Extracellular Matrix Cues Regulate Mechanosensing and Mechanotransduction of Cancer Cells
Abstract
1. Introduction
2. Mechanotransduction Mechanism: Cell–Matrix Force Relationships with Emphasis on Matrix or Tissue Forces
2.1. Tensile Force
2.2. Hydrostatic Pressure
2.3. Fluid Shear Stress
2.4. mTOR-FAK Signaling Axis
3. Mechanical Aspects of the ECM on Cells (Plasticity) and Mechanotransduction
3.1. Regulators of Bulk and Local ECM Stiffness and Material Elasticity
3.1.1. ECM Stiffness Impacts Adhesion, Migration, and Invasion
3.1.2. ECM Stiffness Fosters Drug Resistance
- ECM Stiffness Impacts Chemotherapy
- ECM stiffness impacts radiotherapy
3.2. ECM Mechanical Stress/Loading and Stiffness Alter Cancer Immunity
3.2.1. Force Can Trigger Therapy Resistance
3.2.2. Increased IFP-Based Shear Stress and Resistance to Therapy
3.2.3. Compression-Based Mechanical Stress/Loading and Effect on Resistance to Therapy
3.2.4. Targets for Stiffness Regulation in Tumors
3.3. Tumor Growth and Metastasis Is Controlled by Matrix Stiffness
3.4. Regulation of ECM Composition (Ligand Density) by Matrix Stiffness
3.5. Regulators of Topography
3.6. Regulators of Cancer Cell Transition and Enrichment of CSCs
3.7. ECM Stiffness Regulates Cancer Cell Nuclei Cues
3.7.1. Histone Variants Can Be Altered
3.7.2. ECM Stiffness Impacts the Structure of Chromatin
3.7.3. ECM Stiffness Impacts the Linkage of Chromatin with the Nuclear Membrane
3.7.4. ECM Stiffness Impacts Chromatin Structure That Subsequently Changes Nuclear Mechanics and Mechanosensitivity
3.7.5. ECM Stiffness Impacts other Nuclear Properties
4. Cells Sense and Respond to Mechanical Cues of the ECM
4.1. Cells Can Alter Organization of the ECM in a Biological Manner
4.1.1. Enzymatic Modification of the Cancer ECM
4.1.2. Matricellular Proteins
4.2. Cells Can Sense Mechanical Cues Passively When the ECM Exerts a Force onto Them
4.2.1. How Large Are Cellular Forces?
4.2.2. How Is the Direction of Cellular Forces Regulated?
4.2.3. How Dynamic Are Cellular Forces?
4.3. Cells Can Interact with One Another
4.3.1. Neighboring Cells
4.3.2. Distant Cells (via Traction-Induced ECM Displacements)
5. Emerging New Frontiers in the Field of Mechanotransduction in Cancer
5.1. Systems-Level Knowledge of Mechanosensitive Pathways
5.2. Standardization of Mechanotransduction Models and Approaches
5.3. Translation of Mechanobiology into Clinics
5.3.1. Trials Aiming at Components of Mechanical Sensing and Mechanotransduction
5.3.2. Trials Aiming at the Normalization of the Tumor Microenvironment (TME)
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mak, M. Impact of Crosslink Heterogeneity on Extracellular Matrix Mechanics and Remodeling. Comput. Struct. Biotechnol. J. 2020, 18, 3969–3976. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, A.; Trepat, X.; Kamm, R.D.; Mak, M. Dynamic Filopodial Forces Induce Accumulation, Damage, and Plastic Remodeling of 3D Extracellular Matrices. PLoS Comput. Biol. 2019, 15, e1006684. [Google Scholar] [CrossRef] [PubMed]
- Semkova, M.E.; Hsuan, J.J. TGFβ-1 Induced Cross-Linking of the Extracellular Matrix of Primary Human Dermal Fibroblasts. Int. J. Mol. Sci. 2021, 22, 984. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhou, L.; Li, D.; Andl, T.; Zhang, Y. Cancer-Associated Fibroblasts Build and Secure the Tumor Microenvironment. Front. Cell Dev. Biol. 2019, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Huang, X.; Zhao, Y.; Wu, D.; Xue, K.; Yao, J.; Wang, Y.; Tang, N.; Qiu, Y. The Hippo Pathway Links Adipocyte Plasticity to Adipose Tissue Fibrosis. Nat. Commun. 2022, 13, 6030. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wu, M.; Zeng, N.; Xiong, M.; Hu, W.; Lv, W.; Yi, Y.; Zhang, Q.; Wu, Y. Cancer-Associated Adipocytes: Emerging Supporters in Breast Cancer. J. Exp. Clin. Cancer Res. 2020, 39, 156. [Google Scholar] [CrossRef]
- Carpenco, E. The Role of Tumor-Associated Macrophage in Breast Cancer Biology. Histol. Histopathol. 2017, 33, 133–145. [Google Scholar] [CrossRef]
- Mierke, C.T.; Frey, B.; Fellner, M.; Herrmann, M.; Fabry, B. Integrin A5β1 Facilitates Cancer Cell Invasion through Enhanced Contractile Forces. J. Cell Sci. 2011, 124, 369–383. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, C.; Huang, Z.; Zhou, S.; Yang, Y.; Yin, Z.; Heng, B.C.; Chen, W.; Chen, X.; Shen, W. Extracellular Matrix Remodeling in Stem Cell Culture: A Potential Target for Regulating Stem Cell Function. Tissue Eng. Part B Rev. 2022, 28, 542–554. [Google Scholar] [CrossRef]
- Ghosh, D.; Mejia-Pena, C.; Quach, N.; Xuan, B.; Lee, A.H.; Dawson, M.R. Senescent Mesenchymal Stem Cells Remodel Extracellular Matrix Driving Breast Cancer Cells to More Invasive Phenotype. J. Cell Sci. 2020, 133, jcs.232470. [Google Scholar] [CrossRef]
- Wang, N. Review of Cellular Mechanotransduction. J. Phys. D Appl. Phys. 2017, 50, 233002. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. The Matrix Environmental and Cell Mechanical Properties Regulate Cell Migration and Contribute to the Invasive Phenotype of Cancer Cells. Rep. Prog. Phys. 2019, 82, 064602. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. The Fundamental Role of Mechanical Properties in the Progression of Cancer Disease and Inflammation. Rep. Prog. Phys. 2014, 77, 076602. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. Mechanical Cues Affect Migration and Invasion of Cells From Three Different Directions. Front. Cell Dev. Biol. 2020, 8, 583226. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. Bidirectional Mechanical Response Between Cells and Their Microenvironment. Front. Phys. 2021, 9, 749830. [Google Scholar] [CrossRef]
- Nallanthighal, S.; Heiserman, J.P.; Cheon, D.-J. The Role of the Extracellular Matrix in Cancer Stemness. Front. Cell Dev. Biol. 2019, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Inman, J.L.; Robertson, C.; Mott, J.D.; Bissell, M.J. Mammary Gland Development: Cell Fate Specification, Stem Cells and the Microenvironment. Development 2015, 142, 1028–1042. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Pohl, T.L.M.; Seckinger, A.; Spatz, J.P.; Cavalcanti-Adam, E.A. Regulation of Integrin and Growth Factor Signaling in Biomaterials for Osteodifferentiation. Beilstein J. Org. Chem. 2015, 11, 773–783. [Google Scholar] [CrossRef]
- Sun, Q.; Hou, Y.; Chu, Z.; Wei, Q. Soft Overcomes the Hard: Flexible Materials Adapt to Cell Adhesion to Promote Cell Mechanotransduction. Bioact. Mater. 2022, 10, 397–404. [Google Scholar] [CrossRef]
- Haining, A.W.M.; Rahikainen, R.; Cortes, E.; Lachowski, D.; Rice, A.; von Essen, M.; Hytönen, V.P.; del Río Hernández, A. Mechanotransduction in Talin through the Interaction of the R8 Domain with DLC1. PLoS Biol. 2018, 16, e2005599. [Google Scholar] [CrossRef]
- Goult, B.T.; Brown, N.H.; Schwartz, M.A. Talin in Mechanotransduction and Mechanomemory at a Glance. J. Cell Sci. 2021, 134, jcs258749. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Rojo, R.; Alonso-Caballero, Á.; Fernández, J.M. Talin Folding as the Tuning Fork of Cellular Mechanotransduction. Proc. Natl. Acad. Sci. USA 2020, 117, 21346–21353. [Google Scholar] [CrossRef] [PubMed]
- del Rio, A.; Perez-Jimenez, R.; Liu, R.; Roca-Cusachs, P.; Fernandez, J.M.; Sheetz, M.P. Stretching Single Talin Rod Molecules Activates Vinculin Binding. Science 2009, 323, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T.; Fischer, T.; Puder, S.; Kunschmann, T.; Soetje, B.; Ziegler, W.H. Focal Adhesion Kinase Activity Is Required for Actomyosin Contractility-Based Invasion of Cells into Dense 3D Matrices. Sci. Rep. 2017, 7, 42780. [Google Scholar] [CrossRef] [PubMed]
- Spanjaard, E.; de Rooij, J. Mechanotransduction: Vinculin Provides Stability When Tension Rises. Curr. Biol. 2013, 23, R159–R161. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T.; Kollmannsberger, P.; Zitterbart, D.P.; Diez, G.; Koch, T.M.; Marg, S.; Ziegler, W.H.; Goldmann, W.H.; Fabry, B. Vinculin Facilitates Cell Invasion into Three-Dimensional Collagen Matrices. J. Biol. Chem. 2010, 285, 13121–13130. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T.; Kollmannsberger, P.; Paranhos Zitterbart, D.; Smith, J.; Fabry, B.; Goldmann, W.H. Mechano-Coupling and Regulation of Contractility by the Vinculin Tail Domain. Biophys. J. 2008, 94, 661–670. [Google Scholar] [CrossRef]
- Grashoff, C.; Hoffman, B.D.; Brenner, M.D.; Zhou, R.; Parsons, M.; Yang, M.T.; McLean, M.A.; Sligar, S.G.; Chen, C.S.; Ha, T.; et al. Measuring Mechanical Tension across Vinculin Reveals Regulation of Focal Adhesion Dynamics. Nature 2010, 466, 263–266. [Google Scholar] [CrossRef]
- Tharp, K.M.; Higuchi-Sanabria, R.; Timblin, G.A.; Ford, B.; Garzon-Coral, C.; Schneider, C.; Muncie, J.M.; Stashko, C.; Daniele, J.R.; Moore, A.S.; et al. Adhesion-Mediated Mechanosignaling Forces Mitohormesis. Cell Metab. 2021, 33, 1322–1341.e13. [Google Scholar] [CrossRef]
- Espina, J.A.; Marchant, C.L.; Barriga, E.H. Durotaxis: The Mechanical Control of Directed Cell Migration. FEBS J. 2022, 289, 2736–2754. [Google Scholar] [CrossRef]
- Arnold, M.; Cavalcanti-Adam, E.A.; Glass, R.; Blümmel, J.; Eck, W.; Kantlehner, M.; Kessler, H.; Spatz, J.P. Activation of Integrin Function by Nanopatterned Adhesive Interfaces. ChemPhysChem 2004, 5, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Boudreau, A.; Bissell, M.J. Tissue Architecture and Function: Dynamic Reciprocity via Extra- and Intra-Cellular Matrices. Cancer Metastasis Rev. 2009, 28, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, A.S.; Loe, F.C.; Li, R.; Raghunath, M.; Van Vliet, K.J. Macromolecular Crowding Directs Extracellular Matrix Organization and Mesenchymal Stem Cell Behavior. PLoS ONE 2012, 7, e37904. [Google Scholar] [CrossRef] [PubMed]
- Ricca, B.L.; Venugopalan, G.; Fletcher, D.A. To Pull or Be Pulled: Parsing the Multiple Modes of Mechanotransduction. Curr. Opin. Cell Biol. 2013, 25, 558–564. [Google Scholar] [CrossRef][Green Version]
- Hou, Y.; Yu, L.; Xie, W.; Camacho, L.C.; Zhang, M.; Chu, Z.; Wei, Q.; Haag, R. Surface Roughness and Substrate Stiffness Synergize To Drive Cellular Mechanoresponse. Nano Lett. 2020, 20, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, Q.; Liu, Y.; Chu, Z.; Yu, L.; Hou, Y.; Kang, H.; Wei, Q.; Zhao, W.; Spatz, J.P.; et al. Controllable Ligand Spacing Stimulates Cellular Mechanotransduction and Promotes Stem Cell Osteogenic Differentiation on Soft Hydrogels. Biomaterials 2021, 268, 120543. [Google Scholar] [CrossRef]
- Dhowre, H.S.; Rajput, S.; Russell, N.A.; Zelzer, M. Responsive Cell–Material Interfaces. Nanomedicine 2015, 10, 849–871. [Google Scholar] [CrossRef]
- Li, W.; Yan, Z.; Ren, J.; Qu, X. Manipulating Cell Fate: Dynamic Control of Cell Behaviors on Functional Platforms. Chem. Soc. Rev. 2018, 47, 8639–8684. [Google Scholar] [CrossRef]
- Seo, J.-H.; Yui, N. The Effect of Molecular Mobility of Supramolecular Polymer Surfaces on Fibroblast Adhesion. Biomaterials 2013, 34, 55–63. [Google Scholar] [CrossRef]
- Gonzalez-Molina, J.; Zhang, X.; Borghesan, M.; Mendonça Da Silva, J.; Awan, M.; Fuller, B.; Gavara, N.; Selden, C. Extracellular Fluid Viscosity Enhances Liver Cancer Cell Mechanosensing and Migration. Biomaterials 2018, 177, 113–124. [Google Scholar] [CrossRef]
- Pfeifer, C.R.; Alvey, C.M.; Irianto, J.; Discher, D.E. Genome Variation across Cancers Scales with Tissue Stiffness—An Invasion-Mutation Mechanism and Implications for Immune Cell Infiltration. Curr. Opin. Syst. Biol. 2017, 2, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Haga, H. Matrix Stiffness Contributes to Cancer Progression by Regulating Transcription Factors. Cancers 2022, 14, 1049. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Vidal, L.; Murdica, V.; Venegoni, C.; Pederzoli, F.; Bandini, M.; Necchi, A.; Salonia, A.; Alfano, M. Causal Contributors to Tissue Stiffness and Clinical Relevance in Urology. Commun. Biol. 2021, 4, 1011. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, S.K.; Aryasomayajula, B.; Torchilin, V.P. Barriers to Drug Delivery in Solid Tumors. Tissue Barriers 2014, 2, e29528. [Google Scholar] [CrossRef] [PubMed]
- Furler, R.; Nixon, D.; Brantner, C.; Popratiloff, A.; Uittenbogaart, C. TGF-β Sustains Tumor Progression through Biochemical and Mechanical Signal Transduction. Cancers 2018, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Fontebasso, Y.; Dubinett, S.M. Drug Development for Metastasis Prevention. Crit. Rev. Oncog. 2015, 20, 449–473. [Google Scholar] [CrossRef] [PubMed]
- Gandalovičová, A.; Rosel, D.; Fernandes, M.; Veselý, P.; Heneberg, P.; Čermák, V.; Petruželka, L.; Kumar, S.; Sanz-Moreno, V.; Brábek, J. Migrastatics—Anti-Metastatic and Anti-Invasion Drugs: Promises and Challenges. Trends Cancer 2017, 3, 391–406. [Google Scholar] [CrossRef]
- Gorshtein, G.; Grafinger, O.; Coppolino, M.G. Targeting SNARE-Mediated Vesicle Transport to Block Invadopodium-Based Cancer Cell Invasion. Front. Oncol. 2021, 11, 679955. [Google Scholar] [CrossRef]
- Finn, O.J. Immuno-Oncology: Understanding the Function and Dysfunction of the Immune System in Cancer. Ann. Oncol. 2012, 23, viii6–viii9. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, G.; Feng, J.; Zhang, Z.; Zhang, J.; Guo, H.; Lin, M. T Cell Engineering for Cancer Immunotherapy by Manipulating Mechanosensitive Force-Bearing Receptors. Front. Bioeng. Biotechnol. 2023, 11, 1220074. [Google Scholar] [CrossRef]
- Liu, G.; Rui, W.; Zhao, X.; Lin, X. Enhancing CAR-T Cell Efficacy in Solid Tumors by Targeting the Tumor Microenvironment. Cell Mol. Immunol. 2021, 18, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Conklin, M.W.; Eickhoff, J.C.; Riching, K.M.; Pehlke, C.A.; Eliceiri, K.W.; Provenzano, P.P.; Friedl, A.; Keely, P.J. Aligned Collagen Is a Prognostic Signature for Survival in Human Breast Carcinoma. Am. J. Pathol. 2011, 178, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Conklin, M.W.; Keely, P.J. Why the Stroma Matters in Breast Cancer: Insights into Breast Cancer Patient Outcomes through the Examination of Stromal Biomarkers. Cell Adhes. Migr. 2012, 6, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Coulson-Thomas, V.J.; Coulson-Thomas, Y.M.; Gesteira, T.F.; De Paula, C.A.A.; Mader, A.M.; Waisberg, J.; Pinhal, M.A.; Friedl, A.; Toma, L.; Nader, H.B. Colorectal Cancer Desmoplastic Reaction Up-Regulates Collagen Synthesis and Restricts Cancer Cell Invasion. Cell Tissue Res. 2011, 346, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen Reorganization at the Tumor-Stromal Interface Facilitates Local Invasion. BMC Med. 2006, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Goetz, J.G.; Minguet, S.; Navarro-Lérida, I.; Lazcano, J.J.; Samaniego, R.; Calvo, E.; Tello, M.; Osteso-Ibáñez, T.; Pellinen, T.; Echarri, A.; et al. Biomechanical Remodeling of the Microenvironment by Stromal Caveolin-1 Favors Tumor Invasion and Metastasis. Cell 2011, 146, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A Tense Situation: Forcing Tumour Progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef]
- Kopanska, K.S.; Alcheikh, Y.; Staneva, R.; Vignjevic, D.; Betz, T. Tensile Forces Originating from Cancer Spheroids Facilitate Tumor Invasion. PLoS ONE 2016, 11, e0156442. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Bogaard, H.J.; Condliffe, R.; Frantz, R.; Khanna, D.; Kurzyna, M.; Langleben, D.; Manes, A.; Satoh, T.; Torres, F.; et al. Definitions and Diagnosis of Pulmonary Hypertension. J. Am. Coll. Cardiol. 2013, 62, D42–D50. [Google Scholar] [CrossRef]
- Pullamsetti, S.S.; Nayakanti, S.; Chelladurai, P.; Mamazhakypov, A.; Mansouri, S.; Savai, R.; Seeger, W. Cancer and Pulmonary Hypertension: Learning Lessons and Real-Life Interplay. Glob. Cardiol. Sci. Pract. 2020, 2020, e202010. [Google Scholar] [CrossRef]
- Bertero, T.; Cottrill, K.A.; Lu, Y.; Haeger, C.M.; Dieffenbach, P.; Annis, S.; Hale, A.; Bhat, B.; Kaimal, V.; Zhang, Y.-Y.; et al. Matrix Remodeling Promotes Pulmonary Hypertension through Feedback Mechanoactivation of the YAP/TAZ-miR-130/301 Circuit. Cell Rep. 2015, 13, 1016–1032. [Google Scholar] [CrossRef] [PubMed]
- Bertero, T.; Oldham, W.M.; Cottrill, K.A.; Pisano, S.; Vanderpool, R.R.; Yu, Q.; Zhao, J.; Tai, Y.; Tang, Y.; Zhang, Y.-Y.; et al. Vascular Stiffness Mechanoactivates YAP/TAZ-Dependent Glutaminolysis to Drive Pulmonary Hypertension. J. Clin. Investig. 2016, 126, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Thenappan, T.; Ormiston, M.L.; Ryan, J.J.; Archer, S.L. Pulmonary Arterial Hypertension: Pathogenesis and Clinical Management. BMJ 2018, 360, j5492. [Google Scholar] [CrossRef]
- Frey, B.; Janko, C.; Ebel, N.; Meister, S.; Schlucker, E.; Meyer-Pittroff, R.; Fietkau, R.; Herrmann, M.; Gaipl, U. Cells Under Pressure—Treatment of Eukaryotic Cells with High Hydrostatic Pressure, from Physiologic Aspects to Pressure Induced Cell Death. Curr. Med. Chem. 2008, 15, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Waletzko-Hellwig, J.; Pohl, C.; Riese, J.; Schlosser, M.; Dau, M.; Engel, N.; Springer, A.; Bader, R. Effect of High Hydrostatic Pressure on Human Trabecular Bone Regarding Cell Death and Matrix Integrity. Front. Bioeng. Biotechnol. 2021, 9, 730266. [Google Scholar] [CrossRef] [PubMed]
- Adkins, I.; Hradilova, N.; Palata, O.; Sadilkova, L.; Palova-Jelinkova, L.; Spisek, R. High Hydrostatic Pressure in Cancer Immunotherapy and Biomedicine. Biotechnol. Adv. 2018, 36, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Korn, A.; Frey, B.; Sheriff, A.; Gaipl, U.S.; Franz, S.; Meyer-Pittroff, R.; Bluemelhuberh, G.; Herrmann, M. High Hydrostatic Pressure Inactivated Human Tumour Cells Preserve Their Immunogenicity. Cell Mol. Biol. Noisy-Le-Grand 2004, 50, 469–477. [Google Scholar]
- Jacobo-Velázquez, D.A.; Hernández-Brenes, C. Biochemical Changes during the Storage of High Hydrostatic Pressure Processed Avocado Paste. J. Food Sci. 2010, 75, S264–S270. [Google Scholar] [CrossRef]
- Patterson, M.F. Microbiology of Pressure-Treated Foods. J. Appl. Microbiol. 2005, 98, 1400–1409. [Google Scholar] [CrossRef]
- Weiss, E.M.; Meister, S.; Janko, C.; Ebel, N.; Schlücker, E.; Meyer-Pittroff, R.; Fietkau, R.; Herrmann, M.; Gaipl, U.S.; Frey, B. High Hydrostatic Pressure Treatment Generates Inactivated Mammalian Tumor Cells with Immunogeneic Features. J. Immunotoxicol. 2010, 7, 194–204. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Hashiguchi, K.; Katsuki, S.; Iwamoto, W.; Tsuruhara, S.; Terada, S. Activation of the Intrinsic and Extrinsic Pathways in High Pressure-Induced Apoptosis of Murine Erythroleukemia Cells. Cell. Mol. Biol. Lett. 2008, 13, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Aertsen, A.; Meersman, F.; Hendrickx, M.E.G.; Vogel, R.F.; Michiels, C.W. Biotechnology under High Pressure: Applications and Implications. Trends Biotechnol. 2009, 27, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yan, S.; Ma, Z.; Liu, B. Effective Pressure and Treatment Duration of High Hydrostatic Pressure to Prepare Melanoma Vaccines. Oncol. Lett. 2020, 20, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Seitz, C.; Rückert, M.; Deloch, L.; Weiss, E.-M.; Utz, S.; Izydor, M.; Ebel, N.; Schlücker, E.; Fietkau, R.; Gaipl, U.S.; et al. Tumor Cell-Based Vaccine Generated With High Hydrostatic Pressure Synergizes With Radiotherapy by Generating a Favorable Anti-Tumor Immune Microenvironment. Front. Oncol. 2019, 9, 805. [Google Scholar] [CrossRef]
- Nakamura-López, Y.; Sarmiento-Silva, R.E.; Moran-Andrade, J.; Gómez-García, B. Staurosporine-induced Apoptosis in P388D1 Macrophages Involves Both Extrinsic and Intrinsic Pathways. Cell Biol. Int. 2009, 33, 1026–1031. [Google Scholar] [CrossRef]
- Ravichandran, K.S.; Lorenz, U. Engulfment of Apoptotic Cells: Signals for a Good Meal. Nat. Rev. Immunol. 2007, 7, 964–974. [Google Scholar] [CrossRef]
- Voll, R.E.; Herrmann, M.; Roth, E.A.; Stach, C.; Kalden, J.R.; Girkontaite, I. Immunosuppressive Effects of Apoptotic Cells. Nature 1997, 390, 350–351. [Google Scholar] [CrossRef]
- Locher, C.; Conforti, R.; Aymeric, L.; Ma, Y.; Yamazaki, T.; Rusakiewicz, S.; Tesnière, A.; Ghiringhelli, F.; Apetoh, L.; Morel, Y.; et al. Desirable Cell Death during Anticancer Chemotherapy. Ann. N. Y. Acad. Sci. 2010, 1209, 99–108. [Google Scholar] [CrossRef]
- Fraccaroli, L.; Alfieri, J.; Larocca, L.; Calafat, M.; Mor, G.; Leiros, C.P.; Ramhorst, R. A Potential Tolerogenic Immune Mechanism in a Trophoblast Cell Line through the Activation of Chemokine-Induced T Cell Death and Regulatory T Cell Modulation. Hum. Reprod. 2008, 24, 166–175. [Google Scholar] [CrossRef]
- Griffith, T.S.; Ferguson, T.A. Cell Death in the Maintenance and Abrogation of Tolerance: The Five Ws of Dying Cells. Immunity 2011, 35, 456–466. [Google Scholar] [CrossRef]
- Golstein, P.; Kroemer, G. Cell Death by Necrosis: Towards a Molecular Definition. Trends Biochem. Sci. 2007, 32, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lavric, M.; Miranda-García, M.A.; Holzinger, D.; Foell, D.; Wittkowski, H. Alarmins Firing Arthritis: Helpful Diagnostic Tools and Promising Therapeutic Targets. Jt. Bone Spine 2017, 84, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Martin, S.; Golab, J.; Agostinis, P. Danger Signalling during Cancer Cell Death: Origins, Plasticity and Regulation. Cell Death Differ. 2014, 21, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-A.; Moon, S.Y.; Park, D.; Park, J.B.; Lee, C.S. Apoptotic Cell Clearance in the Tumor Microenvironment: A Potential Cancer Therapeutic Target. Arch. Pharm. Res. 2019, 42, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Balasubramaniam, V.R.M.T.; Othman, I.; Shaikh, M.F. Enlightening the Role of High Mobility Group Box 1 (HMGB1) in Inflammation: Updates on Receptor Signalling. Eur. J. Pharmacol. 2019, 858, 172487. [Google Scholar] [CrossRef] [PubMed]
- Scarlata, S. The Effect of Hydrostatic Pressure on Membrane-Bound Proteins. Braz. J. Med. Biol. Res. 2005, 38, 1203–1208. [Google Scholar] [CrossRef][Green Version]
- Do, J.-L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef]
- Wang, L.; Luo, J.-Y.; Li, B.; Tian, X.Y.; Chen, L.-J.; Huang, Y.; Liu, J.; Deng, D.; Lau, C.W.; Wan, S.; et al. Integrin-YAP/TAZ-JNK Cascade Mediates Atheroprotective Effect of Unidirectional Shear Flow. Nature 2016, 540, 579–582. [Google Scholar] [CrossRef]
- Wang, K.-C.; Yeh, Y.-T.; Nguyen, P.; Limqueco, E.; Lopez, J.; Thorossian, S.; Guan, K.-L.; Li, Y.-S.J.; Chien, S. Flow-Dependent YAP/TAZ Activities Regulate Endothelial Phenotypes and Atherosclerosis. Proc. Natl. Acad. Sci. USA 2016, 113, 11525–11530. [Google Scholar] [CrossRef]
- Xu, S.; Koroleva, M.; Yin, M.; Jin, Z.G. Atheroprotective Laminar Flow Inhibits Hippo Pathway Effector YAP in Endothelial Cells. Transl. Res. 2016, 176, 18–28.e2. [Google Scholar] [CrossRef]
- Li, B.; He, J.; Lv, H.; Liu, Y.; Lv, X.; Zhang, C.; Zhu, Y.; Ai, D. C-Abl Regulates YAPY357 Phosphorylation to Activate Endothelial Atherogenic Responses to Disturbed Flow. J. Clin. Investig. 2019, 129, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Yamamoto, K.; Agarwala, S.; Terai, K.; Fukui, H.; Fukuhara, S.; Ando, K.; Miyazaki, T.; Yokota, Y.; Schmelzer, E.; et al. Flow-Dependent Endothelial YAP Regulation Contributes to Vessel Maintenance. Dev. Cell 2017, 40, 523–536.e6. [Google Scholar] [CrossRef] [PubMed]
- Rausch, V.; Hansen, C.G. The Hippo Pathway, YAP/TAZ, and the Plasma Membrane. Trends Cell Biol. 2020, 30, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Diaz, M.F.; Price, K.M.; Ozuna, J.A.; Zhang, S.; Sevick-Muraca, E.M.; Hagan, J.P.; Wenzel, P.L. Fluid Shear Stress Activates YAP1 to Promote Cancer Cell Motility. Nat. Commun. 2017, 8, 14122. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.-H.; Choi, Y.W.; Park, J.H.; Hong, S.A.; Hong, M.; Chang, I.H.; Lee, H.J. Fluid Shear Stress Facilitates Prostate Cancer Metastasis through Piezo1-Src-YAP Axis. Life Sci. 2022, 308, 120936. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, M.; Rainero, E. Cross-Talk Between the Tumor Microenvironment, Extracellular Matrix, and Cell Metabolism in Cancer. Front. Oncol. 2020, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Di-Luoffo, M.; Ben-Meriem, Z.; Lefebvre, P.; Delarue, M.; Guillermet-Guibert, J. PI3K Functions as a Hub in Mechanotransduction. Trends Biochem. Sci. 2021, 46, 878–888. [Google Scholar] [CrossRef]
- Mousavizadeh, R.; Hojabrpour, P.; Eltit, F.; McDonald, P.C.; Dedhar, S.; McCormack, R.G.; Duronio, V.; Jafarnejad, S.M.; Scott, A. Β1 Integrin, ILK and mTOR Regulate Collagen Synthesis in Mechanically Loaded Tendon Cells. Sci. Rep. 2020, 10, 12644. [Google Scholar] [CrossRef]
- Kim, S.G.; Buel, G.R.; Blenis, J. Nutrient Regulation of the mTOR Complex 1 Signaling Pathway. Mol. Cells 2013, 35, 463–473. [Google Scholar] [CrossRef]
- Takahara, T.; Amemiya, Y.; Sugiyama, R.; Maki, M.; Shibata, H. Amino Acid-Dependent Control of mTORC1 Signaling: A Variety of Regulatory Modes. J. Biomed. Sci. 2020, 27, 87. [Google Scholar] [CrossRef]
- Yim, W.W.-Y.; Mizushima, N. Lysosome Biology in Autophagy. Cell Discov. 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Hall, M.N. Regulation of mTORC2 Signaling. Genes 2020, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Riffo, E.; Farias, A.; Coliboro-Dannich, V.; Espinoza-Francine, L.; Escalona, E.; Amigo, R.; Gutiérrez, J.L.; Pincheira, R.; Castro, A.F. NUAK1 Coordinates Growth Factor-Dependent Activation of mTORC2 and Akt Signaling. Cell Biosci. 2023, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.L.; Collins, O.; Saxena, P.; Buensuceso, A.; Ramos Valdes, Y.; Francis, K.E.; Brown, K.R.; Larsen, B.; Colwill, K.; Gingras, A.-C.; et al. A Novel Role for NUAK1 in Promoting Ovarian Cancer Metastasis through Regulation of Fibronectin Production in Spheroids. Cancers 2020, 12, 1250. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, H.; Jiang, X. mTORC1 beyond Anabolic Metabolism: Regulation of Cell Death. J. Cell Biol. 2022, 221, e202208103. [Google Scholar] [CrossRef] [PubMed]
- Valdembri, D.; Serini, G. The Roles of Integrins in Cancer. Fac. Rev. 2021, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.-Y.; Zhen, Y.-Y.; Yuen, C.-M.; Fan, R.; Chen, Y.-T.; Sheu, J.-J.; Chen, Y.-L.; Wang, C.-J.; Sun, C.-K.; Yip, H.-K. The mTOR-FAK Mechanotransduction Signaling Axis for Focal Adhesion Maturation and Cell Proliferation. Am. J. Transl. Res. 2017, 9, 1603–1617. [Google Scholar]
- Choi, K.; Kuhn, J.L.; Ciarelli, M.J.; Goldstein, S.A. The Elastic Moduli of Human Subchondral, Trabecular, and Cortical Bone Tissue and the Size-Dependency of Cortical Bone Modulus. J. Biomech. 1990, 23, 1103–1113. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional Homeostasis and the Malignant Phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef]
- Janmey, P.A.; Fletcher, D.A.; Reinhart-King, C.A. Stiffness Sensing by Cells. Physiol. Rev. 2020, 100, 695–724. [Google Scholar] [CrossRef]
- Solon, J.; Levental, I.; Sengupta, K.; Georges, P.C.; Janmey, P.A. Fibroblast Adaptation and Stiffness Matching to Soft Elastic Substrates. Biophys. J. 2007, 93, 4453–4461. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Sarangi, B.R.; Deschamps, J.; Nematbakhsh, Y.; Callan-Jones, A.; Margadant, F.; Mège, R.-M.; Lim, C.T.; Voituriez, R.; Ladoux, B. Adaptive Rheology and Ordering of Cell Cytoskeleton Govern Matrix Rigidity Sensing. Nat. Commun. 2015, 6, 7525. [Google Scholar] [CrossRef] [PubMed]
- Merrell, A.J.; Stanger, B.Z. Adult Cell Plasticity in Vivo: De-Differentiation and Transdifferentiation Are Back in Style. Nat. Rev. Mol. Cell Biol. 2016, 17, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Tata, P.R.; Rajagopal, J. Cellular Plasticity: 1712 to the Present Day. Curr. Opin. Cell Biol. 2016, 43, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kopp, J.L.; von Figura, G.; Mayes, E.; Liu, F.-F.; Dubois, C.L.; Morris, J.P.; Pan, F.C.; Akiyama, H.; Wright, C.V.E.; Jensen, K.; et al. Identification of Sox9-Dependent Acinar-to-Ductal Reprogramming as the Principal Mechanism for Initiation of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2012, 22, 737–750. [Google Scholar] [CrossRef]
- Bailey, J.M.; Hendley, A.M.; Lafaro, K.J.; Pruski, M.A.; Jones, N.C.; Alsina, J.; Younes, M.; Maitra, A.; McAllister, F.; Iacobuzio-Donahue, C.A.; et al. P53 Mutations Cooperate with Oncogenic Kras to Promote Adenocarcinoma from Pancreatic Ductal Cells. Oncogene 2016, 35, 4282–4288. [Google Scholar] [CrossRef]
- Kopp, J.L.; Dubois, C.L.; Schaeffer, D.F.; Samani, A.; Taghizadeh, F.; Cowan, R.W.; Rhim, A.D.; Stiles, B.L.; Valasek, M.; Sander, M. Loss of Pten and Activation of Kras Synergistically Induce Formation of Intraductal Papillary Mucinous Neoplasia From Pancreatic Ductal Cells in Mice. Gastroenterology 2018, 154, 1509–1523.e5. [Google Scholar] [CrossRef]
- Fan, B.; Malato, Y.; Calvisi, D.F.; Naqvi, S.; Razumilava, N.; Ribback, S.; Gores, G.J.; Dombrowski, F.; Evert, M.; Chen, X.; et al. Cholangiocarcinomas Can Originate from Hepatocytes in Mice. J. Clin. Investig. 2012, 122, 2911–2915. [Google Scholar] [CrossRef]
- Sekiya, S.; Suzuki, A. Intrahepatic Cholangiocarcinoma Can Arise from Notch-Mediated Conversion of Hepatocytes. J. Clin. Investig. 2012, 122, 3914–3918. [Google Scholar] [CrossRef]
- Ikenoue, T.; Terakado, Y.; Nakagawa, H.; Hikiba, Y.; Fujii, T.; Matsubara, D.; Noguchi, R.; Zhu, C.; Yamamoto, K.; Kudo, Y.; et al. A Novel Mouse Model of Intrahepatic Cholangiocarcinoma Induced by Liver-Specific Kras Activation and Pten Deletion. Sci. Rep. 2016, 6, 23899. [Google Scholar] [CrossRef]
- Guest, R.V.; Boulter, L.; Kendall, T.J.; Minnis-Lyons, S.E.; Walker, R.; Wigmore, S.J.; Sansom, O.J.; Forbes, S.J. Cell Lineage Tracing Reveals a Biliary Origin of Intrahepatic Cholangiocarcinoma. Cancer Res. 2014, 74, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.A.; Alexander, W.B.; Guo, B.; Kato, Y.; Patra, K.; O’Dell, M.R.; McCall, M.N.; Whitney-Miller, C.L.; Bardeesy, N.; Hezel, A.F. Kras and Tp53 Mutations Cause Cholangiocyte- and Hepatocyte-Derived Cholangiocarcinoma. Cancer Res. 2018, 78, 4445–4451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fillmore Brainson, C.; Koyama, S.; Redig, A.J.; Chen, T.; Li, S.; Gupta, M.; Garcia-de-Alba, C.; Paschini, M.; Herter-Sprie, G.S.; et al. Lkb1 Inactivation Drives Lung Cancer Lineage Switching Governed by Polycomb Repressive Complex 2. Nat. Commun. 2017, 8, 14922. [Google Scholar] [CrossRef] [PubMed]
- Loukas, I.; Simeoni, F.; Milan, M.; Inglese, P.; Patel, H.; Goldstone, R.; East, P.; Strohbuecker, S.; Mitter, R.; Talsania, B.; et al. Selective Advantage of Epigenetically Disrupted Cancer Cells via Phenotypic Inertia. Cancer Cell 2023, 41, 70–87.e14. [Google Scholar] [CrossRef] [PubMed]
- Ors, A.; Chitsazan, A.D.; Doe, A.R.; Mulqueen, R.M.; Ak, C.; Wen, Y.; Haverlack, S.; Handu, M.; Naldiga, S.; Saldivar, J.C.; et al. Estrogen Regulates Divergent Transcriptional and Epigenetic Cell States in Breast Cancer. Nucleic Acids Res. 2022, 50, 11492–11508. [Google Scholar] [CrossRef]
- Baslan, T.; Morris, J.P.; Zhao, Z.; Reyes, J.; Ho, Y.-J.; Tsanov, K.M.; Bermeo, J.; Tian, S.; Zhang, S.; Askan, G.; et al. Ordered and Deterministic Cancer Genome Evolution after P53 Loss. Nature 2022, 608, 795–802. [Google Scholar] [CrossRef]
- Zhao, L.-Y.; Mei, J.-X.; Yu, G.; Lei, L.; Zhang, W.-H.; Liu, K.; Chen, X.-L.; Kołat, D.; Yang, K.; Hu, J.-K. Role of the Gut Microbiota in Anticancer Therapy: From Molecular Mechanisms to Clinical Applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef]
- Yuan, S.; Norgard, R.J.; Stanger, B.Z. Cellular Plasticity in Cancer. Cancer Discov. 2019, 9, 837–851. [Google Scholar] [CrossRef]
- Pietras, K.; Östman, A. Hallmarks of Cancer: Interactions with the Tumor Stroma. Exp. Cell Res. 2010, 316, 1324–1331. [Google Scholar] [CrossRef]
- Fan, D.; Takawale, A.; Lee, J.; Kassiri, Z. Cardiac Fibroblasts, Fibrosis and Extracellular Matrix Remodeling in Heart Disease. Fibrogenesis Tissue Repair. 2012, 5, 15. [Google Scholar] [CrossRef]
- Das, T.; Safferling, K.; Rausch, S.; Grabe, N.; Boehm, H.; Spatz, J.P. A Molecular Mechanotransduction Pathway Regulates Collective Migration of Epithelial Cells. Nat. Cell Biol. 2015, 17, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; te Lindert, M.; Krause, M.; Alexander, S.; te Riet, J.; Willis, A.L.; Hoffman, R.M.; Figdor, C.G.; Weiss, S.J.; Friedl, P. Physical Limits of Cell Migration: Control by ECM Space and Nuclear Deformation and Tuning by Proteolysis and Traction Force. J. Cell Biol. 2013, 201, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Bao, M.; Bruekers, S.M.C.; Huck, W.T.S. Collagen Gels with Different Fibrillar Microarchitectures Elicit Different Cellular Responses. ACS Appl. Mater. Interfaces 2017, 9, 19630–19637. [Google Scholar] [CrossRef] [PubMed]
- Barcus, C.E.; Keely, P.J.; Eliceiri, K.W.; Schuler, L.A. Stiff Collagen Matrices Increase Tumorigenic Prolactin Signaling in Breast Cancer Cells. J. Biol. Chem. 2013, 288, 12722–12732. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayi, E.; Mudera, V.; Brown, R.A. Guiding Cell Migration in 3D: A Collagen Matrix with Graded Directional Stiffness. Cell Motil. Cytoskelet. 2009, 66, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Hata, Y.; Sawada, T.; Serizawa, T. Macromolecular Crowding for Materials-Directed Controlled Self-Assembly. J. Mater. Chem. B 2018, 6, 6344–6359. [Google Scholar] [CrossRef] [PubMed]
- Dhand, A.P.; Galarraga, J.H.; Burdick, J.A. Enhancing Biopolymer Hydrogel Functionality through Interpenetrating Networks. Trends Biotechnol. 2021, 39, 519–538. [Google Scholar] [CrossRef]
- Saez, A.; Ghibaudo, M.; Buguin, A.; Silberzan, P.; Ladoux, B. Rigidity-Driven Growth and Migration of Epithelial Cells on Microstructured Anisotropic Substrates. Proc. Natl. Acad. Sci. USA 2007, 104, 8281–8286. [Google Scholar] [CrossRef]
- Isomursu, A.; Park, K.-Y.; Hou, J.; Cheng, B.; Mathieu, M.; Shamsan, G.A.; Fuller, B.; Kasim, J.; Mahmoodi, M.M.; Lu, T.J.; et al. Directed Cell Migration towards Softer Environments. Nat. Mater. 2022, 21, 1081–1090. [Google Scholar] [CrossRef]
- Lo, C.-M.; Wang, H.-B.; Dembo, M.; Wang, Y. Cell Movement Is Guided by the Rigidity of the Substrate. Biophys. J. 2000, 79, 144–152. [Google Scholar] [CrossRef]
- Ladoux, B.; Mège, R.-M. Mechanobiology of Collective Cell Behaviours. Nat. Rev. Mol. Cell Biol. 2017, 18, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, V.; Waterman, C.M. The Molecular Clutch Model for Mechanotransduction Evolves. Nat. Cell Biol. 2016, 18, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A.; Trepat, X.; Roca-Cusachs, P. Control of Mechanotransduction by Molecular Clutch Dynamics. Trends Cell Biol. 2018, 28, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wei, Q.; Zhao, C. How Do the Cells Sense and Respond to the Microenvironment Mechanics? Chin. Sci. Bull. 2021, 66, 2303–2311. [Google Scholar] [CrossRef]
- Paluch, E.K.; Nelson, C.M.; Biais, N.; Fabry, B.; Moeller, J.; Pruitt, B.L.; Wollnik, C.; Kudryasheva, G.; Rehfeldt, F.; Federle, W. Mechanotransduction: Use the Force(s). BMC Biol. 2015, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. The Role of Focal Adhesion Kinase in the Regulation of Cellular Mechanical Properties. Phys. Biol. 2013, 10, 065005. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.G. The Role of Matrix Stiffness in Regulating Cell Behavior. Hepatology 2008, 47, 1394–1400. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Oria, R.; Chen, Y.; Kosmalska, A.; Pérez-González, C.; Castro, N.; Zhu, C.; Trepat, X.; Roca-Cusachs, P. Mechanical Regulation of a Molecular Clutch Defines Force Transmission and Transduction in Response to Matrix Rigidity. Nat. Cell Biol. 2016, 18, 540–548. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Bazellières, E.; Allen, M.D.; Andreu, I.; Oria, R.; Sunyer, R.; Gomm, J.J.; Marshall, J.F.; Jones, J.L.; Trepat, X.; et al. Rigidity Sensing and Adaptation through Regulation of Integrin Types. Nat. Mater. 2014, 13, 631–637. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and Extracellular Matrix Homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the Third Dimension—How 3D Culture Microenvironments Alter Cellular Cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. Viscoelasticity Acts as a Marker for Tumor Extracellular Matrix Characteristics. Front. Cell Dev. Biol. 2021, 9, 785138. [Google Scholar] [CrossRef] [PubMed]
- Mierke, C.T. Viscoelasticity, Like Forces, Plays a Role in Mechanotransduction. Front. Cell Dev. Biol. 2022, 10, 789841. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Doyle, A.D.; Lu, J. Cell–3D Matrix Interactions: Recent Advances and Opportunities. Trends Cell Biol. 2022, 32, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Castillo, A.B.; Jacobs, C.R. Cellular and Molecular Mechanotransduction in Bone. In Osteoporosis; Elsevier: Amsterdam, The Netherlands, 2013; pp. 453–475. ISBN 978-0-12-415853-5. [Google Scholar]
- Taufalele, P.V.; Reinhart-King, C.A. Matrix Stiffness Primes Cells for Future Oxidative Stress. Trends Cancer 2021, 7, 883–885. [Google Scholar] [CrossRef]
- Holle, A.W.; Young, J.L.; Spatz, J.P. In Vitro Cancer Cell–ECM Interactions Inform In Vivo Cancer Treatment. Adv. Drug Deliv. Rev. 2016, 97, 270–279. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.; Jiang, T.; Jin, K.; Luo, Z.; Shi, W.; Mei, H.; Wang, H.; Hu, Y.; Pang, Z.; et al. Cyclopamine Treatment Disrupts Extracellular Matrix and Alleviates Solid Stress to Improve Nanomedicine Delivery for Pancreatic Cancer. J. Drug Target. 2018, 26, 913–919. [Google Scholar] [CrossRef]
- Du Souich, P.; Fradette, C. The Effect and Clinical Consequences of Hypoxia on Cytochrome P450, Membrane Carrier Proteins Activity and Expression. Expert Opin. Drug Metab. Toxicol. 2011, 7, 1083–1100. [Google Scholar] [CrossRef]
- Comerford, K.M.; Wallace, T.J.; Karhausen, J.; Louis, N.A.; Montalto, M.C.; Colgan, S.P. Hypoxia-Inducible Factor-1-Dependent Regulation of the Multidrug Resistance (MDR1) Gene. Cancer Res. 2002, 62, 3387–3394. [Google Scholar]
- Weniger, M.; Honselmann, K.; Liss, A. The Extracellular Matrix and Pancreatic Cancer: A Complex Relationship. Cancers 2018, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Slack-Davis, J.K.; Eblen, S.T.; Zecevic, M.; Boerner, S.A.; Tarcsafalvi, A.; Diaz, H.B.; Marshall, M.S.; Weber, M.J.; Parsons, J.T.; Catling, A.D. PAK1 Phosphorylation of MEK1 Regulates Fibronectin-Stimulated MAPK Activation. J. Cell Biol. 2003, 162, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Yousif, N.G. Fibronectin Promotes Migration and Invasion of Ovarian Cancer Cells through Up-regulation of FAK—PI 3 K/A Kt Pathway. Cell Biol. Int. 2014, 38, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-G.; Gumbiner, B.M. Adhesion to Fibronectin Regulates Hippo Signaling via the FAK–Src–PI3K Pathway. J. Cell Biol. 2015, 210, 503–515. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular Matrix: A Dynamic Microenvironment for Stem Cell Niche. Biochim. Et Biophys. Acta BBA-Gen. Subj. 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- Oskarsson, T.; Batlle, E.; Massagué, J. Metastatic Stem Cells: Sources, Niches, and Vital Pathways. Cell Stem Cell 2014, 14, 306–321. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef]
- Karalis, T.T.; Heldin, P.; Vynios, D.H.; Neill, T.; Buraschi, S.; Iozzo, R.V.; Karamanos, N.K.; Skandalis, S.S. Tumor-Suppressive Functions of 4-MU on Breast Cancer Cells of Different ER Status: Regulation of Hyaluronan/HAS2/CD44 and Specific Matrix Effectors. Matrix Biol. 2019, 78–79, 118–138. [Google Scholar] [CrossRef]
- Jacobetz, M.A.; Chan, D.S.; Neesse, A.; Bapiro, T.E.; Cook, N.; Frese, K.K.; Feig, C.; Nakagawa, T.; Caldwell, M.E.; Zecchini, H.I.; et al. Hyaluronan Impairs Vascular Function and Drug Delivery in a Mouse Model of Pancreatic Cancer. Gut 2013, 62, 112–120. [Google Scholar] [CrossRef]
- Semba, T.; Sammons, R.; Wang, X.; Xie, X.; Dalby, K.N.; Ueno, N.T. JNK Signaling in Stem Cell Self-Renewal and Differentiation. Int. J. Mol. Sci. 2020, 21, 2613. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Munn, L.L.; Jain, R.K. Reengineering the Physical Microenvironment of Tumors to Improve Drug Delivery and Efficacy: From Mathematical Modeling to Bench to Bedside. Trends Cancer 2018, 4, 292–319. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, L.M. Brain Tumors. N. Engl. J. Med. 2001, 344, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, X.; Lu, J.; Salfenmoser, M.; Wirsik, N.M.; Schleussner, N.; Imle, A.; Freire Valls, A.; Radhakrishnan, P.; Liang, J.; et al. Reduction of Liver Metastasis Stiffness Improves Response to Bevacizumab in Metastatic Colorectal Cancer. Cancer Cell 2020, 37, 800–817.e7. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Lv, X.; Li, P.; Yang, R.; Xia, Q.; Chen, Y.; Peng, Y.; Li, L.; Li, S.; Li, T.; et al. Matrix Stiffness Modulates ILK-Mediated YAP Activation to Control the Drug Resistance of Breast Cancer Cells. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2020, 1866, 165625. [Google Scholar] [CrossRef] [PubMed]
- Payen, T.; Oberstein, P.E.; Saharkhiz, N.; Palermo, C.F.; Sastra, S.A.; Han, Y.; Nabavizadeh, A.; Sagalovskiy, I.R.; Orelli, B.; Rosario, V.; et al. Harmonic Motion Imaging of Pancreatic Tumor Stiffness Indicates Disease State and Treatment Response. Clin. Cancer Res. 2020, 26, 1297–1308. [Google Scholar] [CrossRef]
- Feng, J.; Tang, Y.; Xu, Y.; Sun, Q.; Liao, F.; Han, D. Substrate Stiffness Influences the Outcome of Antitumor Drug Screening in Vitro. Clin. Hemorheol. Microcirc. 2013, 55, 121–131. [Google Scholar] [CrossRef]
- Roos, W.P.; Batista, L.F.Z.; Naumann, S.C.; Wick, W.; Weller, M.; Menck, C.F.M.; Kaina, B. Apoptosis in Malignant Glioma Cells Triggered by the Temozolomide-Induced DNA Lesion O6-Methylguanine. Oncogene 2007, 26, 186–197. [Google Scholar] [CrossRef]
- Fan, Y.; Sun, Q.; Li, X.; Feng, J.; Ao, Z.; Li, X.; Wang, J. Substrate Stiffness Modulates the Growth, Phenotype, and Chemoresistance of Ovarian Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 718834. [Google Scholar] [CrossRef]
- Li, S.; Bai, H.; Chen, X.; Gong, S.; Xiao, J.; Li, D.; Li, L.; Jiang, Y.; Li, T.; Qin, X.; et al. Soft Substrate Promotes Osteosarcoma Cell Self-Renewal, Differentiation, and Drug Resistance Through miR-29b and Its Target Protein Spin 1. ACS Biomater. Sci. Eng. 2020, 6, 5588–5598. [Google Scholar] [CrossRef]
- Lee, T.K.W.; Castilho, A.; Ma, S.; Ng, I.O.L. Liver Cancer Stem Cells: Implications for a New Therapeutic Target. Liver Int. 2009, 29, 955–965. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Secomb, T.W. Transport of Drugs from Blood Vessels to Tumour Tissue. Nat. Rev. Cancer 2017, 17, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Viallard, C.; Larrivée, B. Tumor Angiogenesis and Vascular Normalization: Alternative Therapeutic Targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Urbano, R.L.; Furia, C.; Basehore, S.; Clyne, A.M. Stiff Substrates Increase Inflammation-Induced Endothelial Monolayer Tension and Permeability. Biophys. J. 2017, 113, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Bordeleau, F.; Mason, B.N.; Lollis, E.M.; Mazzola, M.; Zanotelli, M.R.; Somasegar, S.; Califano, J.P.; Montague, C.; LaValley, D.J.; Huynh, J.; et al. Matrix Stiffening Promotes a Tumor Vasculature Phenotype. Proc. Natl. Acad. Sci. USA 2017, 114, 492–497. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for Patients with Hepatocellular Carcinoma Who Progressed on Sorafenib Treatment (RESORCE): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Gao, J.; Rong, Y.; Huang, Y.; Shi, P.; Wang, X.; Meng, X.; Dong, J.; Wu, C. Cirrhotic Stiffness Affects the Migration of Hepatocellular Carcinoma Cells and Induces Sorafenib Resistance through YAP. J. Cell. Physiol. 2019, 234, 2639–2648. [Google Scholar] [CrossRef]
- Schwartz, A.D.; Barney, L.E.; Jansen, L.E.; Nguyen, T.V.; Hall, C.L.; Meyer, A.S.; Peyton, S.R. A Biomaterial Screening Approach Reveals Microenvironmental Mechanisms of Drug Resistance. Integr. Biol. 2017, 9, 912–924. [Google Scholar] [CrossRef]
- Lin, C.-H.; Jokela, T.; Gray, J.; LaBarge, M.A. Combinatorial Microenvironments Impose a Continuum of Cellular Responses to a Single Pathway-Targeted Anti-Cancer Compound. Cell Rep. 2017, 21, 533–545. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy Toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef]
- Panzetta, V.; La Verde, G.; Pugliese, M.; Artiola, V.; Arrichiello, C.; Muto, P.; La Commara, M.; Netti, P.A.; Fusco, S. Adhesion and Migration Response to Radiation Therapy of Mammary Epithelial and Adenocarcinoma Cells Interacting with Different Stiffness Substrates. Cancers 2020, 12, 1170. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Keely, P.J. Matrix Density-Induced Mechanoregulation of Breast Cell Phenotype, Signaling and Gene Expression through a FAK–ERK Linkage. Oncogene 2009, 28, 4326–4343. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Kong, W.; Zhao, X.; Chen, S.; Sun, Q.; Feng, J.; Song, D.; Han, D. Substrate Stiffness Affects the Morphology, Proliferation, and Radiosensitivity of Cervical Squamous Carcinoma Cells. Tissue Cell 2022, 74, 101681. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, J.; Harris, A.F.; Zenhausern, R.; Karsunsky, S.; Zenhausern, F. Plant-Based Scaffolds Modify Cellular Response to Drug and Radiation Exposure Compared to Standard Cell Culture Models. Front. Bioeng. Biotechnol. 2020, 8, 932. [Google Scholar] [CrossRef] [PubMed]
- Onwudiwe, K.; Najera, J.; Siri, S.; Datta, M. Do Tumor Mechanical Stresses Promote Cancer Immune Escape? Cells 2022, 11, 3840. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Cachoux, V.M.L.; Narayana, G.H.N.S.; de Beco, S.; D’Alessandro, J.; Cellerin, V.; Chen, T.; Heuzé, M.L.; Marcq, P.; Mège, R.-M.; et al. The Role of Single-Cell Mechanical Behaviour and Polarity in Driving Collective Cell Migration. Nat. Phys. 2020, 16, 802–809. [Google Scholar] [CrossRef]
- Spill, F.; Reynolds, D.S.; Kamm, R.D.; Zaman, M.H. Impact of the Physical Microenvironment on Tumor Progression and Metastasis. Curr. Opin. Biotechnol. 2016, 40, 41–48. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering Nanomedicine to Solid Tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Rajput, S.; Kumar Sharma, P.; Malviya, R. Fluid Mechanics in Circulating Tumour Cells: Role in Metastasis and Treatment Strategies. Med. Drug Discov. 2023, 18, 100158. [Google Scholar] [CrossRef]
- Moose, D.L.; Krog, B.L.; Kim, T.-H.; Zhao, L.; Williams-Perez, S.; Burke, G.; Rhodes, L.; Vanneste, M.; Breheny, P.; Milhem, M.; et al. Cancer Cells Resist Mechanical Destruction in Circulation via RhoA/Actomyosin-Dependent Mechano-Adaptation. Cell Rep. 2020, 30, 3864–3874.e6. [Google Scholar] [CrossRef]
- Blanco, B.; Gomez, H.; Melchor, J.; Palma, R.; Soler, J.; Rus, G. Mechanotransduction in Tumor Dynamics Modeling. Phys. Life Rev. 2023, 44, 279–301. [Google Scholar] [CrossRef]
- Mittler, F.; Obeïd, P.; Haguet, V.; Allier, C.; Gerbaud, S.; Rulina, A.V.; Gidrol, X.; Balakirev, M.Y. Mechanical Stress Shapes the Cancer Cell Response to Neddylation Inhibition. J. Exp. Clin. Cancer Res. 2022, 41, 115. [Google Scholar] [CrossRef] [PubMed]
- Ildiz, E.S.; Gvozdenovic, A.; Kovacs, W.J.; Aceto, N. Travelling under Pressure—Hypoxia and Shear Stress in the Metastatic Journey. Clin. Exp. Metastasis 2023, 40, 375–394. [Google Scholar] [CrossRef] [PubMed]
- Follain, G.; Herrmann, D.; Harlepp, S.; Hyenne, V.; Osmani, N.; Warren, S.C.; Timpson, P.; Goetz, J.G. Fluids and Their Mechanics in Tumour Transit: Shaping Metastasis. Nat. Rev. Cancer 2020, 20, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Codini, M.; Garcia-Gil, M.; Albi, E. Cholesterol and Sphingolipid Enriched Lipid Rafts as Therapeutic Targets in Cancer. Int. J. Mol. Sci. 2021, 22, 726. [Google Scholar] [CrossRef]
- Das, J.; Maji, S.; Agarwal, T.; Chakraborty, S.; Maiti, T.K. Hemodynamic Shear Stress Induces Protective Autophagy in HeLa Cells through Lipid Raft-Mediated Mechanotransduction. Clin. Exp. Metastasis 2018, 35, 135–148. [Google Scholar] [CrossRef]
- Yan, Z.; Su, G.; Gao, W.; He, J.; Shen, Y.; Zeng, Y.; Liu, X. Fluid Shear Stress Induces Cell Migration and Invasion via Activating Autophagy in HepG2 Cells. Cell Adhes. Migr. 2019, 13, 152–163. [Google Scholar] [CrossRef]
- Zhou, Q.; Lin, W.; Wang, C.; Sun, F.; Ju, S.; Chen, Q.; Wang, Y.; Chen, Y.; Li, H.; Wang, L.; et al. Neddylation Inhibition Induces Glutamine Uptake and Metabolism by Targeting CRL3SPOP E3 Ligase in Cancer Cells. Nat. Commun. 2022, 13, 3034. [Google Scholar] [CrossRef]
- Milosevic, M.; Fyles, A.; Hedley, D.; Pintilie, M.; Levin, W.; Manchul, L.; Hill, R. Interstitial Fluid Pressure Predicts Survival in Patients with Cervix Cancer Independent of Clinical Prognostic Factors and Tumor Oxygen Measurements. Cancer Res. 2001, 61, 6400–6405. [Google Scholar]
- Shang, M.; Lim, S.B.; Jiang, K.; Yap, Y.S.; Khoo, B.L.; Han, J.; Lim, C.T. Microfluidic Studies of Hydrostatic Pressure-Enhanced Doxorubicin Resistance in Human Breast Cancer Cells. Lab. Chip 2021, 21, 746–754. [Google Scholar] [CrossRef]
- Ip, C.K.M.; Li, S.-S.; Tang, M.Y.H.; Sy, S.K.H.; Ren, Y.; Shum, H.C.; Wong, A.S.T. Stemness and Chemoresistance in Epithelial Ovarian Carcinoma Cells under Shear Stress. Sci. Rep. 2016, 6, 26788. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Pigula, M.; Khan, A.P.; Hanna, W.; Ruhi, M.K.; Dehkordy, F.M.; Pushpavanam, K.; Rege, K.; Moore, K.; Tsujita, Y.; et al. Flow-Induced Shear Stress Confers Resistance to Carboplatin in an Adherent Three-Dimensional Model for Ovarian Cancer: A Role for EGFR-Targeted Photoimmunotherapy Informed by Physical Stress. J. Clin. Med. 2020, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Novak, C.M.; Horst, E.N.; Taylor, C.C.; Liu, C.Z.; Mehta, G. Fluid Shear Stress Stimulates Breast Cancer Cells to Display Invasive and Chemoresistant Phenotypes While Upregulating PLAU in a 3D Bioreactor. Biotech Bioeng. 2019, 116, 3084–3097. [Google Scholar] [CrossRef] [PubMed]
- Azimi, T.; Loizidou, M.; Dwek, M.V. Cancer Cells Grown in 3D under Fluid Flow Exhibit an Aggressive Phenotype and Reduced Responsiveness to the Anti-Cancer Treatment Doxorubicin. Sci. Rep. 2020, 10, 12020. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Shao, S.; Liu, E.; Li, J.; Tian, Z.; Wu, X.; Zhang, S.; Stover, D.; Wu, H.; Cheng, L.; et al. Subpathway Analysis of Transcriptome Profiles Reveals New Molecular Mechanisms of Acquired Chemotherapy Resistance in Breast Cancer. Cancers 2022, 14, 4878. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Lamhamedi-Cherradi, S.-E.; Menegaz, B.A.; Ludwig, J.A.; Mikos, A.G. Flow Perfusion Effects on Three-Dimensional Culture and Drug Sensitivity of Ewing Sarcoma. Proc. Natl. Acad. Sci. USA 2015, 112, 10304–10309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, D. Exosomes in Cancer Development, Metastasis, and Immunity. Biochim. Et. Biophys. ActaBBA-Rev. Cancer 2019, 1871, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J Extracell. Vesicle 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, M.; Zhang, Y.; Su, Q.; Xie, Z.; Chen, X.; Yan, R.; Li, P.; Li, T.; Qin, X.; et al. Functions and Clinical Significance of Mechanical Tumor Microenvironment: Cancer Cell Sensing, Mechanobiology and Metastasis. Cancer Commun. 2022, 42, 374–400. [Google Scholar] [CrossRef]
- Nia, H.T.; Liu, H.; Seano, G.; Datta, M.; Jones, D.; Rahbari, N.; Incio, J.; Chauhan, V.P.; Jung, K.; Martin, J.D.; et al. Solid Stress and Elastic Energy as Measures of Tumour Mechanopathology. Nat. Biomed. Eng. 2017, 1, 0004. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Martin, J.D.; Chauhan, V.P.; Jain, S.R.; Diop-Frimpong, B.; Bardeesy, N.; Smith, B.L.; Ferrone, C.R.; Hornicek, F.J.; Boucher, Y.; et al. Causes, Consequences, and Remedies for Growth-Induced Solid Stress in Murine and Human Tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 15101–15108. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Tse, J.; Jain, R.K.; Munn, L.L. Micro-Environmental Mechanical Stress Controls Tumor Spheroid Size and Morphology by Suppressing Proliferation and Inducing Apoptosis in Cancer Cells. PLoS ONE 2009, 4, e4632. [Google Scholar] [CrossRef] [PubMed]
- Kalli, M.; Minia, A.; Pliaka, V.; Fotis, C.; Alexopoulos, L.G.; Stylianopoulos, T. Solid Stress-Induced Migration Is Mediated by GDF15 through Akt Pathway Activation in Pancreatic Cancer Cells. Sci. Rep. 2019, 9, 978. [Google Scholar] [CrossRef] [PubMed]
- Delarue, M.; Montel, F.; Vignjevic, D.; Prost, J.; Joanny, J.-F.; Cappello, G. Compressive Stress Inhibits Proliferation in Tumor Spheroids through a Volume Limitation. Biophys. J. 2014, 107, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Desmaison, A.; Frongia, C.; Grenier, K.; Ducommun, B.; Lobjois, V. Mechanical Stress Impairs Mitosis Progression in Multi-Cellular Tumor Spheroids. PLoS ONE 2013, 8, e80447. [Google Scholar] [CrossRef] [PubMed]
- Kalli, M.; Li, R.; Mills, G.B.; Stylianopoulos, T.; Zervantonakis, I.K. Mechanical Stress Signaling in Pancreatic Cancer Cells Triggers P38 MAPK- and JNK-Dependent Cytoskeleton Remodeling and Promotes Cell Migration via Rac1/Cdc42/Myosin II. Mol. Cancer Res. 2022, 20, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Kalli, M.; Papageorgis, P.; Gkretsi, V.; Stylianopoulos, T. Solid Stress Facilitates Fibroblasts Activation to Promote Pancreatic Cancer Cell Migration. Ann. Biomed. Eng. 2018, 46, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Tse, Z.T.H.; Janssen, H.; Hamed, A.; Ristic, M.; Young, I.; Lamperth, M. Magnetic Resonance Elastography Hardware Design: A Survey. Proc. Inst. Mech. Eng. H. 2009, 223, 497–514. [Google Scholar] [CrossRef]
- Chaudhuri, P.K.; Low, B.C.; Lim, C.T. Mechanobiology of Tumor Growth. Chem. Rev. 2018, 118, 6499–6515. [Google Scholar] [CrossRef]
- Rizzuti, I.F.; Mascheroni, P.; Arcucci, S.; Ben-Mériem, Z.; Prunet, A.; Barentin, C.; Rivière, C.; Delanoë-Ayari, H.; Hatzikirou, H.; Guillermet-Guibert, J.; et al. Mechanical Control of Cell Proliferation Increases Resistance to Chemotherapeutic Agents. Phys. Rev. Lett. 2020, 125, 128103. [Google Scholar] [CrossRef]
- Novak, C.M.; Horst, E.N.; Lin, E.; Mehta, G. Compressive Stimulation Enhances Ovarian Cancer Proliferation, Invasion, Chemoresistance, and Mechanotransduction via CDC42 in a 3D Bioreactor. Cancers 2020, 12, 1521. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Massi, D.; Teng, M.W.L.; Mandala, M. PI3K-AKT-mTOR Inhibition in Cancer Immunotherapy, Redux. Semin. Cancer Biol. 2018, 48, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Callahan, M.K.; Postow, M.A.; Wolchok, J.D. Targeting T Cell Co-Receptors for Cancer Therapy. Immunity 2016, 44, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Gunzer, M.; Schäfer, A.; Borgmann, S.; Grabbe, S.; Zänker, K.S.; Bröcker, E.-B.; Kämpgen, E.; Friedl, P. Antigen Presentation in Extracellular Matrix. Immunity 2000, 13, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.H.W.; Norman, M.D.A.; Gentleman, E.; Coppens, M.-O.; Day, R.M. A Hydrogel-Integrated Culture Device to Interrogate T Cell Activation with Physicochemical Cues. ACS Appl. Mater. Interfaces 2020, 12, 47355–47367. [Google Scholar] [CrossRef] [PubMed]
- Rømer, A.M.A.; Thorseth, M.-L.; Madsen, D.H. Immune Modulatory Properties of Collagen in Cancer. Front. Immunol. 2021, 12, 791453. [Google Scholar] [CrossRef]
- Nicolas-Boluda, A.; Vaquero, J.; Vimeux, L.; Guilbert, T.; Barrin, S.; Kantari-Mimoun, C.; Ponzo, M.; Renault, G.; Deptula, P.; Pogoda, K.; et al. Tumor Stiffening Reversion through Collagen Crosslinking Inhibition Improves T Cell Migration and Anti-PD-1 Treatment. eLife 2021, 10, e58688. [Google Scholar] [CrossRef]
- Salmon, H.; Franciszkiewicz, K.; Damotte, D.; Dieu-Nosjean, M.-C.; Validire, P.; Trautmann, A.; Mami-Chouaib, F.; Donnadieu, E. Matrix Architecture Defines the Preferential Localization and Migration of T Cells into the Stroma of Human Lung Tumors. J. Clin. Investig. 2012, 122, 899–910. [Google Scholar] [CrossRef]
- Bougherara, H.; Mansuet-Lupo, A.; Alifano, M.; Ngô, C.; Damotte, D.; Le Frère-Belda, M.-A.; Donnadieu, E.; Peranzoni, E. Real-Time Imaging of Resident T Cells in Human Lung and Ovarian Carcinomas Reveals How Different Tumor Microenvironments Control T Lymphocyte Migration. Front. Immunol. 2015, 6, 500. [Google Scholar] [CrossRef]
- Miyazawa, A.; Ito, S.; Asano, S.; Tanaka, I.; Sato, M.; Kondo, M.; Hasegawa, Y. Regulation of PD-L1 Expression by Matrix Stiffness in Lung Cancer Cells. Biochem. Biophys. Res. Commun. 2018, 495, 2344–2349. [Google Scholar] [CrossRef]
- Peng, D.H.; Rodriguez, B.L.; Diao, L.; Chen, L.; Wang, J.; Byers, L.A.; Wei, Y.; Chapman, H.A.; Yamauchi, M.; Behrens, C.; et al. Collagen Promotes Anti-PD-1/PD-L1 Resistance in Cancer through LAIR1-Dependent CD8+ T Cell Exhaustion. Nat. Commun. 2020, 11, 4520. [Google Scholar] [CrossRef] [PubMed]
- Sangaletti, S.; Chiodoni, C.; Tripodo, C.; Colombo, M.P. Common Extracellular Matrix Regulation of Myeloid Cell Activity in the Bone Marrow and Tumor Microenvironments. Cancer Immunol. Immunother. 2017, 66, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Larionova, I.; Tuguzbaeva, G.; Ponomaryova, A.; Stakheyeva, M.; Cherdyntseva, N.; Pavlov, V.; Choinzonov, E.; Kzhyshkowska, J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front. Oncol. 2020, 10, 566511. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Yang, Z.; Jin, Y.; Qi, X.; Chu, J.; Deng, X. ADM Scaffolds Generate a Pro-Regenerative Microenvironment During Full-Thickness Cutaneous Wound Healing Through M2 Macrophage Polarization via Lamtor1. Front. Physiol. 2018, 9, 657. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Paradise, B.D.; Ma, W.W.; Fernandez-Zapico, M.E. Recent Advances in the Clinical Targeting of Hedgehog/GLI Signaling in Cancer. Cells 2019, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- Mpekris, F.; Papageorgis, P.; Polydorou, C.; Voutouri, C.; Kalli, M.; Pirentis, A.P.; Stylianopoulos, T. Sonic-Hedgehog Pathway Inhibition Normalizes Desmoplastic Tumor Microenvironment to Improve Chemo- and Nanotherapy. J. Control. Release 2017, 261, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Polydorou, C.; Mpekris, F.; Papageorgis, P.; Voutouri, C.; Stylianopoulos, T. Pirfenidone Normalizes the Tumor Microenvironment to Improve Chemotherapy. Oncotarget 2017, 8, 24506–24517. [Google Scholar] [CrossRef]
- Panagi, M.; Mpekris, F.; Chen, P.; Voutouri, C.; Nakagawa, Y.; Martin, J.D.; Hiroi, T.; Hashimoto, H.; Demetriou, P.; Pierides, C.; et al. Polymeric Micelles Effectively Reprogram the Tumor Microenvironment to Potentiate Nano-Immunotherapy in Mouse Breast Cancer Models. Nat. Commun. 2022, 13, 7165. [Google Scholar] [CrossRef]
- Panagi, M.; Voutouri, C.; Mpekris, F.; Papageorgis, P.; Martin, M.R.; Martin, J.D.; Demetriou, P.; Pierides, C.; Polydorou, C.; Stylianou, A.; et al. TGF-β Inhibition Combined with Cytotoxic Nanomedicine Normalizes Triple Negative Breast Cancer Microenvironment towards Anti-Tumor Immunity. Theranostics 2020, 10, 1910–1922. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Jain, R.K. Strategies for Advancing Cancer Nanomedicine. Nat. Mater. 2013, 12, 958–962. [Google Scholar] [CrossRef]
- Mpekris, F.; Panagi, M.; Voutouri, C.; Martin, J.D.; Samuel, R.; Takahashi, S.; Gotohda, N.; Suzuki, T.; Papageorgis, P.; Demetriou, P.; et al. Normalizing the Microenvironment Overcomes Vessel Compression and Resistance to Nano-immunotherapy in Breast Cancer Lung Metastasis. Adv. Sci. 2021, 8, 2001917. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C. Pancreatic Cancer Provides Testbed for First Mechanotherapeutics. Nat. Biotechnol. 2019, 37, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Martin, J.D.; Liu, H.; Lacorre, D.A.; Jain, S.R.; Kozin, S.V.; Stylianopoulos, T.; Mousa, A.S.; Han, X.; Adstamongkonkul, P.; et al. Angiotensin Inhibition Enhances Drug Delivery and Potentiates Chemotherapy by Decompressing Tumour Blood Vessels. Nat. Commun. 2013, 4, 2516. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.D.; Panagi, M.; Wang, C.; Khan, T.T.; Martin, M.R.; Voutouri, C.; Toh, K.; Papageorgis, P.; Mpekris, F.; Polydorou, C.; et al. Dexamethasone Increases Cisplatin-Loaded Nanocarrier Delivery and Efficacy in Metastatic Breast Cancer by Normalizing the Tumor Microenvironment. ACS Nano 2019, 13, 6396–6408. [Google Scholar] [CrossRef]
- Voutouri, C.; Panagi, M.; Mpekris, F.; Stylianou, A.; Michael, C.; Averkiou, M.A.; Martin, J.D.; Stylianopoulos, T. Endothelin Inhibition Potentiates Cancer Immunotherapy Revealing Mechanical Biomarkers Predictive of Response. Adv. Ther. 2021, 4, 2000289. [Google Scholar] [CrossRef]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Clark, J.W.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Ly, L.; Baglini, C.V.; Blaszkowsky, L.S.; et al. Total Neoadjuvant Therapy With FOLFIRINOX in Combination With Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 1020. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular Matrix (ECM) Stiffness and Degradation as Cancer Drivers. J Cell. Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef]
- Azadi, S.; Tafazzoli-Shadpour, M.; Soleimani, M.; Warkiani, M.E. Modulating Cancer Cell Mechanics and Actin Cytoskeleton Structure by Chemical and Mechanical Stimulations. J. Biomed. Mater. Res. 2019, 107, 1569–1581. [Google Scholar] [CrossRef]
- Wei, B.; Zhou, X.; Liang, C.; Zheng, X.; Lei, P.; Fang, J.; Han, X.; Wang, L.; Qi, C.; Wei, H. Human Colorectal Cancer Progression Correlates with LOX-Induced ECM Stiffening. Int. J. Biol. Sci. 2017, 13, 1450–1457. [Google Scholar] [CrossRef]
- Fattet, L.; Jung, H.-Y.; Matsumoto, M.W.; Aubol, B.E.; Kumar, A.; Adams, J.A.; Chen, A.C.; Sah, R.L.; Engler, A.J.; Pasquale, E.B.; et al. Matrix Rigidity Controls Epithelial-Mesenchymal Plasticity and Tumor Metastasis via a Mechanoresponsive EPHA2/LYN Complex. Dev. Cell 2020, 54, 302–316.e7. [Google Scholar] [CrossRef]
- Zhou, J.; Zhan, W.; Chang, C.; Zhang, X.; Jia, Y.; Dong, Y.; Zhou, C.; Sun, J.; Grant, E.G. Breast Lesions: Evaluation with Shear Wave Elastography, with Special Emphasis on the “Stiff Rim” Sign. Radiology 2014, 272, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Whelehan, P.; Thomson, K.; McLean, D.; Brauer, K.; Purdie, C.; Baker, L.; Jordan, L.; Rauchhaus, P.; Thompson, A. Invasive Breast Cancer: Relationship between Shear-Wave Elastographic Findings and Histologic Prognostic Factors. Radiology 2012, 263, 673–677. [Google Scholar] [CrossRef]
- Park, H.S.; Shin, H.J.; Shin, K.C.; Cha, J.H.; Chae, E.Y.; Choi, W.J.; Kim, H.H. Comparison of Peritumoral Stromal Tissue Stiffness Obtained by Shear Wave Elastography between Benign and Malignant Breast Lesions. Acta Radiol. 2018, 59, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.W.; Grant, A.D.; Parker, S.S.; Hill, S.; Whalen, M.B.; Chakrabarti, J.; Harman, M.W.; Roman, M.R.; Forte, B.L.; Gowan, C.C.; et al. Breast Tumor Stiffness Instructs Bone Metastasis via Maintenance of Mechanical Conditioning. Cell Rep. 2021, 35, 109293. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R.; Gartland, A.; Erler, J.T. Lysyl Oxidase, a Targetable Secreted Molecule Involved in Cancer Metastasis. Cancer Res. 2016, 76, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Lucas, M.C.; Leonte, L.E.; Garcia-Montolio, M.; Singh, L.B.; Findlay, A.D.; Deodhar, M.; Foot, J.S.; Jarolimek, W.; Timpson, P.; et al. Pre-Clinical Evaluation of Small Molecule LOXL2 Inhibitors in Breast Cancer. Oncotarget 2017, 8, 26066–26078. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Nielsen, M.J.; Sand, J.M.; Henriksen, K.; Genovese, F.; Bay-Jensen, A.-C.; Smith, V.; Adamkewicz, J.I.; Christiansen, C.; Leeming, D.J. Extracellular Matrix Remodeling: The Common Denominator in Connective Tissue Diseases Possibilities for Evaluation and Current Understanding of the Matrix as More Than a Passive Architecture, but a Key Player in Tissue Failure. ASSAY Drug Dev. Technol. 2013, 11, 70–92. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Heneberg, P. Paracrine Tumor Signaling Induces Transdifferentiation of Surrounding Fibroblasts. Crit. Rev. Oncol./Hematol. 2016, 97, 303–311. [Google Scholar] [CrossRef]
- Barbazán, J.; Matic Vignjevic, D. Cancer Associated Fibroblasts: Is the Force the Path to the Dark Side? Curr. Opin. Cell Biol. 2019, 56, 71–79. [Google Scholar] [CrossRef]
- Dzobo, K.; Dandara, C. Architecture of Cancer-Associated Fibroblasts in Tumor Microenvironment: Mapping Their Origins, Heterogeneity, and Role in Cancer Therapy Resistance. OMICS A J. Integr. Biol. 2020, 24, 314–339. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Wilharm, N.; Hayn, A.; Mierke, C.T. Matrix and Cellular Mechanical Properties Are the Driving Factors for Facilitating Human Cancer Cell Motility into 3D Engineered Matrices. Converg. Sci. Phys. Oncol. 2017, 3, 044003. [Google Scholar] [CrossRef]
- Fischer, T.; Hayn, A.; Mierke, C.T. Fast and Reliable Advanced Two-Step Pore-Size Analysis of Biomimetic 3D Extracellular Matrix Scaffolds. Sci. Rep. 2019, 9, 8352. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Keely, P.J. Mechanical Signaling through the Cytoskeleton Regulates Cell Proliferation by Coordinated Focal Adhesion and Rho GTPase Signaling. J. Cell Sci. 2011, 124, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Provenzano, P.P.; Smith, C.L.; Levchenko, A. Matrix Nanotopography as a Regulator of Cell Function. J. Cell Biol. 2012, 197, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Sciote, J.J.; Morris, T.J. Skeletal Muscle Function and Fibre Types: The Relationship Between Occlusal Function and the Phenotype of Jaw-Closing Muscles in Human. J. Orthod. 2000, 27, 15–30. [Google Scholar] [CrossRef]
- Severs, N.J. The Cardiac Muscle Cell. Bioessays 2000, 22, 188–199. [Google Scholar] [CrossRef]
- Ghorbani, M.; Soleymani, H.; Hashemzadeh, H.; Mortezazadeh, S.; Sedghi, M.; Shojaeilangari, S.; Allahverdi, A.; Naderi-Manesh, H. Microfluidic Investigation of the Effect of Graphene Oxide on Mechanical Properties of Cell and Actin Cytoskeleton Networks: Experimental and Theoretical Approaches. Sci. Rep. 2021, 11, 16216. [Google Scholar] [CrossRef]
- Ermis, M.; Antmen, E.; Hasirci, V. Micro and Nanofabrication Methods to Control Cell-Substrate Interactions and Cell Behavior: A Review from the Tissue Engineering Perspective. Bioact. Mater. 2018, 3, 355–369. [Google Scholar] [CrossRef]
- Werner, M.; Blanquer, S.B.G.; Haimi, S.P.; Korus, G.; Dunlop, J.W.C.; Duda, G.N.; Grijpma, D.W.; Petersen, A. Surface Curvature Differentially Regulates Stem Cell Migration and Differentiation via Altered Attachment Morphology and Nuclear Deformation. Adv. Sci. 2017, 4, 1600347. [Google Scholar] [CrossRef]
- Wu, C.; Chen, M.; Zheng, T.; Yang, X. Effect of Surface Roughness on the Initial Response of MC3T3-E1 Cells Cultured on Polished Titanium Alloy. Bio-Med. Mater. Eng. 2015, 26, S155–S164. [Google Scholar] [CrossRef] [PubMed]
- Riveiro, A.; Maçon, A.L.B.; Del Val, J.; Comesaña, R.; Pou, J. Laser Surface Texturing of Polymers for Biomedical Applications. Front. Phys. 2018, 6, 16. [Google Scholar] [CrossRef]
- Kuzyk, P.R.T.; Schemitsch, E.H. The Basic Science of Peri-Implant Bone Healing. Indian J. Orthop. 2011, 45, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; García, A.J.; Salmeron-Sanchez, M. Receptor Control in Mesenchymal Stem Cell Engineering. Nat. Rev. Mater. 2018, 3, 17091. [Google Scholar] [CrossRef]
- Yang, J.; Weinberg, R.A. Epithelial-Mesenchymal Transition: At the Crossroads of Development and Tumor Metastasis. Dev. Cell 2008, 14, 818–829. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Fattet, L.; Tsai, J.H.; Guo, Y.; Pai, V.H.; Majeski, H.E.; Chen, A.C.; Sah, R.L.; Taylor, S.S.; Engler, A.J.; et al. Matrix Stiffness Drives Epithelial–Mesenchymal Transition and Tumour Metastasis through a TWIST1–G3BP2 Mechanotransduction Pathway. Nat. Cell Biol. 2015, 17, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Emon, M.A.B.; Staudacher, J.J.; Thomas, A.L.; Zessner-Spitzenberg, J.; Mancinelli, G.; Krett, N.; Saif, M.T.; Jung, B. Increased Stiffness of the Tumor Microenvironment in Colon Cancer Stimulates Cancer Associated Fibroblast-Mediated Prometastatic Activin A Signaling. Sci. Rep. 2020, 10, 50. [Google Scholar] [CrossRef]
- Piao, J.; You, K.; Guo, Y.; Zhang, Y.; Li, Z.; Geng, L. Substrate Stiffness Affects Epithelial-Mesenchymal Transition of Cervical Cancer Cells through miR-106b and Its Target Protein DAB2. Int. J. Oncol. 2017, 50, 2033–2042. [Google Scholar] [CrossRef]
- Leight, J.L.; Wozniak, M.A.; Chen, S.; Lynch, M.L.; Chen, C.S. Matrix Rigidity Regulates a Switch between TGF-Β1–Induced Apoptosis and Epithelial–Mesenchymal Transition. Mol. Biol. Cell 2012, 23, 781–791. [Google Scholar] [CrossRef]
- Thodeti, C.K.; Matthews, B.; Ravi, A.; Mammoto, A.; Ghosh, K.; Bracha, A.L.; Ingber, D.E. TRPV4 Channels Mediate Cyclic Strain–Induced Endothelial Cell Reorientation Through Integrin-to-Integrin Signaling. Circ. Res. 2009, 104, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- O’Conor, C.J.; Leddy, H.A.; Benefield, H.C.; Liedtke, W.B.; Guilak, F. TRPV4-Mediated Mechanotransduction Regulates the Metabolic Response of Chondrocytes to Dynamic Loading. Proc. Natl. Acad. Sci. USA 2014, 111, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Goswami, R.; Zhang, D.X.; Rahaman, S.O. TRPV 4 Regulates Matrix Stiffness and TGF Β1-induced Epithelial-mesenchymal Transition. J. Cell. Mol. Medi 2019, 23, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.; Li, Y.; Poh, Y.-C.; Yokohama-Tamaki, T.; Wang, N.; Tanaka, T.S. Soft Substrates Promote Homogeneous Self-Renewal of Embryonic Stem Cells via Downregulating Cell-Matrix Tractions. PLoS ONE 2010, 5, e15655. [Google Scholar] [CrossRef]
- Peiris-Pagès, M.; Martinez-Outschoorn, U.E.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer Stem Cell Metabolism. Breast Cancer Res. 2016, 18, 55. [Google Scholar] [CrossRef]
- Brown, H.K.; Tellez-Gabriel, M.; Heymann, D. Cancer Stem Cells in Osteosarcoma. Cancer Lett. 2017, 386, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Song, S.; Hochster, H.S.; Steinberg, I.B. Cancer Stem Cells: The Promise and the Potential. Semin. Oncol. 2015, 42, S3–S17. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the Role of ABC Transporters in Multidrug-Resistant Cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Hui, L.; Zhang, J.; Ding, X.; Guo, X.; Jiang, X. Matrix Stiffness Regulates the Proliferation, Stemness and Chemoresistance of Laryngeal Squamous Cancer Cells. Int. J. Oncol. 2017, 50, 1439–1447. [Google Scholar] [CrossRef]
- You, Y.; Zheng, Q.; Dong, Y.; Xie, X.; Wang, Y.; Wu, S.; Zhang, L.; Wang, Y.; Xue, T.; Wang, Z.; et al. Matrix Stiffness-Mediated Effects on Stemness Characteristics Occurring in HCC Cells. Oncotarget 2016, 7, 32221–32231. [Google Scholar] [CrossRef]
- Swift, J.; Ivanovska, I.L.; Buxboim, A.; Harada, T.; Dingal, P.C.D.P.; Pinter, J.; Pajerowski, J.D.; Spinler, K.R.; Shin, J.-W.; Tewari, M.; et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-Directed Differentiation. Science 2013, 341, 1240104. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, L.; Holm, E.; Halme, M.; West, G.; Lindholm, F.; Gullmets, J.; Irjala, J.; Heliö, T.; Padzik, A.; Meinander, A.; et al. Lamin A/C Phosphorylation at Serine 22 Is a Conserved Heat Shock Response to Regulate Nuclear Adaptation during Stress. J. Cell Sci. 2023, 136, jcs259788. [Google Scholar] [CrossRef] [PubMed]
- Makarov, A.A.; Zou, J.; Houston, D.R.; Spanos, C.; Solovyova, A.S.; Cardenal-Peralta, C.; Rappsilber, J.; Schirmer, E.C. Lamin A Molecular Compression and Sliding as Mechanisms behind Nucleoskeleton Elasticity. Nat. Commun. 2019, 10, 3056. [Google Scholar] [CrossRef] [PubMed]
- Bednar, J.; Garcia-Saez, I.; Boopathi, R.; Cutter, A.R.; Papai, G.; Reymer, A.; Syed, S.H.; Lone, I.N.; Tonchev, O.; Crucifix, C.; et al. Structure and Dynamics of a 197 Bp Nucleosome in Complex with Linker Histone H1. Mol. Cell 2017, 66, 384–397.e8. [Google Scholar] [CrossRef]
- Bernstein, E.; Hake, S.B. The Nucleosome: A Little Variation Goes a Long wayThis Paper Is One of a Selection of Papers Published in This Special Issue, Entitled 27th International West Coast Chromatin and Chromosome Conference, and Has Undergone the Journal’s Usual Peer Review Process. Biochem. Cell Biol. 2006, 84, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Luger, K.; Mäder, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal Structure of the Nucleosome Core Particle at 2.8 Å Resolution. Nature 1997, 389, 251–260. [Google Scholar] [CrossRef] [PubMed]
- McGinty, R.K.; Tan, S. Nucleosome Structure and Function. Chem. Rev. 2015, 115, 2255–2273. [Google Scholar] [CrossRef]
- Melters, D.; Nye, J.; Zhao, H.; Dalal, Y. Chromatin Dynamics in Vivo: A Game of Musical Chairs. Genes 2015, 6, 751–776. [Google Scholar] [CrossRef]
- Martire, S.; Banaszynski, L.A. The Roles of Histone Variants in Fine-Tuning Chromatin Organization and Function. Nat. Rev. Mol. Cell Biol. 2020, 21, 522–541. [Google Scholar] [CrossRef]
- Sokolova, V.; Sarkar, S.; Tan, D. Histone Variants and Chromatin Structure, Update of Advances. Comput. Struct. Biotechnol. J. 2023, 21, 299–311. [Google Scholar] [CrossRef]
- Melters, D.P.; Pitman, M.; Rakshit, T.; Dimitriadis, E.K.; Bui, M.; Papoian, G.A.; Dalal, Y. Intrinsic Elasticity of Nucleosomes Is Encoded by Histone Variants and Calibrated by Their Binding Partners. Proc. Natl. Acad. Sci. USA 2019, 116, 24066–24074. [Google Scholar] [CrossRef] [PubMed]
- Vardabasso, C.; Gaspar-Maia, A.; Hasson, D.; Pünzeler, S.; Valle-Garcia, D.; Straub, T.; Keilhauer, E.C.; Strub, T.; Dong, J.; Panda, T.; et al. Histone Variant H2A.Z.2 Mediates Proliferation and Drug Sensitivity of Malignant Melanoma. Mol. Cell 2015, 59, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.D.; Kim, P.-J.; Eun, J.W.; Shen, Q.; Kim, H.S.; Shin, W.C.; Ahn, Y.M.; Park, W.S.; Lee, J.Y.; Nam, S.W. Oncogenic Potential of Histone-Variant H2A.Z.1 and Its Regulatory Role in Cell Cycle and Epithelial-Mesenchymal Transition in Liver Cancer. Oncotarget 2016, 7, 11412–11423. [Google Scholar] [CrossRef] [PubMed]
- Svotelis, A.; Gévry, N.; Grondin, G.; Gaudreau, L. H2A.Z Overexpression Promotes Cellular Proliferation of Breast Cancer Cells. Cell Cycle 2010, 9, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Fal, K.; Korsbo, N.; Alonso-Serra, J.; Teles, J.; Liu, M.; Refahi, Y.; Chabouté, M.-E.; Jönsson, H.; Hamant, O. Tissue Folding at the Organ–Meristem Boundary Results in Nuclear Compression and Chromatin Compaction. Proc. Natl. Acad. Sci. USA 2021, 118, e2017859118. [Google Scholar] [CrossRef] [PubMed]
- Stiekema, M.; Van Zandvoort, M.A.M.J.; Ramaekers, F.C.S.; Broers, J.L.V. Structural and Mechanical Aberrations of the Nuclear Lamina in Disease. Cells 2020, 9, 1884. [Google Scholar] [CrossRef]
- Thiagalingam, S. Epigenetic Memory in Development and Disease: Unraveling the Mechanism. Biochim. Biophys. Acta BBA-Rev. Cancer 2020, 1873, 188349. [Google Scholar] [CrossRef]
- Bustin, M.; Misteli, T. Nongenetic Functions of the Genome. Science 2016, 352, aad6933. [Google Scholar] [CrossRef]
- Rodesney, C.A.; Roman, B.; Dhamani, N.; Cooley, B.J.; Katira, P.; Touhami, A.; Gordon, V.D. Mechanosensing of Shear by Pseudomonas Aeruginosa Leads to Increased Levels of the Cyclic-Di-GMP Signal Initiating Biofilm Development. Proc. Natl. Acad. Sci. USA 2017, 114, 5906–5911. [Google Scholar] [CrossRef]
- Wang, N.; Suo, Z. Long-Distance Propagation of Forces in a Cell. Biochem. Biophys. Res. Commun. 2005, 328, 1133–1138. [Google Scholar] [CrossRef]
- Deneke, V.E.; Puliafito, A.; Krueger, D.; Narla, A.V.; De Simone, A.; Primo, L.; Vergassola, M.; De Renzis, S.; Di Talia, S. Self-Organized Nuclear Positioning Synchronizes the Cell Cycle in Drosophila Embryos. Cell 2019, 177, 925–941.e17. [Google Scholar] [CrossRef] [PubMed]
- Tajik, A.; Zhang, Y.; Wei, F.; Sun, J.; Jia, Q.; Zhou, W.; Singh, R.; Khanna, N.; Belmont, A.S.; Wang, N. Transcription Upregulation via Force-Induced Direct Stretching of Chromatin. Nat. Mater. 2016, 15, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, J.; Mohagheghian, E.; Wang, N. Force-Induced Gene up-Regulation Does Not Follow the Weak Power Law but Depends on H3K9 Demethylation. Sci. Adv. 2020, 6, eaay9095. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.B.; Sengupta, P. How Caenorhabditis Elegans Senses Mechanical Stress, Temperature, and Other Physical Stimuli. Genetics 2019, 212, 25–51. [Google Scholar] [CrossRef] [PubMed]
- Zanotelli, M.R.; Zhang, J.; Reinhart-King, C.A. Mechanoresponsive Metabolism in Cancer Cell Migration and Metastasis. Cell Metab. 2021, 33, 1307–1321. [Google Scholar] [CrossRef]
- Purkayastha, P.; Pendyala, K.; Saxena, A.S.; Hakimjavadi, H.; Chamala, S.; Dixit, P.; Baer, C.F.; Lele, T.P. Reverse Plasticity Underlies Rapid Evolution by Clonal Selection within Populations of Fibroblasts Propagated on a Novel Soft Substrate. Mol. Biol. Evol. 2021, 38, 3279–3293. [Google Scholar] [CrossRef]
- Gridina, M.; Fishman, V. Multilevel View on Chromatin Architecture Alterations in Cancer. Front. Genet. 2022, 13, 1059617. [Google Scholar] [CrossRef]
- Brock, M.V.; Herman, J.G.; Baylin, S.B. Cancer as a Manifestation of Aberrant Chromatin Structure. Cancer J. 2007, 13, 3–8. [Google Scholar] [CrossRef]
- Pennacchio, F.A.; Nastały, P.; Poli, A.; Maiuri, P. Tailoring Cellular Function: The Contribution of the Nucleus in Mechanotransduction. Front. Bioeng. Biotechnol. 2021, 8, 596746. [Google Scholar] [CrossRef]
- Li, C.-L.; Pu, M.; Wang, W.; Chaturbedi, A.; Emerson, F.J.; Lee, S.S. Region-Specific H3K9me3 Gain in Aged Somatic Tissues in Caenorhabditis Elegans. PLoS Genet. 2021, 17, e1009432. [Google Scholar] [CrossRef]
- Padeken, J.; Methot, S.P.; Gasser, S.M. Establishment of H3K9-Methylated Heterochromatin and Its Functions in Tissue Differentiation and Maintenance. Nat. Rev. Mol. Cell Biol. 2022, 23, 623–640. [Google Scholar] [CrossRef]
- Jamieson, K.; Wiles, E.T.; McNaught, K.J.; Sidoli, S.; Leggett, N.; Shao, Y.; Garcia, B.A.; Selker, E.U. Loss of HP1 Causes Depletion of H3K27me3 from Facultative Heterochromatin and Gain of H3K27me2 at Constitutive Heterochromatin. Genome Res. 2016, 26, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Igolkina, A.A.; Zinkevich, A.; Karandasheva, K.O.; Popov, A.A.; Selifanova, M.V.; Nikolaeva, D.; Tkachev, V.; Penzar, D.; Nikitin, D.M.; Buzdin, A. H3K4me3, H3K9ac, H3K27ac, H3K27me3 and H3K9me3 Histone Tags Suggest Distinct Regulatory Evolution of Open and Condensed Chromatin Landmarks. Cells 2019, 8, 1034. [Google Scholar] [CrossRef] [PubMed]
- Hsia, C.-R.; Melters, D.P.; Dalal, Y. The Force Is Strong with This Epigenome: Chromatin Structure and Mechanobiology. J. Mol. Biol. 2023, 435, 168019. [Google Scholar] [CrossRef] [PubMed]
- Nicetto, D.; Zaret, K.S. Role of H3K9me3 Heterochromatin in Cell Identity Establishment and Maintenance. Curr. Opin. Genet. Dev. 2019, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, A.; Meshorer, E. Chromatin Remodeling and Bivalent Histone Modifications in Embryonic Stem Cells. EMBO Rep. 2015, 16, 1609–1619. [Google Scholar] [CrossRef]
- Harr, J.C.; Luperchio, T.R.; Wong, X.; Cohen, E.; Wheelan, S.J.; Reddy, K.L. Directed Targeting of Chromatin to the Nuclear Lamina Is Mediated by Chromatin State and A-Type Lamins. J. Cell Biol. 2015, 208, 33–52. [Google Scholar] [CrossRef]
- Loïodice, I.; Garnier, M.; Nikolov, I.; Taddei, A. An Inducible System for Silencing Establishment Reveals a Stepwise Mechanism in Which Anchoring at the Nuclear Periphery Precedes Heterochromatin Formation. Cells 2021, 10, 2810. [Google Scholar] [CrossRef]
- Holla, S.; Dhakshnamoorthy, J.; Folco, H.D.; Balachandran, V.; Xiao, H.; Sun, L.; Wheeler, D.; Zofall, M.; Grewal, S.I.S. Positioning Heterochromatin at the Nuclear Periphery Suppresses Histone Turnover to Promote Epigenetic Inheritance. Cell 2020, 180, 150–164.e15. [Google Scholar] [CrossRef]
- Nava, M.M.; Miroshnikova, Y.A.; Biggs, L.C.; Whitefield, D.B.; Metge, F.; Boucas, J.; Vihinen, H.; Jokitalo, E.; Li, X.; García Arcos, J.M.; et al. Heterochromatin-Driven Nuclear Softening Protects the Genome against Mechanical Stress-Induced Damage. Cell 2020, 181, 800–817.e22. [Google Scholar] [CrossRef]
- Fischer, T.; Hayn, A.; Mierke, C.T. Effect of Nuclear Stiffness on Cell Mechanics and Migration of Human Breast Cancer Cells. Front. Cell Dev. Biol. 2020, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Bizhanova, A.; Kaufman, P.D. Close to the Edge: Heterochromatin at the Nucleolar and Nuclear Peripheries. Biochim. Biophys. Acta BBA-Gene Regul. Mech. 2021, 1864, 194666. [Google Scholar] [CrossRef] [PubMed]
- Van Steensel, B.; Belmont, A.S. Lamina-Associated Domains: Links with Chromosome Architecture, Heterochromatin, and Gene Repression. Cell 2017, 169, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Belton, J.-M.; McCord, R.P.; Gibcus, J.H.; Naumova, N.; Zhan, Y.; Dekker, J. Hi–C: A Comprehensive Technique to Capture the Conformation of Genomes. Methods 2012, 58, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Oluwadare, O.; Highsmith, M.; Cheng, J. An Overview of Methods for Reconstructing 3-D Chromosome and Genome Structures from Hi-C Data. Biol. Proced. Online 2019, 21, 7. [Google Scholar] [CrossRef]
- Stephens, A.D.; Banigan, E.J.; Adam, S.A.; Goldman, R.D.; Marko, J.F. Chromatin and Lamin A Determine Two Different Mechanical Response Regimes of the Cell Nucleus. MBoC 2017, 28, 1984–1996. [Google Scholar] [CrossRef]
- Stephens, A.D.; Liu, P.Z.; Kandula, V.; Chen, H.; Almassalha, L.M.; Herman, C.; Backman, V.; O’Halloran, T.; Adam, S.A.; Goldman, R.D.; et al. Physicochemical Mechanotransduction Alters Nuclear Shape and Mechanics via Heterochromatin Formation. MBoC 2019, 30, 2320–2330. [Google Scholar] [CrossRef]
- MacPherson, Q.; Beltran, B.; Spakowitz, A.J. Chromatin Compaction Leads to a Preference for Peripheral Heterochromatin. Biophys. J. 2020, 118, 1479–1488. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Bisht, M.; Thuma, J.; Tu, L.-C. Single-Chromosome Dynamics Reveals Locus-Dependent Dynamics and Chromosome Territory Orientation. J. Cell Sci. 2023, 136, jcs260137. [Google Scholar] [CrossRef]
- Girelli, G.; Custodio, J.; Kallas, T.; Agostini, F.; Wernersson, E.; Spanjaard, B.; Mota, A.; Kolbeinsdottir, S.; Gelali, E.; Crosetto, N.; et al. GPSeq Reveals the Radial Organization of Chromatin in the Cell Nucleus. Nat. Biotechnol. 2020, 38, 1184–1193. [Google Scholar] [CrossRef]
- Falk, M.; Feodorova, Y.; Naumova, N.; Imakaev, M.; Lajoie, B.R.; Leonhardt, H.; Joffe, B.; Dekker, J.; Fudenberg, G.; Solovei, I.; et al. Heterochromatin Drives Compartmentalization of Inverted and Conventional Nuclei. Nature 2019, 570, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Baldi, S.; Korber, P.; Becker, P.B. Beads on a String—Nucleosome Array Arrangements and Folding of the Chromatin Fiber. Nat. Struct. Mol. Biol. 2020, 27, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Boopathi, R.; Dimitrov, S.; Hamiche, A.; Petosa, C.; Bednar, J. Cryo-Electron Microscopy of the Chromatin Fiber. Curr. Opin. Struct. Biol. 2020, 64, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Rosin, L.F.; Crocker, O.; Isenhart, R.L.; Nguyen, S.C.; Xu, Z.; Joyce, E.F. Chromosome Territory Formation Attenuates the Translocation Potential of Cells. eLife 2019, 8, e49553. [Google Scholar] [CrossRef] [PubMed]
- Amar, K.; Wei, F.; Chen, J.; Wang, N. Effects of Forces on Chromatin. APL Bioeng. 2021, 5, 041503. [Google Scholar] [CrossRef] [PubMed]
- Stephens, A.D.; Liu, P.Z.; Banigan, E.J.; Almassalha, L.M.; Backman, V.; Adam, S.A.; Goldman, R.D.; Marko, J.F. Chromatin Histone Modifications and Rigidity Affect Nuclear Morphology Independent of Lamins. Mol. Biol. Cell 2018, 29, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Driscoll, T.P.; Thorpe, S.D.; Nerurkar, N.L.; Baker, B.M.; Yang, M.T.; Chen, C.S.; Lee, D.A.; Mauck, R.L. Differentiation Alters Stem Cell Nuclear Architecture, Mechanics, and Mechano-Sensitivity. eLife 2016, 5, e18207. [Google Scholar] [CrossRef]
- Gerlitz, G.; Bustin, M. The Role of Chromatin Structure in Cell Migration. Trends Cell Biol. 2011, 21, 6–11. [Google Scholar] [CrossRef]
- Berg, I.K.; Currey, M.L.; Gupta, S.; Berrada, Y.; Viet, B.N.; Pho, M.; Patteson, A.E.; Schwarz, J.M.; Banigan, E.J.; Stephens, A.D. Transcription Regulates Bleb Formation and Stability Independent of Nuclear Rigidity. bioRxiv 2022. [Google Scholar] [CrossRef]
- Shin, Y.; Chang, Y.-C.; Lee, D.S.W.; Berry, J.; Sanders, D.W.; Ronceray, P.; Wingreen, N.S.; Haataja, M.; Brangwynne, C.P. Liquid Nuclear Condensates Mechanically Sense and Restructure the Genome. Cell 2018, 175, 1481–1491.e13. [Google Scholar] [CrossRef]
- Morgan, S.L.; Mariano, N.C.; Bermudez, A.; Arruda, N.L.; Wu, F.; Luo, Y.; Shankar, G.; Jia, L.; Chen, H.; Hu, J.-F.; et al. Manipulation of Nuclear Architecture through CRISPR-Mediated Chromosomal Looping. Nat. Commun. 2017, 8, 15993. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Rege, M.; Valeri, J.; Dunagin, M.C.; Metzger, A.; Titus, K.R.; Gilgenast, T.G.; Gong, W.; Beagan, J.A.; Raj, A.; et al. LADL: Light-Activated Dynamic Looping for Endogenous Gene Expression Control. Nat. Methods 2019, 16, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Keizer, V.I.P.; Grosse-Holz, S.; Woringer, M.; Zambon, L.; Aizel, K.; Bongaerts, M.; Delille, F.; Kolar-Znika, L.; Scolari, V.F.; Hoffmann, S.; et al. Live-Cell Micromanipulation of a Genomic Locus Reveals Interphase Chromatin Mechanics. Science 2022, 377, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Goult, B.T. The Mechanical Basis of Memory—The MeshCODE Theory. Front. Mol. Neurosci. 2021, 14, 592951. [Google Scholar] [CrossRef] [PubMed]
- Castel, S.E.; Martienssen, R.A. RNA Interference in the Nucleus: Roles for Small RNAs in Transcription, Epigenetics and Beyond. Nat. Rev. Genet. 2013, 14, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, L.; Wang, R.R.; Hu, J.-F.; Cui, J. The Effects of Mitochondria-Associated Long Noncoding RNAs in Cancer Mitochondria: New Players in an Old Arena. Crit. Rev. Oncol./Hematol. 2018, 131, 76–82. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Muñoz, A.; Eldridge, W.J.; Jakobsen, N.M.; Sørensen, H.; Wax, A.; Costa, M. Cellular Shear Stiffness Reflects Progression of Arsenic-Induced Transformation during G1. Carcinogenesis 2018, 39, 109–117. [Google Scholar] [CrossRef]
- Gkretsi, V.; Stylianopoulos, T. Cell Adhesion and Matrix Stiffness: Coordinating Cancer Cell Invasion and Metastasis. Front. Oncol. 2018, 8, 145. [Google Scholar] [CrossRef]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Denis, M.; Gregory, A.; Bayat, M.; Fazzio, R.T.; Whaley, D.H.; Ghosh, K.; Shah, S.; Fatemi, M.; Alizad, A. Correlating Tumor Stiffness with Immunohistochemical Subtypes of Breast Cancers: Prognostic Value of Comb-Push Ultrasound Shear Elastography for Differentiating Luminal Subtypes. PLoS ONE 2016, 11, e0165003. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wanggou, S.; Bodalia, A.; Zhu, M.; Dong, W.; Fan, J.J.; Yin, W.C.; Min, H.-K.; Hu, M.; Draghici, D.; et al. A Feedforward Mechanism Mediated by Mechanosensitive Ion Channel PIEZO1 and Tissue Mechanics Promotes Glioma Aggression. Neuron 2018, 100, 799–815.e7. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.; Georgiou, M. Dynamics of Adherens Junctions in Epithelial Establishment, Maintenance, and Remodeling. J. Cell Biol. 2011, 192, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Fortea, M.; Martín-Belmonte, F. Mechanosensitive Adhesion Complexes in Epithelial Architecture and Cancer Onset. Curr. Opin. Cell Biol. 2018, 50, 42–49. [Google Scholar] [CrossRef]
- Sunyer, R.; Conte, V.; Escribano, J.; Elosegui-Artola, A.; Labernadie, A.; Valon, L.; Navajas, D.; Garcia-Aznar, J.M.; Munoz, J.J.; Roca-Cusachs, P.; et al. Collective Cell Durotaxis Emerges from Long-Range Intercellular Force Transmission. Science 2016, 353, 1157–1161. [Google Scholar] [CrossRef]
- Yonemura, S.; Wada, Y.; Watanabe, T.; Nagafuchi, A.; Shibata, M. α-Catenin as a Tension Transducer That Induces Adherens Junction Development. Nat. Cell Biol. 2010, 12, 533–542. [Google Scholar] [CrossRef]
- Leanza, L.; Biasutto, L.; Managò, A.; Gulbins, E.; Zoratti, M.; Szabò, I. Intracellular Ion Channels and Cancer. Front. Physiol. 2013, 4, 227. [Google Scholar] [CrossRef]
- Hoffmann, E.K.; Lambert, I.H. Ion Channels and Transporters in the Development of Drug Resistance in Cancer Cells. Philos. Trans. R. Soc. B 2014, 369, 20130109. [Google Scholar] [CrossRef]
- Munaron, L. Systems Biology of Ion Channels and Transporters in Tumor Angiogenesis: An Omics View. Biochim. Biophys. Acta BBA-Biomembr. 2015, 1848, 2647–2656. [Google Scholar] [CrossRef]
- Yu, J.-L.; Liao, H.-Y. Piezo-Type Mechanosensitive Ion Channel Component 1 (Piezo1) in Human Cancer. Biomed. Pharmacother. 2021, 140, 111692. [Google Scholar] [CrossRef] [PubMed]
- Cawston, T.E.; Young, D.A. Proteinases Involved in Matrix Turnover during Cartilage and Bone Breakdown. Cell Tissue Res. 2010, 339, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Trappmann, B.; Baker, B.M.; Polacheck, W.J.; Choi, C.K.; Burdick, J.A.; Chen, C.S. Matrix Degradability Controls Multicellularity of 3D Cell Migration. Nat. Commun. 2017, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-Based Completely Biodegradable Polymer Materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Griffin, D.R.; Schlosser, J.L.; Lam, S.F.; Nguyen, T.H.; Maynard, H.D.; Kasko, A.M. Synthesis of Photodegradable Macromers for Conjugation and Release of Bioactive Molecules. Biomacromolecules 2013, 14, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Xie, W.; Achazi, K.; Cuellar-Camacho, J.L.; Melzig, M.F.; Chen, W.; Haag, R. Injectable Degradable PVA Microgels Prepared by Microfluidic Technology for Controlled Osteogenic Differentiation of Mesenchymal Stem Cells. Acta Biomater. 2018, 77, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Zhu, M.; Wei, K.; Bian, L. Cell-Mediated Degradation Regulates Human Mesenchymal Stem Cell Chondrogenesis and Hypertrophy in MMP-Sensitive Hyaluronic Acid Hydrogels. PLoS ONE 2014, 9, e99587. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.; Burdick, J.A. Patterning Network Structure to Spatially Control Cellular Remodeling and Stem Cell Fate within 3-Dimensional Hydrogels. Biomaterials 2010, 31, 8228–8234. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New Functions for the Matrix Metalloproteinases in Cancer Progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Prasadam, I.; Farnaghi, S.; Feng, J.Q.; Gu, W.; Perry, S.; Crawford, R.; Xiao, Y. Impact of Extracellular Matrix Derived from Osteoarthritis Subchondral Bone Osteoblasts on Osteocytes: Role of Integrinβ1 and Focal Adhesion Kinase Signaling Cues. Arthritis Res. Ther. 2013, 15, R150. [Google Scholar] [CrossRef]
- Lowin, T.; Straub, R.H. Integrins and Their Ligands in Rheumatoid Arthritis. Arthritis Res. Ther. 2011, 13, 244. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Boyle, D.L.; Aupperle, K.R.; Bennett, B.; Manning, A.M.; Firestein, G.S. Jun N-Terminal Kinase in Rheumatoid Arthritis. J. Pharmacol. Exp. Ther. 1999, 291, 124–130. [Google Scholar] [PubMed]
- Bornstein, P. Diversity of Function Is Inherent in Matricellular Proteins: An Appraisal of Thrombospondin 1. J. Cell Biol. 1995, 130, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Ngov, C.; Henley, N.; Boufaied, N.; Gerarduzzi, C. Characterization of Matricellular Protein Expression Signatures in Mechanistically Diverse Mouse Models of Kidney Injury. Sci. Rep. 2019, 9, 16736. [Google Scholar] [CrossRef] [PubMed]
- Nikoloudaki, G.; Snider, P.; Simmons, O.; Conway, S.J.; Hamilton, D.W. Periostin and Matrix Stiffness Combine to Regulate Myofibroblast Differentiation and Fibronectin Synthesis during Palatal Healing. Matrix Biol. 2020, 94, 31–56. [Google Scholar] [CrossRef] [PubMed]
- González-González, L.; Alonso, J. Periostin: A Matricellular Protein With Multiple Functions in Cancer Development and Progression. Front. Oncol. 2018, 8, 225. [Google Scholar] [CrossRef]
- Mainsiouw, L.; Ryan, M.E.; Hafizi, S.; Fleming, J.C. The Molecular and Clinical Role of Tensin 1/2/3 in Cancer. J. Cell. Mol. Med. 2023, 27, 1763–1774. [Google Scholar] [CrossRef]
- Adams, J.C.; Lawler, J. The Thrombospondins. Cold Spring Harb. Perspect. Biol. 2011, 3, a009712. [Google Scholar] [CrossRef]
- Leckband, D.E.; de Rooij, J. Cadherin Adhesion and Mechanotransduction. Annu. Rev. Cell Dev. Biol. 2014, 30, 291–315. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-Mediated Mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef]
- Kefauver, J.M.; Ward, A.B.; Patapoutian, A. Discoveries in Structure and Physiology of Mechanically Activated Ion Channels. Nature 2020, 587, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Stassen, O.M.J.A.; Ristori, T.; Sahlgren, C.M. Notch in Mechanotransduction—From Molecular Mechanosensitivity to Tissue Mechanostasis. J. Cell Sci. 2020, 133, jcs250738. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Pang, K.-L.; Rozbesky, D.; Nather, K.; Keen, A.; Lachowski, D.; Kong, Y.; Karia, D.; Ameismeier, M.; Huang, J.; et al. The Guidance Receptor Plexin D1 Is a Mechanosensor in Endothelial Cells. Nature 2020, 578, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Lee, S.-F.; Tabuchi, K.; Hao, Y.-H.; Yu, C.; LaPlant, Q.; Ball, H.; Dann, C.E.; Südhof, T.; Yu, G. Nicastrin Functions as a γ-Secretase-Substrate Receptor. Cell 2005, 122, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Kopan, R.; Ilagan, M.X.G. The Canonical Notch Signaling Pathway: Unfolding the Activation Mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Bray, S.J.; Gomez-Lamarca, M. Notch after Cleavage. Curr. Opin. Cell Biol. 2018, 51, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Irianto, J.; Discher, D.E. Mechanosensing by the Nucleus: From Pathways to Scaling Relationships. J. Cell Biol. 2017, 216, 305–315. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, Y.; Ju, L.A. Dynamic Bonds and Their Roles in Mechanosensing. Curr. Opin. Chem. Biol. 2019, 53, 88–97. [Google Scholar] [CrossRef]
- Ehrlicher, A.J.; Nakamura, F.; Hartwig, J.H.; Weitz, D.A.; Stossel, T.P. Mechanical Strain in Actin Networks Regulates FilGAP and Integrin Binding to Filamin A. Nature 2011, 478, 260–263. [Google Scholar] [CrossRef]
- Mack, J.J.; Mosqueiro, T.S.; Archer, B.J.; Jones, W.M.; Sunshine, H.; Faas, G.C.; Briot, A.; Aragón, R.L.; Su, T.; Romay, M.C.; et al. NOTCH1 Is a Mechanosensor in Adult Arteries. Nat. Commun. 2017, 8, 1620. [Google Scholar] [CrossRef]
- Gordon, W.R.; Zimmerman, B.; He, L.; Miles, L.J.; Huang, J.; Tiyanont, K.; McArthur, D.G.; Aster, J.C.; Perrimon, N.; Loparo, J.J.; et al. Mechanical Allostery: Evidence for a Force Requirement in the Proteolytic Activation of Notch. Dev. Cell 2015, 33, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Tamada, M.; Dubin-Thaler, B.J.; Cherniavskaya, O.; Sakai, R.; Tanaka, S.; Sheetz, M.P. Force Sensing by Mechanical Extension of the Src Family Kinase Substrate p130Cas. Cell 2006, 127, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Saotome, K.; Murthy, S.E.; Kefauver, J.M.; Whitwam, T.; Patapoutian, A.; Ward, A.B. Structure of the Mechanically Activated Ion Channel Piezo1. Nature 2018, 554, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Springer, T.A. Energy Landscape Differences among Integrins Establish the Framework for Understanding Activation. J. Cell Biol. 2018, 217, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; García, A.J.; Mould, A.P.; Humphries, M.J.; Zhu, C. Demonstration of Catch Bonds between an Integrin and Its Ligand. J. Cell Biol. 2009, 185, 1275–1284. [Google Scholar] [CrossRef]
- Huang, D.L.; Bax, N.A.; Buckley, C.D.; Weis, W.I.; Dunn, A.R. Vinculin Forms a Directionally Asymmetric Catch Bond with F-Actin. Science 2017, 357, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Dong, J.; Cruz, M.A.; Zhu, C. The N-Terminal Flanking Region of the A1 Domain Regulates the Force-Dependent Binding of von Willebrand Factor to Platelet Glycoprotein Ibα. J. Biol. Chem. 2013, 288, 32289–32301. [Google Scholar] [CrossRef]
- Yao, M.; Goult, B.T.; Chen, H.; Cong, P.; Sheetz, M.P.; Yan, J. Mechanical Activation of Vinculin Binding to Talin Locks Talin in an Unfolded Conformation. Sci. Rep. 2015, 4, 4610. [Google Scholar] [CrossRef]
- Gong, Z.; Szczesny, S.E.; Caliari, S.R.; Charrier, E.E.; Chaudhuri, O.; Cao, X.; Lin, Y.; Mauck, R.L.; Janmey, P.A.; Burdick, J.A.; et al. Matching Material and Cellular Timescales Maximizes Cell Spreading on Viscoelastic Substrates. Proc. Natl. Acad. Sci. USA 2018, 115, E2686–E2695. [Google Scholar] [CrossRef]
- Mège, R.M.; Ishiyama, N. Integration of Cadherin Adhesion and Cytoskeleton at Adherens Junctions. Cold Spring Harb. Perspect. Biol. 2017, 9, a028738. [Google Scholar] [CrossRef]
- Röper, J.-C.; Mitrossilis, D.; Stirnemann, G.; Waharte, F.; Brito, I.; Fernandez-Sanchez, M.-E.; Baaden, M.; Salamero, J.; Farge, E. The Major β-Catenin/E-Cadherin Junctional Binding Site Is a Primary Molecular Mechano-Transductor of Differentiation in Vivo. eLife 2018, 7, e33381. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.; Etienne-Manneville, S. Integrin Diversity Brings Specificity in Mechanotransduction. Biol. Cell 2018, 110, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Austen, K.; Ringer, P.; Mehlich, A.; Chrostek-Grashoff, A.; Kluger, C.; Klingner, C.; Sabass, B.; Zent, R.; Rief, M.; Grashoff, C. Extracellular Rigidity Sensing by Talin Isoform-Specific Mechanical Linkages. Nat. Cell Biol. 2015, 17, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Liu, W.; Zhou, H.; Zhang, T.; Wang, L.; Zhang, M.; Li, Y.; Shen, B.; Li, X.; Xiao, B. A Plug-and-Latch Mechanism for Gating the Mechanosensitive Piezo Channel. Neuron 2020, 106, 438–451.e6. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, K.; Himoto, T.; Mochizuki, Y.; Ikenouchi, J. α-Catenin Controls the Anisotropy of Force Distribution at Cell-Cell Junctions during Collective Cell Migration. Cell Rep. 2018, 23, 3447–3456. [Google Scholar] [CrossRef] [PubMed]
- Hart, K.C.; Tan, J.; Siemers, K.A.; Sim, J.Y.; Pruitt, B.L.; Nelson, W.J.; Gloerich, M. E-Cadherin and LGN Align Epithelial Cell Divisions with Tissue Tension Independently of Cell Shape. Proc. Natl. Acad. Sci. USA 2017, 114, E5845–E5853. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Pasapera, A.M.; Koretsky, A.P.; Waterman, C.M. Orientation-Specific Responses to Sustained Uniaxial Stretching in Focal Adhesion Growth and Turnover. Proc. Natl. Acad. Sci. USA 2013, 110, E2352–E2361. [Google Scholar] [CrossRef] [PubMed]
- Kale, G.R.; Yang, X.; Philippe, J.-M.; Mani, M.; Lenne, P.-F.; Lecuit, T. Distinct Contributions of Tensile and Shear Stress on E-Cadherin Levels during Morphogenesis. Nat. Commun. 2018, 9, 5021. [Google Scholar] [CrossRef]
- Jetta, D.; Shireen, T.; Hua, S.Z. Epithelial Cells Sense Local Stiffness via Piezo1 Mediated Cytoskeletal Reorganization. Front. Cell Dev. Biol. 2023, 11, 1198109. [Google Scholar] [CrossRef]
- Gudipaty, S.A.; Lindblom, J.; Loftus, P.D.; Redd, M.J.; Edes, K.; Davey, C.F.; Krishnegowda, V.; Rosenblatt, J. Mechanical Stretch Triggers Rapid Epithelial Cell Division through Piezo1. Nature 2017, 543, 118–121. [Google Scholar] [CrossRef]
- Gaub, B.M.; Müller, D.J. Mechanical Stimulation of Piezo1 Receptors Depends on Extracellular Matrix Proteins and Directionality of Force. Nano Lett. 2017, 17, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Brazin, K.N.; Kobayashi, E.; Mallis, R.J.; Reinherz, E.L.; Lang, M.J. Mechanosensing Drives Acuity of Aβ T-Cell Recognition. Proc. Natl. Acad. Sci. USA 2017, 114, E8204–E8213. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chennupati, R.; Kaur, H.; Iring, A.; Wettschureck, N.; Offermanns, S. Endothelial Cation Channel PIEZO1 Controls Blood Pressure by Mediating Flow-Induced ATP Release. J. Clin. Investig. 2016, 126, 4527–4536. [Google Scholar] [CrossRef] [PubMed]
- Xanthis, I.; Souilhol, C.; Serbanovic-Canic, J.; Roddie, H.; Kalli, A.C.; Fragiadaki, M.; Wong, R.; Shah, D.R.; Askari, J.A.; Canham, L.; et al. Β1 Integrin Is a Sensor of Blood Flow Direction. J. Cell Sci. 2019, 132, jcs.229542. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-P.; Pokutta, S.; Torres, M.; Swift, M.F.; Hanein, D.; Volkmann, N.; Weis, W.I. Structural Basis of αE-Catenin–F-Actin Catch Bond Behavior. eLife 2020, 9, e60878. [Google Scholar] [CrossRef]
- Kluger, C.; Braun, L.; Sedlak, S.M.; Pippig, D.A.; Bauer, M.S.; Miller, K.; Milles, L.F.; Gaub, H.E.; Vogel, V. Different Vinculin Binding Sites Use the Same Mechanism to Regulate Directional Force Transduction. Biophys. J. 2020, 118, 1344–1356. [Google Scholar] [CrossRef]
- Kumar, A.; Shutova, M.S.; Tanaka, K.; Iwamoto, D.V.; Calderwood, D.A.; Svitkina, T.M.; Schwartz, M.A. Filamin A Mediates Isotropic Distribution of Applied Force across the Actin Network. J. Cell Biol. 2019, 218, 2481–2491. [Google Scholar] [CrossRef]
- Wyatt, T.; Baum, B.; Charras, G. A Question of Time: Tissue Adaptation to Mechanical Forces. Curr. Opin. Cell Biol. 2016, 38, 68–73. [Google Scholar] [CrossRef]
- Price, C.C.; Mathur, J.; Boerckel, J.D.; Pathak, A.; Shenoy, V.B. Dynamic Self-Reinforcement of Gene Expression Determines Acquisition of Cellular Mechanical Memory. Biophys. J. 2021, 120, 5074–5089. [Google Scholar] [CrossRef]
- Plotnikov, S.V.; Pasapera, A.M.; Sabass, B.; Waterman, C.M. Force Fluctuations within Focal Adhesions Mediate ECM-Rigidity Sensing to Guide Directed Cell Migration. Cell 2012, 151, 1513–1527. [Google Scholar] [CrossRef]
- Solis, A.G.; Bielecki, P.; Steach, H.R.; Sharma, L.; Harman, C.C.D.; Yun, S.; de Zoete, M.R.; Warnock, J.N.; To, S.D.F.; York, A.G.; et al. Mechanosensation of Cyclical Force by PIEZO1 Is Essential for Innate Immunity. Nature 2019, 573, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Hameed, F.M.; Yang, B.; Lee, K.; Pan, C.Q.; Park, S.; Sheetz, M. Cyclic Stretching of Soft Substrates Induces Spreading and Growth. Nat. Commun. 2015, 6, 6333. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Christensen, L.P.; Tomanek, R.J. Differential Effects of Cyclic Stretch and Static Stretch on Angiogenic Responses of Microvascular Endothelial Cells. FASEB J. 2007, 21, A138. [Google Scholar] [CrossRef]
- Liu, B.; Qu, M.-J.; Qin, K.-R.; Li, H.; Li, Z.-K.; Shen, B.-R.; Jiang, Z.-L. Role of Cyclic Strain Frequency in Regulating the Alignment of Vascular Smooth Muscle Cells In Vitro. Biophys. J. 2008, 94, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Li, Z.; Parks, W.M.; Dumbauld, D.W.; García, A.J.; Mould, A.P.; Humphries, M.J.; Zhu, C. Cyclic Mechanical Reinforcement of Integrin–Ligand Interactions. Mol. Cell 2013, 49, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.H.; Cui, A.F.; McDonald, M.F.; Grandl, J. Transduction of Repetitive Mechanical Stimuli by Piezo1 and Piezo2 Ion Channels. Cell Rep. 2017, 19, 2572–2585. [Google Scholar] [CrossRef]
- Bennett, M.; Cantini, M.; Reboud, J.; Cooper, J.M.; Roca-Cusachs, P.; Salmeron-Sanchez, M. Molecular Clutch Drives Cell Response to Surface Viscosity. Proc. Natl. Acad. Sci. USA 2018, 115, 1192–1197. [Google Scholar] [CrossRef]
- Esfahani, A.M.; Rosenbohm, J.; Safa, B.T.; Lavrik, N.V.; Minnick, G.; Zhou, Q.; Kong, F.; Jin, X.; Kim, E.; Liu, Y.; et al. Characterization of the Strain-Rate–Dependent Mechanical Response of Single Cell–Cell Junctions. Proc. Natl. Acad. Sci. USA 2021, 118, e2019347118. [Google Scholar] [CrossRef]
- Le, S.; Yu, M.; Yan, J. Direct Single-Molecule Quantification Reveals Unexpectedly High Mechanical Stability of Vinculin—Talin/α-Catenin Linkages. Sci. Adv. 2019, 5, eaav2720. [Google Scholar] [CrossRef]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef]
- Benham-Pyle, B.W.; Pruitt, B.L.; Nelson, W.J. Mechanical Strain Induces E-Cadherin–Dependent Yap1 and β-Catenin Activation to Drive Cell Cycle Entry. Science 2015, 348, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Rauskolb, C.; Sun, S.; Sun, G.; Pan, Y.; Irvine, K.D. Cytoskeletal Tension Inhibits Hippo Signaling through an Ajuba-Warts Complex. Cell 2014, 158, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Totaro, A.; Castellan, M.; Battilana, G.; Zanconato, F.; Azzolin, L.; Giulitti, S.; Cordenonsi, M.; Piccolo, S. YAP/TAZ Link Cell Mechanics to Notch Signalling to Control Epidermal Stem Cell Fate. Nat. Commun. 2017, 8, 15206. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.S.; Lopez, J.I.; McGhee, E.J.; Croft, D.R.; Strachan, D.; Timpson, P.; Munro, J.; Schröder, E.; Zhou, J.; Brunton, V.G.; et al. Actomyosin-Mediated Cellular Tension Drives Increased Tissue Stiffness and β-Catenin Activation to Induce Epidermal Hyperplasia and Tumor Growth. Cancer Cell 2011, 19, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Zuidema, A.; Wang, W.; Sonnenberg, A. Crosstalk between Cell Adhesion Complexes in Regulation of Mechanotransduction. BioEssays 2020, 42, 2000119. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Schlue, K.T.; Kniffin, A.F.; Mayer, C.R.; Duke, A.A.; Narayanan, V.; Arsenovic, P.T.; Bathula, K.; Danielsson, B.E.; Dumbali, S.P.; et al. Spatial Proliferation of Epithelial Cells Is Regulated by E-Cadherin Force. Biophys. J. 2018, 115, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, L.; Doostmohammadi, A.; Saw, T.B.; Narayana, G.H.N.S.; Mueller, R.; Dang, T.; Thomas, M.; Gupta, S.; Sonam, S.; Yap, A.S.; et al. Investigating the Nature of Active Forces in Tissues Reveals How Contractile Cells Can Form Extensile Monolayers. Nat. Mater. 2021, 20, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Maruthamuthu, V.; Sabass, B.; Schwarz, U.S.; Gardel, M.L. Cell-ECM Traction Force Modulates Endogenous Tension at Cell-Cell Contacts. Proc. Natl. Acad. Sci. USA 2011, 108, 4708–4713. [Google Scholar] [CrossRef]
- Muhamed, I.; Wu, J.; Sehgal, P.; Kong, X.; Tajik, A.; Wang, N.; Leckband, D.E. E-Cadherin-Mediated Force Transduction Signals Regulate Global Cell Mechanics. J. Cell Sci. 2016, 129, 1843–1854. [Google Scholar] [CrossRef]
- Mertz, A.F.; Che, Y.; Banerjee, S.; Goldstein, J.M.; Rosowski, K.A.; Revilla, S.F.; Niessen, C.M.; Marchetti, M.C.; Dufresne, E.R.; Horsley, V. Cadherin-Based Intercellular Adhesions Organize Epithelial Cell–Matrix Traction Forces. Proc. Natl. Acad. Sci. USA 2013, 110, 842–847. [Google Scholar] [CrossRef]
- Ellefsen, K.L.; Holt, J.R.; Chang, A.C.; Nourse, J.L.; Arulmoli, J.; Mekhdjian, A.H.; Abuwarda, H.; Tombola, F.; Flanagan, L.A.; Dunn, A.R.; et al. Myosin-II Mediated Traction Forces Evoke Localized Piezo1-Dependent Ca2+ Flickers. Commun. Biol. 2019, 2, 298. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Pastor, C.; Rubio-Moscardo, F.; Vogel-González, M.; Serra, S.A.; Afthinos, A.; Mrkonjic, S.; Destaing, O.; Abenza, J.F.; Fernández-Fernández, J.M.; Trepat, X.; et al. Piezo2 Channel Regulates RhoA and Actin Cytoskeleton to Promote Cell Mechanobiological Responses. Proc. Natl. Acad. Sci. USA 2018, 115, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Polacheck, W.J.; Kutys, M.L.; Yang, J.; Eyckmans, J.; Wu, Y.; Vasavada, H.; Hirschi, K.K.; Chen, C.S. A Non-Canonical Notch Complex Regulates Adherens Junctions and Vascular Barrier Function. Nature 2017, 552, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Caolo, V.; Debant, M.; Endesh, N.; Futers, T.S.; Lichtenstein, L.; Bartoli, F.; Parsonage, G.; Jones, E.A.; Beech, D.J. Shear Stress Activates ADAM10 Sheddase to Regulate Notch1 via the Piezo1 Force Sensor in Endothelial Cells. eLife 2020, 9, e50684. [Google Scholar] [CrossRef] [PubMed]
- Tzima, E.; Irani-Tehrani, M.; Kiosses, W.B.; Dejana, E.; Schultz, D.A.; Engelhardt, B.; Cao, G.; DeLisser, H.; Schwartz, M.A. A Mechanosensory Complex That Mediates the Endothelial Cell Response to Fluid Shear Stress. Nature 2005, 437, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zuidema, A.; Te Molder, L.; Nahidiazar, L.; Hoekman, L.; Schmidt, T.; Coppola, S.; Sonnenberg, A. Hemidesmosomes Modulate Force Generation via Focal Adhesions. J. Cell Biol. 2020, 219, e201904137. [Google Scholar] [CrossRef] [PubMed]
- Sarker, F.A.; Prior, V.G.; Bax, S.; O’Neill, G.M. Forcing a Growth Factor Response—Tissue-Stiffness Modulation of Integrin Signaling and Crosstalk with Growth Factor Receptors. J. Cell Sci. 2020, 133, jcs242461. [Google Scholar] [CrossRef]
- Hinz, B. Tissue Stiffness, Latent TGF-Β1 Activation, and Mechanical Signal Transduction: Implications for the Pathogenesis and Treatment of Fibrosis. Curr. Rheumatol. Rep. 2009, 11, 120–126. [Google Scholar] [CrossRef]
- Rao, T.C.; Pui-Yan Ma, V.; Blanchard, A.; Urner, T.M.; Grandhi, S.; Salaita, K.; Mattheyses, A.L. EGFR Activation Attenuates the Mechanical Threshold for Integrin Tension and Focal Adhesion Formation. J. Cell Sci. 2020, 133, jcs.238840. [Google Scholar] [CrossRef]
- Hino, N.; Rossetti, L.; Marín-Llauradó, A.; Aoki, K.; Trepat, X.; Matsuda, M.; Hirashima, T. ERK-Mediated Mechanochemical Waves Direct Collective Cell Polarization. Dev. Cell 2020, 53, 646–660.e8. [Google Scholar] [CrossRef]
- Shi, Q.; Ghosh, R.P.; Engelke, H.; Rycroft, C.H.; Cassereau, L.; Sethian, J.A.; Weaver, V.M.; Liphardt, J.T. Rapid Disorganization of Mechanically Interacting Systems of Mammary Acini. Proc. Natl. Acad. Sci. USA 2014, 111, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Steinwachs, J.; Metzner, C.; Skodzek, K.; Lang, N.; Thievessen, I.; Mark, C.; Münster, S.; Aifantis, K.E.; Fabry, B. Three-Dimensional Force Microscopy of Cells in Biopolymer Networks. Nat. Methods 2016, 13, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.D.; Yamada, K.M. Mechanosensing via Cell-Matrix Adhesions in 3D Microenvironments. Exp. Cell Res. 2016, 343, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Pakshir, P.; Alizadehgiashi, M.; Wong, B.; Coelho, N.M.; Chen, X.; Gong, Z.; Shenoy, V.B.; McCulloch, C.A.; Hinz, B. Dynamic Fibroblast Contractions Attract Remote Macrophages in Fibrillar Collagen Matrix. Nat. Commun. 2019, 10, 1850. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, H.; Zhao, H.; Wu, Z.; Long, Y.; Zhang, J.; Yan, X.; You, Z.; Zhou, L.; Xia, T.; et al. Matrix-Transmitted Paratensile Signaling Enables Myofibroblast—Fibroblast Cross Talk in Fibrosis Expansion. Proc. Natl. Acad. Sci. USA 2020, 117, 10832–10838. [Google Scholar] [CrossRef] [PubMed]
- Davidson, C.D.; DePalma, S.J.; Wang, W.Y.; Kamen, J.L.; Jayco, D.K.P.; Baker, B.M. Mechanical Intercellular Communication via Matrix-Borne Cell Force Transmission during Vascular Network Formation. Adv. Sci. 2023. [Google Scholar] [CrossRef]
- Guo, C.-L.; Ouyang, M.; Yu, J.-Y.; Maslov, J.; Price, A.; Shen, C.-Y. Long-Range Mechanical Force Enables Self-Assembly of Epithelial Tubular Patterns. Proc. Natl. Acad. Sci. USA 2012, 109, 5576–5582. [Google Scholar] [CrossRef]
- Hughes, A.J.; Miyazaki, H.; Coyle, M.C.; Zhang, J.; Laurie, M.T.; Chu, D.; Vavrušová, Z.; Schneider, R.A.; Klein, O.D.; Gartner, Z.J. Engineered Tissue Folding by Mechanical Compaction of the Mesenchyme. Dev. Cell 2018, 44, 165–178.e6. [Google Scholar] [CrossRef]
- Kim, J.; Feng, J.; Jones, C.A.R.; Mao, X.; Sander, L.M.; Levine, H.; Sun, B. Stress-Induced Plasticity of Dynamic Collagen Networks. Nat. Commun. 2017, 8, 842. [Google Scholar] [CrossRef]
- Wells, R.G. Tissue Mechanics and Fibrosis. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2013, 1832, 884–890. [Google Scholar] [CrossRef]
- Sohrabi Kashani, A.; Packirisamy, M. Cancer-Nano-Interaction: From Cellular Uptake to Mechanobiological Responses. Int. J. Mol. Sci. 2021, 22, 9587. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Trappmann, B.; Wang, W.Y.; Sakar, M.S.; Kim, I.L.; Shenoy, V.B.; Burdick, J.A.; Chen, C.S. Cell-Mediated Fibre Recruitment Drives Extracellular Matrix Mechanosensing in Engineered Fibrillar Microenvironments. Nat. Mater. 2015, 14, 1262–1268. [Google Scholar] [CrossRef]
- Liu, X.; Liu, R.; Cao, B.; Ye, K.; Li, S.; Gu, Y.; Pan, Z.; Ding, J. Subcellular Cell Geometry on Micropillars Regulates Stem Cell Differentiation. Biomaterials 2016, 111, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Tusamda Wakhloo, N.; Anders, S.; Badique, F.; Eichhorn, M.; Brigaud, I.; Petithory, T.; Vassaux, M.; Milan, J.-L.; Freund, J.-N.; Rühe, J.; et al. Actomyosin, Vimentin and LINC Complex Pull on Osteosarcoma Nuclei to Deform on Micropillar Topography. Biomaterials 2020, 234, 119746. [Google Scholar] [CrossRef] [PubMed]
- Carthew, J.; Abdelmaksoud, H.H.; Hodgson-Garms, M.; Aslanoglou, S.; Ghavamian, S.; Elnathan, R.; Spatz, J.P.; Brugger, J.; Thissen, H.; Voelcker, N.H.; et al. Precision Surface Microtopography Regulates Cell Fate via Changes to Actomyosin Contractility and Nuclear Architecture. Adv. Sci. 2021, 8, 2003186. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Q.; Ronaldson, K.; Park, M.; Taylor, G.; Zhang, Y.; Gimble, J.M.; Vunjak-Novakovic, G. Micropatterned Mammalian Cells Exhibit Phenotype-Specific Left-Right Asymmetry. Proc. Natl. Acad. Sci. USA 2011, 108, 12295–12300. [Google Scholar] [CrossRef] [PubMed]
- Beil, M.; Micoulet, A.; von Wichert, G.; Paschke, S.; Walther, P.; Omary, M.B.; Van Veldhoven, P.P.; Gern, U.; Wolff-Hieber, E.; Eggermann, J.; et al. Sphingosylphosphorylcholine Regulates Keratin Network Architecture and Visco-Elastic Properties of Human Cancer Cells. Nat. Cell Biol. 2003, 5, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, A.S.; Liu, F.D.; Durham, J.T.; Jagielska, A.; Mahmoodian, R.; Van Vliet, K.J.; Herman, I.M. Static Mechanical Strain Induces Capillary Endothelial Cell Cycle Re-Entry and Sprouting. Phys. Biol. 2016, 13, 046006. [Google Scholar] [CrossRef]
- Bell-McGuinn, K.M.; Matthews, C.M.; Ho, S.N.; Barve, M.; Gilbert, L.; Penson, R.T.; Lengyel, E.; Palaparthy, R.; Gilder, K.; Vassos, A.; et al. A Phase II, Single-Arm Study of the Anti-A5β1 Integrin Antibody Volociximab as Monotherapy in Patients with Platinum-Resistant Advanced Epithelial Ovarian or Primary Peritoneal Cancer. Gynecol. Oncol. 2011, 121, 273–279. [Google Scholar] [CrossRef]
- Haddad, T.; Qin, R.; Lupu, R.; Satele, D.; Eadens, M.; Goetz, M.P.; Erlichman, C.; Molina, J. A Phase I Study of Cilengitide and Paclitaxel in Patients with Advanced Solid Tumors. Cancer Chemother. Pharmacol. 2017, 79, 1221–1227. [Google Scholar] [CrossRef]
- McNeel, D.G.; Eickhoff, J.; Lee, F.T.; King, D.M.; Alberti, D.; Thomas, J.P.; Friedl, A.; Kolesar, J.; Marnocha, R.; Volkman, J.; et al. Phase I Trial of a Monoclonal Antibody Specific for Avβ3 Integrin (MEDI-522) in Patients with Advanced Malignancies, Including an Assessment of Effect on Tumor Perfusion. Clin. Cancer Res. 2005, 11, 7851–7860. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Merritt, R.; Fu, L.; Pan, Z. Targeting Calcium Signaling in Cancer Therapy. Acta Pharm. Sin. B 2017, 7, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Kischel, P.; Girault, A.; Rodat-Despoix, L.; Chamlali, M.; Radoslavova, S.; Abou Daya, H.; Lefebvre, T.; Foulon, A.; Rybarczyk, P.; Hague, F.; et al. Ion Channels: New Actors Playing in Chemotherapeutic Resistance. Cancers 2019, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.W.; Powell, D.P.; Blake, S.D.; Hoffman, J.L.; Hopkins, M.M.; Feng, X. Enhanced Cytotoxicity in Triple-Negative and Estrogen Receptor-Positive Breast Adenocarcinoma Cells Due to Inhibition of the Transient Receptor Potential Melastatin-2 Channel. Oncol. Rep. 2015, 34, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, X.; Li, H.; Chen, Z.; Yao, X.; Jin, J.; Ma, X. TRPC5-Induced Autophagy Promotes Drug Resistance in Breast Carcinoma via CaMKKβ/AMPKα/mTOR Pathway. Sci. Rep. 2017, 7, 3158. [Google Scholar] [CrossRef] [PubMed]
- Gautier, M.; Dhennin-Duthille, I.; Ay, A.S.; Rybarczyk, P.; Korichneva, I.; Ouadid-Ahidouch, H. New Insights into Pharmacological Tools to TR (i) P Cancer Up. Br. J. Pharmacol. 2014, 171, 2582–2592. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.A.; Hwang, M.K.; Jang, Y.; Lee, E.J.; Kim, J.-E.; Oh, M.H.; Shin, D.J.; Lim, S.; Ji, G.O.; Oh, U.; et al. 20-O-β-d-Glucopyranosyl-20(S)-Protopanaxadiol, a Metabolite of Ginseng, Inhibits Colon Cancer Growth by Targeting TRPC Channel-Mediated Calcium Influx. J. Nutr. Biochem. 2013, 24, 1096–1104. [Google Scholar] [CrossRef]
- Cai, R.; Ding, X.; Zhou, K.; Shi, Y.; Ge, R.; Ren, G.; Jin, Y.; Wang, Y. Blockade of TRPC6 Channels Induced G2/M Phase Arrest and Suppressed Growth in Human Gastric Cancer Cells. Int. J. Cancer 2009, 125, 2281–2287. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, S.H.; Yang, E.K. RNA Interference Mediated Suppression of TRPV6 Inhibits the Progression of Prostate Cancer In Vitro by Modulating Cathepsin B and MMP9 Expression. Investig. Clin. Urol. 2021, 62, 447. [Google Scholar] [CrossRef]
- Song, H.; Dong, M.; Zhou, J.; Sheng, W.; Li, X.; Gao, W. Expression and Prognostic Significance of TRPV6 in the Development and Progression of Pancreatic Cancer. Oncol. Rep. 2018, 48, 180. [Google Scholar] [CrossRef]
- Bowman, C.L.; Gottlieb, P.A.; Suchyna, T.M.; Murphy, Y.K.; Sachs, F. Mechanosensitive Ion Channels and the Peptide Inhibitor GsMTx-4: History, Properties, Mechanisms and Pharmacology. Toxicon 2007, 49, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Cisowski, J.; O’Callaghan, K.; Kuliopulos, A.; Yang, J.; Nguyen, N.; Deng, Q.; Yang, E.; Fogel, M.; Tressel, S.; Foley, C.; et al. Targeting Protease-Activated Receptor-1 with Cell-Penetrating Pepducins in Lung Cancer. Am. J. Pathol. 2011, 179, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Chen, S.; Zhang, Q.; Xiao, T.; Qin, Y.; Gu, J.; Sun, B.; Liu, Y.; Jing, X.; Hu, X.; et al. Doxycycline Directly Targets PAR1 to Suppress Tumor Progression. Oncotarget 2017, 8, 16829–16842. [Google Scholar] [CrossRef] [PubMed]
- Hitchman, T.D.; Bayshtok, G.; Ceraudo, E.; Moore, A.R.; Lee, C.; Jia, R.; Wang, N.; Pachai, M.R.; Shoushtari, A.N.; Francis, J.H.; et al. Combined Inhibition of Gαq and MEK Enhances Therapeutic Efficacy in Uveal Melanoma. Clin. Cancer Res. 2021, 27, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Tsou, L.K.; Yeh, S.-H.; Ueng, S.-H.; Chang, C.-P.; Song, J.-S.; Wu, M.-H.; Chang, H.-F.; Chen, S.-R.; Shih, C.; Chen, C.-T.; et al. Comparative Study between Deep Learning and QSAR Classifications for TNBC Inhibitors and Novel GPCR Agonist Discovery. Sci. Rep. 2020, 10, 16771. [Google Scholar] [CrossRef] [PubMed]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR Drug Discovery: New Agents, Targets and Indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Skandalis, S.S.; Karalis, T.T.; Chatzopoulos, A.; Karamanos, N.K. Hyaluronan-CD44 Axis Orchestrates Cancer Stem Cell Functions. Cell. Signal. 2019, 63, 109377. [Google Scholar] [CrossRef]
- Lompardía, S.L.; Díaz, M.; Papademetrio, D.L.; Pibuel, M.; Álvarez, É.; Hajos, S.E. 4-Methylumbelliferone and Imatinib Combination Enhances Senescence Induction in Chronic Myeloid Leukemia Cell Lines. Investig. New Drugs 2017, 35, 1–10. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Tempero, M.A.; Sigal, D.; Oh, D.-Y.; Fazio, N.; Macarulla, T.; Hitre, E.; Hammel, P.; Hendifar, A.E.; Bates, S.E.; et al. Randomized Phase III Trial of Pegvorhyaluronidase Alfa With Nab-Paclitaxel Plus Gemcitabine for Patients With Hyaluronan-High Metastatic Pancreatic Adenocarcinoma. J. Clin. Oncol. 2020, 38, 3185–3194. [Google Scholar] [CrossRef]
- Siegel, R.C. Biosynthesis of Collagen Crosslinks: Increased Activity of Purified Lysyl Oxidase with Reconstituted Collagen Fibrils. Proc. Natl. Acad. Sci. USA 1974, 71, 4826–4830. [Google Scholar] [CrossRef]
- Rossow, L.; Veitl, S.; Vorlová, S.; Wax, J.K.; Kuhn, A.E.; Maltzahn, V.; Upcin, B.; Karl, F.; Hoffmann, H.; Gätzner, S.; et al. LOX-Catalyzed Collagen Stabilization Is a Proximal Cause for Intrinsic Resistance to Chemotherapy. Oncogene 2018, 37, 4921–4940. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Wainberg, Z.A.; Hecht, J.R.; Vyushkov, D.; Dong, H.; Bendell, J.; Kudrik, F. A Phase II Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab or Placebo in Combination with Gemcitabine for the First-Line Treatment of Pancreatic Adenocarcinoma. Oncologist 2017, 22, 241-e15. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, G.; Destrempes, F.; Yu, F.; Tang, A. Quantitative Ultrasound Imaging of Soft Biological Tissues: A Primer for Radiologists and Medical Physicists. Insights Imaging 2021, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- Brito, B.; Price, T.W.; Gallo, J.; Bañobre-López, M.; Stasiuk, G.J. Smart Magnetic Resonance Imaging-Based Theranostics for Cancer. Theranostics 2021, 11, 8706–8737. [Google Scholar] [CrossRef]

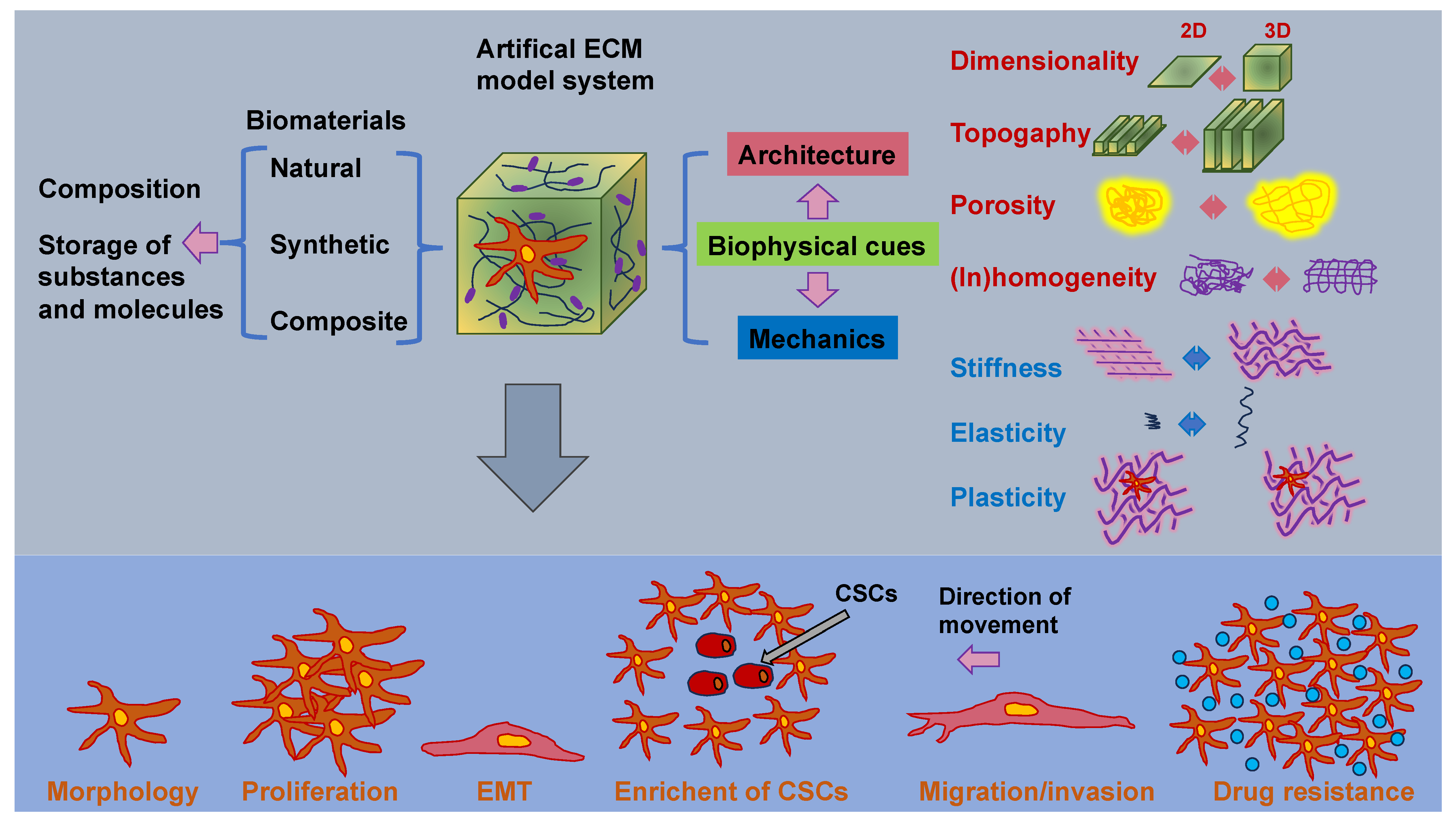
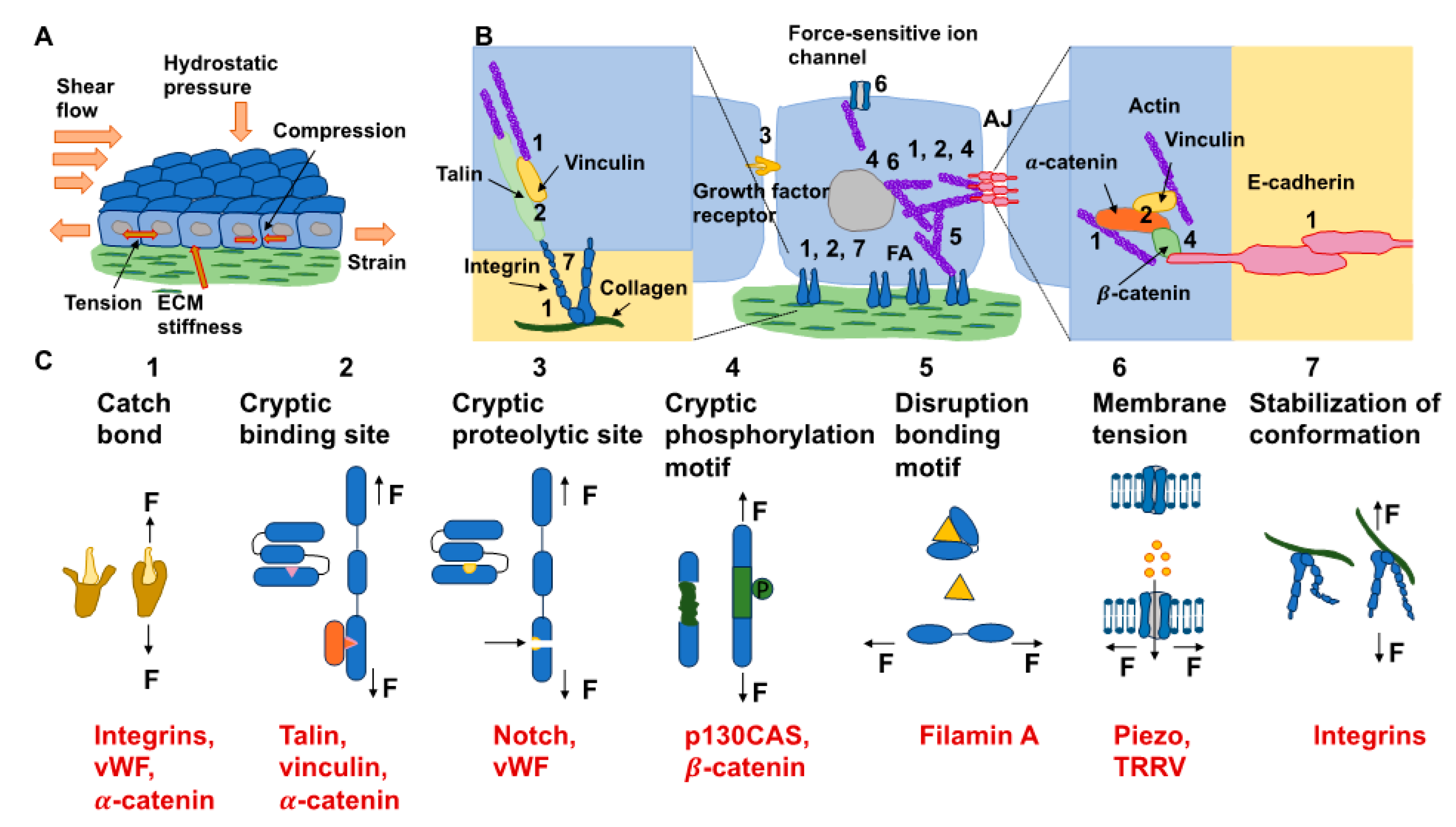

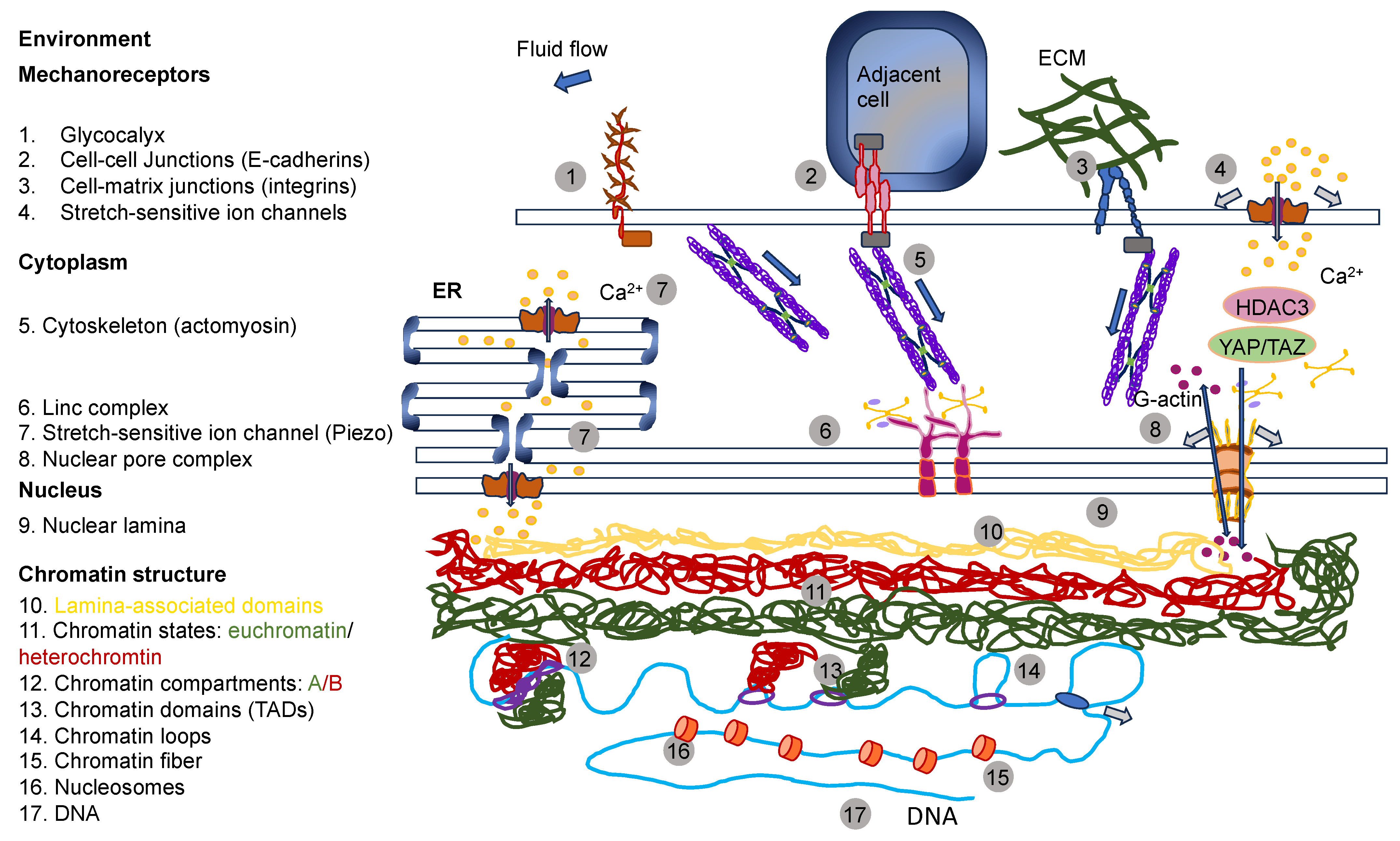

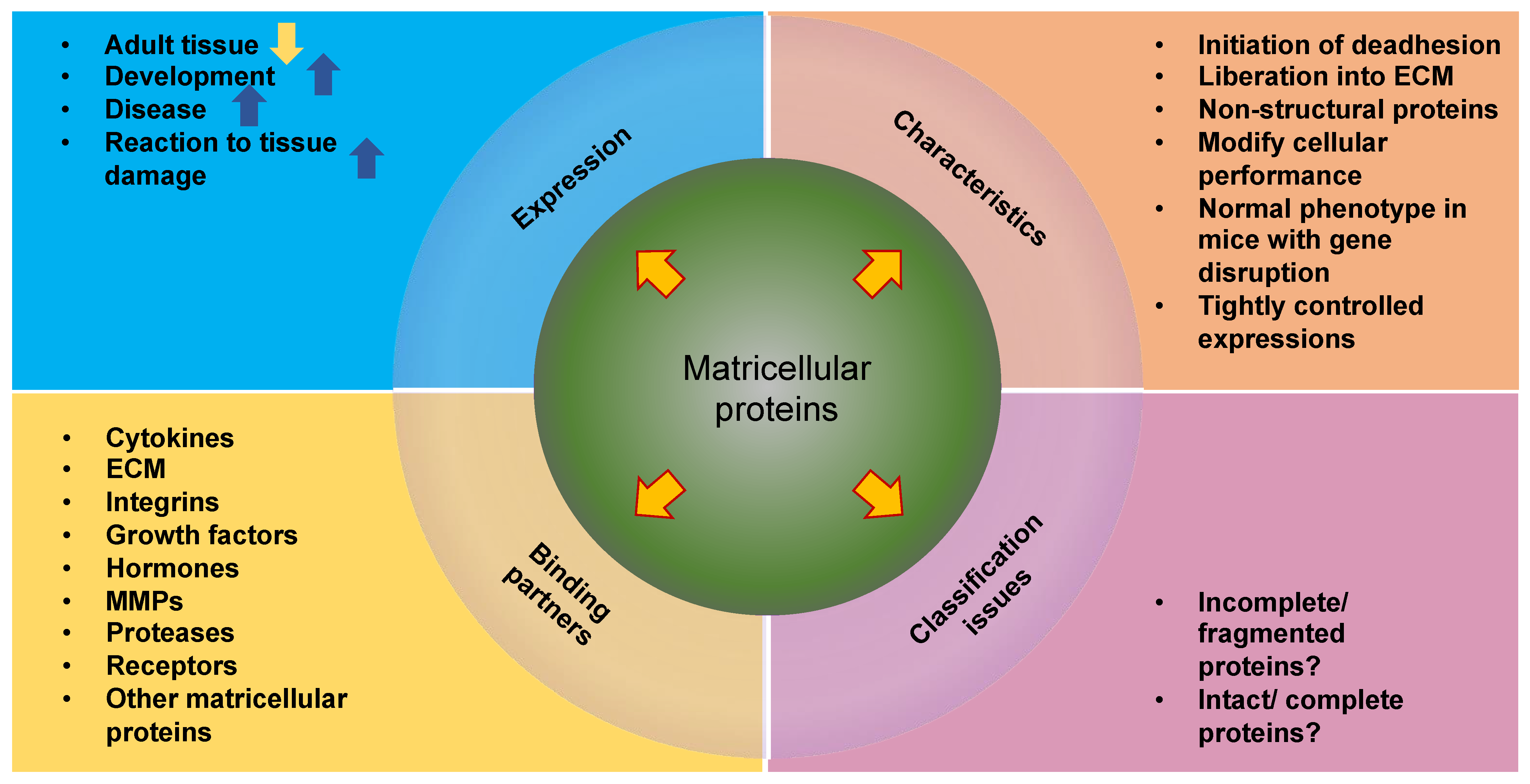
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mierke, C.T. Extracellular Matrix Cues Regulate Mechanosensing and Mechanotransduction of Cancer Cells. Cells 2024, 13, 96. https://doi.org/10.3390/cells13010096
Mierke CT. Extracellular Matrix Cues Regulate Mechanosensing and Mechanotransduction of Cancer Cells. Cells. 2024; 13(1):96. https://doi.org/10.3390/cells13010096
Chicago/Turabian StyleMierke, Claudia Tanja. 2024. "Extracellular Matrix Cues Regulate Mechanosensing and Mechanotransduction of Cancer Cells" Cells 13, no. 1: 96. https://doi.org/10.3390/cells13010096
APA StyleMierke, C. T. (2024). Extracellular Matrix Cues Regulate Mechanosensing and Mechanotransduction of Cancer Cells. Cells, 13(1), 96. https://doi.org/10.3390/cells13010096






