Exposure to Environmental Toxins: Potential Implications for Stroke Risk via the Gut– and Lung–Brain Axis
Abstract
:1. Introduction
2. Environmental Toxins and Stroke Risk
2.1. Persistent Organic Pollutants
2.1.1. Polycyclic Aromatic Hydrocarbons
2.1.2. Polychlorinated Biphenyls
2.2. Per- and Polyfluorinated Substances
2.3. Air Pollutants
2.4. Nano- and Microplastics
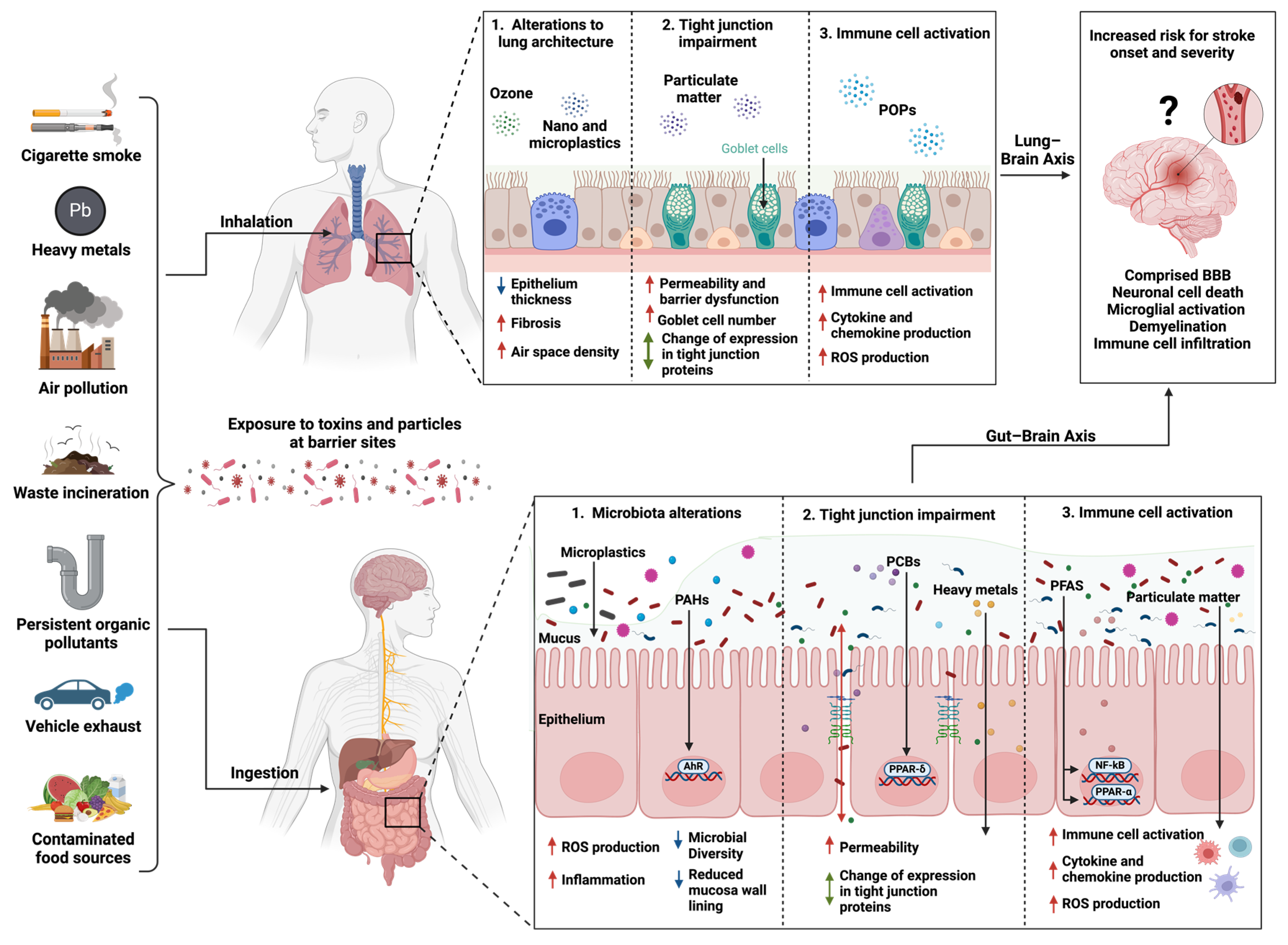
3. Exposure to Environmental Toxins at Barrier Sites
3.1. Lung–Brain Axis and Environmental Toxins
3.1.1. Environmental Toxins and Immune Response in the Lungs
3.1.2. Effect of Environmental Toxins on the Lung Microbiome
3.2. Gut–Brain Axis and Environmental Toxins
Exposure to Environmental Toxins and Stroke Risk
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Iwasaki, M.; Itoh, H.; Sawada, N.; Tsugane, S. Exposure to Environmental Chemicals and Cancer Risk: Epidemiological Evidence from Japanese Studies. Genes Environ. 2023, 45, 10. [Google Scholar] [CrossRef]
- Béjot, Y.; Reis, J.; Giroud, M.; Feigin, V. A Review of Epidemiological Research on Stroke and Dementia and Exposure to Air Pollution. Int. J. Stroke 2018, 13, 687–695. [Google Scholar] [CrossRef]
- Kurt, O.K.; Zhang, J.; Pinkerton, K.E. Pulmonary Health Effects of Air Pollution. Curr. Opin. Pulm. Med. 2016, 22, 138–143. [Google Scholar] [CrossRef]
- Yang, A.-M.; Lo, K.; Zheng, T.-Z.; Yang, J.-L.; Bai, Y.-N.; Feng, Y.-Q.; Cheng, N.; Liu, S.-M. Environmental Heavy Metals and Cardiovascular Diseases: Status and Future Direction. Chronic Dis. Transl. Med. 2020, 6, 251–259. [Google Scholar] [CrossRef]
- Steinmetz, J.D.; Seeher, K.M.; Schiess, N.; Nichols, E.; Cao, B.; Servili, C.; Cavallera, V.; Cousin, E.; Hagins, H.; Moberg, M.E.; et al. Global, Regional, and National Burden of Disorders Affecting the Nervous System, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 4, 344–381. [Google Scholar] [CrossRef]
- Krishnamurthi, R.V.; Moran, A.E.; Feigin, V.L.; Barker-Collo, S.; Norrving, B.; Mensah, G.A.; Taylor, S.; Naghavi, M.; Forouzanfar, M.H.; Nguyen, G.; et al. Stroke Prevalence, Mortality and Disability-Adjusted Life Years in Adults Aged 20–64 Years in 1990-2013: Data from the Global Burden of Disease 2013 Study. Neuroepidemiology 2015, 45, 190–202. [Google Scholar] [CrossRef]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2021, 17, 18–29. [Google Scholar] [CrossRef]
- Feigin, V.L.; Roth, G.A.; Naghavi, M.; Parmar, P.; Krishnamurthi, R.; Chugh, S.; Mensah, G.A.; Norrving, B.; Shiue, I.; Ng, M.; et al. Global Burden of Stroke and Risk Factors in 188 Countries, during 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016, 15, 913–924. [Google Scholar] [CrossRef]
- Genuis, S.J.; Kelln, K.L. Toxicant Exposure and Bioaccumulation: A Common and Potentially Reversible Cause of Cognitive Dysfunction and Dementia. Behav. Neurol. 2015, 2015, 620143. [Google Scholar] [CrossRef]
- Cresto, N.; Forner-Piquer, I.; Baig, A.; Chatterjee, M.; Perroy, J.; Goracci, J.; Marchi, N. Pesticides at Brain Borders: Impact on the Blood-Brain Barrier, Neuroinflammation, and Neurological Risk Trajectories. Chemosphere 2023, 324, 138251. [Google Scholar] [CrossRef]
- Ribière, C.; Peyret, P.; Parisot, N.; Darcha, C.; Déchelotte, P.J.; Barnich, N.; Peyretaillade, E.; Boucher, D. Oral Exposure to Environmental Pollutant Benzo[a]Pyrene Impacts the Intestinal Epithelium and Induces Gut Microbial Shifts in Murine Model. Sci. Rep-UK 2016, 6, 31027. [Google Scholar] [CrossRef]
- Yang, Q.; Dai, H.; Cheng, Y.; Wang, B.; Xu, J.; Zhang, Y.; Chen, Y.; Xu, F.; Ma, Q.; Lin, F.; et al. Oral Feeding of Nanoplastics Affects Brain Function of Mice by Inducing Macrophage IL-1 Signal in the Intestine. Cell Rep. 2023, 42, 112346. [Google Scholar] [CrossRef]
- Michaudel, C.; Fauconnier, L.; Julé, Y.; Ryffel, B. Functional and Morphological Differences of the Lung upon Acute and Chronic Ozone Exposure in Mice. Sci. Rep. 2018, 8, 10611. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, R.; Wang, M.; Bai, R.; Cui, X.; Liu, Y.; Wu, C.; Chen, C. Perturbation of Gut Microbiota Plays an Important Role in Micro/Nanoplastics-Induced Gut Barrier Dysfunction. Nanoscale 2021, 13, 8806–8816. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and Health: A Progress Update. Lancet Planet. Health 2022, 6, e535–e547. [Google Scholar] [CrossRef]
- Thompson, L.A.; Darwish, W.S. Environmental Chemical Contaminants in Food: Review of a Global Problem. J. Toxicol. 2019, 2019, 2345283. [Google Scholar] [CrossRef]
- Nabi, M.; Tabassum, N. Role of Environmental Toxicants on Neurodegenerative Disorders. Front. Toxicol. 2022, 4, 837579. [Google Scholar] [CrossRef]
- Lanphear, B.P. The Impact of Toxins on the Developing Brain. Annu. Rev. Public Health 2015, 36, 1–20. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J. The Immunology of Stroke: From Mechanisms to Translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Calderón-Garcidueñas, L. Air Pollution: Mechanisms of Neuroinflammation and CNS Disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.R.; Greenamyre, J.T. The Role of Environmental Exposures in Neurodegeneration and Neurodegenerative Diseases. Toxicol. Sci. 2011, 124, 225–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Deng, X.; Li, Z.; Zhou, W.; Wang, G.; Zhan, J.; Hu, B. Environmental Cadmium Exposure Alters the Internal Microbiota and Metabolome of Sprague–Dawley Rats. Front. Vet. Sci. 2023, 10, 1219729. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Brejnrod, A.D.; Ernst, M.; Rykær, M.; Herschend, J.; Olsen, N.M.C.; Dorrestein, P.C.; Rensing, C.; Sørensen, S.J. Heavy Metal Exposure Causes Changes in the Metabolic Health-Associated Gut Microbiome and Metabolites. Environ. Int. 2019, 126, 454–467. [Google Scholar] [CrossRef]
- Ersbøll, A.K.; Monrad, M.; Sørensen, M.; Baastrup, R.; Hansen, B.; Bach, F.W.; Tjønneland, A.; Overvad, K.; Raaschou-Nielsen, O. Low-level exposure to arsenic in drinking water and incidence rate of stroke: A cohort study in Denmark. Environ. Int. 2018, 120, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, A.; Molinero, N.; Reinosa, J.J.; Alcolea-Rodriguez, V.; Portela, R.; Bañares, M.A.; Fernández, J.F.; Moreno-Arribas, M.V. PET microplastics affect human gut microbiota communities during simulated gastrointestinal digestion, first evidence of plausible polymer biodegradation during human digestion. Sci. Rep. 2022, 12, 528. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- Choi, J.J.; Eum, S.Y.; Rampersaud, E.; Daunert, S.; Abreu, M.T.; Toborek, M. Exercise Attenuates PCB-Induced Changes in the Mouse Gut Microbiome. Environ. Health Perspect. 2013, 121, 725–730. [Google Scholar] [CrossRef]
- Bergkvist, C.; Kippler, M.; Larsson, S.C.; Berglund, M.; Glynn, A.; Wolk, A.; Åkesson, A. Dietary Exposure to Polychlorinated Biphenyls Is Associated with Increased Risk of Stroke in Women. J. Intern. Med. 2014, 276, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Petriello, M.C.; Hoffman, J.B.; Vsevolozhskaya, O.; Morris, A.J.; Hennig, B. Dioxin-like PCB 126 Increases Intestinal Inflammation and Disrupts Gut Microbiota and Metabolic Homeostasis. Environ. Pollut. 2018, 242, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C.; Dheer, R.; Santaolalla, R.; Davies, J.M.; Burgueño, J.; Lang, J.K.; Toborek, M.; Abreu, M.T. Intestinal exposure to PCB 153 induces inflammation via the ATM/NEMO pathway. Toxicol. Appl. Pharmacol. 2018, 339, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Vignal, C.; Pichavant, M.; Alleman, L.Y.; Djouina, M.; Dingreville, F.; Perdrix, E.; Waxin, C.; Alami, A.O.; Gower-Rousseau, C.; Desreumaux, P.; et al. Effects of Urban Coarse Particles Inhalation on Oxidative and Inflammatory Parameters in the Mouse Lung and Colon. Part. Fibre Toxicol. 2017, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.A.; Engen, P.A.; Soberanes, S.; Urich, D.; Forsyth, C.B.; Nigdelioglu, R.; Chiarella, S.E.; Radigan, K.A.; Gonzalez, A.; Jakate, S.; et al. Particulate Matter Air Pollution Causes Oxidant-Mediated Increase in Gut Permeability in Mice. Part. Fibre Toxicol. 2011, 8, 19. [Google Scholar] [CrossRef]
- Woodby, B.; Schiavone, M.L.; Pambianchi, E.; Mastaloudis, A.; Hester, S.N.; Wood, S.M.; Pecorelli, A.; Valacchi, G. Particulate Matter Decreases Intestinal Barrier-Associated Proteins Levels in 3D Human Intestinal Model. Int. J. Environ. Res. Public Health 2020, 17, 3234. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.-J.; Jeon, S.; Yu, H.-S.; Cho, W.-S.; Lee, S.; Kang, D.; Kim, Y.; Kim, Y.-J.; Kim, S.-Y. Exposure to Nickel Oxide Nanoparticles Induces Acute and Chronic Inflammatory Responses in Rat Lungs and Perturbs the Lung Microbiome. Int. J. Environ. Res. Public Health 2022, 19, 522. [Google Scholar] [CrossRef]
- Li, X.; Zhang, T.; Lv, W.; Wang, H.; Chen, H.; Xu, Q.; Cai, H.; Dai, J. Intratracheal administration of polystyrene microplastics induces pulmonary fibrosis by activating oxidative stress and Wnt/β-catenin signaling pathway in mice. Ecotoxicol. Environ. Saf. 2022, 232, 113238. [Google Scholar] [CrossRef]
- Michaudel, C.; Mackowiak, C.; Maillet, I.; Fauconnier, L.; Akdis, C.A.; Sokolowska, M.; Dreher, A.; Tan, H.-T.T.; Quesniaux, V.F.; Ryffel, B.; et al. Ozone Exposure Induces Respiratory Barrier Biphasic Injury and Inflammation Controlled by IL-33. J. Allergy Clin. Immunol. 2018, 142, 942–958. [Google Scholar] [CrossRef]
- Gentner, N.J.; Weber, L.P. Intranasal benzo[a]pyrene alters circadian blood pressure patterns and causes lung inflammation in rats. Arch. Toxicol. 2011, 85, 337–346. [Google Scholar] [CrossRef]
- Bai, H.; Wu, M.; Zhang, H.; Tang, G. Chronic polycyclic aromatic hydrocarbon exposure causes DNA damage and genomic instability in lung epithelial cells. Oncotarget 2017, 8, 79034–79045. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, Y.; Liu, L.; Wang, Q.; Zeng, J.; Chen, C. PM2.5 Exposure Perturbs Lung Microbiome and Its Metabolic Profile in Mice. Sci. Total Environ. 2020, 721, 137432. [Google Scholar] [CrossRef] [PubMed]
- Kaspersen, K.A.; Antonsen, S.; Horsdal, H.T.; Kjerulff, B.; Brandt, J.; Geels, C.; Christensen, J.H.; Frohn, L.M.; Sabel, C.E.; Dinh, K.M.; et al. Exposure to Air Pollution and Risk of Respiratory Tract Infections in the Adult Danish Population—A Nationwide Study. Clin. Microbiol. Infect. 2024, 30, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xun, P.; Tsinovoi, C.; McClure, L.A.; Brockman, J.; MacDonald, L.; Cushman, M.; Cai, J.; Kamendulis, L.; Mackey, J.; et al. Urinary cadmium concentration and the risk of ischemic stroke. Neurology. 2018, 91, e382–e391. [Google Scholar] [CrossRef]
- Wen, Y.; Huang, S.; Zhang, Y.; Zhang, H.; Zhou, L.; Li, D.; Xie, C.; Lv, Z.; Guo, Y.; Ke, Y.; et al. Associations of multiple plasma metals with the risk of ischemic stroke: A case-control study. Environ. Int. 2019, 125, 125–134. [Google Scholar] [CrossRef]
- Kopf, P.G.; Walker, M.K. 2,3,7,8-tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicol. Appl. Pharmacol. 2010, 245, 91–99. [Google Scholar] [CrossRef]
- Tzeng, H.P.; Yang, T.H.; Wu, C.T.; Chiu, H.C.; Liu, S.H.; Lan, K.C. Benzo[a]pyrene alters vascular function in rat aortas ex vivo and in vivo. Vasc. Pharmacol. 2019, 121, 106578. [Google Scholar] [CrossRef]
- Lee, D.-H.; Lind, P.M.; Jacobs, D.R., Jr.; Salihovic, S.; van Bavel, B.; Lind, L. Background exposure to persistent organic pollutants predicts stroke in the elderly. Environ. Int. 2012, 47, 115–1120. [Google Scholar] [CrossRef] [PubMed]
- Toborek, M.; Barger, S.W.; Mattson, M.P.; Espandiari, P.; Robertson, L.W.; Hennig, B. Exposure to polychlorinated biphenyls causes endothelial cell dysfunction. J. Biochem. Toxicol. 1995, 10, 219–226. [Google Scholar] [CrossRef]
- Gaur, N.; Dutta, D.; Singh, A.; Dubey, R.; Kamboj, D.V. Recent Advances in the Elimination of Persistent Organic Pollutants by Photocatalysis. Front. Environ. Sci. 2022, 10, 872514. [Google Scholar] [CrossRef]
- Samanta, S.K.; Singh, O.V.; Jain, R.K. Polycyclic Aromatic Hydrocarbons: Environmental Pollution and Bioremediation. Trends Biotechnol. 2002, 20, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Levels of Polycyclic Aromatic Hydrocarbons in the Water and Sediment of Buffalo River Estuary, South Africa and Their Health Risk Assessment. Arch. Environ. Contam. Toxicol. 2019, 76, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Rodgman, A.; Smith, C.J.; Perfetti, T.A. The Composition of Cigarette Smoke: A Retrospective, with Emphasis on Polycyclic Components. Hum. Exp. Toxicol. 2000, 19, 573–595. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.M.; Bharat, G.K.; Tayal, S.; Nizzetto, L.; Čupr, P.; Larssen, T. Environment and Human Exposure to Persistent Organic Pollutants (POPs) in India: A Systematic Review of Recent and Historical Data. Environ. Int. 2014, 66, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Daley, J.M.; Paterson, G.; Drouillard, K.G. Bioamplification as a Bioaccumulation Mechanism for Persistent Organic Pollutants (POPs) in Wildlife. Rev. Environ. Contam. Toxicol. 2013, 227, 107–155. [Google Scholar] [CrossRef] [PubMed]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR Signaling Pathways and Regulatory Functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J. Xenobiotics 2021, 11, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B.; Mokra, K.; Michałowicz, J. Benzo[a]Pyrene—Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. Int. J. Mol. Sci. 2022, 23, 6348. [Google Scholar] [CrossRef] [PubMed]
- Knecht, A.L.; Goodale, B.C.; Truong, L.; Simonich, M.T.; Swanson, A.J.; Matzke, M.M.; Anderson, K.A.; Waters, K.M.; Tanguay, R.L. Comparative Developmental Toxicity of Environmentally Relevant Oxygenated PAHs. Toxicol. Appl. Pharmacol. 2013, 271, 266–275. [Google Scholar] [CrossRef]
- Mandal, P.K. Dioxin: A Review of Its Environmental Effects and Its Aryl Hydrocarbon Receptor Biology. J. Comp. Physiol. B 2005, 175, 221–230. [Google Scholar] [CrossRef]
- Shimizu, Y.; Nakatsuru, Y.; Ichinose, M.; Takahashi, Y.; Kume, H.; Mimura, J.; Fujii-Kuriyama, Y.; Ishikawa, T. Benzo[a]Pyrene Carcinogenicity Is Lost in Mice Lacking the Aryl Hydrocarbon Receptor. Proc Natl. Acad. Sci. USA 2000, 97, 779–782. [Google Scholar] [CrossRef]
- Gonzalez, F.J.; Fernandez-Salguero, P. The Aryl Hydrocarbon Receptor: Studies Using the AHR-Null Mice. Drug Metab. Dispos. Biol. Fate Chem. 1998, 26, 1194–1198. [Google Scholar]
- Ranjbar, M.; Rotondi, M.A.; Ardern, C.I.; Kuk, J.L. Urinary Biomarkers of Polycyclic Aromatic Hydrocarbons Are Associated with Cardiometabolic Health Risk. PLoS ONE 2015, 10, e0137536. [Google Scholar] [CrossRef]
- Xu, X.; Cook, R.L.; Ilacqua, V.A.; Kan, H.; Talbott, E.O.; Kearney, G. Studying Associations between Urinary Metabolites of Polycyclic Aromatic Hydrocarbons (PAHs) and Cardiovascular Diseases in the United States. Sci. Total Environ. 2010, 408, 4943–4948. [Google Scholar] [CrossRef]
- Mallah, M.A.; Mallah, M.A.; Liu, Y.; Xi, H.; Wang, W.; Feng, F.; Zhang, Q. Relationship Between Polycyclic Aromatic Hydrocarbons and Cardiovascular Diseases: A Systematic Review. Front. Public Health 2021, 9, 763706. [Google Scholar] [CrossRef]
- Rahman, H.H.; Sheikh, S.P.; Munson-McGee, S.H. Arsenic, Polycyclic Aromatic Hydrocarbons, and Metal Exposure and Risk Assessment of Stroke. Environ. Sci. Pollut. Res. 2023, 30, 86973–86986. [Google Scholar] [CrossRef]
- Curfs, D.M.J.; Lutgens, E.; Gijbels, M.J.J.; Kockx, M.M.; Daemen, M.J.A.P.; van Schooten, F.J. Chronic Exposure to the Carcinogenic Compound Benzo[a]Pyrene Induces Larger and Phenotypically Different Atherosclerotic Plaques in ApoE-Knockout Mice. Am. J. Pathol. 2003, 164, 101–108. [Google Scholar] [CrossRef]
- Grova, N.; Schroeder, H.; Farinelle, S.; Prodhomme, E.; Valley, A.; Muller, C.P. Sub-Acute Administration of Benzo[a]Pyrene (B[a]P) Reduces Anxiety-Related Behaviour in Adult Mice and Modulates Regional Expression of N-Methyl-d-Aspartate (NMDA) Receptors Genes in Relevant Brain Regions. Chemosphere 2008, 73, S295–S302. [Google Scholar] [CrossRef]
- Ho, D.H.; Burggren, W.W. Blood-Brain Barrier Function, Cell Viability, and Gene Expression of Tight Junction-Associated Proteins in the Mouse Are Disrupted by Crude Oil, Benzo[a]Pyrene, and the Dispersant COREXIT. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 223, 96–105. [Google Scholar] [CrossRef]
- Tanaka, M.; Okuda, T.; Itoh, K.; Ishihara, N.; Oguro, A.; Fujii-Kuriyama, Y.; Nabetani, Y.; Yamamoto, M.; Vogel, C.F.A.; Ishihara, Y. Polycyclic Aromatic Hydrocarbons in Urban Particle Matter Exacerbate Movement Disorder after Ischemic Stroke via Potentiation of Neuroinflammation. Part. Fibre Toxicol. 2023, 20, 6. [Google Scholar] [CrossRef]
- Gu, X.; Manautou, J.E. Molecular Mechanisms Underlying Chemical Liver Injury. Expert Rev. Mol. Med. 2012, 14, e4. [Google Scholar] [CrossRef]
- Erickson, M.D. Analytical Chemistry of PCBs; Routledge: London, UK, 1997; pp. 125–151. [Google Scholar] [CrossRef]
- Raffetti, E.; Donato, F.; Palma, G.D.; Leonardi, L.; Sileo, C.; Magoni, M. Polychlorinated Biphenyls (PCBs) and Risk of Hypertension: A Population-Based Cohort Study in a North Italian Highly Polluted Area. Sci. Total Environ. 2020, 714, 136660. [Google Scholar] [CrossRef]
- Goncharov, A.; Pavuk, M.; Foushee, H.R.; Carpenter, D.O.; Consortium, A.E.H.R. Blood Pressure in Relation to Concentrations of PCB Congeners and Chlorinated Pesticides. Environ. Health Perspect. 2011, 119, 319–325. [Google Scholar] [CrossRef]
- Lim, J.; Lee, S.; Lee, S.; Jee, S.H. Serum Persistent Organic Pollutants Levels and Stroke Risk. Environ. Pollut. 2018, 233, 855–861. [Google Scholar] [CrossRef]
- Schlezinger, J.J.; Struntz, W.D.J.; Goldstone, J.V.; Stegeman, J.J. Uncoupling of Cytochrome P450 1A and Stimulation of Reactive Oxygen Species Production by Co-Planar Polychlorinated Biphenyl Congeners. Aquat. Toxicol. 2006, 77, 422–432. [Google Scholar] [CrossRef]
- Hennig, B.; Meerarani, P.; Slim, R.; Toborek, M.; Daugherty, A.; Silverstone, A.E.; Robertson, L.W. Proinflammatory Properties of Coplanar PCBs: In Vitro and in Vivo Evidence. Toxicol. Appl. Pharmacol. 2002, 181, 174–183. [Google Scholar] [CrossRef]
- Evich, M.G.; Davis, M.J.B.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and Polyfluoroalkyl Substances in the Environment. Science 2022, 375, eabg9065. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An Overview of the Uses of Per- and Polyfluoroalkyl Substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef]
- Cousins, I.T.; DeWitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Scheringer, M.; Wang, Z. The High Persistence of PFAS Is Sufficient for Their Management as a Chemical Class. Environ. Sci. Process. Impacts 2020, 22, 2307–2312. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Human Exposure to Per- and Polyfluoroalkyl Substances (PFAS) through Drinking Water: A Review of the Recent Scientific Literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef]
- Roth, K.; Imran, Z.; Liu, W.; Petriello, M.C. Diet as an Exposure Source and Mediator of Per- and Polyfluoroalkyl Substance (PFAS) Toxicity. Front. Toxicol. 2020, 2, 601149. [Google Scholar] [CrossRef]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-Life of Serum Elimination of Perfluorooctanesulfonate, Perfluorohexanesulfonate, and Perfluorooctanoate in Retired Fluorochemical Production Workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef]
- Xiao, F.; An, Z.; Lv, J.; Sun, X.; Sun, H.; Liu, Y.; Liu, X.; Guo, H. Association between Per- and Polyfluoroalkyl Substances and Risk of Hypertension: A Systematic Review and Meta-Analysis. Front. Public Health 2023, 11, 1173101. [Google Scholar] [CrossRef]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S.V. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef]
- Simpson, C.; Winquist, A.; Lally, C.; Steenland, K. Relation between Perfluorooctanoic Acid Exposure and Strokes in a Large Cohort Living near a Chemical Plant. Environ. Res. 2013, 127, 22–28. [Google Scholar] [CrossRef]
- Feng, X.; Long, G.; Zeng, G.; Zhang, Q.; Song, B.; Wu, K.-H. Association of Increased Risk of Cardiovascular Diseases with Higher Levels of Perfluoroalkylated Substances in the Serum of Adults. Environ. Sci. Pollut. Res. 2022, 29, 89081–89092. [Google Scholar] [CrossRef]
- Schillemans, T.; Donat-Vargas, C.; Lindh, C.H.; de Faire, U.; Wolk, A.; Leander, K.; Åkesson, A. Per- and Polyfluoroalkyl Substances and Risk of Myocardial Infarction and Stroke: A Nested Case–Control Study in Sweden. Environ. Health Perspect. 2022, 130, 037007. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, L.; Fang, W.; Zhang, X.; Zhong, Y. Perfluorooctanoic Acid-Induced Immunotoxicity via NF-Kappa B Pathway in Zebrafish (Danio Rerio) Kidney. Fish Shellfish Immunol. 2021, 113, 9–19. [Google Scholar] [CrossRef]
- Vemuganti, R. Therapeutic Potential of PPAR γ Activation in Stroke. PPAR Res. 2008, 2008, 461981. [Google Scholar] [CrossRef]
- Satriotomo, I.; Bowen, K.K.; Vemuganti, R. JAK2 and STAT3 Activation Contributes to Neuronal Damage Following Transient Focal Cerebral Ischemia. J. Neurochem. 2006, 98, 1353–1368. [Google Scholar] [CrossRef]
- Meneguzzi, A.; Fava, C.; Castelli, M.; Minuz, P. Exposure to Perfluoroalkyl Chemicals and Cardiovascular Disease: Experimental and Epidemiological Evidence. Front. Endocrinol. 2021, 12, 706352. [Google Scholar] [CrossRef]
- Verhoeven, J.I.; Allach, Y.; Vaartjes, I.C.H.; Klijn, C.J.M.; de Leeuw, F.-E. Ambient Air Pollution and the Risk of Ischaemic and Haemorrhagic Stroke. Lancet Planet. Health 2021, 5, e542–e552. [Google Scholar] [CrossRef]
- Strosnider, H.; Kennedy, C.; Monti, M.; Yip, F. Rural and Urban Differences in Air Quality, 2008–2012, and Community Drinking Water Quality, 2010–2015—United States. MMWR Surveill. Summ. 2017, 66, 1–10. [Google Scholar] [CrossRef]
- Mannucci, P.M.; Franchini, M. Health Effects of Ambient Air Pollution in Developing Countries. Int. J. Environ. Res. Public Health 2017, 14, 1048. [Google Scholar] [CrossRef]
- Schwarze, P.E.; Øvrevik, J.; Låg, M.; Refsnes, M.; Nafstad, P.; Hetland, R.B.; Dybing, E. Particulate Matter Properties and Health Effects: Consistency of Epidemiological and Toxicological Studies. Hum. Exp. Toxicol. 2006, 25, 559–579. [Google Scholar] [CrossRef]
- Poh, T.Y.; Ali, N.A.B.M.; Aogáin, M.M.; Kathawala, M.H.; Setyawati, M.I.; Ng, K.W.; Chotirmall, S.H. Inhaled Nanomaterials and the Respiratory Microbiome: Clinical, Immunological and Toxicological Perspectives. Part. Fibre Toxicol. 2018, 15, 46. [Google Scholar] [CrossRef]
- Mühlfeld, C.; Rothen-Rutishauser, B.; Blank, F.; Vanhecke, D.; Ochs, M.; Gehr, P. Interactions of Nanoparticles with Pulmonary Structures and Cellular Responses. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 294, L817–L829. [Google Scholar] [CrossRef]
- Hameed, S.; Zhao, J.; Zare, R.N. Ambient PM Particles Reach Mouse Brain, Generate Ultrastructural Hallmarks of Neuroinflammation, and Stimulate Amyloid Deposition, Tangles, and Plaque Formation. Talanta. Open 2020, 2, 100013. [Google Scholar] [CrossRef]
- Hartz, A.M.S.; Bauer, B.; Block, M.L.; Hong, J.-S.; Miller, D.S. Diesel Exhaust Particles Induce Oxidative Stress, Proinflammatory Signaling, and P-glycoprotein Up-regulation at the Blood-brain Barrier. FASEB J. 2008, 22, 2723–2733. [Google Scholar] [CrossRef]
- Block, M.L.; Wu, X.; Pei, Z.; Li, G.; Wang, T.; Qin, L.; Wilson, B.; Yang, J.; Hong, J.S.; Veronesi, B. Nanometer Size Diesel Exhaust Particles Are Selectively Toxic to Dopaminergic Neurons: The Role of Microglia, Phagocytosis, and NADPH Oxidase. FASEB J. 2004, 18, 1618–1620. [Google Scholar] [CrossRef]
- Gulisano, M.; Pacini, P.; Marceddu, S.; Orlandini, G.E. Scanning Electron Microscopic Evaluation of the Alterations Induced by Polluted Air in the Rabbit Bronchial Epithelium. Ann. Anat. Anat. Anz. 1995, 177, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, M.T.; Iley, R.M.; Chang, Y.-T.C.; Clark, K.W.; Jones, M.P.; Linn, W.S.; Hackney, J.D. Exposures of Human Volunteers to a Controlled Atmospheric Mixture of Ozone, Sulfur Dioxide and Sulfuric Acid. AIHA J. 1981, 42, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Son, J.-Y.; Peng, R.D.; Wang, Y.; Dominici, F. Ambient PM2.5 and Risk of Hospital Admissions: Do Risks Differ for Men and Women? Epidemiology 2015, 26, 575–579. [Google Scholar] [CrossRef]
- Andersen, Z.J.; Olsen, T.S.; Andersen, K.K.; Loft, S.; Ketzel, M.; Raaschou-Nielsen, O. Association between Short-Term Exposure to Ultrafine Particles and Hospital Admissions for Stroke in Copenhagen, Denmark. Eur. Heart, J. 2010, 31, 2034–2040. [Google Scholar] [CrossRef]
- Li, B.; Ma, Y.; Zhou, Y.; Chai, E. Research Progress of Different Components of PM2.5 and Ischemic Stroke. Sci. Rep. 2023, 13, 15965. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, G.; Jiang, Y.; Li, G.; Pan, Y.; Wang, Y.; Wei, Z.; Wang, J.; Wang, Y. Acute Effects of Particulate Air Pollution on Ischemic Stroke and Hemorrhagic Stroke Mortality. Front. Neurol. 2018, 9, 827. [Google Scholar] [CrossRef]
- Szyszkowicz, M.; Rowe, B.H.; Brook, R.D. Even Low Levels of Ambient Air Pollutants Are Associated With Increased Emergency Department Visits for Hypertension. Can. J. Cardiol. 2012, 28, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Liu, X.; Qu, M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials 2022, 12, 1298. [Google Scholar] [CrossRef]
- Ghosh, S.; Sinha, J.K.; Ghosh, S.; Vashisth, K.; Han, S.; Bhaskar, R. Microplastics as an Emerging Threat to the Global Environment and Human Health. Sustainability 2023, 15, 10821. [Google Scholar] [CrossRef]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic Polymer Contamination in Bottled Water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in Air: Are We Breathing It In? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; Luca, C.D.; D’Avino, S.; Gulotta, A.; et al. Raman Microspectroscopy Detection and Characterisation of Microplastics in Human Breastmilk. Polymers 2022, 14, 2700. [Google Scholar] [CrossRef] [PubMed]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Meaza, I.; Toyoda, J.H.; Wise, J.P. Microplastics in Sea Turtles, Marine Mammals and Humans: A One Environmental Health Perspective. Front. Environ. Sci. 2020, 8, 575614. [Google Scholar] [CrossRef] [PubMed]
- Nelms, S.E.; Barnett, J.; Brownlow, A.; Davison, N.J.; Deaville, R.; Galloway, T.S.; Lindeque, P.K.; Santillo, D.; Godley, B.J. Microplastics in Marine Mammals Stranded around the British Coast: Ubiquitous but Transitory? Sci. Rep. 2019, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; Grotta, R.L.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and Maintenance of Skin Barrier Function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Brune, K.; Frank, J.; Schwingshackl, A.; Finigan, J.; Sidhaye, V.K. Pulmonary Epithelial Barrier Function: Some New Players and Mechanisms. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 308, L731–L745. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal Mucosal Barrier Function in Health and Disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Ganesan, S.; Comstock, A.T.; Sajjan, U.S. Barrier Function of Airway Tract Epithelium. Tissue Barriers 2013, 1, e24997. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Dou, M.; He, H.; Ju, M.; Ji, S.; Zhou, J.; Chen, C.; Zhang, D.; Miao, C.; et al. Particulate Matter Disrupts Airway Epithelial Barrier via Oxidative Stress to Promote Pseudomonas Aeruginosa Infection. J. Thorac. Dis. 2019, 11, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, J.C.; Yshii, C.; Westphal, W.; Moninger, T.; Comellas, A.P. Ambient Particulate Matter Affects Occludin Distribution and Increases Alveolar Transepithelial Electrical Conductance. Respirology 2011, 16, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.-S.; Choi, I.-S.; Koh, Y.-I.; Park, C.-S.; Lee, J.-S. The Relationship between Alveolar Epithelial Proliferation and Airway Obstruction after Ozone Exposure. Allergy 2002, 57, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Hess, C.; Hess, M. Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens. Toxins 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, C. The Relationship between Intestinal Goblet Cells and the Immune Response. Biosci. Rep. 2020, 40, BSR20201471. [Google Scholar] [CrossRef] [PubMed]
- Iacob, S.; Iacob, D.G.; Luminos, L.M. Intestinal Microbiota as a Host Defense Mechanism to Infectious Threats. Front. Microbiol. 2019, 9, 3328. [Google Scholar] [CrossRef] [PubMed]
- Crapser, J.; Ritzel, R.; Verma, R.; Venna, V.R.; Liu, F.; Chauhan, A.; Koellhoffer, E.; Patel, A.; Ricker, A.; Maas, K.; et al. Ischemic Stroke Induces Gut Permeability and Enhances Bacterial Translocation Leading to Sepsis in Aged Mice. Aging Albany Ny 2016, 8, 1049–1060. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, C.; Zhao, J.; Li, D.; Chen, J. Exposure to Concentrated Ambient PM2.5 (CAPM) Induces Intestinal Disturbance via Inflammation and Alternation of Gut Microbiome. Environ. Int. 2022, 161, 107138. [Google Scholar] [CrossRef]
- Saitou, M.; Furuse, M.; Sasaki, H.; Schulzke, J.-D.; Fromm, M.; Takano, H.; Noda, T.; Tsukita, S. Complex Phenotype of Mice Lacking Occludin, a Component of Tight Junction Strands. Mol. Biol. Cell 2000, 11, 4131–4142. [Google Scholar] [CrossRef]
- Nouri, M.; Bredberg, A.; Weström, B.; Lavasani, S. Intestinal Barrier Dysfunction Develops at the Onset of Experimental Autoimmune Encephalomyelitis, and Can Be Induced by Adoptive Transfer of Auto-Reactive T Cells. PLoS ONE 2014, 9, e106335. [Google Scholar] [CrossRef]
- Li, C.; Chen, W.; Lin, F.; Li, W.; Wang, P.; Liao, G.; Zhang, L. Functional Two-Way Crosstalk Between Brain and Lung: The Brain–Lung Axis. Cell. Mol. Neurobiol. 2023, 43, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Hosang, L.; Canals, R.C.; van der Flier, F.J.; Hollensteiner, J.; Daniel, R.; Flügel, A.; Odoardi, F. The Lung Microbiome Regulates Brain Autoimmunity. Nature 2022, 603, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Aslanyan, S.; Weir, C.J.; Diener, H.-C.; Kaste, M.; Lees, K.R.; GAIN International Steering Committee and Investigators. Pneumonia and Urinary Tract Infection after Acute Ischaemic Stroke: A Tertiary Analysis of the GAIN International Trial. Eur. J. Neurol. 2004, 11, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Faura, J.; Ramiro, L.; Simats, A.; Ma, F.; Penalba, A.; Gasull, T.; Rosell, A.; Montaner, J.; Bustamante, A. Evaluation and Characterization of Post-Stroke Lung Damage in a Murine Model of Cerebral Ischemia. Int. J. Mol. Sci. 2022, 23, 8093. [Google Scholar] [CrossRef] [PubMed]
- Faura, J.; Bustamante, A.; Miró-Mur, F.; Montaner, J. Stroke-Induced Immunosuppression: Implications for the Prevention and Prediction of Post-Stroke Infections. J. Neuroinflamm. 2021, 18, 127. [Google Scholar] [CrossRef] [PubMed]
- Katzan, I.L.; Cebul, R.D.; Husak, S.H.; Dawson, N.V.; Baker, D.W. The Effect of Pneumonia on Mortality among Patients Hospitalized for Acute Stroke. Neurology 2003, 60, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Sigaud, S.; Goldsmith, C.-A.W.; Zhou, H.; Yang, Z.; Fedulov, A.; Imrich, A.; Kobzik, L. Air Pollution Particles Diminish Bacterial Clearance in the Primed Lungs of Mice. Toxicol. Appl. Pharmacol. 2007, 223, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, K.E.; Green, F.H.; Saiki, C.; Vallyathan, V.; Plopper, C.G.; Gopal, V.; Hung, D.; Bahne, E.B.; Lin, S.S.; Ménache, M.G.; et al. Distribution of Particulate Matter and Tissue Remodeling in the Human Lung. Environ. Health Perspect. 2000, 108, 1063–1069. [Google Scholar] [CrossRef]
- Kurmi, O.P.; Semple, S.; Simkhada, P.; Smith, W.C.S.; Ayres, J.G. COPD and Chronic Bronchitis Risk of Indoor Air Pollution from Solid Fuel: A Systematic Review and Meta-Analysis. Thorax 2010, 65, 221. [Google Scholar] [CrossRef]
- Elonheimo, H.M.; Mattila, T.; Andersen, H.R.; Bocca, B.; Ruggieri, F.; Haverinen, E.; Tolonen, H. Environmental Substances Associated with Chronic Obstructive Pulmonary Disease—A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 3945. [Google Scholar] [CrossRef] [PubMed]
- O’Piela, D.R.; Durisek, G.R.; Escobar, Y.-N.H.; Mackos, A.R.; Wold, L.E. Particulate Matter and Alzheimer’s Disease: An Intimate Connection. Trends Mol. Med. 2022, 28, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Ural, B.B.; Caron, D.P.; Dogra, P.; Wells, S.B.; Szabo, P.A.; Granot, T.; Senda, T.; Poon, M.M.L.; Lam, N.; Thapa, P.; et al. Inhaled Particulate Accumulation with Age Impairs Immune Function and Architecture in Human Lung Lymph Nodes. Nat. Med. 2022, 28, 2622–2632. [Google Scholar] [CrossRef] [PubMed]
- Samary, C.S.; Ramos, A.B.; Maia, L.A.; Rocha, N.N.; Santos, C.L.; Magalhães, R.F.; Clevelario, A.L.; Pimentel-Coelho, P.M.; Mendez-Otero, R.; Cruz, F.F.; et al. Focal Ischemic Stroke Leads to Lung Injury and Reduces Alveolar Macrophage Phagocytic Capability in Rats. Crit. Care 2018, 22, 249. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C.; et al. Air Pollution and Cardiovascular Disease. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef] [PubMed]
- Hozawa, A.; Billings, J.L.; Shahar, E.; Ohira, T.; Rosamond, W.D.; Folsom, A.R. Lung Function and Ischemic Stroke Incidence The Atherosclerosis Risk in Communities Study. Chest 2006, 130, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P.; Erb-Downward, J.R.; Martinez, F.J.; Huffnagle, G.B. The Microbiome and the Respiratory Tract. Annu. Rev. Physiol. 2015, 78, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P.; Erb-Downward, J.R.; Freeman, C.M.; McCloskey, L.; Falkowski, N.R.; Huffnagle, G.B.; Curtis, J.L. Bacterial Topography of the Healthy Human Lower Respiratory Tract. mBio 2017, 8, e02287-16. [Google Scholar] [CrossRef]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Carloni, S.; Rescigno, M. The Gut-Brain Vascular Axis in Neuroinflammation. Semin. Immunol. 2023, 69, 101802. [Google Scholar] [CrossRef]
- Johnson, C.H.; Patterson, A.D.; Idle, J.R.; Gonzalez, F.J. Xenobiotic Metabolomics: Major Impact on the Metabolome. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 37–56. [Google Scholar] [CrossRef]
- Koppel, N.; Rekdal, V.M.; Balskus, E.P. Chemical Transformation of Xenobiotics by the Human Gut Microbiota. Science 2018, 356, eaag2770. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Q.; Fu, J.; Li, X.; Mao, H.; Wang, T. Influence of Exposure Pathways on Tissue Distribution and Health Impact of Polycyclic Aromatic Hydrocarbon Derivatives. Environ. Health 2023, 1, 150–167. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef]
- Gacesa, R.; Kurilshikov, A.; Vila, A.V.; Sinha, T.; Klaassen, M.A.Y.; Bolte, L.A.; Andreu-Sánchez, S.; Chen, L.; Collij, V.; Hu, S.; et al. Environmental Factors Shaping the Gut Microbiome in a Dutch Population. Nature 2022, 604, 732–739. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and Clinical Implications of the Brain–Gut–Enteric Microbiota Axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef]
- Lefever, D.E.; Xu, J.; Chen, Y.; Huang, G.; Tamas, N.; Guo, T.L. TCDD Modulation of Gut Microbiome Correlated with Liver and Immune Toxicity in Streptozotocin (STZ)-Induced Hyperglycemic Mice. Toxicol. Appl. Pharmacol. 2016, 304, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A.; Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of Ischaemic Stroke: An Integrated View. Trends Neurosci 1999, 22, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Buckwalter, M.S.; Anrather, J. Immune Responses to Stroke: Mechanisms, Modulation, and Therapeutic Potential. J. Clin. Investig. 2020, 130, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Simats, A.; Liesz, A. Systemic Inflammation after Stroke: Implications for Post-stroke Comorbidities. Embo Mol. Med. 2022, 14, e16219. [Google Scholar] [CrossRef] [PubMed]
- Benakis, C.; Brea, D.; Caballero, S.; Faraco, G.; Moore, J.; Murphy, M.; Sita, G.; Racchumi, G.; Ling, L.; Pamer, E.G.; et al. Commensal Microbiota Affects Ischemic Stroke Outcome by Regulating Intestinal Γδ T Cells. Nat. Med. 2016, 22, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Brea, D.; Poon, C.; Benakis, C.; Lubitz, G.; Murphy, M.; Iadecola, C.; Anrather, J. Stroke Affects Intestinal Immune Cell Trafficking to the Central Nervous System. Brain Behav. Immun. 2021, 96, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Roth, S.; Llovera, G.; Sadler, R.; Garzetti, D.; Stecher, B.; Dichgans, M.; Liesz, A. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J. Neurosci. 2016, 36, 7428–7440. [Google Scholar] [CrossRef] [PubMed]
- Houlden, A.; Goldrick, M.; Brough, D.; Vizi, E.S.; Lénárt, N.; Martinecz, B.; Roberts, I.S.; Denes, A. Brain Injury Induces Specific Changes in the Caecal Microbiota of Mice via Altered Autonomic Activity and Mucoprotein Production. Brain Behav. Immun. 2016, 57, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Gao, X.; Xia, G.; Chen, M.; Zeng, N.; Wang, S.; You, C.; Tian, X.; Di, H.; Tang, W.; et al. Rapid Gut Dysbiosis Induced by Stroke Exacerbates Brain Infarction in Turn. Gut 2021, 70, 1486–1494. [Google Scholar] [CrossRef]
- Haak, B.W.; Westendorp, W.F.; van Engelen, T.S.R.; Brands, X.; Brouwer, M.C.; Vermeij, J.-D.; Hugenholtz, F.; Verhoeven, A.; Derks, R.J.; Giera, M.; et al. Disruptions of Anaerobic Gut Bacteria Are Associated with Stroke and Post-Stroke Infection: A Prospective Case–Control Study. Transl. Stroke Res. 2021, 12, 581–592. [Google Scholar] [CrossRef]
- Benakis, C.; Poon, C.; Lane, D.; Brea, D.; Sita, G.; Moore, J.; Murphy, M.; Racchumi, G.; Iadecola, C.; Anrather, J. Distinct Commensal Bacterial Signature in the Gut Is Associated With Acute and Long-Term Protection From Ischemic Stroke. Stroke 2020, 51, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Strickland, P.; Kang, D.; Sithisarankul, P. Polycyclic Aromatic Hydrocarbon Metabolites in Urine as Biomarkers of Exposure and Effect. Environ. Health Perspect. 1996, 104, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-C.; Lai, C.-S.; Tsai, M.-L.; Ho, C.-T.; Wang, Y.-J.; Pan, M.-H. Chemopreventive Effect of Natural Dietary Compounds on Xenobiotic-Induced Toxicity. J. Food Drug Anal. 2017, 25, 176–186. [Google Scholar] [CrossRef]
- Stavric, B.; Klassen, R. Dietary Effects on the Uptake of Benzo[a]Pyrene. Food Chem. Toxicol. 1994, 32, 727–734. [Google Scholar] [CrossRef] [PubMed]
| Environmental Toxins | Compound/ Substance | Model | Dosage | Exposure Method and Duration | Effect | References |
|---|---|---|---|---|---|---|
| Gut | ||||||
| Heavy Metal | Cadmium | Sprague–Dawley rats | 5 mg/kg | Gastric infusion daily for 30 days | ↓ Expression of the tight junction protein ZO-1 ↑ Expression of TNF-α, and IL-6 in the gut | [25] |
| Heavy Metals | Cadmium/Arsenic | C57BL/6 mice | 50 ppm | Dietary exposure through drinking water for 2 weeks | ↓ Alpha diversity of the microbiota | [26] |
| Heavy Metal | Arsenic | Participants in cohort study | <50 μg/L | 20-year exposure window | Higher incidence of stroke | [27] |
| Microplastics and nanoplastics | Micro/nanoplastics | C57/B6 mice | 0.2 and 2 mg/kg | 28 days | High dose led to morphological changes along the GI including the followings: -Absence of villi -Damaged crypts ↓ Mucosa wall lining ↓ Tight junction expression. ↓ Microbial diversity and ratio of bacteria | [15] |
| Microplastics | Polyethylene terephthalate (PET) | In vitro simulation | 0.166 g/intake | 72 h | Shift in the relative abundance of bacteria in the upper region of the colon. Specifically, ↑ Desulfobacterota and ↓ Proteobacteria over time. ↓ Viable bacterial count | [28] |
| Microplastics | PS-MPs | Male mice | 0.1mg/day of 5 or 20 μm PS-MPs | 28 days via oral gavage | Accumulation of PS-MPs in the gut, liver, and kidney. ↑ Inflammation and lipid accumulation in the liver | [29] |
| PAH | BaP | C57BL/6 mice | 10 mL/kg of BW | 28 days oral exposure | ↑ Inflammation in ileal segments -Altered relative abundance of fecal and mucosa associated microbiota ↓ Lactobacillus | [12] |
| PCBs | PCB153, PCB138, and PCB180 | C57BL/6 mice | 150 µmol/kg | 2 days | ↓ Proteobacteria | [30] |
| PCBs | Prospective population based Follow up | Middle-aged and elderly women | Passive dietary exposure | 12 years of follow-up | Positive association observed between stroke risk and PCB exposure. | [31] |
| PCB | PCB 126 | Ldlr−/− mice | 1 μmol/kg of PCB 126 | 14-week atherogenic diet and exposed to PCB 126 (1 μmol/kg) at weeks 2 and 4 | ↑ Expression of TNF-α, IL-6, and interleukin IL-18 in the jejunum ↑ Tight junction proteins: Occludin and claudin in the colon ↓ Expression of PPAR-δ in the colon ↓ Abundance of Clostridiales, Bifidobacterium, Lactobacillus, Ruminococcus, and Oscillospira. ↑ Abundance of Akkermansia. ↓ Alpha diversity in cecum contents | [32] |
| PCB | PCB153 | C57BL/6 mice | 300 μmol/kg | 1× per day for 2-days | ↑ Expression of TNF-α and IL-6 in the intestinal epithelial cells of the small intestines -Activates NF-κB pathway -Induces DNA damage | [33] |
| Particulate matter | Urban particulate matter | C57BL/6 mice | 40 μg course particulate matter/mL | Mice were placed 4 h/day or 5 days/week for 2 weeks in inhalation chamber | ↑ Expression of TNF-α, IFN-γ, CXCL10, and IL-10 ↓ Expression of IL-5 | [34] |
| Particulate matter | Urban particulate matter | C57BL/6 mice | 200 μg | Gastric gavage | ↑ ROS production ↑ Colonic epithelial cell death | [35] |
| Particulate matter | Atmosphericparticulate matter | Intestinal tissue | 50–500 µg/cm2 | 1 week and 2 weeks | ↓ ZO−1 and claudin−1 expression level | [36] |
| Lungs | ||||||
| Nanoparticles | Nickel Oxide Nanoparticles | Rats | 50 and 150 cm2 for 10 min | Intratracheally instilled 1 day and 4 weeks | -Narrowed alveolar ducts and alveoli ↑ Levels of neutrophils and cytokines -Induced pulmonary microbiome dysbiosis in the acute phase | [37] |
| Nano and microplastics | Polyethylene particles | BALB/c mice | 10 mg/kg 1× daily for 1 week | Oral administration | ↑ Percentage of Th17, Tregs, and Th2 cells ↓ Colon length ↑ Percentages of IL-4+, Foxp3+(Tregs), and IL-17 in CD4+T cells ↑ Secretion of IL-4 and IL-17 cytokines | [13] |
| Microplastics | PS-MPs | C57BL/6 mice | 6.25 mg/kg PS-MPs | Intratracheally instilled 3× per week for 3 weeks | ↑ Expression of collagen ↑ Fibrosis with increased exposure ↑ Oxidative stress | [38] |
| Ozone | Ozone exposure | C57BL/6 mice | 1 ppm ozone for 1 h | Inhalation chamber 1× | ↑ Barrier disruption and epithelial cell permeability | [39] |
| Ozone | Ozone exposure | C57BL/6 mice | 1 ppm for the acute model and 1.5 ppm for the chronic model. | Acute phase: 1 h Chronic phase: 2 h, 2× per week for 6 weeks | Acute exposure induced the following: Disrupted tight junctions Desquamation of epithelial layer Chronic exposure to ozone particles remodeled the airway in mice using the following: ↑ Airspace density and diameter ↓ Number of airspaces ↓ Epithelium thickness | [14] |
| PAH | BaP | Sprague Dawley rats | 0.01 mg/kg | Intratracheally instilled for 7 days | ↑ Neutrophil recruitment ↑ Lung inflammation | [40] |
| PAH | Atmospheric PAHs | Human lung epithelial cell lines | Low dose | 30 days | Chronic exposure: Induced DNA damage Altered cellular homeostasis and ↑ ROS production | [41] |
| Particulate matter | PM2.5 | C57BL/6N mice | 1.8, 5.4, and 16.2 mg/kg | Intratracheally instilled for 1 week | PM2.5 exposure led to the following: Infiltration of inflammatory cells, ↑ serum cytokine levels in the serum ↑ Lung microbiome diversity ↓ Relative abundance of proteobacteria post-exposure ↑ Number of goblet cells | [42] |
| Particulate matter | Various | 18–64 years | Passive exposure | Average daily concentration | Exposure to air pollutants increased the risk for respiratory tract infections. | [43] |
| Circulatory system | ||||||
| Heavy Metal | Cadmium | Cohort study | Passive exposure | Measured urinary cadmium concentration | May increase the incidence of ischemic stroke | [44] |
| Heavy Metal | Mixed metals | Ischemic stroke patients | Passive exposure | Fasting blood concentration within 48 h post-diagnosis | Higher plasma concentrations of aluminum, arsenic, and cadmium may increase the risk of ischemic stroke. | [45] |
| Dioxin | TCDD | Primary human aortic endothelial cell | 0.1% TCDD | 24 h | ↑ Hypertension and endothelial dysfunction. | [46] |
| PAH | BaP | Sprague Dawley rats | 0.01 mg/kg | Intratracheally instilled for 7 days | ↑ Systolic and diastolic pressure and heart rate | [40] |
| PAH | BaP | Wistar rats | 20 mg/kg for 4 and 8 weeks | Intra-peritoneal. injection | ↑ Blood pressure after 8 weeks in vivo ↑ Vasoconstriction ex vivo | [47] |
| POPs | Passive exposure | Aged 70 yrs. in Sweden. 5-year follow-up | Passive exposure | Baseline plasma samples | PCB congeners, organochlorine pesticides, and octachlorodibenzo-p-dioxin showed increased risk of developing stroke in elderly population. | [48] |
| PCBs | Various PCBs | Endothelial cells from porcine pulmonary arteries | PCB 77, PCB 153, and PCB114 | 24 h | Exposure led to the development of atherosclerosis and endothelial barrier dysfunction. ↑ Albumin flux and oxidative stress | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggles, A.; Benakis, C. Exposure to Environmental Toxins: Potential Implications for Stroke Risk via the Gut– and Lung–Brain Axis. Cells 2024, 13, 803. https://doi.org/10.3390/cells13100803
Ruggles A, Benakis C. Exposure to Environmental Toxins: Potential Implications for Stroke Risk via the Gut– and Lung–Brain Axis. Cells. 2024; 13(10):803. https://doi.org/10.3390/cells13100803
Chicago/Turabian StyleRuggles, Alexandria, and Corinne Benakis. 2024. "Exposure to Environmental Toxins: Potential Implications for Stroke Risk via the Gut– and Lung–Brain Axis" Cells 13, no. 10: 803. https://doi.org/10.3390/cells13100803
APA StyleRuggles, A., & Benakis, C. (2024). Exposure to Environmental Toxins: Potential Implications for Stroke Risk via the Gut– and Lung–Brain Axis. Cells, 13(10), 803. https://doi.org/10.3390/cells13100803






