Biological Functions and Potential Therapeutic Significance of O-GlcNAcylation in Hepatic Cellular Stress and Liver Diseases
Abstract
1. Introduction
2. The Involvement of O-GlcNAcylation in Hepatic Cellular Stress
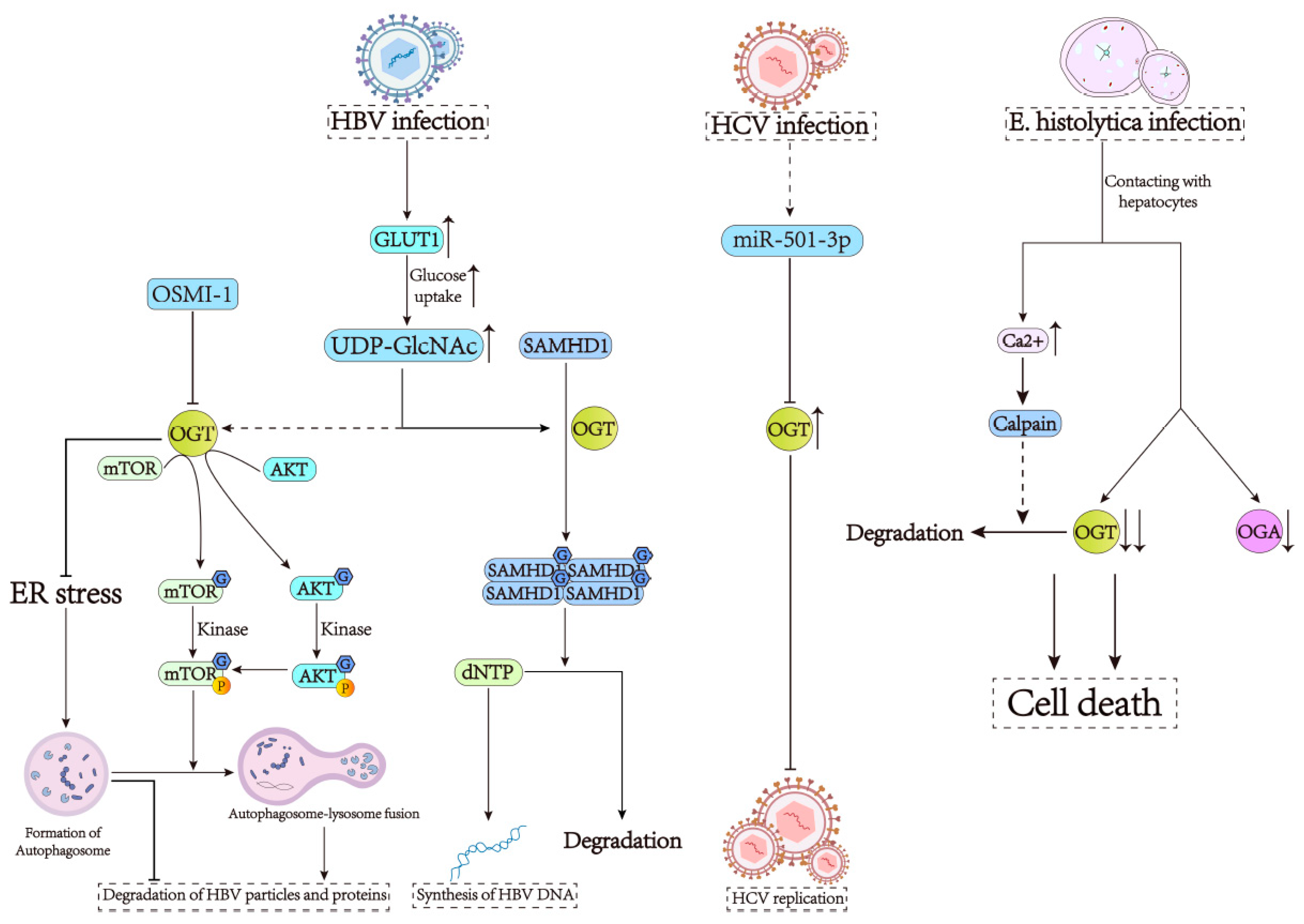
3. O-GlcNAcylation Is Required for the Development of Infectious Liver Diseases
4. O-GlcNAcylation Contributes to the Progression of NAFLD
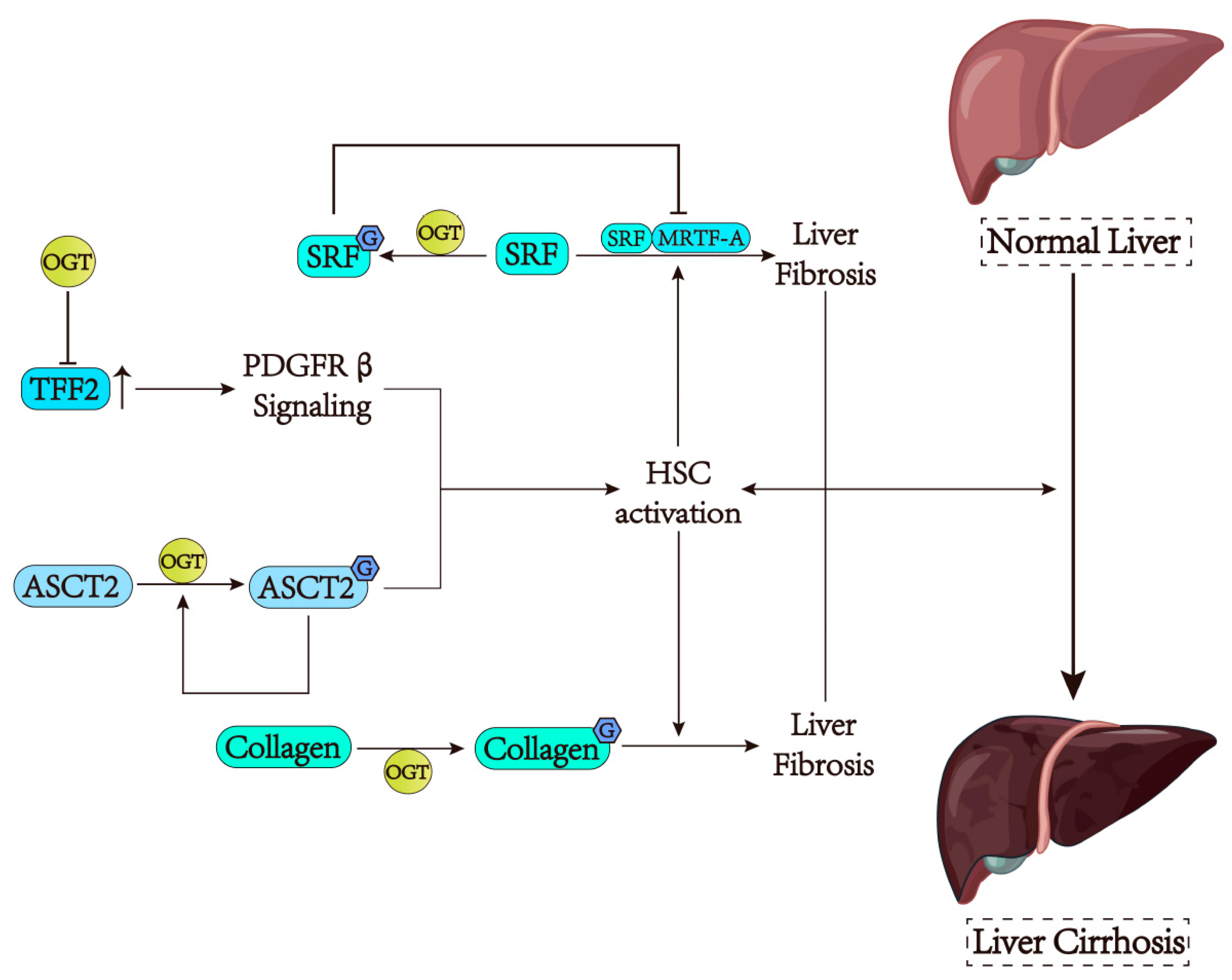
5. O-GlcNAcylation Has a Dual Influence on Liver Fibrosis
6. The Biological Function of O-GlcNAcylation in HCC
6.1. The Role of O-GlcNAcylation in the Carcinogenesis of HCC
6.2. The Effect of O-GlcNAcylation on the Proliferation of HCC
6.3. The Regulation of O-GlcNAcylation in the Metastasis of HCC
6.4. The Influence of O-GlcNAcylation on the Progression and Recurrence of HCC
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Spaner, D.E. O-GlcNAcylation in chronic lymphocytic leukemia and other blood cancers. Front. Immunol. 2021, 12, 772304. [Google Scholar] [CrossRef] [PubMed]
- McClain, D.A.; Crook, E.D. Hexosamines and insulin resistance. Diabetes 1996, 45, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.R.; Hart, G.W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 1984, 259, 3308–3317. [Google Scholar] [CrossRef] [PubMed]
- Lubas, W.A.; Hanover, J.A. Functional expression of O-linked GlcNAc transferase. Domain structure and substrate specificity. J. Biol. Chem. 2000, 275, 10983–10988. [Google Scholar] [CrossRef] [PubMed]
- Hart, G.W.; Housley, M.P.; Slawson, C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 2007, 446, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.J.; Zhang, X.; Lv, H.M.; Liu, Y.; Li, S.Z. Protein O-GlcNAcylation: The sweet hub in liver metabolic flexibility from a (patho)physiological perspective. Liver Int. 2024, 44, 293–315. [Google Scholar] [CrossRef] [PubMed]
- Robarts, D.R.; Kotulkar, M.; Paine-Cabrera, D.; Venneman, K.K.; Hanover, J.A.; Zachara, N.E.; Slawson, C.; Apte, U. The essential role of O-GlcNAcylation in hepatic differentiation. Hepatol. Commun. 2023, 7, e0283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, M.D.; Yin, R.; Liu, Y.; Yang, Y.; Mitchell-Richards, K.A.; Nam, J.H.; Li, R.; Wang, L.; Iwakiri, Y.; et al. O-GlcNAc transferase suppresses necroptosis and liver fibrosis. JCI Insight 2019, 4, e127709. [Google Scholar] [CrossRef]
- Walter, L.A.; Lin, Y.H.; Halbrook, C.J.; Chuh, K.N.; He, L.; Pedowitz, N.J.; Batt, A.R.; Brennan, C.K.; Stiles, B.L.; Lyssiotis, C.A.; et al. Inhibiting the hexosamine biosynthetic pathway lowers O-GlcNAcylation levels and sensitizes cancer to environmental stress. Biochemistry 2020, 59, 3169–3179. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, Y.; Wu, Q.; Chen, Y.; Zou, S.; Liu, X.; Zhu, G.; Zhao, Y.; Chen, Y.; Yu, Y.; et al. The essential role of YAP O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nat. Commun. 2017, 8, 15280. [Google Scholar] [CrossRef]
- You, Z.; Peng, D.; Cao, Y.; Zhu, Y.; Yin, J.; Zhang, G.; Peng, X. P53 suppresses the progression of hepatocellular carcinoma via miR-15a by decreasing OGT expression and EZH2 stabilization. J. Cell Mol. Med. 2021, 25, 9168–9182. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ong, Q.; Wong, C.C.; Chu, E.S.H.; Sung, J.J.Y.; Yang, X.; Yu, J. O-GlcNAcylation inhibits hepatic stellate cell activation. J. Gastroenterol. Hepatol. 2021, 36, 3477–3486. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Miao, Y.; Liu, Y.; Liu, X.; Fan, X.; Lin, Y.; Qian, P.; Zhou, J.; Dai, Y.; Xia, L.; et al. Reciprocal regulation between O-GlcNAcylation and beta-catenin facilitates cell viability and inhibits apoptosis in liver cancer. DNA Cell Biol. 2019, 38, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhang, X.; Zhang, Y.; Wang, Y.; Xu, Y.; Liu, X.; Sun, F.; Wang, J. High glucose stimulates tumorigenesis in hepatocellular carcinoma cells through AGER-dependent O-GlcNAcylation of c-Jun. Diabetes 2016, 65, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Lee, S.J.; Kim, S.J.; Lee, H.S.; Kwon, O.S. Curcumin ameliorates nonalcoholic fatty liver disease through inhibition of O-GlcNAcylation. Nutrients 2019, 11, 2702. [Google Scholar] [CrossRef]
- Baldini, S.F.; Wavelet, C.; Hainault, I.; Guinez, C.; Lefebvre, T. The nutrient-dependent O-GlcNAc modification controls the expression of liver fatty acid synthase. J. Mol. Biol. 2016, 428, 3295–3304. [Google Scholar] [CrossRef]
- Liu, Q.; Tao, T.; Liu, F.; Ni, R.; Lu, C.; Shen, A. Hyper-O-GlcNAcylation of YB-1 affects Ser102 phosphorylation and promotes cell proliferation in hepatocellular carcinoma. Exp. Cell Res. 2016, 349, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gou, D.; Xiang, J.; Pan, X.; Gao, Q.; Zhou, P.; Liu, Y.; Hu, J.; Wang, K.; Tang, N. O-GlcNAc modified-TIP60/KAT5 is required for PCK1 deficiency-induced HCC metastasis. Oncogene 2021, 40, 6707–6719. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Jiang, M.; Wu, N.; Xu, B.; Li, W.; Liu, H.; Su, S.; Shi, Y.; Liu, H.; Gao, X.; et al. O-GlcNAcylation of SIX1 enhances its stability and promotes hepatocellular carcinoma proliferation. Theranostics 2020, 10, 9830–9842. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Xiao, M.; Zhu, Y.; Gong, R.; Liu, J.; Zeng, Q.; Xu, C.; Chen, X.; Wang, F.; et al. RANBP2 activates O-GlcNAcylation through inducing CEBPalpha-dependent OGA downregulation to promote hepatocellular carcinoma malignant phenotypes. Cancers 2021, 13, 3475. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; White, E. Autophagy and metabolism. Science 2010, 330, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Wang, K. Autophagy and apoptosis in liver injury. Cell Cycle 2015, 14, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Guicciardi, M.E.; Malhi, H.; Mott, J.L.; Gores, G.J. Apoptosis and necrosis in the liver. Compr. Physiol. 2013, 3, 977–1010. [Google Scholar] [PubMed]
- Michalopoulos, G.K.; Bhushan, B. Liver regeneration: Biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.P.; Geisler, T.S.; Chambers, J.H.; McClain, D.A. Up-regulation of O-GlcNAc transferase with glucose deprivation in HepG2 cells is mediated by decreased hexosamine pathway flux. J. Biol. Chem. 2009, 284, 3425–3432. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.B.; Ma, Y.; Torres, S.; Zhang, B.; Feriod, C.; Heck, R.M.; Qian, K.; Fu, M.; Li, X.; Nathanson, M.H.; et al. Calcium-dependent O-GlcNAc signaling drives liver autophagy in adaptation to starvation. Genes Dev. 2017, 31, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Schworer, C.M.; Mortimore, G.E. Glucagon-induced autophagy and proteolysis in rat liver: Mediation by selective deprivation of intracellular amino acids. Proc. Natl. Acad. Sci. USA 1979, 76, 3169–3173. [Google Scholar] [CrossRef] [PubMed]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Mair, W.; Vasquez, D.S.; Joshi, A.; Gwinn, D.M.; Taylor, R.; et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 2011, 331, 456–461. [Google Scholar] [CrossRef]
- Ferrer, C.M.; Lynch, T.P.; Sodi, V.L.; Falcone, J.N.; Schwab, L.P.; Peacock, D.L.; Vocadlo, D.J.; Seagroves, T.N.; Reginato, M.J. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol. Cell 2014, 54, 820–831. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Yun, C.Y.; Oyadomari, S.; Novoa, I.; Zhang, Y.; Jungreis, R.; Nagata, K.; Harding, H.P.; Ron, D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004, 18, 3066–3077. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.; Kim, H.B.; Seo, H.; Kim, J.Y.; Choi, H.; Yoo, J.S.; Kim, J.W.; Cho, J.W. O-GlcNAcylation of eIF2alpha regulates the phospho-eIF2alpha-mediated ER stress response. Biochim. Biophys. Acta 2015, 1853, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Brunet, M.; Didelot, C.; Zermati, Y.; Schmitt, E.; Kroemer, G. Heat shock proteins 27 and 70: Anti-apoptotic proteins with tumorigenic properties. Cell Cycle 2006, 5, 2592–2601. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Biswas, M.H.; Zhang, C.; Du, C.; Balaji, K.C. Heat shock protein 27 mediates repression of androgen receptor function by protein kinase D1 in prostate cancer cells. Oncogene 2009, 28, 4386–4396. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Gan, L.; Zhang, S.; Cui, F.J.; Cun, W.; Li, Y.; Kang, N.X.; Gao, M.D.; Liu, K.Y. Translocation of HSP27 into liver cancer cell nucleus may be associated with phosphorylation and O-GlcNAc glycosylation. Oncol. Rep. 2012, 28, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Li, Y.; Wang, J.; Ma, J.; Zhao, S.; Ye, X.; He, L.; Yang, J.; Gao, M.; Xiao, F.; et al. Deficient O-GlcNAc glycosylation impairs regulatory T cell differentiation and notch signaling in autoimmune hepatitis. Front. Immunol. 2018, 9, 2089. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Luedde, T. Apoptosis and necroptosis in the liver: A matter of life and death. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.B.; Rodrigues, P.M.; Carvalho, T.; Caridade, M.; Borralho, P.; Cortez-Pinto, H.; Castro, R.E.; Rodrigues, C.M. Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis. Clin. Sci. 2015, 129, 721–739. [Google Scholar] [CrossRef]
- Gautheron, J.; Vucur, M.; Reisinger, F.; Cardenas, D.V.; Roderburg, C.; Koppe, C.; Kreggenwinkel, K.; Schneider, A.T.; Bartneck, M.; Neumann, U.P.; et al. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol. Med. 2014, 6, 1062–1074. [Google Scholar] [CrossRef]
- Linkermann, A.; Green, D.R. Necroptosis. N. Engl. J. Med. 2014, 370, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Robarts, D.R.; McGreal, S.R.; Umbaugh, D.S.; Parkes, W.S.; Kotulkar, M.; Abernathy, S.; Lee, N.; Jaeschke, H.; Gunewardena, S.; Whelan, S.A.; et al. Regulation of liver regeneration by hepatocyte O-GlcNAcylation in mice. Cell Mol. Gastroenterol. Hepatol. 2022, 13, 1510–1529. [Google Scholar] [CrossRef] [PubMed]
- Huck, I.; Gunewardena, S.; Espanol-Suner, R.; Willenbring, H.; Apte, U. Hepatocyte nuclear factor 4 alpha activation is essential for termination of liver regeneration in mice. Hepatology 2019, 70, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.A.; Weinstein, M.L.; Lindenmayer, G.E.; Buse, M.G. Effects of diabetes and hyperglycemia on the hexosamine synthesis pathway in rat muscle and liver. Diabetes 1995, 44, 1438–1446. [Google Scholar] [CrossRef]
- Taylor, R.P.; Parker, G.J.; Hazel, M.W.; Soesanto, Y.; Fuller, W.; Yazzie, M.J.; McClain, D.A. Glucose deprivation stimulates O-GlcNAc modification of proteins through up-regulation of O-linked N-acetylglucosaminyltransferase. J. Biol. Chem. 2008, 283, 6050–6057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, W.; Cao, Y.; Kou, L.; Liu, C.; Li, X.; Zhang, B.; Guo, W.; Xu, B.; Li, S. O-GlcNAcylation regulates long-chain fatty acid metabolism by inhibiting ACOX1 ubiquitination-dependent degradation. Int. J. Biol. Macromol. 2024, 266, 131151. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Yang, Y.; Lian, S.; Shi, H.; Liu, P.; Liu, Y.; Yang, H.; Li, S. Effects of acute cold stress on liver O-GlcNAcylation and glycometabolism in mice. Int. J. Mol. Sci. 2018, 19, 2815. [Google Scholar] [CrossRef]
- Chen, C.; Liu, T.S.; Zhao, S.C.; Yang, W.Z.; Chen, Z.P.; Yan, Y. XIAP impairs mitochondrial function during apoptosis by regulating the Bcl-2 family in renal cell carcinoma. Exp. Ther. Med. 2018, 15, 4587–4593. [Google Scholar] [CrossRef]
- Yang, J.; Pi, C.; Wang, G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed. Pharmacother. 2018, 103, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Liu, B.B.; Qian, X.D.; Li, L.Q.; Cao, H.B.; Guo, Q.S.; Zhou, G.F. Crocin induces autophagic apoptosis in hepatocellular carcinoma by inhibiting Akt/mTOR activity. Onco Targets Ther. 2018, 11, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.P.; McGreal, S.R.; Graw, S.; Tessman, R.; Koppel, S.J.; Dhakal, P.; Zhang, Z.; Machacek, M.; Zachara, N.E.; Koestler, D.C.; et al. O-GlcNAcylation reprograms mitochondrial function to regulate energy metabolism. J. Biol. Chem. 2017, 292, 14940–14962. [Google Scholar] [CrossRef]
- Enomoto, A.; Itoh, K.; Nagayoshi, E.; Haruta, J.; Kimura, T.; O’Connor, T.; Harada, T.; Yamamoto, M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 2001, 59, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.C.; Mulcahy, R.T. Regulation of gamma-glutamylcysteine synthetase subunit gene expression: Insights into transcriptional control of antioxidant defenses. Free Radic. Res. 2000, 32, 281–301. [Google Scholar] [CrossRef] [PubMed]
- McGreal, S.R.; Bhushan, B.; Walesky, C.; McGill, M.R.; Lebofsky, M.; Kandel, S.E.; Winefield, R.D.; Jaeschke, H.; Zachara, N.E.; Zhang, Z.; et al. Modulation of O-GlcNAc levels in the liver impacts acetaminophen-induced liver injury by affecting protein adduct formation and glutathione synthesis. Toxicol. Sci. 2018, 162, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Ramachandran, A.; Jaeschke, H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 2016, 10, 148–156. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; Williams, C.D.; Xie, Y.; Ramachandran, A.; Jaeschke, H. Acetaminophen-induced liver injury in rats and mice: Comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol. Appl. Pharmacol. 2012, 264, 387–394. [Google Scholar] [CrossRef]
- Han, D.; Shinohara, M.; Ybanez, M.D.; Saberi, B.; Kaplowitz, N. Signal transduction pathways involved in drug-induced liver injury. Handb. Exp. Pharmacol. 2010, 196, 267–310. [Google Scholar]
- European Association for the Study of the Liver. Electronic address: Easloffice@easloffice.eu; European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.M. Hepatitis C Virus resistance to direct-acting antiviral drugs in interferon-free regimens. Gastroenterology 2016, 151, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Y.; Liu, S.; Zhu, Y.; Lu, K.; Broering, R.; Lu, M. O-GlcNAcylation modulates HBV replication through regulating cellular autophagy at multiple levels. FASEB J. 2020, 34, 14473–14489. [Google Scholar] [CrossRef] [PubMed]
- Sze, A.; Olagnier, D.; Lin, R.; van Grevenynghe, J.; Hiscott, J. SAMHD1 host restriction factor: A link with innate immune sensing of retrovirus infection. J. Mol. Biol. 2013, 425, 4981–4994. [Google Scholar] [CrossRef]
- Hu, J.; Gao, Q.; Yang, Y.; Xia, J.; Zhang, W.; Chen, Y.; Zhou, Z.; Chang, L.; Hu, Y.; Zhou, H.; et al. Hexosamine biosynthetic pathway promotes the antiviral activity of SAMHD1 by enhancing O-GlcNAc transferase-mediated protein O-GlcNAcylation. Theranostics 2021, 11, 805–823. [Google Scholar] [CrossRef]
- Jeong, G.U.; Park, I.H.; Ahn, K.; Ahn, B.Y. Inhibition of hepatitis B virus replication by a dNTPase-dependent function of the host restriction factor SAMHD1. Virology 2016, 495, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, J.; Mell, J.C.; Bouchard, M.J. Transcriptome-wide analysis of hepatitis B virus-mediated changes to normal hepatocyte gene expression. PLoS Pathog. 2016, 12, e1005438. [Google Scholar] [CrossRef] [PubMed]
- Housley, M.P.; Rodgers, J.T.; Udeshi, N.D.; Kelly, T.J.; Shabanowitz, J.; Hunt, D.F.; Puigserver, P.; Hart, G.W. O-GlcNAc regulates FoxO activation in response to glucose. J. Biol. Chem. 2008, 283, 16283–16292. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Wang, Z.; Liu, K.; Wang, Y.; Liu, J.; Ding, H.; Yuan, Z. Subversion of cellular autophagy machinery by hepatitis B virus for viral envelopment. J. Virol. 2011, 85, 6319–6333. [Google Scholar] [CrossRef]
- Tang, H.; Da, L.; Mao, Y.; Li, Y.; Li, D.; Xu, Z.; Li, F.; Wang, Y.; Tiollais, P.; Li, T.; et al. Hepatitis B virus X protein sensitizes cells to starvation-induced autophagy via up-regulation of beclin 1 expression. Hepatology 2009, 49, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Greis, K.D.; Gibson, W.; Hart, G.W. Site-specific glycosylation of the human cytomegalovirus tegument basic phosphoprotein (UL32) at serine 921 and serine 952. J. Virol. 1994, 68, 8339–8349. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.A.; Burrone, O.R. Rotavirus NS26 is modified by addition of single O-linked residues of N-acetylglucosamine. Virology 1991, 182, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, M.B.; Crouchet, E.; Baumert, T.F.; Schuster, C. Host-targeting agents to prevent and cure hepatitis c virus infection. Viruses 2015, 7, 5659–5685. [Google Scholar] [CrossRef]
- Herzog, K.; Bandiera, S.; Pernot, S.; Fauvelle, C.; Juhling, F.; Weiss, A.; Bull, A.; Durand, S.C.; Chane-Woon-Ming, B.; Pfeffer, S.; et al. Functional microRNA screen uncovers O-linked N-acetylglucosamine transferase as a host factor modulating hepatitis C virus morphogenesis and infectivity. Gut 2020, 69, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Min, A.; Shin, M.H. O-deGlcNAcylation is required for Entamoeba histolytica-induced HepG2 cell death. Microb. Pathog. 2018, 123, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Huston, C.D.; Houpt, E.R.; Mann, B.J.; Hahn, C.S.; Petri, W.A., Jr. Caspase 3-dependent killing of host cells by the parasite Entamoeba histolytica. Cell Microbiol. 2000, 2, 617–625. [Google Scholar] [CrossRef]
- Kim, K.A.; Lee, Y.A.; Shin, M.H. Calpain-dependent calpastatin cleavage regulates caspase-3 activation during apoptosis of Jurkat T cells induced by Entamoeba histolytica. Int. J. Parasitol. 2007, 37, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Banerjee, R.; Nandi, N.; Sardar, A.H.; Das, P. Anoikis potential of Entameba histolytica secretory cysteine proteases: Evidence of contact independent host cell death. Microb. Pathog. 2012, 52, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Villalba-Magdaleno, J.D.; Perez-Ishiwara, G.; Serrano-Luna, J.; Tsutsumi, V.; Shibayama, M. In vivo programmed cell death of Entamoeba histolytica trophozoites in a hamster model of amoebic liver abscess. Microbiology 2011, 157, 1489–1499. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Champattanachai, V.; Marchase, R.B.; Chatham, J.C. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am. J. Physiol. Cell Physiol. 2007, 292, C178–C187. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Yang, S.; Champattanachai, V.; Hu, S.; Chaudry, I.H.; Marchase, R.B.; Chatham, J.C. Glucosamine improves cardiac function following trauma-hemorrhage by increased protein O-GlcNAcylation and attenuation of NF-kappaB signaling. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H515–H523. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Love, D.C.; Hanover, J.A. Elevated O-GlcNAc-dependent signaling through inducible mOGT expression selectively triggers apoptosis. Amino Acids 2011, 40, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, K.; Hernandez-Ramirez, V.I.; Rios, A.; Mondragon, R.; Talamas-Rohana, P. Entamoeba histolytica: Monoclonal antibody against the beta1 integrin-like molecule (140 kDa) inhibits cell adhesion to extracellular matrix components. Exp. Parasitol. 2001, 98, 83–89. [Google Scholar] [CrossRef]
- Lee, Y.A.; Kim, K.A.; Shin, M.H. Calpain mediates degradation of cytoskeletal proteins during Jurkat T-cell death induced by Entamoeba histolytica. Parasite Immunol. 2011, 33, 349–356. [Google Scholar] [CrossRef]
- Perla, F.M.; Prelati, M.; Lavorato, M.; Visicchio, D.; Anania, C. The role of lipid and lipoprotein metabolism in non-alcoholic fatty liver disease. Children 2017, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Khamphaya, T.; Chukijrungroat, N.; Saengsirisuwan, V.; Mitchell-Richards, K.A.; Robert, M.E.; Mennone, A.; Ananthanarayanan, M.; Nathanson, M.H.; Weerachayaphorn, J. Nonalcoholic fatty liver disease impairs expression of the type II inositol 1,4,5-trisphosphate receptor. Hepatology 2018, 67, 560–574. [Google Scholar] [CrossRef] [PubMed]
- Lockridge, A.; Hanover, J.A. A nexus of lipid and O-Glcnac metabolism in physiology and disease. Front. Endocrinol. 2022, 13, 943576. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, S.; Mallanna, S.H.; Nandini, C.D. Diet-inducing hypercholesterolemia show decreased O-GlcNAcylation of liver proteins through modulation of AMPK. J. Physiol. Biochem. 2024, 80, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Repa, J.J. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J. Biol. Chem. 2007, 282, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Bindesboll, C.; Fan, Q.; Norgaard, R.C.; MacPherson, L.; Ruan, H.B.; Wu, J.; Pedersen, T.A.; Steffensen, K.R.; Yang, X.; Matthews, J.; et al. Liver X receptor regulates hepatic nuclear O-GlcNAc signaling and carbohydrate responsive element-binding protein activity. J. Lipid Res. 2015, 56, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Anthonisen, E.H.; Berven, L.; Holm, S.; Nygard, M.; Nebb, H.I.; Gronning-Wang, L.M. Nuclear receptor liver X receptor is O-GlcNAc-modified in response to glucose. J. Biol. Chem. 2010, 285, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Guinez, C.; Filhoulaud, G.; Rayah-Benhamed, F.; Marmier, S.; Dubuquoy, C.; Dentin, R.; Moldes, M.; Burnol, A.F.; Yang, X.; Lefebvre, T.; et al. O-GlcNAcylation increases ChREBP protein content and transcriptional activity in the liver. Diabetes 2011, 60, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.L.; Liu, H.; Peng, J.; Gan, L.; Lu, L.; Zhang, Q.; Li, L.; He, F.; Jiang, Y. Effects of farnesoid X receptor on the expression of the fatty acid synthetase and hepatic lipase. Mol. Biol. Rep. 2011, 38, 553–559. [Google Scholar] [CrossRef]
- Berrabah, W.; Aumercier, P.; Gheeraert, C.; Dehondt, H.; Bouchaert, E.; Alexandre, J.; Ploton, M.; Mazuy, C.; Caron, S.; Tailleux, A.; et al. Glucose sensing O-GlcNAcylation pathway regulates the nuclear bile acid receptor farnesoid X receptor (FXR). Hepatology 2014, 59, 2022–2033. [Google Scholar] [CrossRef]
- Sangwan, S.; Bhattacharyya, R.; Banerjee, D. Plastic compounds and liver diseases: Whether bisphenol A is the only culprit. Liver Int. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Tovar, E.; Muriel, P. NLRP3 inflammasome in hepatic diseases: A pharmacological target. Biochem. Pharmacol. 2023, 217, 115861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, S.; Li, T.; Zhu, L.; Wei, F. Bisphenol A induces non-alcoholic fatty liver disease by promoting the O-GlcNAcylation of NLRP3. Arch. Physiol. Biochem. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lebrecht, D.; Vargas-Infante, Y.A.; Setzer, B.; Kirschner, J.; Walker, U.A. Uridine supplementation antagonizes zalcitabine-induced microvesicular steatohepatitis in mice. Hepatology 2007, 45, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Urasaki, Y.; Pizzorno, G. Uridine prevents fenofibrate-induced fatty liver. PLoS ONE 2014, 9, e87179. [Google Scholar] [CrossRef] [PubMed]
- Urasaki, Y.; Pizzorno, G.; Le, T.T. Chronic Uridine Administration induces fatty liver and pre-diabetic conditions in mice. PLoS ONE 2016, 11, e0146994. [Google Scholar] [CrossRef] [PubMed]
- Guzman, C.; Benet, M.; Pisonero-Vaquero, S.; Moya, M.; Garcia-Mediavilla, M.V.; Martinez-Chantar, M.L.; Gonzalez-Gallego, J.; Castell, J.V.; Sanchez-Campos, S.; Jover, R. The human liver fatty acid binding protein (FABP1) gene is activated by FOXA1 and PPARalpha; and repressed by C/EBPalpha: Implications in FABP1 down-regulation in nonalcoholic fatty liver disease. Biochim. Biophys. Acta 2013, 1831, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridi, A.M.; Chrysavgis, L.; Koutsilieris, M.; Chatzigeorgiou, A. The role of senescence in the development of nonalcoholic fatty liver disease and progression to nonalcoholic steatohepatitis. Hepatology 2020, 71, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Yang, X.; Qian, K. Protein O-GlcNAcylation: Emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2017, 18, 452–465. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Tian, M.; Zhang, H.; Lou, L.; Liu, K.; Zhang, J.; Zhao, Y.; Zhang, J.; Le, S.; et al. Comparative proteomic profiling reveals a pathogenic role for the O-GlcNAcylated AIMP2-PARP1 complex in aging-related hepatic steatosis in mice. FEBS Lett. 2022, 596, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E. NAD+ in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Hirschey, M.D.; Shimazu, T.; Jing, E.; Grueter, C.A.; Collins, A.M.; Aouizerat, B.; Stancakova, A.; Goetzman, E.; Lam, M.M.; Schwer, B.; et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell 2011, 44, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Pfluger, P.T.; Herranz, D.; Velasco-Miguel, S.; Serrano, M.; Tschop, M.H. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl. Acad. Sci. USA 2008, 105, 9793–9798. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Kim, E.R.; Lee, M.; Choi, D.H.; Kim, S.H.; Shin, E.; Kim, J.H.; Cho, J.W.; Han, D.H.; Cha, B.S.; et al. Increased expression of sodium-glucose cotransporter 2 and O-GlcNAcylation in hepatocytes drives non-alcoholic steatohepatitis. Metabolism 2023, 145, 155612. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rellan, M.J.; Parracho, T.; Heras, V.; Rodriguez, A.; Fondevila, M.F.; Novoa, E.; Lima, N.; Varela-Rey, M.; Senra, A.; Chantada-Vazquez, M.D.P.; et al. Hepatocyte-specific O-GlcNAc transferase downregulation ameliorates nonalcoholic steatohepatitis by improving mitochondrial function. Mol. Metab. 2023, 75, 101776. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, L.; Issad, T. O-GlcNAcylation and inflammation: A vast territory to explore. Front. Endocrinol. 2014, 5, 235. [Google Scholar] [CrossRef]
- Sanchez-Valle, V.; Chavez-Tapia, N.C.; Uribe, M.; Mendez-Sanchez, N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Yang, N.; Huang, Y.; Hu, T.; Rao, C. Endoplasmic reticulum stress-mediated cell death in liver injury. Cell Death Dis. 2022, 13, 1051. [Google Scholar] [CrossRef] [PubMed]
- So, J.S.; Hur, K.Y.; Tarrio, M.; Ruda, V.; Frank-Kamenetsky, M.; Fitzgerald, K.; Koteliansky, V.; Lichtman, A.H.; Iwawaki, T.; Glimcher, L.H.; et al. Silencing of lipid metabolism genes through IRE1alpha-mediated mRNA decay lowers plasma lipids in mice. Cell Metab. 2012, 16, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Nam, M.J.; Lee, D.E.; Park, J.W.; Kang, B.S.; Lee, D.S.; Lee, H.S.; Kwon, O.S. Silibinin ameliorates O-GlcNAcylation and inflammation in a mouse model of nonalcoholic steatohepatitis. Int. J. Mol. Sci. 2018, 19, 2165. [Google Scholar] [CrossRef] [PubMed]
- Visinoni, S.; Khalid, N.F.; Joannides, C.N.; Shulkes, A.; Yim, M.; Whitehead, J.; Tiganis, T.; Lamont, B.J.; Favaloro, J.M.; Proietto, J.; et al. The role of liver fructose-1,6-bisphosphatase in regulating appetite and adiposity. Diabetes 2012, 61, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis—A common pathway to organ injury and failure. N. Engl. J. Med. 2015, 373, 96. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Hong, W.; Shao, Y.; Lv, F.; Fan, Z.; Li, P.; Xu, Y.; Guo, J. Ablation of serum response factor in hepatic stellate cells attenuates liver fibrosis. J. Mol. Med. 2019, 97, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Ren, M.; Rockey, D.C. Myocardin and myocardin-related transcription factor—A synergistically mediate actin cytoskeletal-dependent inhibition of liver fibrogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G504–G517. [Google Scholar] [CrossRef] [PubMed]
- Ise, H.; Araki, Y.; Song, I.; Akatsuka, G. N-acetylglucosamine-bearing polymers mimicking O-GlcNAc-modified proteins elicit anti-fibrotic activities in myofibroblasts and activated stellate cells. Glycobiology 2022, 33, 17–37. [Google Scholar] [CrossRef]
- Zhang, B.; Lapenta, K.; Wang, Q.; Nam, J.H.; Chung, D.; Robert, M.E.; Nathanson, M.H.; Yang, X. Trefoil factor 2 secreted from damaged hepatocytes activates hepatic stellate cells to induce fibrogenesis. J. Biol. Chem. 2021, 297, 100887. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Chuan, S.; Hongshan, W. Protein O glycosylation regulates activation of hepatic stellate cells. Inflammation 2013, 36, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Harvey, L.D.; Chan, S.Y. YAPping about glutaminolysis in hepatic fibrosis. Gastroenterology 2018, 154, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, L.; Zhang, B.; Li, Z.; Shen, M.; Tang, L.; Zhang, Z.; Shao, J.; Zhang, F.; Zheng, S.; et al. O-GlcNAcylation coordinates glutaminolysis by regulating the stability and membrane trafficking of ASCT2 in hepatic stellate cells. J. Clin. Transl. Hepatol. 2022, 10, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Roberts, L.R. Hepatocellular carcinoma: A global view. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Fasani, P.; Sangiovanni, A.; De Fazio, C.; Borzio, M.; Bruno, S.; Ronchi, G.; Del Ninno, E.; Colombo, M. High prevalence of multinodular hepatocellular carcinoma in patients with cirrhosis attributable to multiple risk factors. Hepatology 1999, 29, 1704–1707. [Google Scholar] [CrossRef] [PubMed]
- Harbour, J.W.; Dean, D.C. Rb function in cell-cycle regulation and apoptosis. Nat. Cell Biol. 2000, 2, E65–E67. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.; Chen, J.; Du, P.; Liu, Q.; Cheng, Q.; Li, X.; Zhang, B.; Chen, X.; Jiang, L. The crosstalk network of XIST/miR-424-5p/OGT mediates RAF1 glycosylation and participates in the progression of liver cancer. Liver Int. 2021, 41, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhou, L.; Yang, Z.; Lai, M.; Xie, H.; Wu, L.; Xing, C.; Zhang, F.; Zheng, S. O-GlcNAcylation plays a role in tumor recurrence of hepatocellular carcinoma following liver transplantation. Med. Oncol. 2012, 29, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.J.; McDonough, P.M.; Swanson, E.; Trost, S.U.; Suzuki, M.; Fukuda, M.; Dillmann, W.H. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J. Biol. Chem. 2003, 278, 44230–44237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef] [PubMed]
- Overholtzer, M.; Zhang, J.; Smolen, G.A.; Muir, B.; Li, W.; Sgroi, D.C.; Deng, C.X.; Brugge, J.S.; Haber, D.A. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc. Natl. Acad. Sci. USA 2006, 103, 12405–12410. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Shimotohno, K. Ubiquitination and proteasome-dependent degradation of human eukaryotic translation initiation factor 4E. J. Biol. Chem. 2006, 281, 20788–20800. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Duan, M.; Xing, Y.; Liu, C.; Yang, F.; Li, Y.; Yang, T.; Wei, Y.; Gao, Q.; Jiang, J. O-GlcNAc transferase activates stem-like cell potential in hepatocarcinoma through O-GlcNAcylation of eukaryotic initiation factor 4E. J. Cell Mol. Med. 2019, 23, 2384–2398. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, G.; Liu, Y.; Wu, Q.; Zhang, X.; Bian, Z.; Zhang, Y.; Pan, Q.; Sun, F. O-GlcNAcylated c-Jun antagonizes ferroptosis via inhibiting GSH synthesis in liver cancer. Cell Signal. 2019, 63, 109384. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.B.; Han, X.; Li, M.D.; Singh, J.P.; Qian, K.; Azarhoush, S.; Zhao, L.; Bennett, A.M.; Samuel, V.T.; Wu, J.; et al. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1alpha stability. Cell Metab. 2012, 16, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.J.; Feng, Y.H.; Gu, B.H.; Li, Y.M.; Chen, H. The post-translational modification, SUMOylation, and cancer (Review). Int. J. Oncol. 2018, 52, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Augustin, R. The protein family of glucose transport facilitators: It’s not only about glucose after all. IUBMB Life. 2010, 62, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.K.; Hwang, S.Y.; Park, M.K.; Bae, H.I.; Kim, W.H.; Kim, J.C.; Kim, M. Fatty acid-CoA ligase 4 is overexpressed in human hepatocellular carcinoma. Cancer Sci. 2003, 94, 421–424. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Yuan, J.; Wang, J.; Shen, X. The positive feedback between ACSL4 expression and O-GlcNAcylation contributes to the growth and survival of hepatocellular carcinoma. Aging 2020, 12, 7786–7800. [Google Scholar] [CrossRef]
- Peneff, C.; Ferrari, P.; Charrier, V.; Taburet, Y.; Monnier, C.; Zamboni, V.; Winter, J.; Harnois, M.; Fassy, F.; Bourne, Y. Crystal structures of two human pyrophosphorylase isoforms in complexes with UDPGlc(Gal)NAc: Role of the alternatively spliced insert in the enzyme oligomeric assembly and active site architecture. EMBO J. 2001, 20, 6191–6202. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S. Role of insulin, adipocyte hormones, and nutrient-sensing pathways in regulating fuel metabolism and energy homeostasis: A nutritional perspective of diabetes, obesity, and cancer. Sci. STKE 2006, 2006, re7. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liang, Y.; Kang, L.; Liu, Y.; Gao, S.; Chen, S.; Li, Y.; You, W.; Dong, Q.; Hong, T.; et al. Transcriptional regulation of the warburg effect in cancer by SIX1. Cancer Cell 2018, 33, 368–385 e367. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Davicioni, E.; Triche, T.J.; Merlino, G. The homeoprotein six1 transcriptionally activates multiple protumorigenic genes but requires ezrin to promote metastasis. Cancer Res. 2006, 66, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.T.; Lee, T.K.; Cheng, Q.; Wo, J.Y.; Sun, C.K.; Guo, D.Y.; Lim, Z.X.; Lo, C.M.; Poon, R.T.; Fan, S.T.; et al. Suppression of tumorigenesis and metastasis of hepatocellular carcinoma by shRNA interference targeting on homeoprotein Six1. Int. J. Cancer 2010, 127, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Shtraizent, N.; DeRossi, C.; Nayar, S.; Sachidanandam, R.; Katz, L.S.; Prince, A.; Koh, A.P.; Vincek, A.; Hadas, Y.; Hoshida, Y.; et al. MPI depletion enhances O-GlcNAcylation of p53 and suppresses the Warburg effect. Elife 2017, 6, e22477. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.S.; La, Z.; Yang, L.; He, Q.; Li, P. p53 gene in treatment of hepatic carcinoma: Status quo. World J. Gastroenterol. 2007, 13, 985–992. [Google Scholar] [CrossRef]
- Bensaad, K.; Tsuruta, A.; Selak, M.A.; Vidal, M.N.; Nakano, K.; Bartrons, R.; Gottlieb, E.; Vousden, K.H. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 2006, 126, 107–120. [Google Scholar] [CrossRef]
- Pepe, F.; Pagotto, S.; Soliman, S.; Rossi, C.; Lanuti, P.; Braconi, C.; Mariani-Costantini, R.; Visone, R.; Veronese, A. Regulation of miR-483-3p by the O-linked N-acetylglucosamine transferase links chemosensitivity to glucose metabolism in liver cancer cells. Oncogenesis 2017, 6, e328. [Google Scholar] [CrossRef] [PubMed]
- Gartel, A.L.; Najmabadi, F.; Goufman, E.; Tyner, A.L. A role for E2F1 in Ras activation of p21(WAF1/CIP1) transcription. Oncogene 2000, 19, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Gui, C.Y.; Ngo, L.; Xu, W.S.; Richon, V.M.; Marks, P.A. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc. Natl. Acad. Sci. USA 2004, 101, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Tao, T.; Zhang, D.; Liu, X.; Qiu, H.; Han, L.; Xu, Z.; Xiao, Y.; Cheng, C.; Shen, A. O-GlcNAcylation of histone deacetylases 1 in hepatocellular carcinoma promotes cancer progression. Glycobiology 2016, 26, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jonasch, E.; Alexander, A.; Short, J.D.; Cai, S.; Wen, S.; Tsavachidou, D.; Tamboli, P.; Czerniak, B.A.; Do, K.A.; et al. Cytoplasmic sequestration of p27 via AKT phosphorylation in renal cell carcinoma. Clin. Cancer Res. 2009, 15, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Liu, F.; Tao, T.; Zhang, D.; Liu, X.; Zhu, G.; Xu, Z.; Ni, R.; Shen, A. Modification of p27 with O-linked N-acetylglucosamine regulates cell proliferation in hepatocellular carcinoma. Mol. Carcinog. 2017, 56, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Buren, S.; Gomes, A.L.; Teijeiro, A.; Fawal, M.A.; Yilmaz, M.; Tummala, K.S.; Perez, M.; Rodriguez-Justo, M.; Campos-Olivas, R.; Megias, D.; et al. Regulation of OGT by URI in response to glucose confers c-MYC-dependent survival mechanisms. Cancer Cell 2016, 30, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Liu, H.; Tin, K.X.; Huang, Y.; Yeh, K.H.; Peng, H.W.; Chen, H.D.; He, J.Y.; Chiang, Y.J.; Liu, C.S.; et al. Identification of UAP1L1 as a critical factor for protein O-GlcNAcylation and cell proliferation in human hepatoma cells. Oncogene 2019, 38, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, X.; Wu, J.L.; Fu, L.; Liu, K.; Liu, D.; Chen, G.G.; Lai, P.B.; Wong, N.; Yu, J. O-GlcNAc transferase promotes fatty liver-associated liver cancer through inducing palmitic acid and activating endoplasmic reticulum stress. J. Hepatol. 2017, 67, 310–320. [Google Scholar] [CrossRef]
- Vucur, M.; Reisinger, F.; Gautheron, J.; Janssen, J.; Roderburg, C.; Cardenas, D.V.; Kreggenwinkel, K.; Koppe, C.; Hammerich, L.; Hakem, R.; et al. RIP3 inhibits inflammatory hepatocarcinogenesis but promotes cholestasis by controlling caspase-8- and JNK-dependent compensatory cell proliferation. Cell Rep. 2013, 4, 776–790. [Google Scholar] [CrossRef]
- Jhu, J.W.; Yan, J.B.; Lin, Z.H.; Lin, S.C.; Peng, I.C. SREBP1-induced glutamine synthetase triggers a feedforward loop to upregulate SREBP1 through Sp1 O-GlcNAcylation and augments lipid droplet formation in cancer cells. Int. J. Mol. Sci. 2021, 22, 9814. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Vleminckx, K.; Vakaet, L., Jr.; Mareel, M.; Fiers, W.; van Roy, F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 1991, 66, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wu, Q.; Guo, X.; Huang, T.; Xie, X.; Wang, L.; Liu, Y.; Shi, L.; Li, W.; Zhang, J.; et al. O-GlcNAcylation promotes the migratory ability of hepatocellular carcinoma cells via regulating FOXA2 stability and transcriptional activity. J. Cell Physiol. 2021, 236, 7491–7503. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Y.; Zhang, C.; Chen, X.; Huang, H.; Li, W.; Zhang, J.; Liu, Y. Upregulation of OGT by Caveolin-1 promotes hepatocellular carcinoma cell migration and invasion. Cell Biol. Int. 2021, 45, 2251–2263. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, H.; Liu, M.; Wu, Q.; Li, W.; Zhang, J. MicroRNA-24-1 suppresses mouse hepatoma cell invasion and metastasis via directly targeting O-GlcNAc transferase. Biomed. Pharmacother. 2017, 91, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Wu, H.; Jia, D.; Wu, W.; Ren, S.; Wang, L.; Song, S.; Guo, X.; Liu, F.; Ruan, Y.; et al. O-GlcNAcylation of RACK1 promotes hepatocellular carcinogenesis. J. Hepatol. 2018, 68, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bei, J.; Liu, M.; Huang, J.; Xie, L.; Huang, W.; Cai, M.; Guo, Y.; Lin, L.; Zhu, K. Sublethal heat stress-induced O-GlcNAcylation coordinates the Warburg effect to promote hepatocellular carcinoma recurrence and metastasis after thermal ablation. Cancer Lett. 2021, 518, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Bieging, K.T.; Mello, S.S.; Attardi, L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer 2014, 14, 359–370. [Google Scholar] [CrossRef]
- Lee, S.J.; Kwon, O.S. O-GlcNAc transferase inhibitor synergistically enhances doxorubicin-induced apoptosis in HepG2 cells. Cancers 2020, 12, 3154. [Google Scholar] [CrossRef]
- Zhang, C.; Niu, Y.; Wang, Z.; Xu, X.; Li, Y.; Ma, L.; Wang, J.; Yu, Y. Corosolic acid inhibits cancer progression by decreasing the level of CDK19-mediated O-GlcNAcylation in liver cancer cells. Cell Death Dis. 2021, 12, 889. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wu, C.; Hart, G.W. Analytical and biochemical perspectives of protein O-GlcNAcylation. Chem. Rev. 2021, 121, 1513–1581. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Z.; Mu, J.; Gao, Z.; Huang, S.; Chen, L. Biological Functions and Potential Therapeutic Significance of O-GlcNAcylation in Hepatic Cellular Stress and Liver Diseases. Cells 2024, 13, 805. https://doi.org/10.3390/cells13100805
Mao Z, Mu J, Gao Z, Huang S, Chen L. Biological Functions and Potential Therapeutic Significance of O-GlcNAcylation in Hepatic Cellular Stress and Liver Diseases. Cells. 2024; 13(10):805. https://doi.org/10.3390/cells13100805
Chicago/Turabian StyleMao, Zun, Junpeng Mu, Zhixiang Gao, Shile Huang, and Long Chen. 2024. "Biological Functions and Potential Therapeutic Significance of O-GlcNAcylation in Hepatic Cellular Stress and Liver Diseases" Cells 13, no. 10: 805. https://doi.org/10.3390/cells13100805
APA StyleMao, Z., Mu, J., Gao, Z., Huang, S., & Chen, L. (2024). Biological Functions and Potential Therapeutic Significance of O-GlcNAcylation in Hepatic Cellular Stress and Liver Diseases. Cells, 13(10), 805. https://doi.org/10.3390/cells13100805





