Cytoprotective Small Compound M109S Attenuated Retinal Ganglion Cell Degeneration Induced by Optic Nerve Crush in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. ONC Procedure
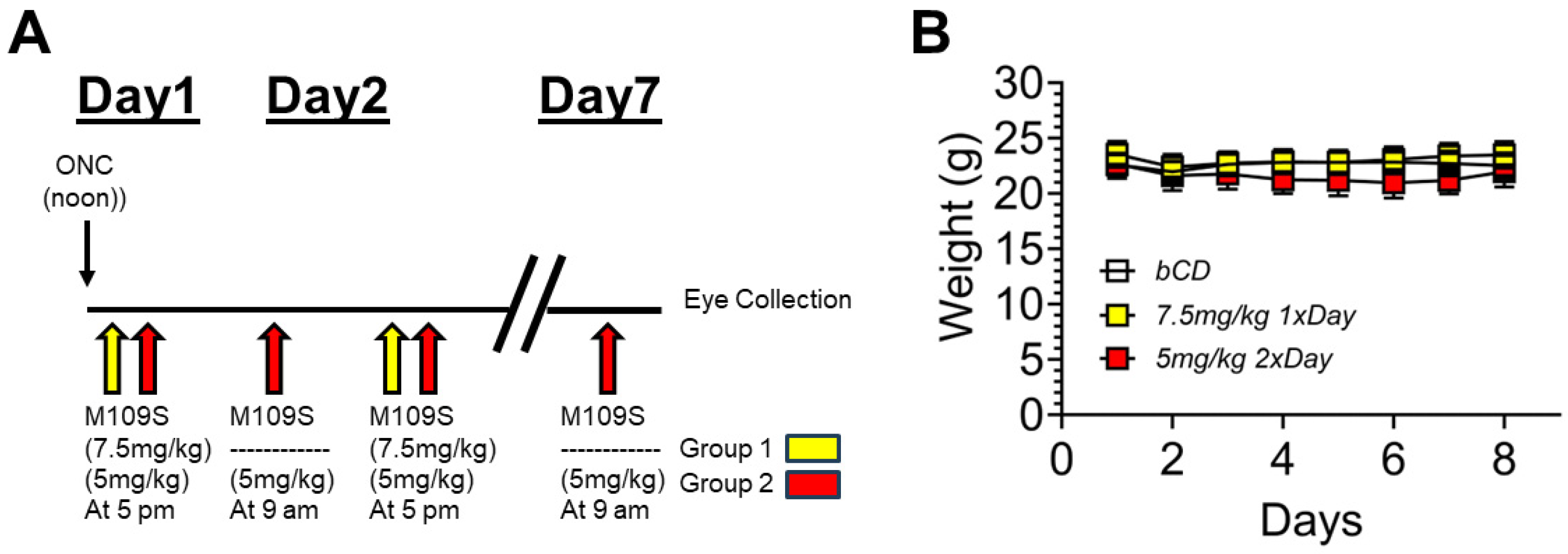
2.3. M109S Treatment
2.4. Immunohistochemistry (IHC)
2.5. Image Analysis
2.6. Histological Analysis of the Lungs and Kidney
2.7. Data Analysis
3. Results
3.1. The Time Schedule of ONC and M109S Treatment
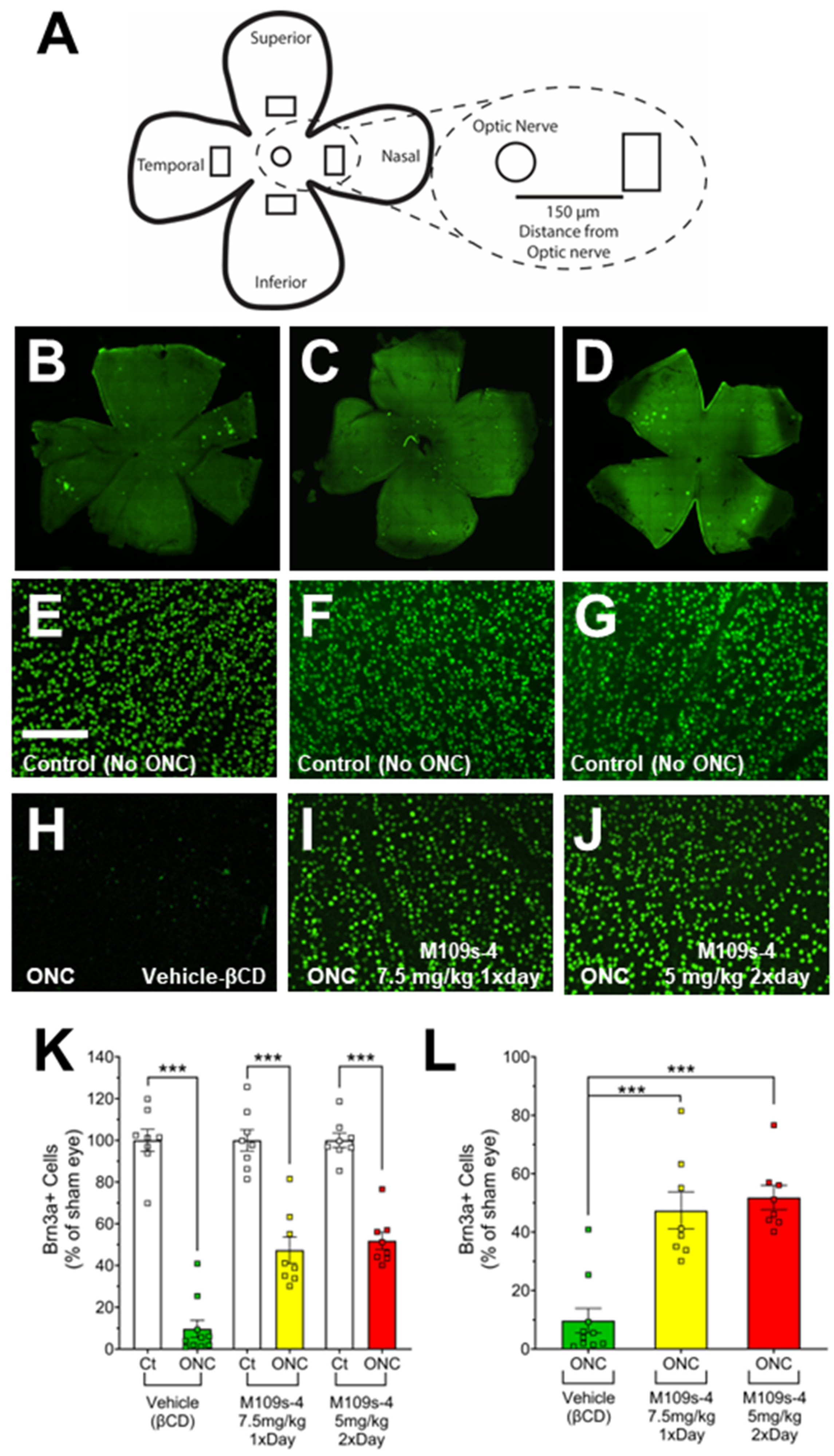
3.2. M109S Attenuated the Decrease of BRN3A-Positive (BRN3A+) Cells in the Retinal of ONC-Treated Mice
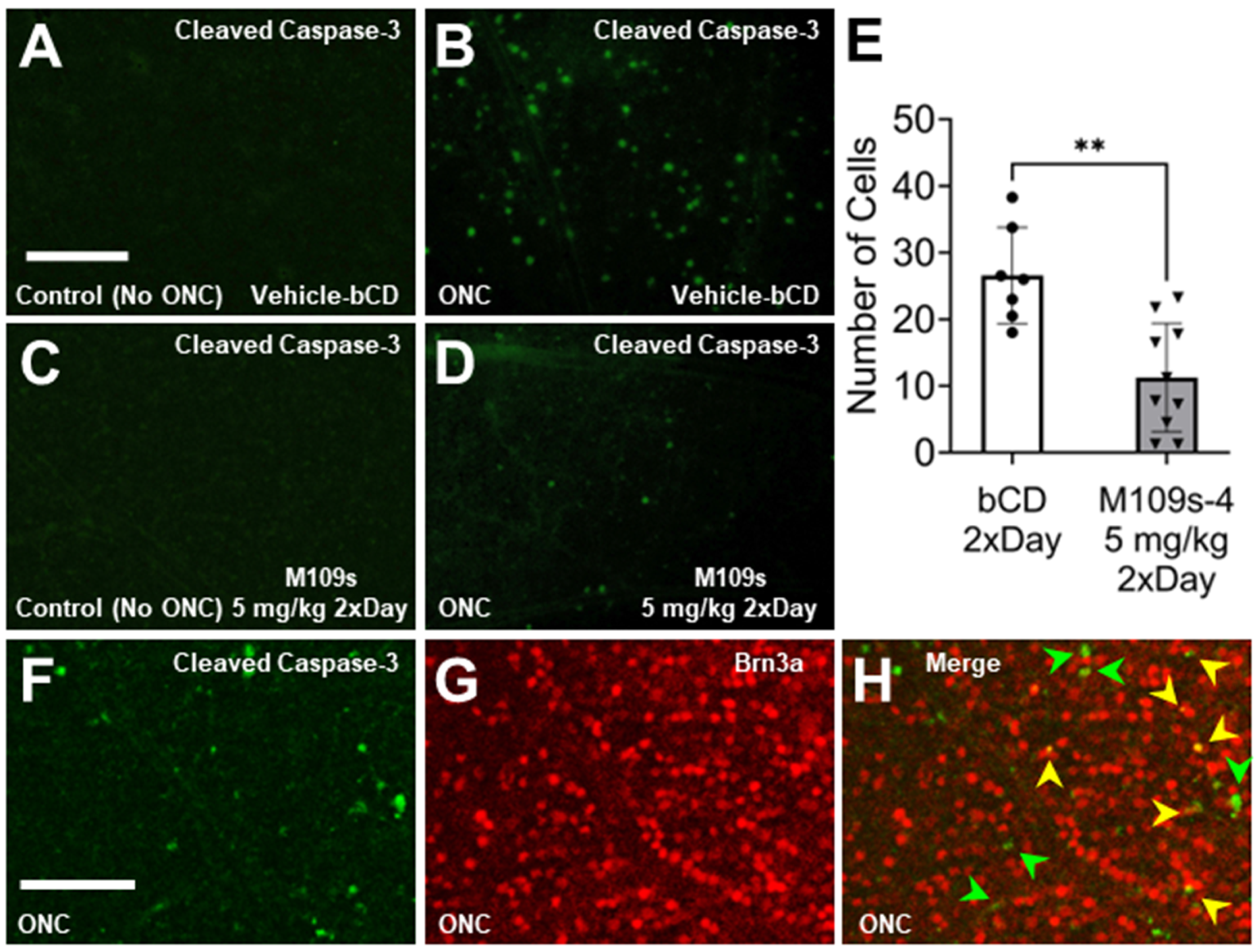
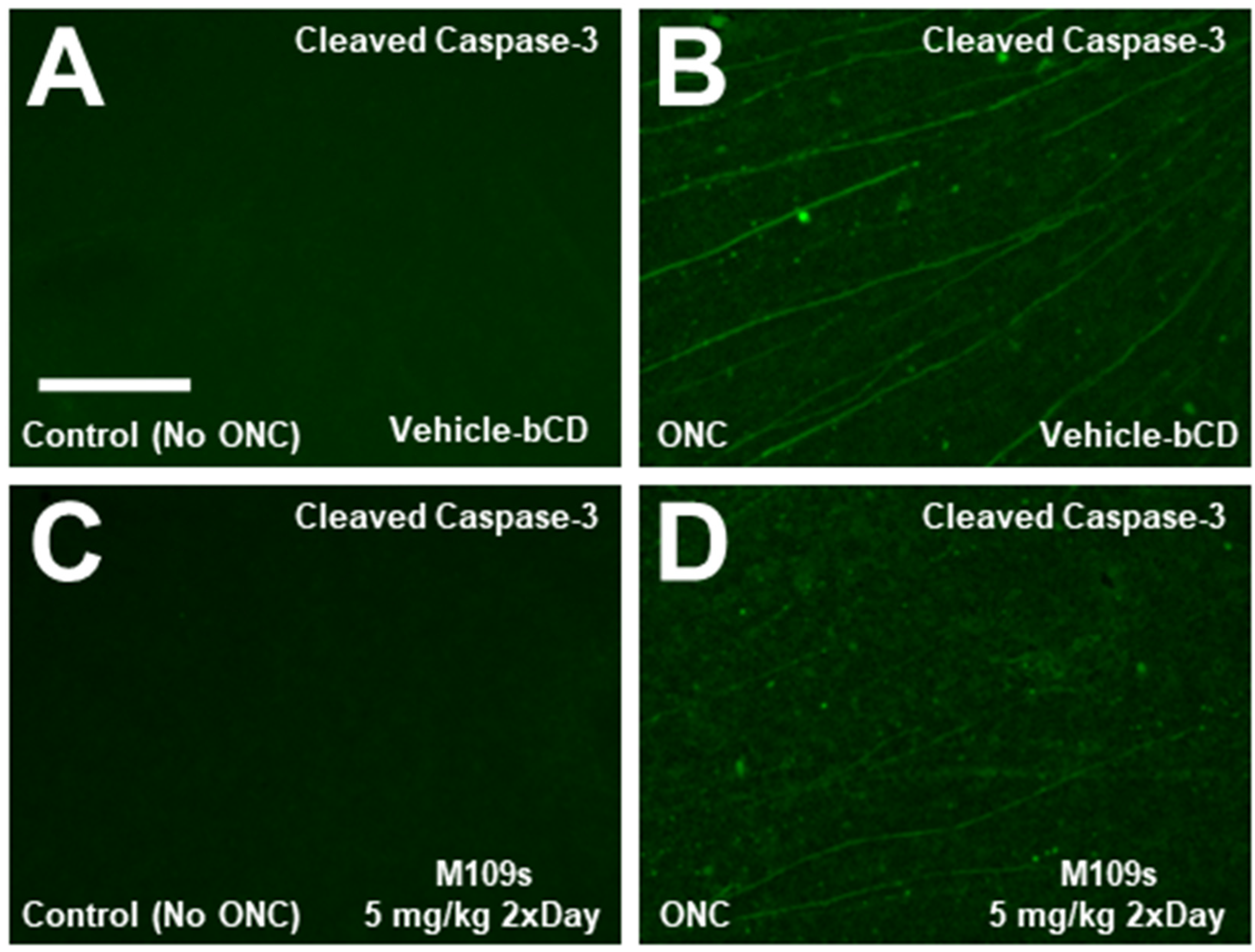
3.3. M109S Inhibited ONC-Induced Caspase-3 Activation in the Retina
3.4. M109S Did Not Induce Abnormal Histology in the Lungs and Kidneys
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boccaccini, A.; Cavaterra, D.; Carnevale, C.; Tanga, L.; Marini, S.; Bocedi, A.; Lacal, P.M.; Manni, G.; Graziani, G.; Sbardella, D.; et al. Novel frontiers in neuroprotective therapies in glaucoma: Molecular and clinical aspects. Mol. Aspects Med. 2023, 94, 101225. [Google Scholar] [CrossRef]
- Quigley, H.A.; Nickells, R.W.; Kerrigan, L.A.; Pease, M.E.; Thibault, D.J.; Zack, D.J. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest. Ophthalmol. Vis. Sci. 1995, 36, 774–786. [Google Scholar] [PubMed]
- Donahue, R.J.; Maes, M.E.; Grosser, J.A.; Nickells, R.W. BAX-Depleted Retinal Ganglion Cells Survive and Become Quiescent Following Optic Nerve Damage. Mol. Neurobiol. 2020, 57, 1070–1084. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.C.; Zong, W.X.; Cheng, E.H.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef]
- Li, Y.; Schlamp, C.L.; Poulsen, K.P.; Nickells, R.W. Bax-dependent and independent pathways of retinal ganglion cell death induced by different damaging stimuli. Exp. Eye Res. 2000, 71, 209–213. [Google Scholar] [CrossRef]
- Libby, R.T.; Li, Y.; Savinova, O.V.; Barter, J.; Smith, R.S.; Nickells, R.W.; John, S.W. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005, 1, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Semaan, S.J.; Li, Y.; Nickells, R.W. A single nucleotide polymorphism in the Bax gene promoter affects transcription and influences retinal ganglion cell death. ASN Neuro 2010, 2, e00032. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Maes, M.E.; Donahue, R.J.; Schlamp, C.L.; Marola, O.J.; Libby, R.T.; Nickells, R. BAX activation in mouse retinal ganglion cells occurs in two temporally and mechanistically distinct steps. Mol. Neurodegener. 2023, 18, 67. [Google Scholar] [CrossRef]
- Maes, M.E.; Schlamp, C.L.; Nickells, R.W. BAX to basics: How the BCL2 gene family controls the death of retinal ganglion cells. Prog. Retin. Eye Res. 2017, 57, 1–25. [Google Scholar] [CrossRef]
- Risner, M.L.; Pasini, S.; McGrady, N.R.; Calkins, D.J. Bax Contributes to Retinal Ganglion Cell Dendritic Degeneration During Glaucoma. Mol. Neurobiol. 2022, 59, 1366–1380. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, M.; Ortega, J.T.; Fedorov, Y.; Scott-McKean, J.; Muller-Greven, J.; Buck, M.; Adams, D.; Jastrzebska, B.; Greenlee, W.; Matsuyama, S. Development of novel cytoprotective small compounds inhibiting mitochondria-dependent cell death. iScience 2023, 26, 107916. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.G.; Xia, X.; Galvao, J.; Ashouri, M.; Kapiloff, M.S.; Goldberg, J.L. Optic Nerve Crush in Mice to Study Retinal Ganglion Cell Survival and Regeneration. Bio Protoc. 2020, 10, e3559. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schlamp, C.L.; Nickells, R.W. Experimental induction of retinal ganglion cell death in adult mice. Invest. Ophthalmol. Vis. Sci. 1999, 40, 1004–1008. [Google Scholar]
- Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Sobrado-Calvo, P.; Nieto-Lopez, L.; Canovas-Martinez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo, M. Brn3a as a marker of retinal ganglion cells: Qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Invest. Ophthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.N.; Berry, M.; Logan, A.; Blanch, R.J.; Ahmed, Z. Caspases in retinal ganglion cell death and axon regeneration. Cell Death Discov. 2017, 3, 17032. [Google Scholar] [CrossRef]
- Wei, Q.; Dong, G.; Franklin, J.; Dong, Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int. 2007, 72, 53–62. [Google Scholar] [CrossRef]
- Paller, M.S.; Hoidal, J.R.; Ferris, T.F. Oxygen free radicals in ischemic acute renal failure in the rat. J. Clin. Investig. 1984, 74, 1156–1164. [Google Scholar] [CrossRef]
- Ngo, J.; Matsuyama, M.; Kim, C.; Poventud-Fuentes, I.; Bates, A.; Siedlak, S.L.; Lee, H.G.; Doughman, Y.Q.; Watanabe, M.; Liner, A.; et al. Bax deficiency extends the survival of Ku70 knockout mice that develop lung and heart diseases. Cell Death Dis. 2015, 6, e1706. [Google Scholar] [CrossRef]
- Perez, G.I.; Jurisicova, A.; Wise, L.; Lipina, T.; Kanisek, M.; Bechard, A.; Takai, Y.; Hunt, P.; Roder, J.; Grynpas, M.; et al. Absence of the proapoptotic Bax protein extends fertility and alleviates age-related health complications in female mice. Proc. Natl. Acad. Sci. USA 2007, 104, 5229–5234. [Google Scholar] [CrossRef]
- Mead, B.; Thompson, A.; Scheven, B.A.; Logan, A.; Berry, M.; Leadbeater, W. Comparative evaluation of methods for estimating retinal ganglion cell loss in retinal sections and wholemounts. PLoS ONE 2014, 9, e110612. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Salinas-Navarro, M.; Sobrado-Calvo, P.; Alburquerque-Bejar, J.J.; Vidal-Sanz, M.; Agudo-Barriuso, M. Whole number, distribution and co-expression of brn3 transcription factors in retinal ganglion cells of adult albino and pigmented rats. PLoS ONE 2012, 7, e49830. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Fouda, A.Y.; Lemtalsi, T.; Shosha, E.; Rojas, M.; Liu, F.; Patel, C.; Caldwell, R.W.; Narayanan, S.P.; Caldwell, R.B. Retinal Neuroprotection From Optic Nerve Trauma by Deletion of Arginase 2. Front. Neurosci. 2018, 12, 970. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.M.; Zeng, L.P.; Zeng, J.H.; Tang, L.; Chen, X.M.; Wei, X. beta-III-Tubulin: A reliable marker for retinal ganglion cell labeling in experimental models of glaucoma. Int. J. Ophthalmol. 2015, 8, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Tomarev, S. Evaluating retinal ganglion cell loss and dysfunction. Exp. Eye Res. 2016, 151, 96–106. [Google Scholar] [CrossRef]
- Au, N.P.B.; Chand, R.; Kumar, G.; Asthana, P.; Tam, W.Y.; Tang, K.M.; Ko, C.C.; Ma, C.H.E. A small molecule M1 promotes optic nerve regeneration to restore target-specific neural activity and visual function. Proc. Natl. Acad. Sci. USA 2022, 119, e2121273119. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.M.; Shevtsova, Z.; Bahr, M.; Kugler, S. Long-term in vivo inhibition of CNS neurodegeneration by Bcl-XL gene transfer. Mol. Ther. 2005, 11, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, J.M.; Soane, L. Multiple functions of BCL-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008722. [Google Scholar] [CrossRef] [PubMed]
- Rohn, T.T.; Head, E. Caspases as therapeutic targets in Alzheimer’s disease: Is it time to “cut” to the chase? Int. J. Clin. Exp. Pathol. 2009, 2, 108–118. [Google Scholar]
- Perier, C.; Bove, J.; Wu, D.C.; Dehay, B.; Choi, D.K.; Jackson-Lewis, V.; Rathke-Hartlieb, S.; Bouillet, P.; Strasser, A.; Schulz, J.B.; et al. Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2007, 104, 8161–8166. [Google Scholar] [CrossRef]
- Gould, T.W.; Buss, R.R.; Vinsant, S.; Prevette, D.; Sun, W.; Knudson, C.M.; Milligan, C.E.; Oppenheim, R.W. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J. Neurosci. 2006, 26, 8774–8786. [Google Scholar] [CrossRef] [PubMed]
- Reyes, N.A.; Fisher, J.K.; Austgen, K.; VandenBerg, S.; Huang, E.J.; Oakes, S.A. Blocking the mitochondrial apoptotic pathway preserves motor neuron viability and function in a mouse model of amyotrophic lateral sclerosis. J. Clin. Investig. 2010, 120, 3673–3679. [Google Scholar] [CrossRef] [PubMed]
- Steele, A.D.; Yi, C.H. Neuromuscular denervation: Bax up against the wall in amyotrophic lateral sclerosis. J. Neurosci. 2006, 26, 12849–12851. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.A.; Chesselet, M.F. Apoptosis in Huntington’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Oltersdorf, T.; Elmore, S.W.; Shoemaker, A.R.; Armstrong, R.C.; Augeri, D.J.; Belli, B.A.; Bruncko, M.; Deckwerth, T.L.; Dinges, J.; Hajduk, P.J.; et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005, 435, 677–681. [Google Scholar] [CrossRef]
- Raghupathi, R.; Graham, D.I.; McIntosh, T.K. Apoptosis after traumatic brain injury. J. Neurotrauma 2000, 17, 927–938. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott-McKean, J.J.; Matsuyama, M.; Guo, C.W.; Ni, L.; Sassouni, B.; Kurup, S.; Nickells, R.; Matsuyama, S. Cytoprotective Small Compound M109S Attenuated Retinal Ganglion Cell Degeneration Induced by Optic Nerve Crush in Mice. Cells 2024, 13, 911. https://doi.org/10.3390/cells13110911
Scott-McKean JJ, Matsuyama M, Guo CW, Ni L, Sassouni B, Kurup S, Nickells R, Matsuyama S. Cytoprotective Small Compound M109S Attenuated Retinal Ganglion Cell Degeneration Induced by Optic Nerve Crush in Mice. Cells. 2024; 13(11):911. https://doi.org/10.3390/cells13110911
Chicago/Turabian StyleScott-McKean, Jonah J., Mieko Matsuyama, Charles W. Guo, Lin Ni, Brandon Sassouni, Shree Kurup, Robert Nickells, and Shigemi Matsuyama. 2024. "Cytoprotective Small Compound M109S Attenuated Retinal Ganglion Cell Degeneration Induced by Optic Nerve Crush in Mice" Cells 13, no. 11: 911. https://doi.org/10.3390/cells13110911
APA StyleScott-McKean, J. J., Matsuyama, M., Guo, C. W., Ni, L., Sassouni, B., Kurup, S., Nickells, R., & Matsuyama, S. (2024). Cytoprotective Small Compound M109S Attenuated Retinal Ganglion Cell Degeneration Induced by Optic Nerve Crush in Mice. Cells, 13(11), 911. https://doi.org/10.3390/cells13110911





