Long-Term Maintenance of Viable Human Endometrial Epithelial Cells to Analyze Estrogen and Progestin Effects
Abstract
:1. Introduction
2. Material and Methods
2.1. Cell Culture of Mesothelial Cells
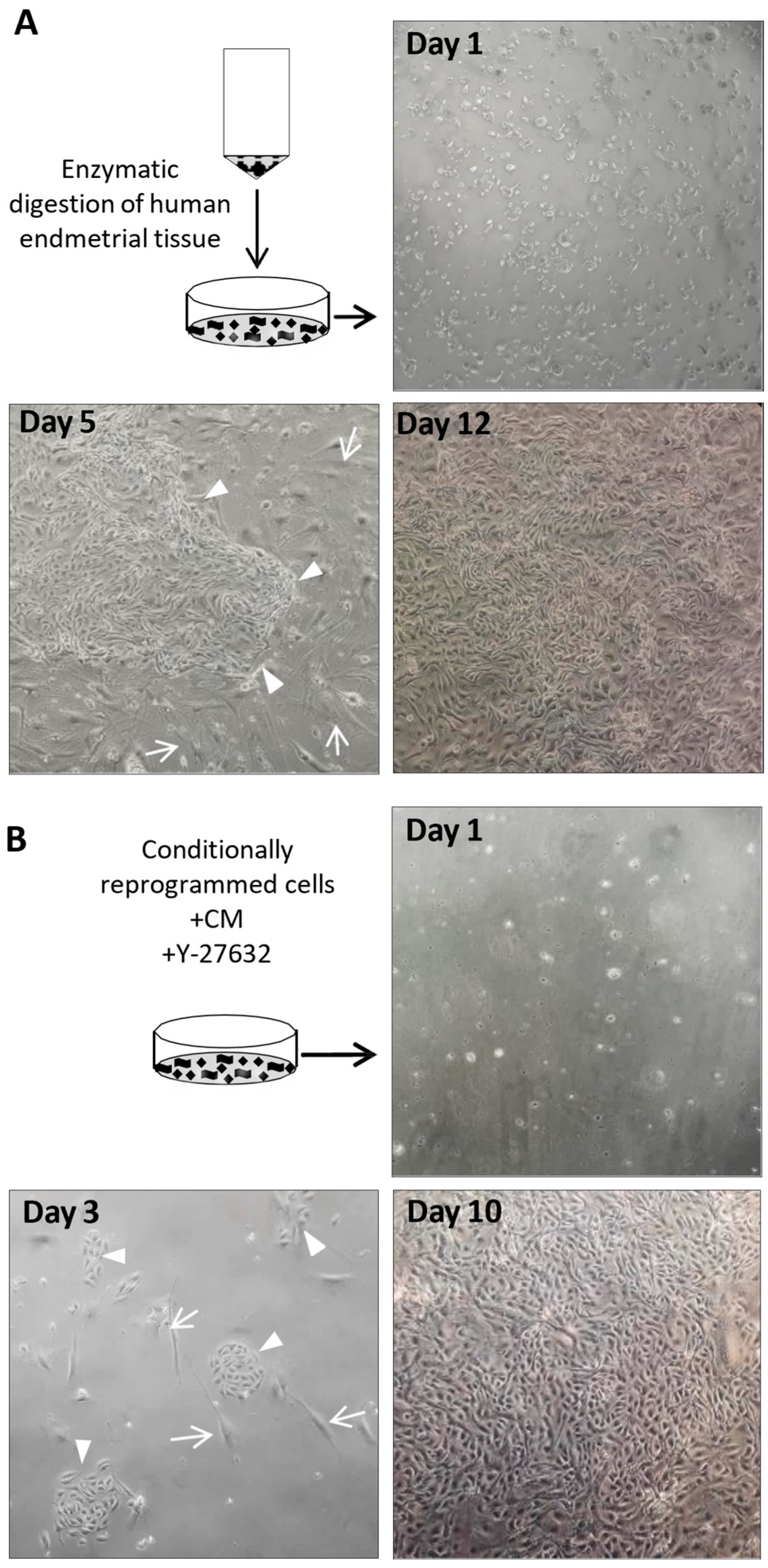
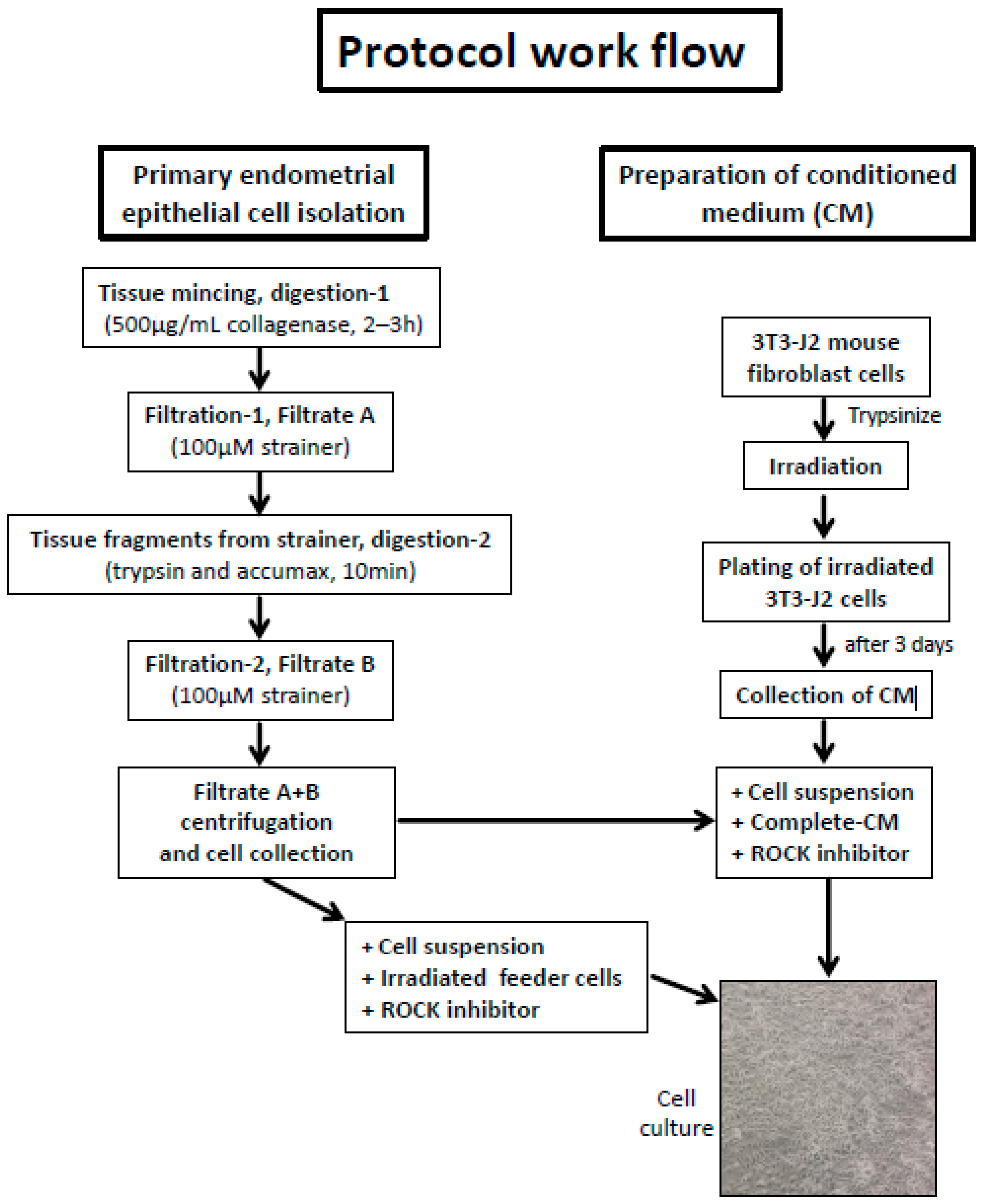
2.2. Isolation of Human Primary Endometrial Stromal and Epithelial Cells
2.3. Irradiation of Feeder Cells and Preparation of Conditioned Medium (CM)
2.4. Induction of CRP in HPEECs with Irradiated Feeder Cells
2.5. Separation of Epithelial Cells and Feeder Cells
2.6. Cell Culture and Treatment of Cells with Various Agents
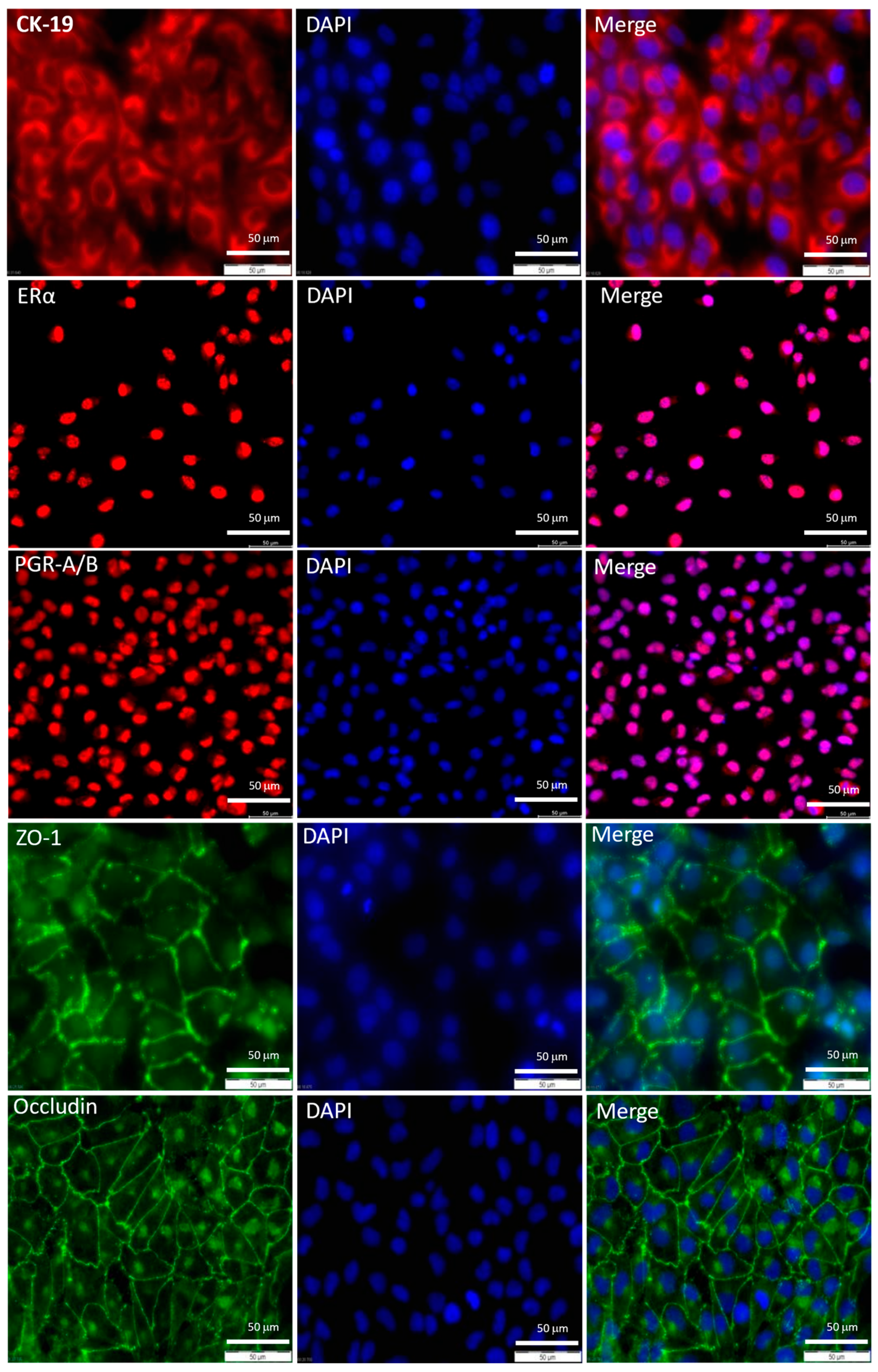
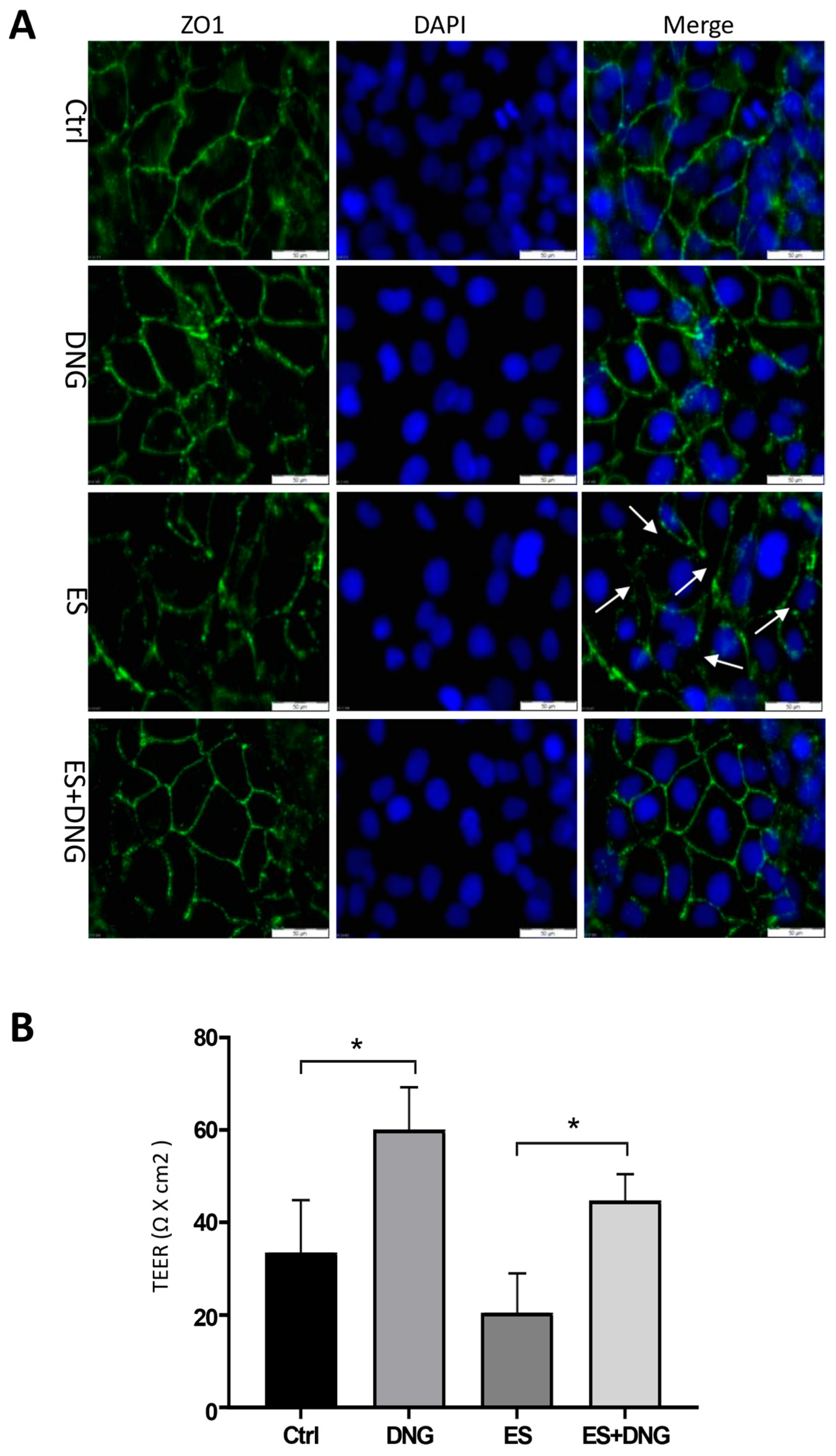
2.7. Immunofluorescence (IF)
2.8. Western Blot
2.9. Measurement of Transepithelial Electrical Resistance (TEER)
2.10. Cell Proliferation, Migration, and Invasion Assays
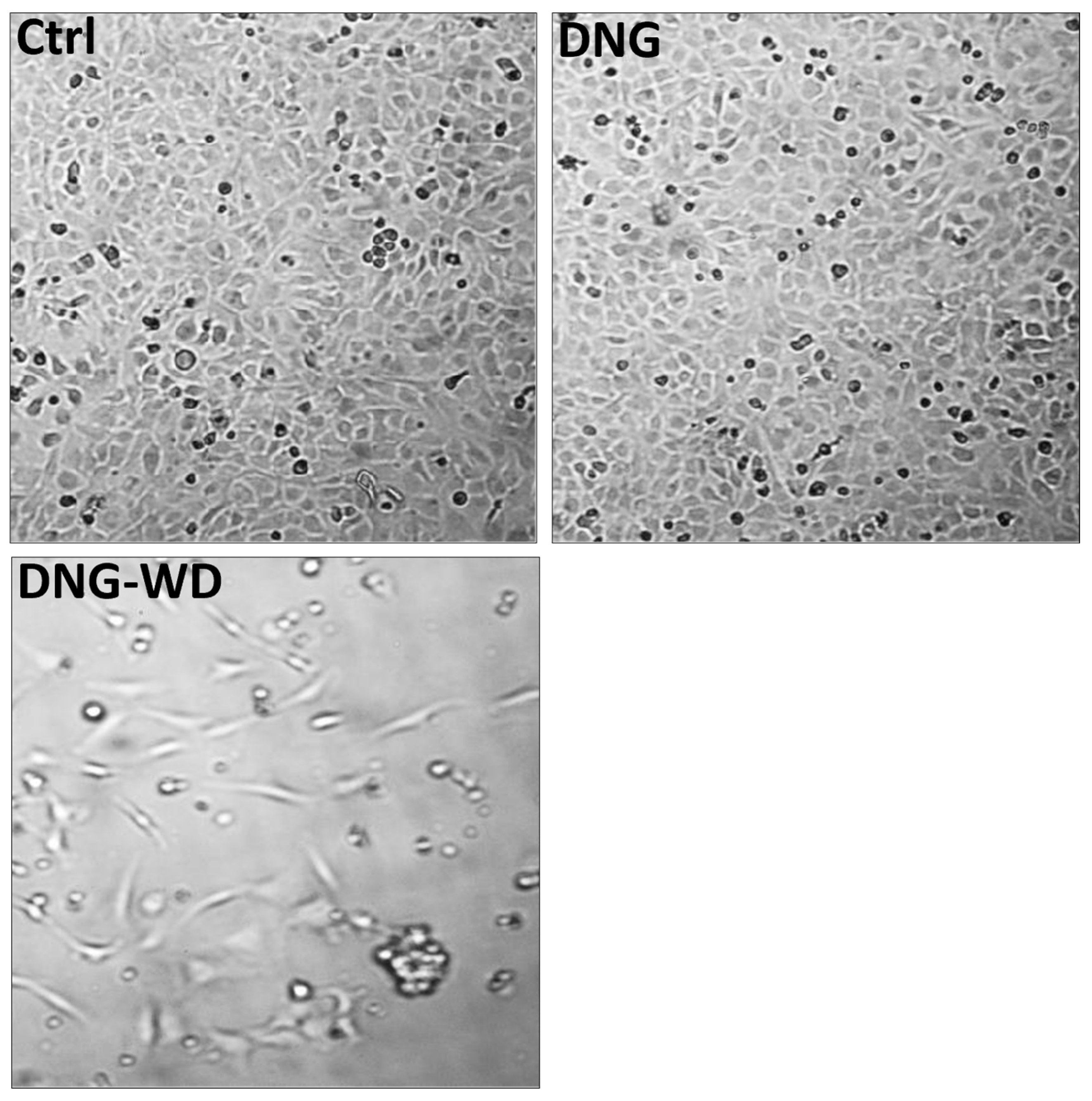
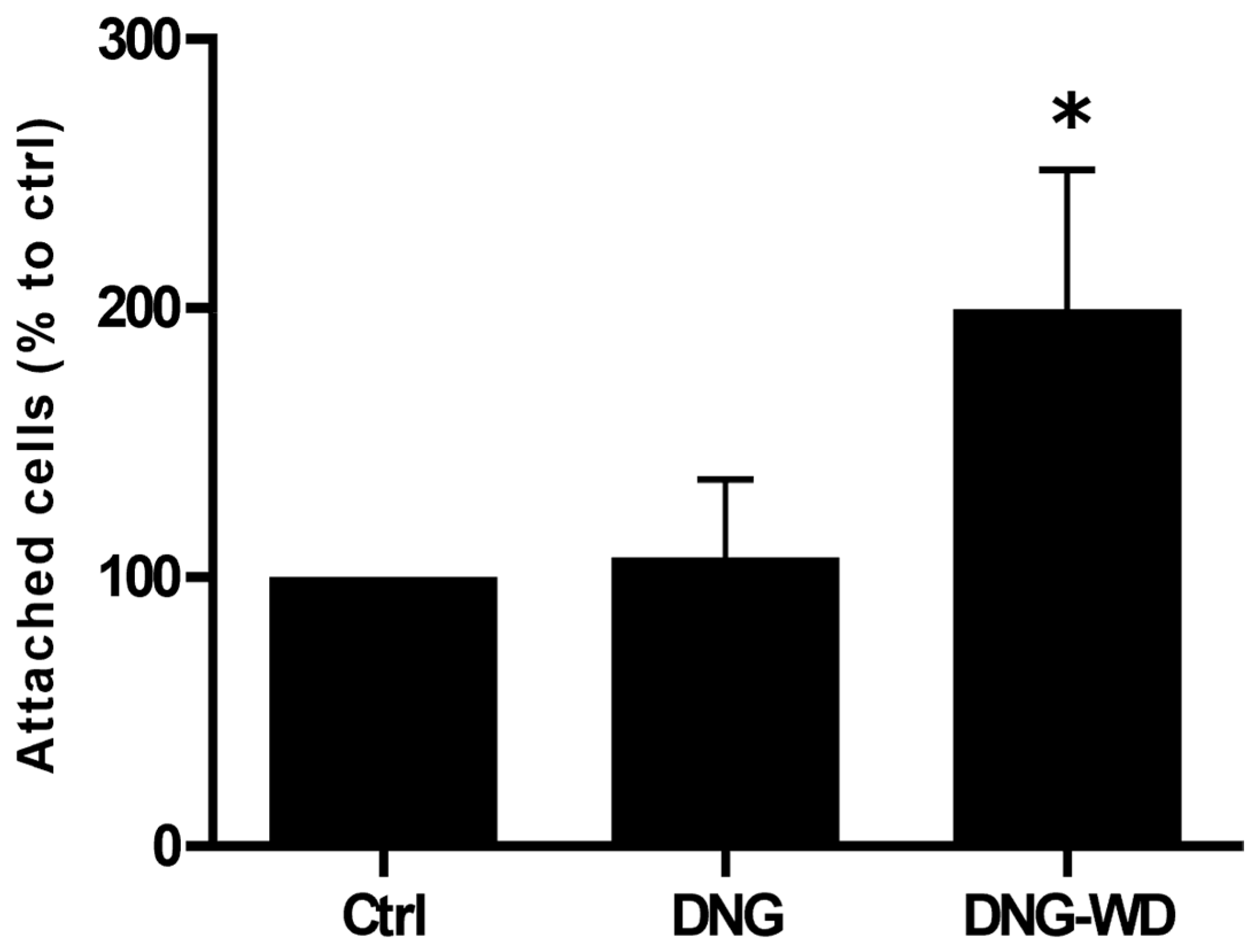
2.11. Hormone Withdrawal and Endometrial–Mesothelial Cell Attachment Assay
2.12. Statistical Analysis
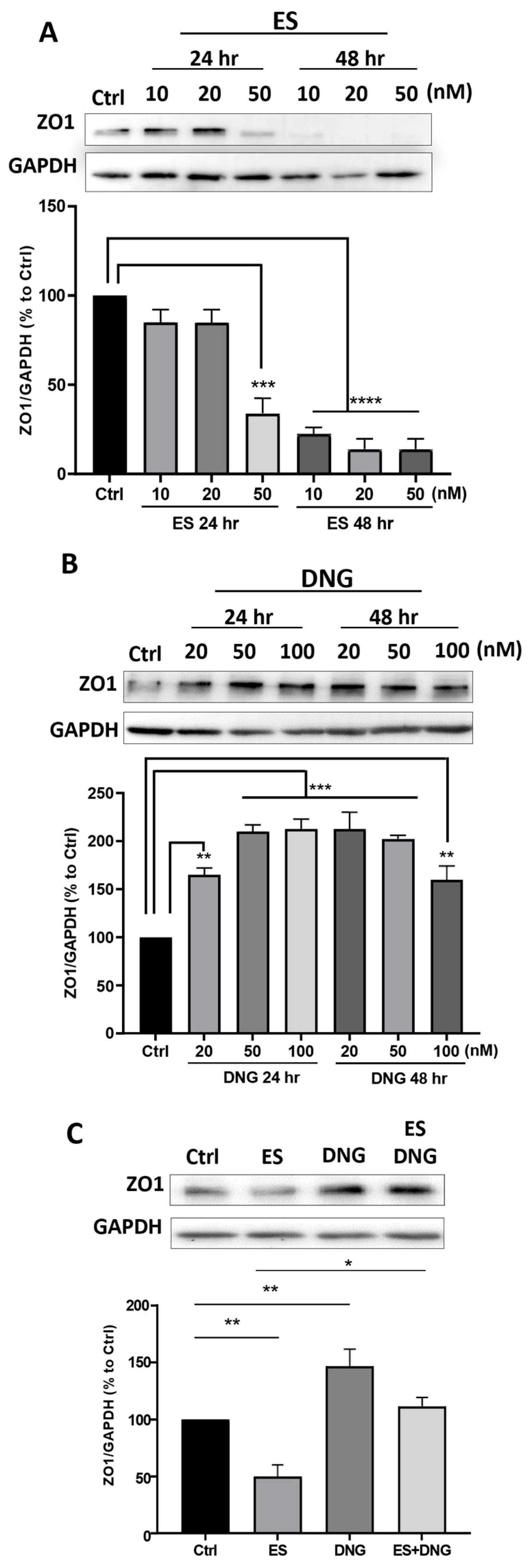
3. Results
4. Discussion
5. Strength and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartosch, C.; Lopes, J.M.; Beires, J.; Sousa, M. Human endometrium ultrastructure during the implantation window: A new perspective of the human epithelium cell types. Reprod. Sci. 2011, 18, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vilella, F.; Alama, P.; Moreno, I.; Mignardi, M.; Isakova, A.; Pan, W.; Simon, C.; Quake, S.R. Single-cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat. Med. 2020, 26, 1644–1653. [Google Scholar] [CrossRef]
- Queckbörner, S.; von Grothusen, C.; Boggavarapu, N.R.; Francis, R.M.; Davies, L.C.; Gemzell-Danielson, K. Stromal heterogeneity in the human proliferative endometrium—A single-cell RNA sequencing study. J. Pers. Med. 2021, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alonso, L.; Handfield, L.F.; Roberts, K.; Nikolakopoulou, K.; Fernando, R.C.; Gardner, L.; Woodhams, B.; Arutyunyan, A.; Polanski, K.; Hoo, R.; et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat. Genet. 2021, 53, 1698–1711. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, L.; Zhan, H.; Mo, Y.; Ren, Z.; Shao, A.; Lin, J. Single-cell transcriptomic analysis of endometriosis provides insights into fibroblast fates and immune cell heterogeneity. Cell Biosci. 2021, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Flynn, W.F.; Sivajothi, S.; Luo, D.; Bozal, S.B.; Davé, M.; Luciano, A.A.; Robson, P.; Luciano, D.E.; Courtois, E.T. Single-cell analysis of endometriosis reveals a coordinated transcriptional programme driving immunotolerance and angiogenesis across eutopic and ectopic tissues. Nat. Cell Biol. 2022, 24, 1306–1318. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, G.; Jiang, P.; Wang, Z.; Yao, S.; Zhou, Z.; Wang, L.; Liu, D.; Deng, W.; Dai, J.; et al. Deciphering the endometrial niche of human thin endometrium at single-cell resolution. Proc. Natl. Acad. Sci. USA 2022, 119, e2115912119. [Google Scholar] [CrossRef]
- Fonseca, M.A.S.; Haro, M.; Wright, K.N.; Lin, X.; Abbasi, F.; Sun, J.; Hernandez, L.; Orr, N.L.; Hong, J.; Choi-Kuaea, Y.; et al. Single-cell transcriptomic analysis of endometriosis. Nat. Genet. 2023, 55, 255–267. [Google Scholar] [CrossRef]
- Shin, S.; Chung, Y.J.; Moon, S.W.; Choi, E.J.; Kim, M.R.; Chung, Y.J.; Lee, S.H. Single-cell profiling identifies distinct hormonal, immunological, and inflammatory signatures of endometriosis-constituting cells. J. Pathol. 2023, 261, 323–334. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, L.; Liu, M.; Zhu, H.; Zhang, X.; Cai, E.; Xu, X.; Chen, T.; Cheng, H.; Liu, J.; et al. Single-cell analysis reveals insights into epithelial abnormalities in ovarian endometriosis. Cell Rep. 2024, 43, 113716. [Google Scholar] [CrossRef]
- Ryan, I.P.; Schriock, E.D.; Taylor, R.N. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J. Clin. Endocrinol. Metab. 1994, 78, 642–649. [Google Scholar] [PubMed]
- Li, T.; He, H.; Liu, R.; Wang, S.X.; Pu, D.M. Isolation and identification of epithelial and stromal stem cells from eutopic endometrium of women with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 178, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Masuda, A.; Katoh, N.; Nakabayashi, K.; Kato, K.; Sonoda, K.; Kitade, M.; Takeda, S.; Hata, K.; Tonikawa, J. An improved method for isolation of epithelial and stromal cells from the human endometrium. J. Reprod. Dev. 2016, 62, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Hoffman, J.R.; Arora, R.; Perrone, L.A.; Gonzalez-Gomez, C.J.; Chi Vo, K.; Laird, D.J.; Irwin, J.C.; Giudice, L.C. Cryopreservation and recovery of human endometrial epithelial cells with high viability, purity, and functional fidelity. Fertil. Steril. 2016, 105, 501–510.e1. [Google Scholar] [CrossRef]
- He, W.; Zhu, X.; Xin, A.; Zhang, H.; Sun, Y.; Xu, H.; Li, H.; Yang, T.; Zhou, D.; Yan, H.; et al. Long-term maintenance of human endometrial epithelial stem cells and their therapeutic effects on intrauterine adhesion. Cell Biosci. 2022, 12, 175. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, H.C.; Schust, D.J.; Spencer, T.E. In vitro models of the human endometrium: Evolution and application for women’s health. Biol. Reprod. 2021, 104, 282–293. [Google Scholar] [CrossRef]
- Boretto, M.; Cox, B.; Noben, M.; Hendriks, N.; Fassbender, A.; Roose, H.; Amant, F.; Timmerman, D.; Tomassetti, C.; Vanhie, A.; et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 2017, 144, 1775–1786. [Google Scholar] [CrossRef]
- Turco, M.Y.; Gardner, L.; Hughes, J.; Cindrovs-Davies, T.; Gomez, M.J.; Farrell, L.; Hollinshead, M.; Marsh, S.G.E.; Brosens, J.J.; Critchley, H.O.; et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 2017, 19, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, H.C.; Dhakal, P.; Behura, S.K.; Schust, D.J.; Spencer, T.E. Self-renewing endometrial epithelial organoids of the human uterus. Proc. Natl. Acad. Sci. USA 2019, 116, 23132–23142. [Google Scholar] [CrossRef]
- Xia, S.; Wu, M.; Zhou, X.; Zhang, X.; Ye, L.; Zhang, K.; Kang, Y.; Liu, J.; Zhang, Y.; Wu, W.; et al. Treating intrauterine adhesion using conditionally reprogrammed physiological endometrial epithelial cells. Stem Cell Res. Ther. 2022, 13, 178. [Google Scholar] [CrossRef]
- Fraser, R.; Smith, R.; Lin, C.J. A 3D endometrial organotypic model simulating the acute inflammatory decidualisation initiation phase with epithelial induction of the key endometrial receptivity marker, integrin αvβ3. Hum. Reprod. Open 2021, 2021, hoab034. [Google Scholar] [CrossRef]
- Yokomizo, R.; Fujiki, Y.; Kishigami, H.; Kishi, H.; Kiyono, T.; Nakayama, S.; Sago, H.; Okamoto, A.; Umezawa, A. Endometrial regeneration with endometrial epithelium: Homologous orchestration with endometrial stroma as a feeder. Stem Cell Res. Ther. 2021, 12, 130. [Google Scholar] [CrossRef]
- Chapman, S.; Liu, X.; Meyers, C.; Schlegel, R.; McBride, A.A. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J. Clin. Investig. 2010, 120, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Suprynowicz, F.A.; Upadhyay, G.; Krawczyk, E.; Kramer, S.C.; Hebert, J.D.; Liu, X.; Yuan, H.; Cheluvaraju, C.; Clapp, P.W.; Boucher, R.C., Jr.; et al. Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells. Proc. Natl. Acad. Sci. USA 2012, 109, 20035–20040. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ory, V.; Chapman, S.; Yuan, H.; Albanese, C.; Kallakury, B.; Timofeeva, O.; Nealon, C.; Dakic, A.; Simic, V.; et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am. J. Pathol. 2012, 180, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Krawczyk, E.; Suprynowicz, F.; Palechor-Ceron, N.; Yuan, H.; Dakic, A.; Simic, V.; Zheng, Y.L.; Sripadhan, P.; Chen, C.; et al. Conditional reprogramming and long-term expansion of normal and tumor cells from human biospecimens. Nat. Protoc. 2017, 12, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Rheinwald, J.G.; Green, H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Terunuma, A.; Limgala, R.P.; Park, C.J.; Choudhary, I.; Vogel, J.C. Efficient procurement of epithelial stem cells from human tissue specimens using a Rho-associated protein kinase inhibitor Y-27632. Tissue Eng. Part A 2010, 16, 1363–1368. [Google Scholar] [CrossRef]
- Yue, J.; Shukla, R.; Accardi, R.; Zanella-Cleon, I.; Siouda, M.; Cros, M.P.; Krutovskikh, V.; Hussain, I.; Niu, Y.; Hu, S.; et al. Cutaneous human papillomavirus type 38 E7 regulates actin cytoskeleton structure for increasing cell proliferation through CK2 and the eukaryotic elongation factor 1A. J. Virol. 2011, 85, 8477–8494. [Google Scholar] [CrossRef]
- Palechor-Ceron, N.; Suprynowicz, F.A.; Upadhyay, G.; Dakic, A.; Minas, T.; Simic, V.; Johnson, M.; Albanese, C.; Schlegel, R.; Liu, X. Radiation induces diffusible feeder cell factor(s) that cooperate with ROCK inhibitor to conditionally reprogram and immortalize epithelial cells. Am. J. Pathol. 2013, 183, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Ochiel, D.O.; Ochsenbauer, C.; Kappes, J.C.; Ghosh, M.; Fahey, J.V.; Wira, C.R. Uterine epithelial cell regulation of DC-SIGN expression inhibits transmitted/founder HIV-1 trans infection by immature dendritic cells. PLoS ONE 2010, 5, e14306. [Google Scholar] [CrossRef] [PubMed]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef]
- Tempest, N.; Hill, C.J.; Maclean, A.; Marston, K.; Powell, S.G.; Al-Lamee, H.; Hapangama, D.K. Novel microarchitecture of human endometrial glands: Implications in endometrial regeneration and pathologies. Hum. Reprod. Update 2022, 28, 153–171. [Google Scholar] [CrossRef]
- Garry, R.; Hart, R.; Karthigasu, K.A.; Burke, C. Structural changes in endometrial basal glands during menstruation. BJOG 2010, 117, 1175–1185. [Google Scholar] [CrossRef]
- Swangchan-Uthai, T.; Lavender, C.R.M.; Cheng, Z.; Fouladi-Nashta, A.A.; Wathes, D.C. Time course of defense mechanisms in bovine endometrium in response to lipopolysaccharide. Biol. Reprod. 2012, 87, 135. [Google Scholar] [CrossRef]
- Raheem, K.; Fouladi-Nashta, A. Isolation and characterization of endometrial luminal epithelial and stromal cells in vitro. Sokoto. J. Vet. Sci. 2014, 12, 1–8. [Google Scholar] [CrossRef]
- Fortier, M.A.; Guilbault, L.A.; Grasso, F. Specific properties of epithelial and stromal cells from the endometrium of cows. Reproduction 1988, 83, 239–248. [Google Scholar] [CrossRef]
- Konrad, L.; Gronbach, J.; Horné, F.; Mecha, E.O.; Berkes, E.; Frank, M.; Gattenlöhner, S.; Omwandho, C.O.; Oehmke, F.; Tinneberg, H.R. Similar characteristics of the endometrial and endometriotic epithelium. Reprod. Sci. 2019, 26, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.A.; Sak, A.; Bahadir Erol, Y.; Groneberg, M.; Thomale, J.; Stuschke, M. Metformin enhances the radiosensitizing effect of cisplatin in non-small cell lung cancer cell lines with different cisplatin sensitivities. Sci. Rep. 2019, 9, 1282. [Google Scholar] [CrossRef]
- Mwaura, A.N.; Riaz, M.A.; Maoga, J.B.; Mecha, E.; Omwandho, C.O.A.; Scheiner-Bobis, G.; Meinhold-Heerlein, I.; Konrad, L. Role of betaglycan in TGF-β signaling and wound healing in human endometriotic epithelial cells and in endometriosis. Biology 2022, 11, 513. [Google Scholar] [CrossRef]
- Aslam, M.; Härtel, F.V.; Arshad, M.; Gündüz, D.; Abdallah, Y.; Sauer, H. cAMP/PKA antagonizes thrombin-induced inactivation of endothelial myosin light chain phosphatase: Role of CPI-17. Cardiovasc. Res. 2010, 87, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Kabbesh, H.; Riaz, M.A.; Jensen, A.D.; Scheiner-Bobis, G.; Konrad, L. Long-term maintenance of viable adult rat Sertoli cells able to establish testis barrier components and function in response to androgens. Cells 2021, 10, 2405. [Google Scholar] [CrossRef]
- Edlund, M.; Andersson, E.; Fried, G. Progesterone withdrawal causes endothelin release from cultured human uterine microvascular endothelial cells. Hum. Reprod. 2004, 19, 1272–1280. [Google Scholar] [CrossRef]
- Nair, A.S.; Nair, H.B.; Lucidi, R.S.; Kirchner, A.J.; Schenken, R.S.; Tekmal, R.R.; Witz, C.A. Modeling the early endometriotic lesion: Mesothelium-endometrial cell co-culture increases endometrial invasion and alters mesothelial and endometrial gene transcription. Fertil. Steril. 2008, 90 (Suppl. S4), 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.H.; Wilde, M.I. Dienogest. Drugs 1998, 56, 834–835. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Fanning, A.S.; Bridges, A.; Anderson, J.M. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell 2009, 20, 3930–3940. [Google Scholar] [CrossRef] [PubMed]
- Mehasseb, M.K.; Panchal, R.; Taylor, A.H.; Brown, L.; Bell, S.C.; Habiba, M. Estrogen and progesterone isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil. Steril. 2011, 95, 2228–2235. [Google Scholar] [CrossRef]
- Grant-Tschudy, K.S.; Wira, C.R. Effect of estradiol on mouse uterine epithelial cell transepithelial resistance (TER). Am. J. Reprod. Immunol. 2004, 52, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Kurita, T.; Young, P.; Brody, J.R.; Lydon, J.P.; O’Malley, B.W.; Cunha, G.R. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology 1998, 139, 4708–4713. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.A.; Newton, G.R.; Weise, D.W.; Bazer, F.W.; Burghardt, R.C. Characterization of a polarized porcine uterine epithelial model system. Biol. Reprod. 1996, 55, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Salazar, J.; Posadas-Rodriguez, P.; Lazzarini-Lechuga, R.C.; Luna-López, A.; Zentella-Dehesa, A.; Gómez-Quiroz, L.E.; Königsberg, M.; Domínguez-Gómez, G.; Damián-Matsumura, P. Membrane-initiated estradiol signaling of epithelial-mesenchymal transition-associated mechanisms through regulation of tight junctions in human breast cancer cells. Horm. Cancer 2014, 5, 161–173. [Google Scholar] [CrossRef]
- Buck, V.U.; Windoffer, R.; Leube, R.E.; Classen-Linke, I. Redistribution of adhering junctions in human endometrial epithelial cells during the implantation window of the menstrual cycle. Histochem. Cell Biol. 2012, 137, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C.; Pouly, J.L.; Canis, M. Effects of matrix stiffness on epithelial to mesenchymal transition-like processes of endometrial epithelial cells. Implications for the pathogenesis of endometriosis. Sci. Rep. 2017, 7, 44616. [Google Scholar] [CrossRef] [PubMed]
- Ando-Akatsuka, Y.; Yonemura, S.; Itoh, M.; Furuse, M.; Tsukita, S. Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J. Cell. Physiol. 1999, 179, 115–125. [Google Scholar] [CrossRef]
- Müller, S.L.; Portwich, M.; Schmidt, A.; Utepbergenov, D.I.; Huber, O.; Blasig, I.E.; Krause, G. The tight junction protein occludin and the adherens junction protein alpha-catenin share a common interaction mechanism with ZO-1. J. Biol. Chem. 2005, 280, 3747–3756. [Google Scholar] [CrossRef]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef]
- Díaz-Coránguez, M.; Liu, X.; Antonetti, D.A. Tight junctions in cell proliferation. Int. J. Mol. Sci. 2019, 20, 5972. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Betanzos, A.; Ávila-Flores, A. MAGUK proteins: Structure and role in the tight junction. Semin. Cell Dev. Biol. 2000, 11, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.V.; Garwood, C.J.; Jennings, L.; Simpson, J.E.; Castelli, L.M.; Heath, P.R.; Mihaylov, S.R.; Vaquéz-Villaseñor, I.; Minshull, T.C.; Ine, P.G.; et al. Proteomic and cellular localization studies suggest non-tight junction cytoplasmic and nuclear roles for occluding in astrocytes. Eur. J. Neurosci. 2018, 47, 1444–1456. [Google Scholar] [CrossRef]
- Zeng, H.; Safratowich, B.D.; Cheng, W.H.; Larson, K.J.; Briske-Anderson, M. Deoxycholin acid modulates cell-junction gene expression and increases intestinal barrier dysfunction. Molecules 2022, 27, 723. [Google Scholar] [CrossRef]
- Castellani, S.; Guerra, L.; Favia, M.; Di Gioia, S.; Casavola, V.; Conese, M. NHERF1 and CFTR restore tight junction organization and function in cystic fibrosis airway epithelial cells: Role of ezrin and the RhoA/ROCK pathway. Lab. Investig. 2012, 92, 1527–1540. [Google Scholar] [CrossRef]
- Runkle, A.; Sundstrom, J.M.; Runkle, K.B.; Liu, X.; Antonetti, D.A. Occludin localizes to centrosomes and modifies mitotic entry. J. Biol. Chem. 2011, 286, 30847–30858. [Google Scholar] [CrossRef]
- Pierro, E.; Minici, F.; Alesiani, O.; Miceli, F.; Proto, C.; Screpanti, I.; Mancuso, S.; Lanzone, A. Stromal-epithelial interactions modulate estrogen responsiveness in normal human endometrium. Biol. Reprod. 2001, 64, 831–838. [Google Scholar] [CrossRef]
- Qi, S.; Yan, L.; Liu, Z.; Mu, Y.L.; Li, M.; Zhao, X.; Chen, Z.J.; Zhang, H. Melatonin inhibits 17β-estradiol-induced migration, invasion and epithelial-mesenchymal transition in normal and endometriotic endometrial epithelial cells. Reprod. Biol. Endocrinol. 2018, 23, 62. [Google Scholar] [CrossRef] [PubMed]
- Witz, C.A.; Monotoya-Rodriguez, I.A.; Schenken, R.S. Whole explants of peritoneum and endometrium: A novel model of the early endometriosis lesion. Fertil. Steril. 1999, 71, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Witz, C.A.; Thomas, M.R.; Monotoya-Rodriguez, I.A.; Nair, A.S.; Centonze, V.E.; Schenken, R.S. Short-term culture of peritoneum explants confirms attachment of endometrium to intact peritoneal mesothelium. Fertil. Steril. 2001, 75, 385–390. [Google Scholar] [CrossRef]
- Lucidi, R.S.; Witz, C.A.; Chrisco, M.; Binkley, P.A.; Shain, S.A.; Schenken, R.S. A novel in vitro model of the early endometriotic lesion demonstrates that attachment of endometrial cells to mesothelial cells is dependent on the source of endometrial cells. Fertil. Steril. 2005, 84, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Gaide Chevronnay, H.P.; Selvais, C.; Emonard, H.; Galant, C.; Marbaix, E.; Henriet, P. Regulation of matrix metalloproteinases activity studied in human endometrium as a paradigm of cyclic tissue breakdown and regeneration. Biochim. Biophys. Acta 2012, 1824, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Nap, A.W.; Dunselman, G.A.J.; de Goeij, A.F.P.M.; Evers, J.L.H.; Groothuis, P.G. Inhibiting MMP activity prevents the development of endometriosis in the chicken chorioallantoic membrane model. Hum. Reprod. 2004, 19, 2180–2187. [Google Scholar] [CrossRef]
- Song, Y.; Burns, G.W.; Joshii, N.B.; Arora, R.; Kim, J.J.; Fazleabas, A.T. Spheroids as a model for endometriotic lesions. JCI Insight 2023, 8, e160815. [Google Scholar] [CrossRef]
- Flores, V.A.; Vanhie, A.; Dang, T.; Taylor, H.S. Progesterone receptor status predicts response to progestin therapy in endometriosis. J. Clin. Endocrinol. Metab. 2018, 103, 4561–4568. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.M.; Coutinho, L.M.; Vannuccini, S.; Batteux, F.; Chapron, C.; Petraglia, F. Progesterone receptor ligands for the treatment of endometriosis: The mechanisms behind therapeutic success and failure. Hum. Reprod. Update 2020, 26, 565–585. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaz, M.A.; Kary, F.L.; Jensen, A.; Zeppernick, F.; Meinhold-Heerlein, I.; Konrad, L. Long-Term Maintenance of Viable Human Endometrial Epithelial Cells to Analyze Estrogen and Progestin Effects. Cells 2024, 13, 811. https://doi.org/10.3390/cells13100811
Riaz MA, Kary FL, Jensen A, Zeppernick F, Meinhold-Heerlein I, Konrad L. Long-Term Maintenance of Viable Human Endometrial Epithelial Cells to Analyze Estrogen and Progestin Effects. Cells. 2024; 13(10):811. https://doi.org/10.3390/cells13100811
Chicago/Turabian StyleRiaz, Muhammad Assad, Franziska Louisa Kary, Alexandra Jensen, Felix Zeppernick, Ivo Meinhold-Heerlein, and Lutz Konrad. 2024. "Long-Term Maintenance of Viable Human Endometrial Epithelial Cells to Analyze Estrogen and Progestin Effects" Cells 13, no. 10: 811. https://doi.org/10.3390/cells13100811
APA StyleRiaz, M. A., Kary, F. L., Jensen, A., Zeppernick, F., Meinhold-Heerlein, I., & Konrad, L. (2024). Long-Term Maintenance of Viable Human Endometrial Epithelial Cells to Analyze Estrogen and Progestin Effects. Cells, 13(10), 811. https://doi.org/10.3390/cells13100811







