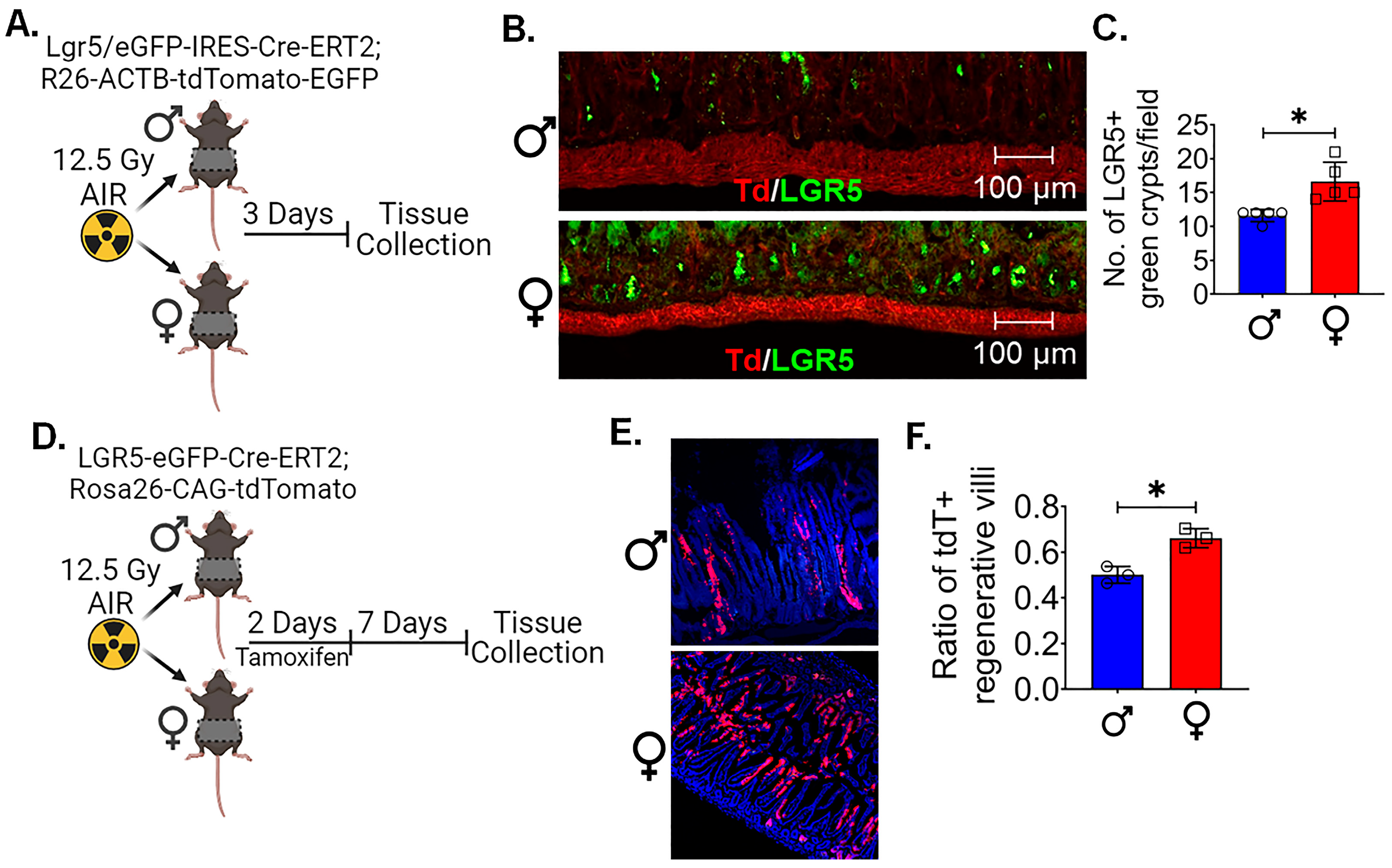
3.2. LGR5+ ISCs in Females Are More Radioresistant Compared to Males
3.3. Female LGR5+ Cells Are More Capable of Proliferation Following Radiation
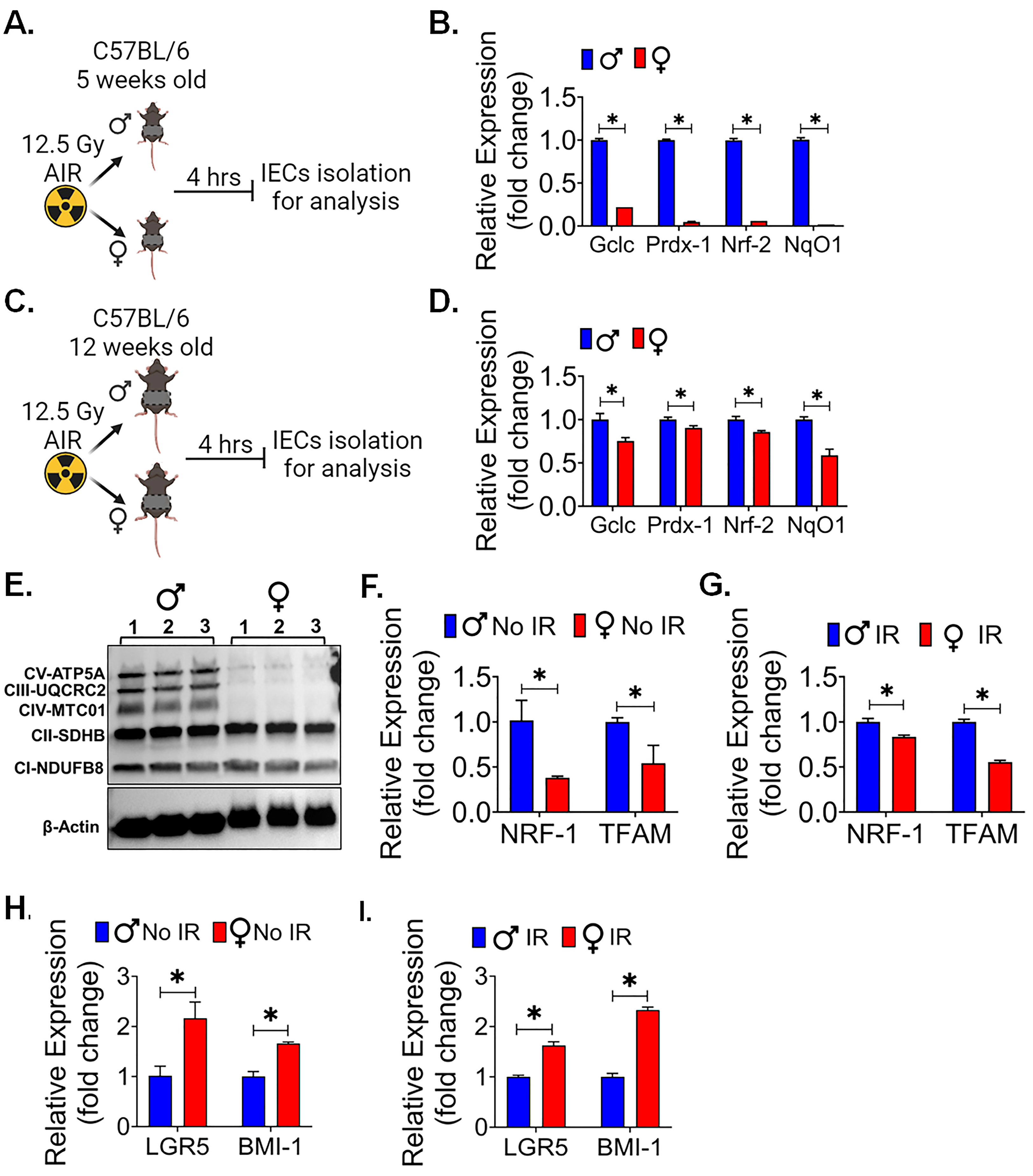
3.4. Female Intestinal Epithelium Showed Less Oxidative Stress, Mitochondrial Biogenesis and Better Stemness
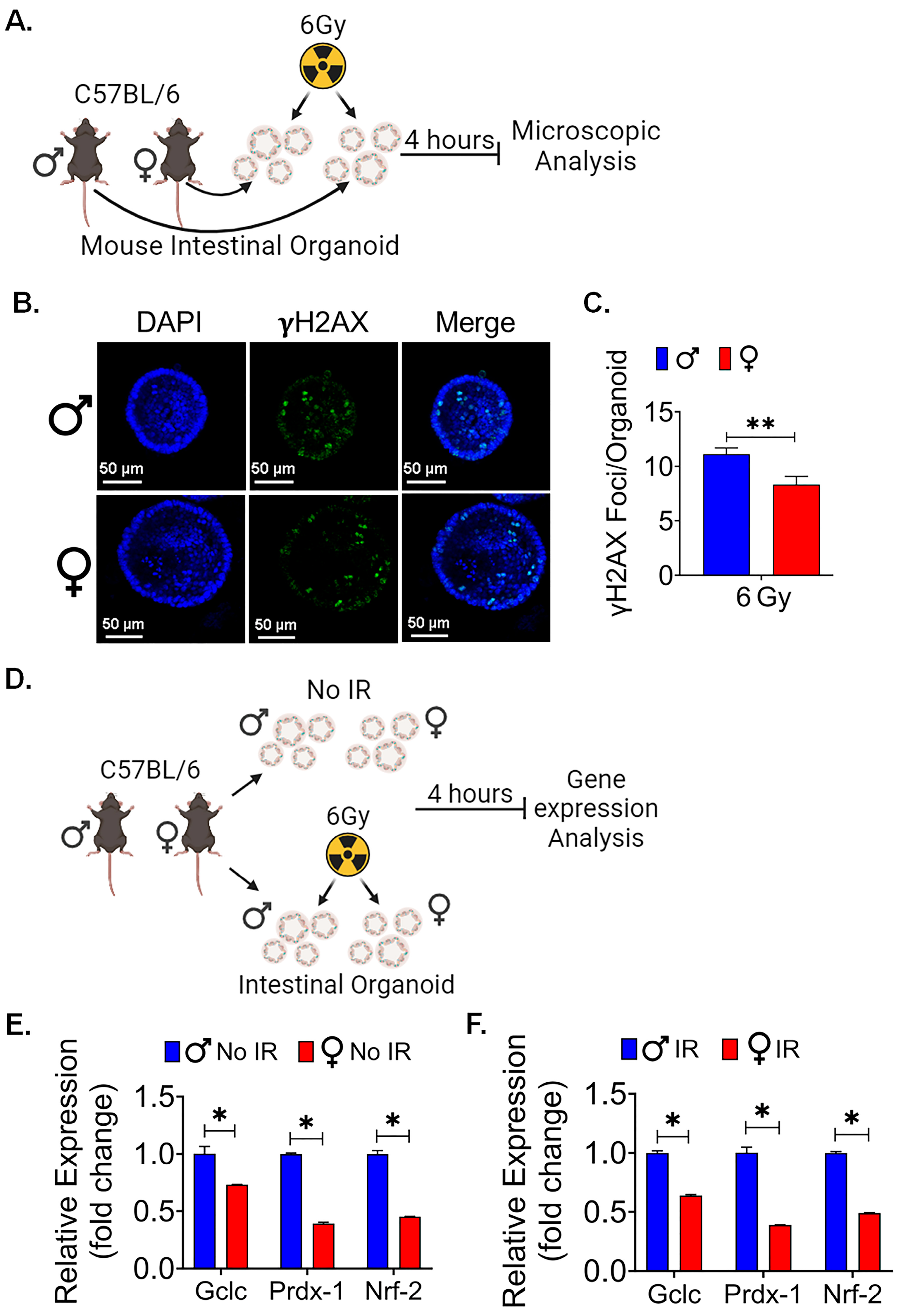
3.5. Female Intestinal Epithelium Shows Less Radiationinduced DNA Damage
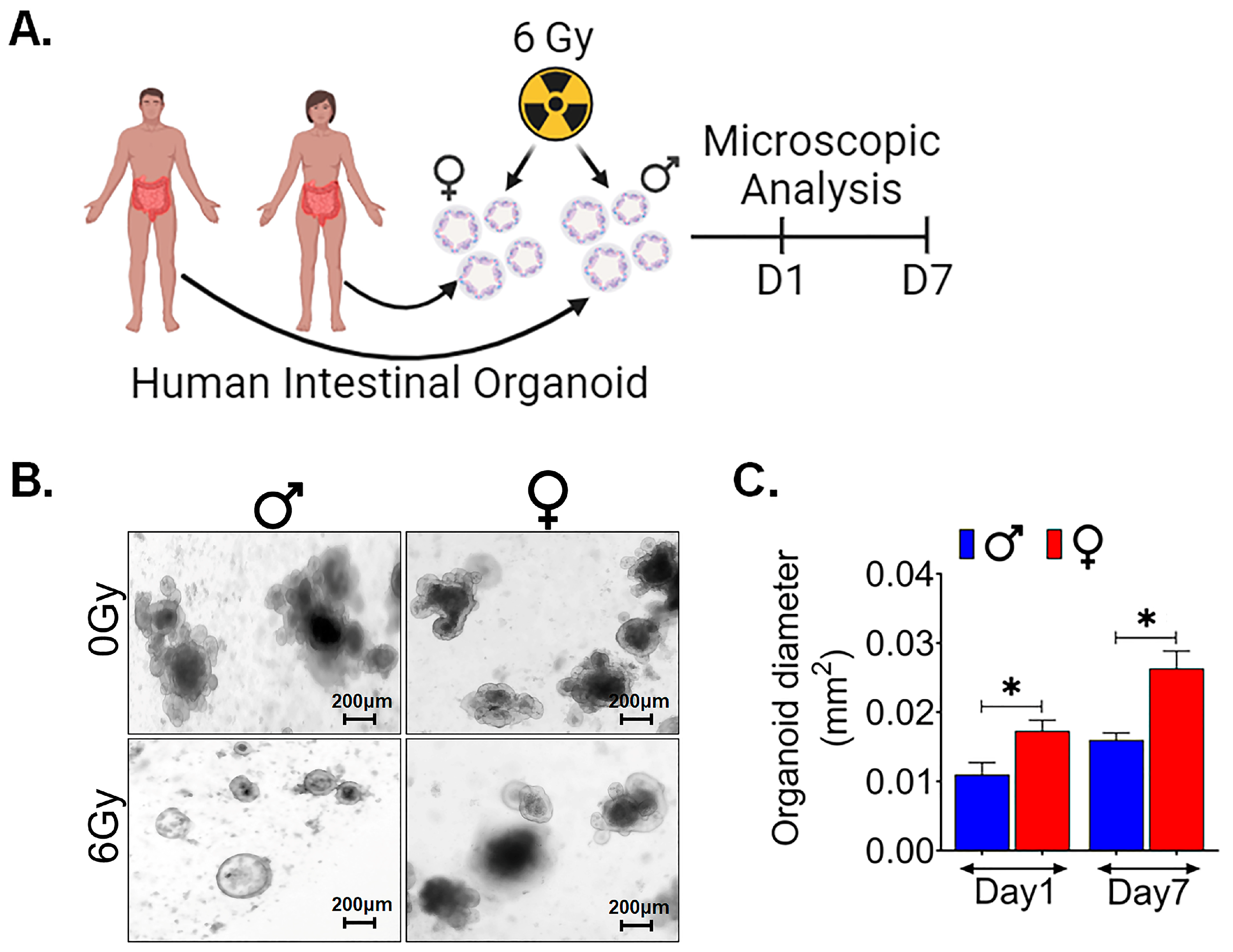
3.6. Female Ex Vivo Intestinal Organoid Is More RadioResistant
3.7. Female Intestinal Organoid Mimics the In Vivo Characteristics

3.8. Human Intestinal Organoids Derived from Females Are Radioresistant Compared to Those Derived from Males
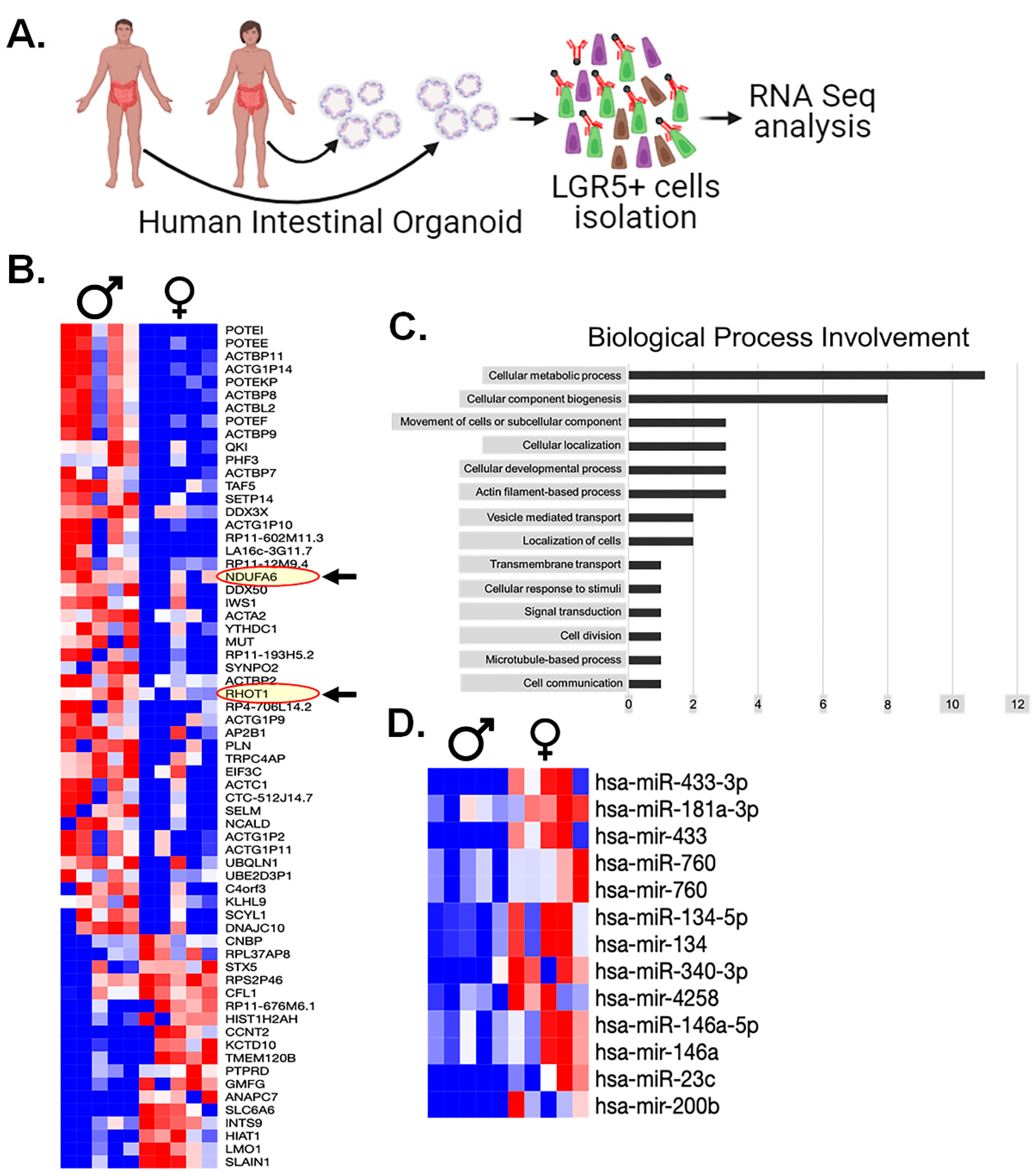
3.9. RNAseq Analysis of Human LGR5+ Cells Shows Sex-Dependent Differences in Mitochondrial Gene Expression
3.10. miRNA Analysis of Human Serum Highlights Several Biomarkers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, T.; Baumann, M. Prolongation of latency or overall treatment time by unplanned radiation pauses. The clinical importance of compensation. Strahlenther Onkol. 2005, 181, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Matsuyama, R.; Koike, I.; Kumamoto, T.; Kaizu, H.; Homma, Y.; Takano, S.; Sawada, Y.; Sugiura, M.; Yabushita, Y.; et al. Outcome of postoperative radiation therapy for cholangiocarcinoma and analysis of dose-volume histogram of remnant liver. Medicine 2019, 98, e16673. [Google Scholar] [CrossRef] [PubMed]
- Siala, W.; Mnejja, W.; Abid, M.; Ghorbel, A.; Frikha, M.; Daoud, J. Thyroid toxicity after radiotherapy of nasopharyngeal carcinoma. Ann. D’endocrinologie 2011, 72, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski-Stivers, N.; Petit, B.; Ollivier, J.; Monceau, V.; Tsoutsou, P.; Pousa, A.Q.; Lin, X.; Limoli, C.; Vozenin, M.-C. Sex-Specific Differences in Toxicity Following Systemic Paclitaxel Treatment and Localized Cardiac Radiotherapy. Cancers 2021, 13, 3973. [Google Scholar] [CrossRef]
- Rodman, S.N.; Kluz, P.N.; Hines, M.R.; Oberley-Deegan, R.E.; Coleman, M.C. Sex-based differences in the severity of radiation-induced arthrofibrosis. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2022, 40, 2586–2596. [Google Scholar] [CrossRef]
- Stacey, R.; Green, J.T. Radiation-induced small bowel disease: Latest developments and clinical guidance. Ther. Adv. Chronic Dis. 2014, 5, 15–29. [Google Scholar] [CrossRef]
- Andreyev, H.J.N.; Vlavianos, P.; Blake, P.; Dearnaley, D.; Norman, A.R.; Tait, D. Gastrointestinal symptoms after pelvic radiotherapy: Role for the gastroenterologist? Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1464–1471. [Google Scholar] [CrossRef]
- Do, N.L.; Nagle, D.; Poylin, V.Y. Radiation proctitis: Current strategies in management. Gastroenterol. Res. Pract. 2011, 2011, 917941. [Google Scholar] [CrossRef]
- Roberts, S.A.; Hendry, J.H.; Potten, C.S. Intestinal crypt clonogens: A new interpretation of radiation survival curve shape and clonogenic cell number. Cell Prolif. 2003, 36, 215–231. [Google Scholar] [CrossRef]
- Paris, F.; Fuks, Z.; Kang, A.; Capodieci, P.; Juan, G.; Ehleiter, D.; Haimovitz-Friedman, A.; Cordon-Cardo, C.; Kolesnick, R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 2001, 293, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Schuller, B.W.; Rogers, A.B.; Cormier, K.S.; Riley, K.J.; Binns, P.J.; Julius, R.; Hawthorne, M.F.; Coderre, J.A. No significant endothelial apoptosis in the radiation-Induced gastrointestinal syndrome. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Cannon, G.; Olson, M.G.; Smith, J.T.; Anderson, M.N.; Zhai, M.; Umali, M.V.; Ho, K.; Ho, C.; Cui, W.; et al. Female Mice are More Resistant to the Mixed-Field (67% Neutron + 33% Gamma) Radiation-Induced Injury in Bone Marrow and Small Intestine than Male Mice due to Sustained Increases in G-CSF and the Bcl-2/Bax Ratio and Lower miR-34a and MAPK Activation. Radiat. Res. 2022, 198, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Heath, J.P. Epithelial cell migration in the Intestine. Cell Biol. Int. 1996, 20, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Van Es, J.H.; Kuipers, J.; Kujala, P.; Van Den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Ayyaz, A.; Kumar, S.; Sangiorgi, B.; Ghoshal, B.; Gosio, J.; Ouladan, S.; Fink, M.; Barutcu, S.; Trcka, D.; Shen, J.; et al. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 2019, 569, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.; Kljavin, N.M.; Ybarra, R.; de Sauvage, F.J. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 2014, 14, 149–159. [Google Scholar] [CrossRef]
- Liu, Y.K.; Harty, J.I.; Steinbock, G.S.; Holt, H.A.; Goldstein, D.H.; Amin, M. Treatment of Radiation or cyclophosphamide induced hemorrhagic cystitis using conjugated estrogen. J. Urol. 1990, 144, 41–43. [Google Scholar] [CrossRef]
- Myrehaug, S.; Pintilie, M.; Tsang, R.; Mackenzie, R.; Crump, M.; Chen, Z.; Sun, A.; Hodgson, D.C. Cardiac morbidity following modern treatment for Hodgkin lymphoma: Supra-additive cardiotoxicity of doxorubicin and radiation therapy. Leuk. Lymphoma 2008, 49, 1486–1493. [Google Scholar] [CrossRef]
- Zárate, S.; Stevnsner, T.; Gredilla, R. Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Front. Aging Neurosci. 2017, 9, 430. [Google Scholar] [CrossRef]
- Tran, A.; Scholtes, C.; Songane, M.; Champagne, C.; Galarneau, L.; Levasseur, M.-P.; Fodil, N.; Dufour, C.R.; Giguère, V.; Saleh, M. Estrogen-related receptor alpha (ERRα) is a key regulator of intestinal homeostasis and protects against colitis. Sci. Rep. 2021, 11, 15073. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Davis, E.A.; Li, K.; Nowak, R.A.; Dailey, M.J. Sex differences influence intestinal epithelial stem cell proliferation independent of obesity. Physiol. Rep. 2018, 6, e13746. [Google Scholar] [CrossRef] [PubMed]
- Bhanja, P.; Norris, A.; Gupta-Saraf, P.; Hoover, A.; Saha, S. BCN057 induces intestinal stem cell repair and mitigates radiation-induced intestinal injury. Stem Cell Res. Ther. 2018, 9, 26. [Google Scholar] [CrossRef]
- Muzumdar, M.D.; Tasic, B.; Miyamichi, K.; Li, L.; Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 2007, 45, 593–605. [Google Scholar] [CrossRef]
- Saha, S.; Aranda, E.; Hayakawa, Y.; Bhanja, P.; Atay, S.; Brodin, N.P.; Li, J.; Asfaha, S.; Liu, L.; Tailor, Y.; et al. Macrophage-derived extracellular vesicle-packaged WNTs rescue intestinal stem cells and enhance survival after radiation injury. Nat. Commun. 2016, 7, 13096. [Google Scholar] [CrossRef]
- Sato, T.; Van Es, J.H.; Snippert, H.J.; Stange, D.E.; Vries, R.G.; van den Born, M.; Barker, N.; Shroyer, N.F.; Van De Wetering, M.; Clevers, H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011, 469, 415–418. [Google Scholar] [CrossRef]
- Saha, S.; Bhanja, P.; Kabarriti, R.; Liu, L.; Alfieri, A.A.; Guha, C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS ONE 2011, 6, e24072. [Google Scholar] [CrossRef]
- Potten, C.S.; Booth, C.; Pritchard, D.M. The intestinal epithelial stem cell: The mucosal governor. Int. J. Exp. Pathol. 1997, 78, 219–243. [Google Scholar] [CrossRef]
- Wiechelman, K.J.; Braun, R.D.; Fitzpatrick, J.D. Investigation of the bicinchoninic acid protein assay: Identification of the groups responsible for color formation. Anal. Biochem. 1988, 175, 231–237. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Warren, S.L.; Whipple, G.H. Roentgen ray intoxication: III. speed of autolysis of various body tissues after lethal x-ray exposures. the remarkable disturbance in the epithelium of the small intestine. J. Exp. Med. 1922, 35, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-K.; Yang, V.W.; Bialkowska, A.B. The Role of Intestinal Stem Cells in Epithelial Regeneration Following Radiation-Induced Gut Injury. Curr. Stem Cell Rep. 2017, 3, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. /Fundam. Mol. Mech. Mutagen. 2011, 711, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Barsoumian, H.B.; Fischer, G.; Yang, L.; Verma, V.; I Younes, A.; Hu, Y.; Masropour, F.; Klein, K.; Vellano, C.; et al. Combination treatment with radiotherapy and a novel oxidative phosphorylation inhibitor overcomes PD-1 resistance and enhances antitumor immunity. J. Immunother. Cancer 2020, 8, e000289. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Sasatani, M.; Kawai, H.; Kamiya, K.; Kobayashi, J.; Komatsu, K.; Kunugita, N. A comparison of radiation-induced mitochondrial damage between neural progenitor stem cells and differentiated cells. Cell Cycle 2017, 16, 565–573. [Google Scholar] [CrossRef]
- Schell, J.C.; Wisidagama, D.R.; Bensard, C.; Zhao, H.; Wei, P.; Tanner, J.; Flores, A.; Mohlman, J.; Sorensen, L.K.; Earl, C.S.; et al. Control of intestinal stem cell function and proliferation by mitochondrial pyruvate metabolism. Nat. Cell Biol. 2017, 19, 1027–1036. [Google Scholar] [CrossRef]
- Rodríguez-Colman, M.J.; Schewe, M.; Meerlo, M.; Stigter, E.; Gerrits, J.; Pras-Raves, M.; Sacchetti, A.; Hornsveld, M.; Oost, K.C.; Snippert, H.J.; et al. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature 2017, 543, 424–427. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Y.; Wei, R.; Napier, D.L.; Sengoku, T.; Alstott, M.C.; Liu, J.; Wang, C.; Zaytseva, Y.Y.; Weiss, H.L.; et al. Glycolytic Regulation of Intestinal Stem Cell Self-Renewal and Differentiation. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 931–947. [Google Scholar] [CrossRef]
- Wanet, A.; Arnould, T.; Najimi, M.; Renard, P. Connecting Mitochondria, Metabolism, and Stem Cell Fate. Stem Cells Dev. 2015, 24, 1957–1971. [Google Scholar] [CrossRef]
- Firsanov, D.V.; Solovjeva, L.V.; Svetlova, M.P. H2AX phosphorylation at the sites of DNA double-strand breaks in cultivated mammalian cells and tissues. Clin. Epigenetics 2011, 2, 283–297. [Google Scholar] [CrossRef]
- Reynolds, A.; Wharton, N.; Parris, A.; Mitchell, E.; Sobolewski, A.; Kam, C.; Bigwood, L.; El Hadi, A.; Münsterberg, A.; Lewis, M.; et al. Canonical Wnt signals combined with suppressed TGFβ/BMP pathways promote renewal of the native human colonic epithelium. Gut 2014, 63, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Matano, M.; Toshimitsu, K.; Takano, A.; Mikami, Y.; Nishikori, S.; Sugimoto, S.; Sato, T. Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell 2018, 23, 787–793.e6. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos-Manzano, C.; Puschhof, J.; van den Brink, S.; Geurts, V.; Beumer, J.; Clevers, H. Establishment and Culture of Human Intestinal Organoids Derived from Adult Stem Cells. Curr. Protoc. Immunol. 2020, 130, e106. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, Y.; Wang, D.; Huang, L.; Li, F.; Liu, J.; Zhang, C.; Shen, Z.; Gao, Q.; Yuan, W.; et al. MiR-760 suppresses human colorectal cancer growth by targeting BATF3/AP-1/cyclinD1 signaling. J. Exp. Clin. Cancer Res. 2018, 37, 83. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Bian, Y.; Liu, L.; Liu, L.; Liu, X.; Ma, S. Molecular pathways associated with oxidative stress and their potential applications in radiotherapy (Review). Int. J. Mol. Med. 2022, 49, 65. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular Stress Responses in Radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef]
- Folmes, C.D.; Nelson, T.J.; Dzeja, P.P.; Terzic, A. Energy metabolism plasticity enables stemness programs. Ann. N. Y. Acad. Sci. 2012, 1254, 82–89. [Google Scholar] [CrossRef]
- Hu, C.; Fan, L.; Cen, P.; Chen, E.; Jiang, Z.; Li, L. Energy Metabolism Plays a Critical Role in Stem Cell Maintenance and Differentiation. Int. J. Mol. Sci. 2016, 17, 253. [Google Scholar] [CrossRef]
- Rigaud, V.O.C.; Hoy, R.; Mohsin, S.; Khan, M. Stem Cell Metabolism: Powering Cell-Based Therapeutics. Cells 2020, 9, 2490. [Google Scholar] [CrossRef]
- Morganti, C.; Cabezas-Wallscheid, N.; Ito, K. Metabolic Regulation of Hematopoietic Stem Cells. HemaSphere 2022, 6, e740. [Google Scholar] [CrossRef]
- Park, I.-H.; Song, Y.-S.; Joo, H.-W.; Shen, G.-Y.; Seong, J.-H.; Shin, N.-K.; Cho, Y.J.; Lee, Y.; Shin, J.H.; Lim, Y.-H.; et al. Role of MicroRNA-34a in Anti-Apoptotic Effects of Granulocyte-Colony Stimulating Factor in Diabetic Cardiomyopathy. Diabetes Metab. J. 2020, 44, 173–185. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zitter, R.C.; Chugh, R.M.; Bhanja, P.; Kimler, B.F.; Saha, S. LGR5+ Intestinal Stem Cells Display Sex-Dependent Radiosensitivity. Cells 2024, 13, 46. https://doi.org/10.3390/cells13010046
Zitter RC, Chugh RM, Bhanja P, Kimler BF, Saha S. LGR5+ Intestinal Stem Cells Display Sex-Dependent Radiosensitivity. Cells. 2024; 13(1):46. https://doi.org/10.3390/cells13010046
Chicago/Turabian StyleZitter, Ryan C., Rishi Man Chugh, Payel Bhanja, Bruce F. Kimler, and Subhrajit Saha. 2024. "LGR5+ Intestinal Stem Cells Display Sex-Dependent Radiosensitivity" Cells 13, no. 1: 46. https://doi.org/10.3390/cells13010046




