Inhibition of PIKfyve Leads to Lysosomal Disorders via Dysregulation of mTOR Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Lines
2.2. LysoTracker Red Staining
2.3. Cell Culture
2.4. Western Blot Analysis
2.5. Drug Treatment
2.6. Immunofluorescence Staining
2.7. Real-Time Quantitative PCR Analysis
2.8. Microscopy and Fluorescent Pixel Count
2.9. Neutral Red Staining
2.10. Statistical Analysis
3. Results
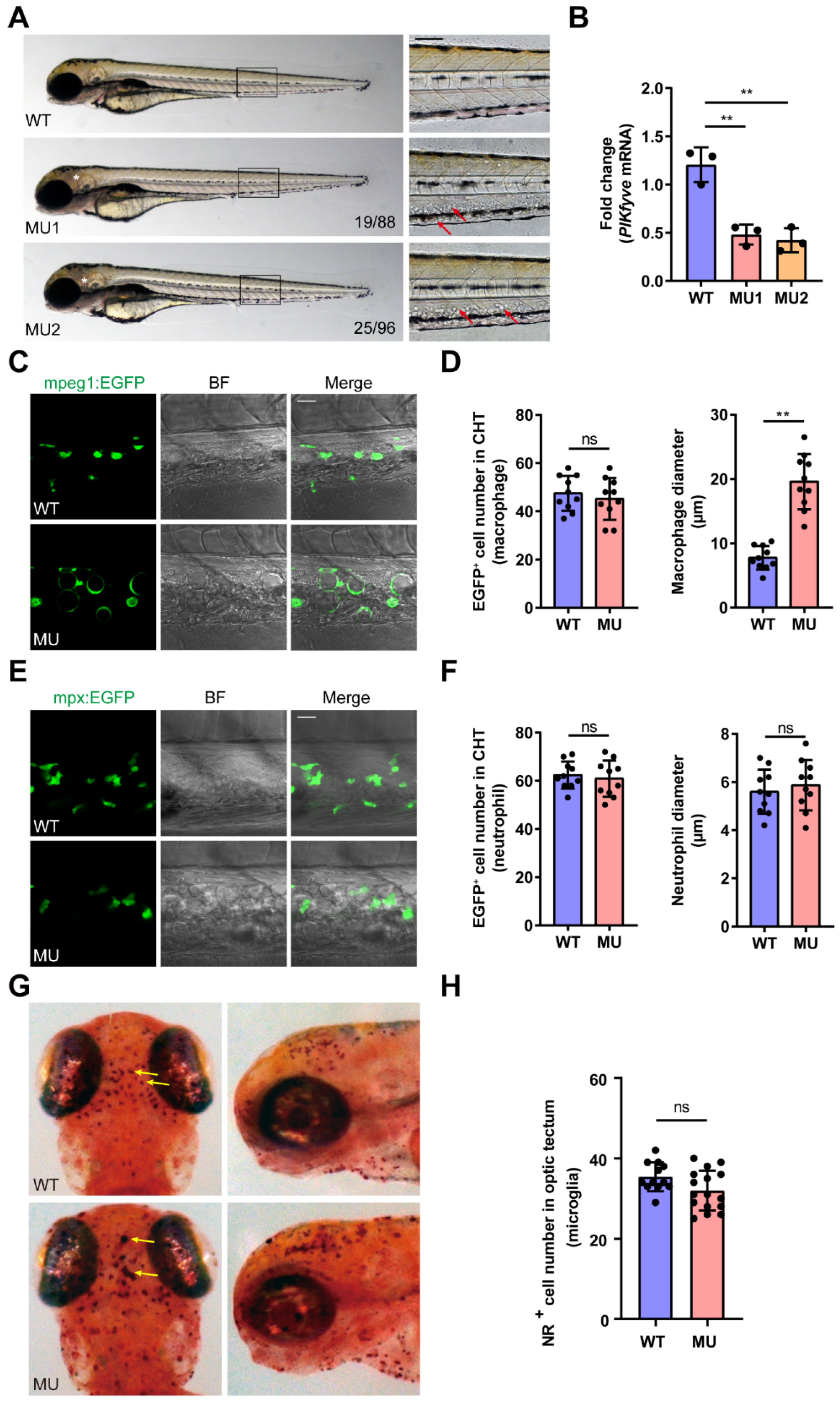
3.1. Generation of PIKfyve Mutant Zebrafish Using CRISPR/Cas9
3.2. General Morphological Defects in PIKfyve Mutant Zebrafish
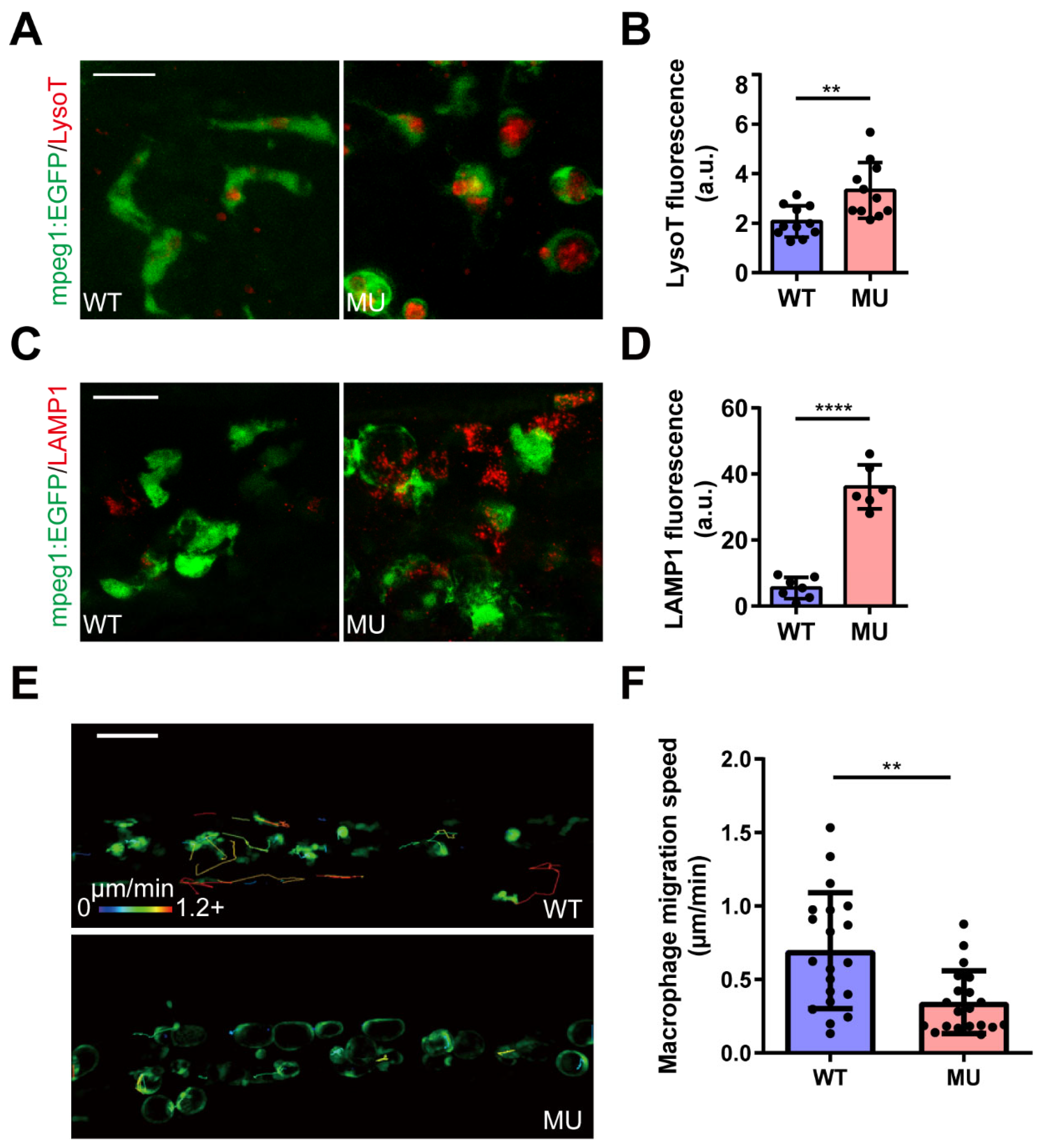
3.3. PIKfyve Mutation Leads to Lysosomal Disorders in Macrophages
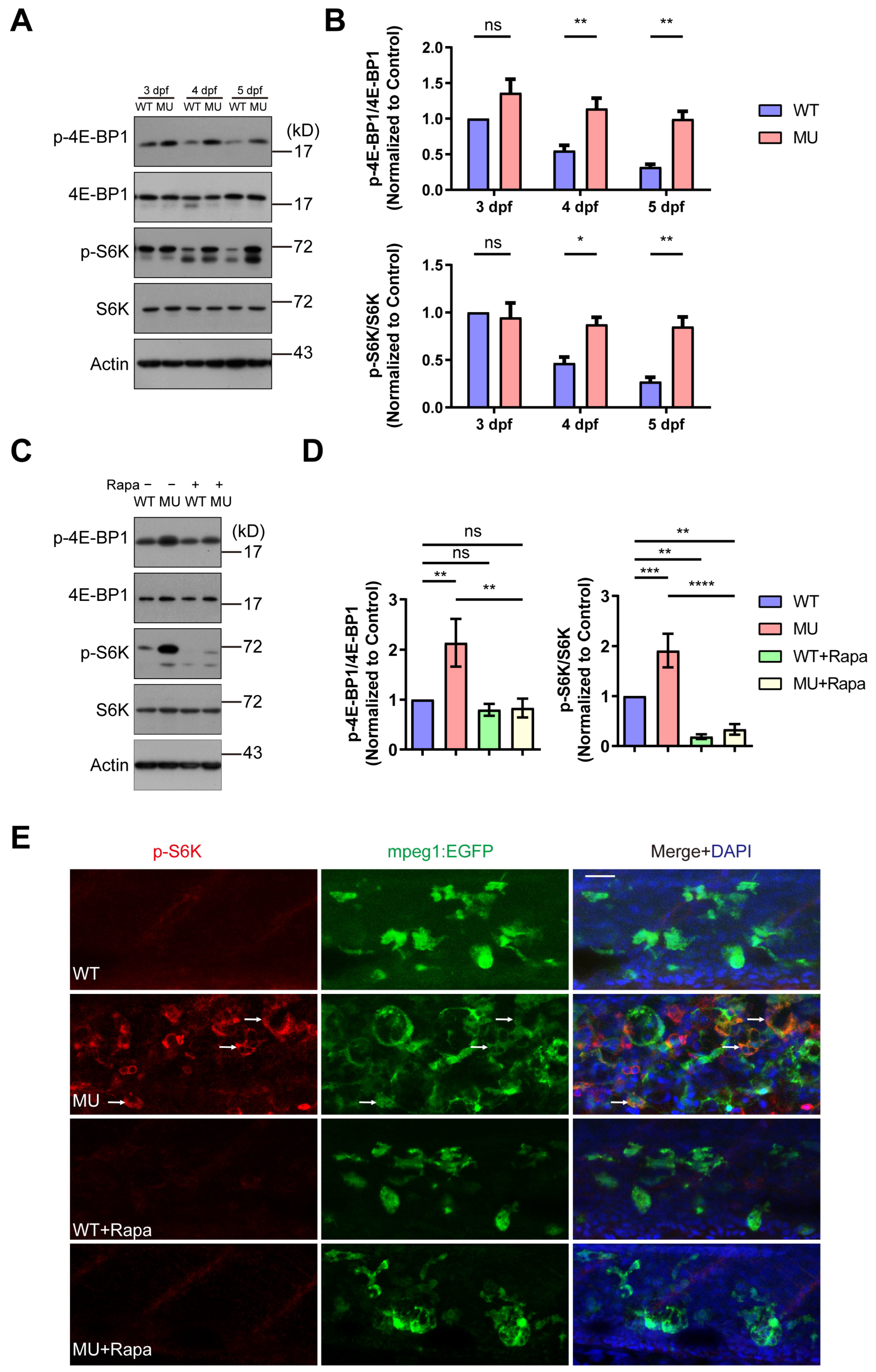
3.4. The Defects in PIKfyve Mutants Are Dependent on Sustained mTOR Activity
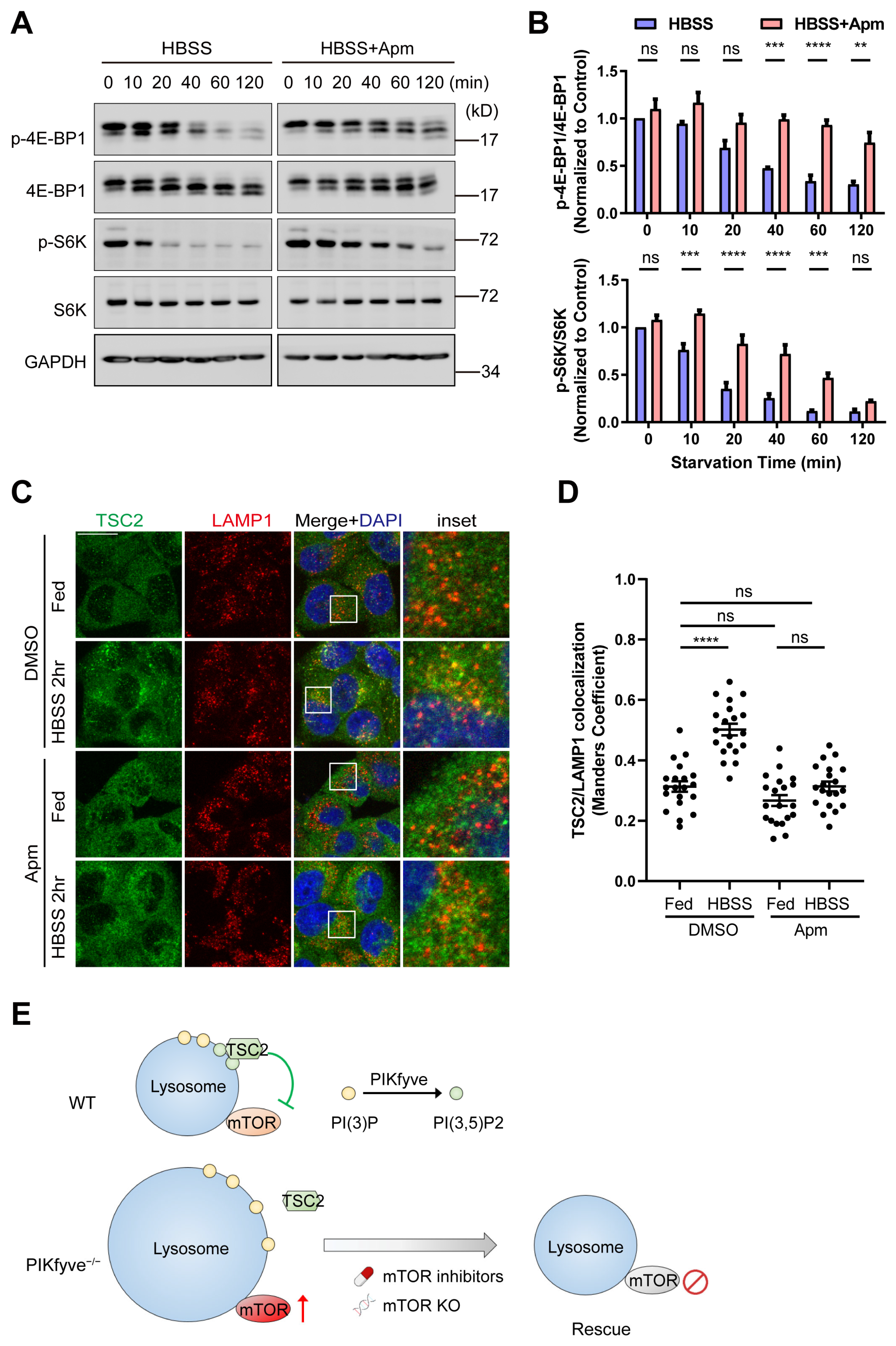
3.5. PIKfyve Is Required for Starvation-Induced Shutdown of mTOR in Cultured Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Huang, P.-T.; Einav, S.; Asquith, C.R.M. PIKfyve: A Lipid Kinase Target for COVID-19, Cancer and Neurodegenerative Disorders. Nat. Rev. Drug Discov. 2021, 20, 730. [Google Scholar] [CrossRef] [PubMed]
- Zolov, S.N.; Bridges, D.; Zhang, Y.; Lee, W.-W.; Riehle, E.; Verma, R.; Lenk, G.M.; Converso-Baran, K.; Weide, T.; Albin, R.L.; et al. In Vivo, Pikfyve Generates PI(3,5)P2, Which Serves as Both a Signaling Lipid and the Major Precursor for PI5P. Proc. Natl. Acad. Sci. USA 2012, 109, 17472–17477. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jahng, W.J.; Di Vizio, D.; Lee, J.S.; Jhaveri, R.; Rubin, M.A.; Shisheva, A.; Freeman, M.R. The Phosphoinositide Kinase PIKfyve Mediates Epidermal Growth Factor Receptor Trafficking to the Nucleus. Cancer Res. 2007, 67, 9229–9237. [Google Scholar] [CrossRef] [PubMed]
- de Lartigue, J.; Polson, H.; Feldman, M.; Shokat, K.; Tooze, S.A.; Urbé, S.; Clague, M.J. PIKfyve Regulation of Endosome-Linked Pathways. Traffic 2009, 10, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.C.; Ferreira, A.; Mariën, J.; Delay, C.; Lee, E.; Trojanowski, J.Q.; Moechars, D.; Annaert, W.; De Muynck, L. PIKfyve Activity Is Required for Lysosomal Trafficking of Tau Aggregates and Tau Seeding. J. Biol. Chem. 2021, 296, 100636. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, J.; Tokuda, E.; Yao, Y.; Sasaki, T.; Inoki, K.; Weisman, L.S. PP2A-Dependent TFEB Activation Is Blocked by PIKfyve-Induced MTORC1 Activity. Mol. Biol. Cell 2022, 33, jcs213587. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.H.; Saffi, G.; Gray, M.A.; Wallace, C.; Dayam, R.M.; Ou, Z.-Y.A.; Lenk, G.; Puertollano, R.; Watkins, S.C.; Botelho, R.J. Lysosome Enlargement during Inhibition of the Lipid Kinase PIKfyve Proceeds through Lysosome Coalescence. J. Cell Sci. 2018, 131, jcs213587. [Google Scholar] [CrossRef] [PubMed]
- Dukes, J.D.; Whitley, P.; Chalmers, A.D. The PIKfyve Inhibitor YM201636 Blocks the Continuous Recycling of the Tight Junction Proteins Claudin-1 and Claudin-2 in MDCK Cells. PLoS ONE 2012, 7, e28659. [Google Scholar] [CrossRef]
- Sharma, G.; Guardia, C.M.; Roy, A.; Vassilev, A.; Saric, A.; Griner, L.N.; Marugan, J.; Ferrer, M.; Bonifacino, J.S.; DePamphilis, M.L. A Family of PIKFYVE Inhibitors with Therapeutic Potential against Autophagy-Dependent Cancer Cells Disrupt Multiple Events in Lysosome Homeostasis. Autophagy 2019, 8627, 1694–1718. [Google Scholar] [CrossRef]
- Qiao, Y.; Choi, J.E.; Tien, J.C.; Simko, S.A.; Rajendiran, T.; Vo, J.N.; Delekta, A.D.; Wang, L.; Xiao, L.; Hodge, N.B.; et al. Autophagy Inhibition by Targeting PIKfyve Potentiates Response to Immune Checkpoint Blockade in Prostate Cancer. Nat. Cancer 2021, 2, 978–993. [Google Scholar] [CrossRef]
- Lakkaraju, A.K.K.; Frontzek, K.; Lemes, E.; Herrmann, U.; Losa, M.; Marpakwar, R.; Aguzzi, A. Loss of PIKfyve Drives the Spongiform Degeneration in Prion Diseases. EMBO Mol. Med. 2021, 13, e14714. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Wang, X.; Luo, L.; Ni, R. The Lysosomal Storage Disorder Due to Fig4a Mutation Causes Robust Liver Vacuolation in Zebrafish. Zebrafish 2021, 18, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-L.; Chou, Y.; Rothlauf, P.W.; Liu, Z.; Soh, T.K.; Cureton, D.; Case, J.B.; Chen, R.E.; Diamond, M.S.; Whelan, S.P.J.; et al. Inhibition of PIKfyve Kinase Prevents Infection by Zaire Ebolavirus and SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 20803–20813. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.M.; Heath, V.L.; Guého, A.; Bosmani, C.; Knobloch, P.; Sikakana, P.; Personnic, N.; Dove, S.K.; Michell, R.H.; Meier, R.; et al. Pikfyve/Fab1 Is Required for Efficient V-ATPase and Hydrolase Delivery to Phagosomes, Phagosomal Killing, and Restriction of Legionella Infection. PLoS Pathog. 2019, 15, e1007551. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Leung, A.; Bo, Y.; Kozak, R.A.; Anand, S.P.; Warkentin, C.; Salambanga, F.D.R.; Cui, J.; Kobinger, G.; Kobasa, D.; et al. Ebola Virus Requires Phosphatidylinositol (3,5) Bisphosphate Production for Efficient Viral Entry. Virology 2018, 513, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.C.; Wang, J.T.H.H.; Castro, N.A.; Hamilton, N.A.; Town, L.; Brown, D.L.; Meunier, F.A.; Brown, N.F.; Stow, J.L.; Teasdale, R.D. Inhibition of the PtdIns(5) Kinase PIKfyve Disrupts Intracellular Replication of Salmonella. EMBO J. 2010, 29, 1331–1347. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tiab, L.; Jiao, X.; Munier, F.L.; Zografos, L.; Frueh, B.E.; Sergeev, Y.; Smith, J.; Rubin, B.; Meallet, M.A.; et al. Mutations in PIP5K3 Are Associated with François-Neetens Mouchetée Fleck Corneal Dystrophy. Am. J. Hum. Genet. 2005, 77, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Boisset, G.; Polok, B.K.; Schorderet, D.F. Characterization of Pip5k3 Fleck Corneal Dystrophy-Linked Gene in Zebrafish. Gene Expr. Patterns 2008, 8, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Wu, Y.; Wang, Y.; Cui, Y.; Zhang, M.; Zhang, T.; Huang, X.; Yu, S.; Yu, T.; Zhao, J. Disruption of PIKFYVE Causes Congenital Cataract in Human and Zebrafish. eLife 2022, 11, e71256. [Google Scholar] [CrossRef]
- Takasuga, S.; Horie, Y.; Sasaki, J.; Sun-Wada, G.-H.; Kawamura, N.; Iizuka, R.; Mizuno, K.; Eguchi, S.; Kofuji, S.; Kimura, H.; et al. Critical Roles of Type III Phosphatidylinositol Phosphate Kinase in Murine Embryonic Visceral Endoderm and Adult Intestine. Proc. Natl. Acad. Sci. USA 2013, 110, 1726–1731. [Google Scholar] [CrossRef]
- Ikonomov, O.C.; Sbrissa, D.; Delvecchio, K.; Xie, Y.; Jin, J.-P.; Rappolee, D.; Shisheva, A. The Phosphoinositide Kinase PIKfyve Is Vital in Early Embryonic Development. J. Biol. Chem. 2011, 286, 13404–13413. [Google Scholar] [CrossRef] [PubMed]
- Ikonomov, O.C.; Sbrissa, D.; Delvecchio, K.; Feng, H.-Z.; Cartee, G.D.; Jin, J.-P.; Shisheva, A. Muscle-Specific Pikfyve Gene Disruption Causes Glucose Intolerance, Insulin Resistance, Adiposity, and Hyperinsulinemia but Not Muscle Fiber-Type Switching. Am. J. Physiol. Metab. 2013, 305, E119–E131. [Google Scholar] [CrossRef]
- Liggins, M.C.; Flesher, J.L.; Jahid, S.; Vasudeva, P.; Eby, V.; Takasuga, S.; Sasaki, J.; Sasaki, T.; Boissy, R.E.; Ganesan, A.K. PIKfyve Regulates Melanosome Biogenesis. PLoS Genet. 2018, 14, e1007290. [Google Scholar] [CrossRef] [PubMed]
- Min, S.H.; Suzuki, A.; Stalker, T.J.; Zhao, L.; Wang, Y.; McKennan, C.; Riese, M.J.; Guzman, J.F.; Zhang, S.; Lian, L.; et al. Loss of PIKfyve in Platelets Causes a Lysosomal Disease Leading to Inflammation and Thrombosis in Mice. Nat. Commun. 2014, 5, 4691. [Google Scholar] [CrossRef] [PubMed]
- Min, S.H.; Suzuki, A.; Weaver, L.; Guzman, J.; Chung, Y.; Jin, H.; Gonzalez, F.; Trasorras, C.; Zhao, L.; Spruce, L.A.; et al. PIKfyve Deficiency in Myeloid Cells Impairs Lysosomal Homeostasis in Macrophages and Promotes Systemic Inflammation in Mice. Mol. Cell. Biol. 2019, 39, e00158-19. [Google Scholar] [CrossRef] [PubMed]
- Jhanwar-Uniyal, M.; Zeller, S.L.; Spirollari, E.; Das, M.; Hanft, S.J.; Gandhi, C.D. Discrete Mechanistic Target of Rapamycin Signaling Pathways, Stem Cells, and Therapeutic Targets. Cells 2024, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Bridges, D.; Ma, J.-T.; Park, S.; Inoki, K.; Weisman, L.S.; Saltiel, A.R. Phosphatidylinositol 3,5-Bisphosphate Plays a Role in the Activation and Subcellular Localization of Mechanistic Target of Rapamycin 1. Mol. Biol. Cell 2012, 23, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Palm, W.; Lee, Y.; Yang, W.; Bandyopadhyay, U.; Xu, H.; Florey, O.; Thompson, C.B.; Overholtzer, M. PIKfyve Regulates Vacuole Maturation and Nutrient Recovery Following Engulfment. Dev. Cell 2016, 38, 536–547. [Google Scholar] [CrossRef]
- Fitzian, K.; Brückner, A.; Brohée, L.; Zech, R.; Antoni, C.; Kiontke, S.; Gasper, R.; Linard Matos, A.L.; Beel, S.; Wilhelm, S.; et al. TSC1 Binding to Lysosomal PIPs Is Required for TSC Complex Translocation and MTORC1 Regulation. Mol. Cell 2021, 81, 2705–2721.e8. [Google Scholar] [CrossRef]
- Zhao, S.; Xia, J.; Wu, X.; Zhang, L.; Wang, P.; Wang, H.; Li, H.; Wang, X.; Chen, Y.; Agnetti, J.; et al. Deficiency in Class III PI3-Kinase Confers Postnatal Lethality with IBD-like Features in Zebrafish. Nat. Commun. 2018, 9, 2639. [Google Scholar] [CrossRef]
- Takaki, K.; Cosma, C.L.; Troll, M.A.; Ramakrishnan, L. An In Vivo Platform for Rapid High-Throughput Antitubercular Drug Discovery. Cell Rep. 2012, 2, 175–184. [Google Scholar] [CrossRef]
- Lindeboom, R.G.H.; Vermeulen, M.; Lehner, B.; Supek, F. The Impact of Nonsense-Mediated mRNA Decay on Genetic Disease, Gene Editing and Cancer Immunotherapy. Nat. Genet. 2019, 51, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- El-Brolosy, M.A.; Kontarakis, Z.; Rossi, A.; Kuenne, C.; Günther, S.; Fukuda, N.; Kikhi, K.; Boezio, G.L.M.; Takacs, C.M.; Lai, S.-L.; et al. Genetic Compensation Triggered by Mutant mRNA Degradation. Nature 2019, 568, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Gorgoni, B.; Zhao, Y.-B.; Krishnan, J.; Stansfield, I. Destabilization of Eukaryote mRNAs by 5′ Proximal Stop Codons Can Occur Independently of the Nonsense-Mediated mRNA Decay Pathway. Cells 2019, 8, 800. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Singer, R.H. Cellular Variability of Nonsense-Mediated mRNA Decay. Nat. Commun. 2021, 12, 7203. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Lv, J.; Zhang, C.; Wang, L.; Ma, D.; Liu, F. The Vascular Niche Regulates Hematopoietic Stem and Progenitor Cell Lodgment and Expansion via Klf6a-Ccl25b. Dev. Cell 2017, 42, 349–362.e4. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.D.; Levitte, S.; O’Sullivan, M.P.; O’Leary, S.M.; Cambier, C.J.J.; Cameron, J.; Takaki, K.K.; Moens, C.B.; Tobin, D.M.; Keane, J.; et al. Lysosomal Disorders Drive Susceptibility to Tuberculosis by Compromising Macrophage Migration. Cell 2016, 165, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Idol, R.A.; Wozniak, D.F.; Fujiwara, H.; Yuede, C.M.; Ory, D.S.; Kornfeld, S.; Vogel, P. Neurologic Abnormalities in Mouse Models of the Lysosomal Storage Disorders Mucolipidosis II and Mucolipidosis III γ. PLoS ONE 2014, 9, e109768. [Google Scholar] [CrossRef]
- Kieseier, B.C.; Wisniewski, K.E.; Goebel, H.H. The Monocyte-Macrophage System Is Affected in Lysosomal Storage Diseases: An Immunoelectron Microscopic Study. Acta Neuropathol. 1997, 94, 359–362. [Google Scholar] [CrossRef]
- Ata, H.; Ekstrom, T.L.; Martínez-Gálvez, G.; Mann, C.M.; Dvornikov, A.V.; Schaefbauer, K.J.; Ma, A.C.; Dobbs, D.; Clark, K.J.; Ekker, S.C. Robust Activation of Microhomology-Mediated End Joining for Precision Gene Editing Applications. PLOS Genet. 2018, 14, e1007652. [Google Scholar] [CrossRef]
- Bu, H.; Ding, Y.; Li, J.; Zhu, P.; Shih, Y.-H.; Wang, M.; Zhang, Y.; Lin, X.; Xu, X. Inhibition of MTOR or MAPK Ameliorates Vmhcl/Myh7 Cardiomyopathy in Zebrafish. JCI Insight 2021, 6, e154215. [Google Scholar] [CrossRef] [PubMed]
- Demetriades, C.; Plescher, M.; Teleman, A.A. Lysosomal Recruitment of TSC2 Is a Universal Response to Cellular Stress. Nat. Commun. 2016, 7, 10662. [Google Scholar] [CrossRef] [PubMed]
- Wible, D.J.; Parikh, Z.; Cho, E.J.; Chen, M.-D.; Jeter, C.R.; Mukhopadhyay, S.; Dalby, K.N.; Varadarajan, S.; Bratton, S.B. Unexpected Inhibition of the Lipid Kinase PIKfyve Reveals an Epistatic Role for P38 MAPKs in Endolysosomal Fission and Volume Control. Cell Death Dis. 2024, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Zhang, X.; Zhao, M.; Tsang, W.L.; Zhang, Y.; Yau, R.G.W.; Weisman, L.S.; Xu, H. Genetically Encoded Fluorescent Probe to Visualize Intracellular Phosphatidylinositol 3,5-Bisphosphate Localization and Dynamics. Proc. Natl. Acad. Sci. USA 2013, 110, 21165–21170. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Shen, Y.; Dokmanovic, M.; Endo, Y.; Hirsch, D.S.; Wu, W.J. VPS34 Regulates TSC1/TSC2 Heterodimer to Mediate RheB and MTORC1/S6K1 Activation and Cellular Transformation. Oncotarget 2016, 7, 52239–52254. [Google Scholar] [CrossRef] [PubMed]
- MacRae, C.A.; Peterson, R.T. Zebrafish as a Mainstream Model for In Vivo Systems Pharmacology and Toxicology. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Sun, X.; Huang, W.; Hoage, T.; Redfield, M.; Kushwaha, S.; Sivasubbu, S.; Lin, X.; Ekker, S.; Xu, X. Haploinsufficiency of Target of Rapamycin Attenuates Cardiomyopathies in Adult Zebrafish. Circ. Res. 2011, 109, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Jia, D.; Cao, L.; Lu, W.; Liu, X.; Liang, C.; Pan, Z.; Fang, Z. Akebia Saponin E, as a Novel PIKfyve Inhibitor, Induces Lysosome-Associated Cytoplasmic Vacuolation to Inhibit Proliferation of Hepatocellular Carcinoma Cells. J. Ethnopharmacol. 2021, 266, 113446. [Google Scholar] [CrossRef] [PubMed]
- Karabiyik, C.; Vicinanza, M.; Son, S.M.; Rubinsztein, D.C. Glucose Starvation Induces Autophagy via ULK1-Mediated Activation of PIKfyve in an AMPK-Dependent Manner. Dev. Cell 2021, 56, 1961–1975.e5. [Google Scholar] [CrossRef]
- Sharma, V.; Makhdoomi, M.; Singh, L.; Kumar, P.; Khan, N.; Singh, S.; Verma, H.N.; Luthra, K.; Sarkar, S.; Kumar, D. Trehalose Limits Opportunistic Mycobacterial Survival during HIV Co-Infection by Reversing HIV-Mediated Autophagy Block. Autophagy 2021, 17, 476–495. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, J.; Wang, H.; Zhong, Z.; Jiang, J. Inhibition of PIKfyve Leads to Lysosomal Disorders via Dysregulation of mTOR Signaling. Cells 2024, 13, 953. https://doi.org/10.3390/cells13110953
Xia J, Wang H, Zhong Z, Jiang J. Inhibition of PIKfyve Leads to Lysosomal Disorders via Dysregulation of mTOR Signaling. Cells. 2024; 13(11):953. https://doi.org/10.3390/cells13110953
Chicago/Turabian StyleXia, Jianhong, Haiyun Wang, Zhihang Zhong, and Jun Jiang. 2024. "Inhibition of PIKfyve Leads to Lysosomal Disorders via Dysregulation of mTOR Signaling" Cells 13, no. 11: 953. https://doi.org/10.3390/cells13110953



