Embryos from Prepubertal Hyperglycemic Female Mice Respond Differentially to Oxygen Tension In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethical Clearance
2.2. Experimental Design
2.3. Ovarian Stimulation
2.4. Assessment of Spindle Morphology
2.5. In Vitro Fertilization and Preimplantation Embryo Development
2.6. TUNEL Assay
2.7. ICM Outgrowth Assay
2.8. Isolation of Total RNA, cDNA Synthesis, and Gene Expression Analysis
2.9. Statistical Analysis
3. Results
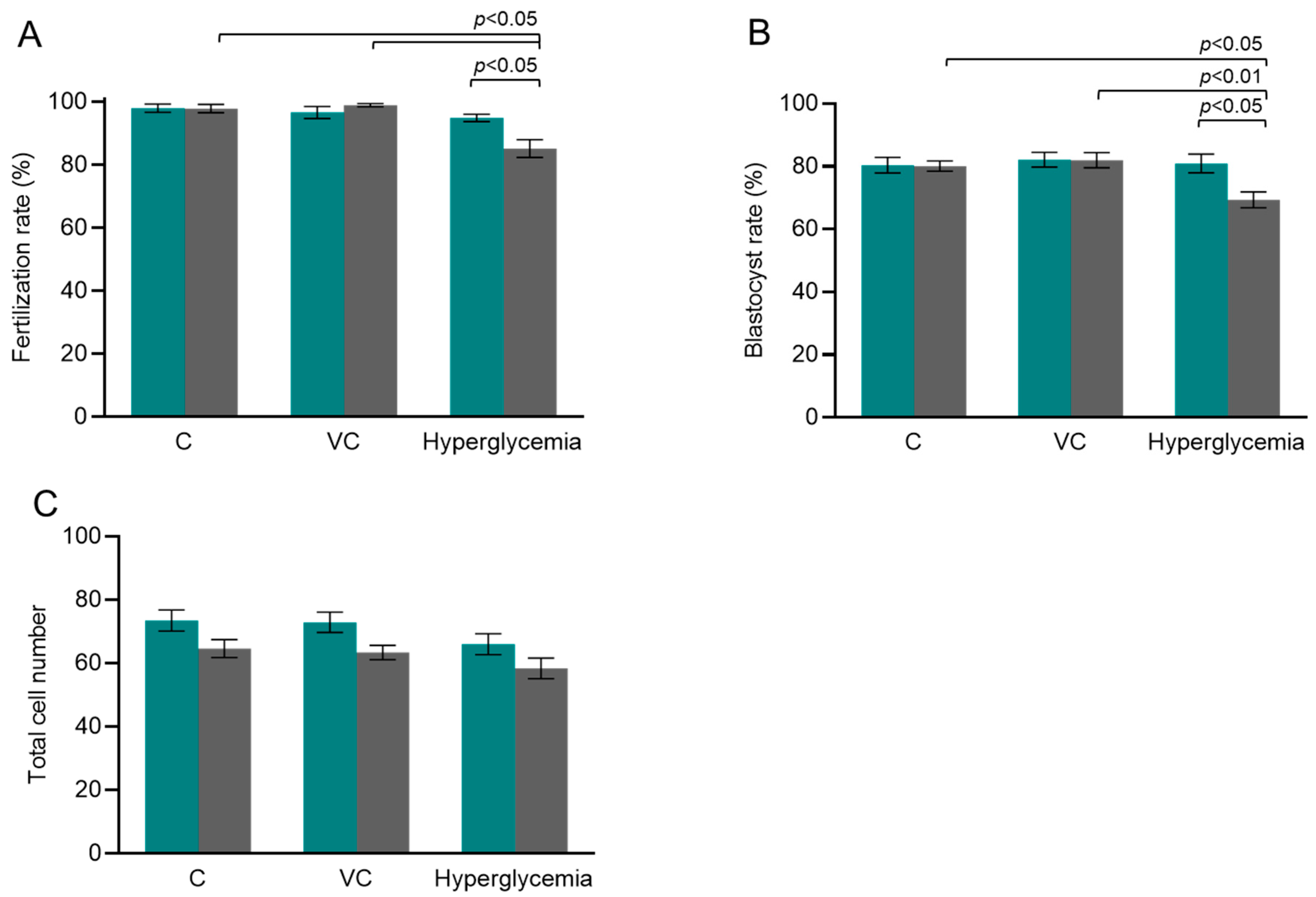
3.1. Fertilization, Developmental Competence, and Meiotic Integrity of Hyperglycemic Oocytes
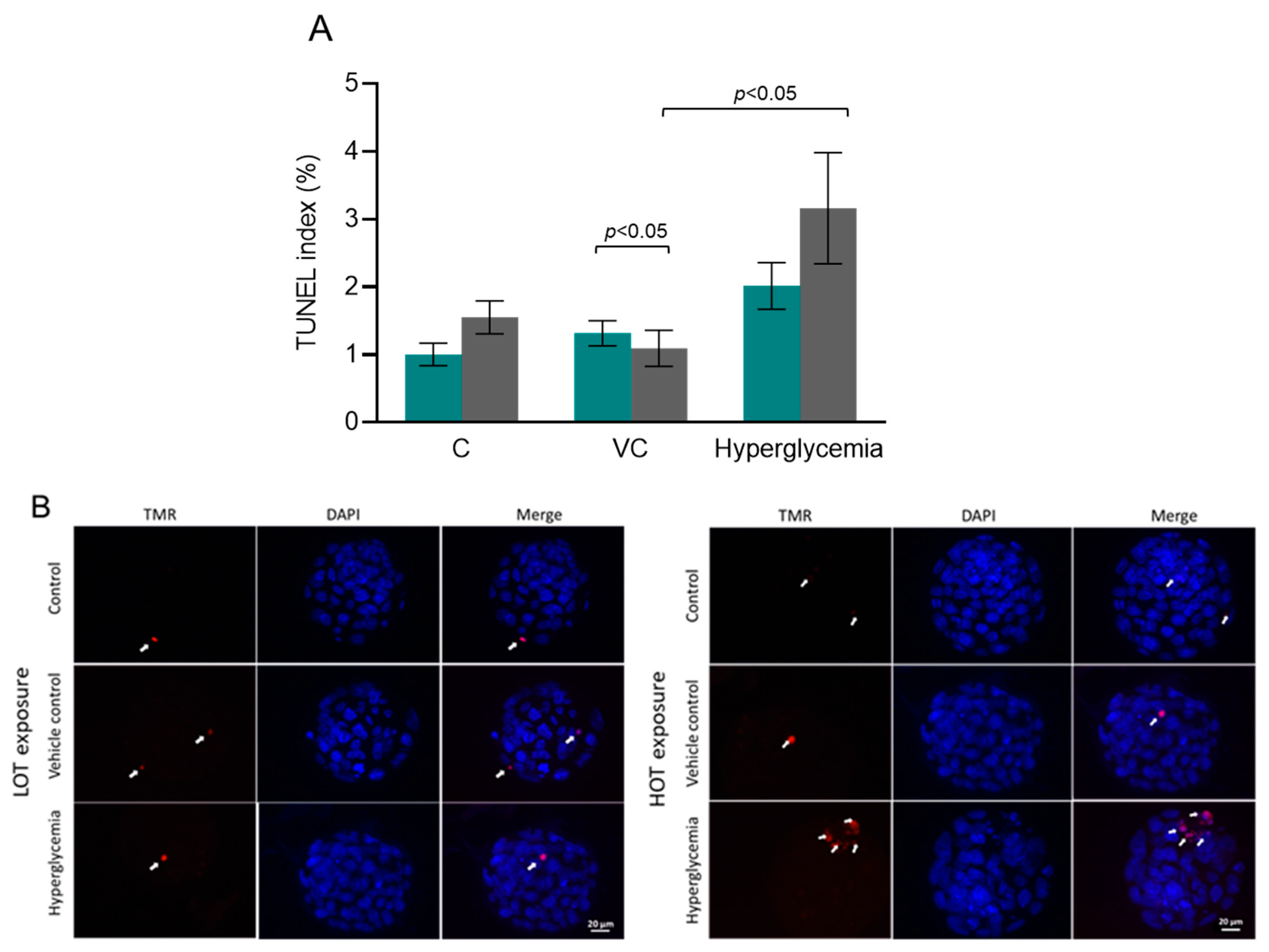
3.2. Increased Incidence of Apoptosis in Blastocysts from HOT Culture
3.3. HOT Impaired ICM Expansion and Hif1α Expression at 96 h of Extended In Vitro Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wale, P.L.; Gardner, D.K. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum. Reprod. Update 2016, 22, 2–22. [Google Scholar] [CrossRef]
- Sciorio, R.; Smith, G.D. Embryo culture at a reduced oxygen concentration of 5%: A mini review. Zygote 2019, 27, 355–361. [Google Scholar] [CrossRef]
- Morin, S.J. Oxygen tension in embryo culture: Does a shift to 2% O2 in extended culture represent the most physiologic system? J. Assist. Reprod. Genet. 2017, 34, 309–314. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Chen, H.W.; Tzeng, C.R. Low oxygen tension increases mitochondrial membrane potential and enhances expression of antioxidant genes and implantation protein of mouse blastocyst cultured in vitro. J. Ovarian Res. 2017, 10, 47. [Google Scholar] [CrossRef]
- Gomes Sobrinho, D.B.; Oliveira, J.B.; Petersen, C.G.; Mauri, A.L.; Silva, L.F.; Massaro, F.C.; Baruffi, R.L.; Cavagna, M.; Franco, J.G., Jr. IVF/ICSI outcomes after culture of human embryos at low oxygen tension: A meta-analysis. Reprod. Biol. Endocrinol. 2011, 9, 143. [Google Scholar] [CrossRef]
- Nastri, C.O.; Nóbrega, B.N.; Teixeira, D.M.; Amorim, J.; Diniz, L.M.M.; Barbosa, M.W.P.; Giorgi, V.S.I.; Pileggi, V.N.; Martins, W.P. Low versus atmospheric oxygen tension for embryo culture in assisted reproduction: A systematic review and meta-analysis. Fertil. Steril. 2016, 106, 95–104. [Google Scholar] [CrossRef]
- Thong, E.P.; Codner, E.; Laven, J.S.E.; Teede, H. Diabetes: A metabolic and reproductive disorder in women. Lancet Diabetes Endocrinol. 2020, 8, 134–149. [Google Scholar] [CrossRef]
- Maresch, C.C.; Stute, D.C.; Alves, M.G.; Oliveira, P.F.; de Kretser, D.M.; Linn, T. Diabetes-induced hyperglycemia impairs male reproductive function: A systematic review. Hum. Reprod. Update 2018, 24, 86–105. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chen, K.J.; Peng, Y.S.; Chen, P.C.; Yang, Y.H. Type 1 diabetes impairs female fertility even before it is diagnosed. Diabetes Res. Clin. Pract. 2018, 143, 151–158. [Google Scholar] [CrossRef]
- Wiebe, J.C.; Santana, A.; Medina-Rodríguez, N.; Hernández, M.; Nóvoa, J.; Mauricio, D.; Wägner, A.M. Fertility is reduced in women and in men with type 1 diabetes: Results from the Type 1 Diabetes Genetics Consortium (T1DGC). Diabetologia 2014, 57, 2501–2504. [Google Scholar] [CrossRef]
- Predheepan, D.; Daddangadi, A.; Uppangala, S.; Laxminarayana, S.L.K.; Raval, K.; Kalthur, G.; Kovačič, B.; Adiga, S.K. Experimentally Induced Hyperglycemia in Prepubertal Phase Impairs Oocyte Quality and Functionality in Adult Mice. Endocrinology 2022, 163, bqac121. [Google Scholar] [CrossRef]
- Belli, M.; Antonouli, S.; Palmerini, M.G.; Bianchi, S.; Bernardi, S.; Khalili, M.A.; Donfrancesco, O.; Nottola, S.A.; Macchiarelli, G. The effect of low and ultra-low oxygen tensions on mammalian embryo culture and development in experimental and clinical IVF. Syst. Biol. Reprod. Med. 2020, 66, 229–235. [Google Scholar] [CrossRef]
- Li, W.; Goossens, K.; Van Poucke, M.; Forier, K.; Braeckmans, K.; Van Soom, A.; Peelman, L.J. High oxygen tension increases global methylation in bovine 4-cell embryos and blastocysts but does not affect general retrotransposon expression. Reprod. Fertil. Dev. 2016, 28, 948–959. [Google Scholar] [CrossRef]
- Leite, R.F.; Annes, K.; Ispada, J.; de Lima, C.B.; Dos Santos, É.C.; Fontes, P.K.; Nogueira, M.F.G.; Milazzotto, M.P. Oxidative Stress Alters the Profile of Transcription Factors Related to Early Development on In Vitro Produced Embryos. Oxid. Med. Cell Longev. 2017, 217, 1502489. [Google Scholar] [CrossRef]
- Marín, R.; Abad, C.; Rojas, D.; Chiarello, D.I.; Alejandro, T.G. Biomarkers of oxidative stress and reproductive complications. Adv. Clin. Chem. 2023, 113, 157–233. [Google Scholar] [CrossRef]
- Martin, P.M.; Roon, P.; Van Ells, T.K.; Ganapathy, V.; Smith, S.B. Death of retinal neurons in streptozotocin-induced diabetic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3330–3336. [Google Scholar] [CrossRef]
- D’Souza, F.; Uppangala, S.; Asampille, G.; Salian, S.R.; Kalthur, G.; Talevi, R.; Atreya, H.S.; Adiga, S.K. Spent embryo culture medium metabolites are related to the in vitro attachment ability of blastocysts. Sci. Rep. 2018, 8, 17025. [Google Scholar] [CrossRef]
- Mantikou, E.; Youssef, M.A.; van Wely, M.; van der Veen, F.; Al-Inany, H.G.; Repping, S.; Mastenbroek, S. Embryo culture media and IVF/ICSI success rates: A systematic review. Hum. Reprod. Update 2013, 19, 210–220. [Google Scholar] [CrossRef]
- Bontekoe, S.; Mantikou, E.; van Wely, M.; Seshadri, S.; Repping, S.; Mastenbroek, S. Low oxygen concentrations for embryo culture in assisted reproductive technologies. Cochrane Database Syst. Rev. 2012, 7, CD008950. [Google Scholar] [CrossRef]
- Konstantogianni, O.; Panou, T.; Zikopoulos, A.; Skentou, C.; Stavros, S.; Asimakopoulos, B. Culture of Human Embryos at High and Low Oxygen Levels. J. Clin. Med. 2024, 13, 2222. [Google Scholar] [CrossRef]
- Johnson, M.H.; Nasr-Esfahani, M.H. Radical solutions and cultural problems: Could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays 1994, 16, 31–38. [Google Scholar] [CrossRef]
- Goto, Y.; Noda, Y.; Mori, T.; Nakano, M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free Radic. Biol. Med. 1993, 15, 69–75. [Google Scholar] [CrossRef]
- Wang, Q.; Ratchford, A.M.; Chi, M.M.; Schoeller, E.; Frolova, A.; Schedl, T.; Moley, K.H. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol. Endocrinol. 2009, 23, 1603–1612. [Google Scholar] [CrossRef]
- Uppangala, S.; Dhiman, S.; Salian, S.R.; Singh, V.J.; Kalthur, G.; Adiga, S.K. In vitro matured oocytes are more susceptible than in vivo matured oocytes to mock ICSI induced functional and genetic changes. PLoS ONE 2015, 18, e0119735. [Google Scholar] [CrossRef]
- Ramin, N.; Thieme, R.; Fischer, S.; Schindler, M.; Schmidt, T.; Fischer, B.; Navarrete Santos, A. Maternal diabetes impairs gastrulation and insulin and IGF-I receptor expression in rabbit blastocysts. Endocrinology 2015, 151, 4158–4167. [Google Scholar] [CrossRef]
- Makarevich, A.V.; Markkula, M. Apoptosis and cell proliferation potential of bovine embryos stimulated with insulin-like growth factor I during in vitro maturation and culture. Biol. Reprod. 2002, 66, 386–392. [Google Scholar] [CrossRef]
- Chi, M.M.; Pingsterhaus, J.; Carayannopoulos, M.; Moley, K.H. Decreased glucose transporter expression triggers BAX-dependent apoptosis in the murine blastocyst. J. Biol. Chem. 2000, 275, 40252–40257. [Google Scholar] [CrossRef]
- Moley, K.H.; Chi, M.M.; Knudson, C.M.; Korsmeyer, S.J.; Mueckler, M.M. Hyperglycemia induces apoptosis in pre-implantation embryos through cell death effector pathways. Nat. Med. 1998, 4, 1421–1424. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best. Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Adiga, S.K.; Toyoshima, M.; Shiraishi, K.; Shimura, T.; Takeda, J.; Taga, M.; Nagai, H.; Kumar, P.; Niwa, O. p21 provides stage specific DNA damage control to preimplantation embryos. Oncogene 2007, 26, 6141–6149. [Google Scholar] [CrossRef] [PubMed]
- Forristal, C.E.; Christensen, D.R.; Chinnery, F.E.; Petruzzelli, R.; Parry, K.L.; Sanchez-Elsner, T.; Houghton, F.D. Environmental oxygen tension regulates the energy metabolism and self-renewal of human embryonic stem cells. PLoS ONE 2013, 8, e62507. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Jun, J.H. Advantages of the outgrowth model for evaluating the implantation competence of blastocysts. Clin. Exp. Reprod. Med. 2020, 47, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Davy, P.M.; Gardner, L.H.; Mathews, J.; Yamazaki, Y.; Allsopp, R.C. Hypoxia Inducible Factor 1 Alpha Is Expressed in Germ Cells throughout the Murine Life Cycle. PLoS ONE 2016, 11, e0154309. [Google Scholar] [CrossRef] [PubMed]
- Cerychova, J.; Pavlinkova, G. HIF-1, Metabolism, and Diabetes in the Embryonic and Adult Heart. Front. Endocrinol. 2018, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Dunwoodie, S.L. The role of hypoxia in development of the Mammalian embryo. Dev. Cell 2009, 17, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, W.; Yoon, H.; Lee, J.; Jun, J.H. Dynamic Oxygen Conditions Promote the Translocation of HIF-1α to the Nucleus in Mouse Blastocysts. Biomed. Res. Int. 2021, 2021, 5050527. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Nandi, S.; Gupta, P.S.P.; Mondal, S. Antioxidants supplementation improves the quality of in vitro produced ovine embryos with amendments in key development gene expressions. Theriogenology 2023, 201, 41–52. [Google Scholar] [CrossRef]
- Zarbakhsh, S. Effect of antioxidants on preimplantation embryo development in vitro: A review. Zygote 2021, 29, 179–193. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predheepan, D.; Salian, S.R.; Uppangala, S.; Lakshmi R, V.; Kalthur, G.; Kovačič, B.; Adiga, S.K. Embryos from Prepubertal Hyperglycemic Female Mice Respond Differentially to Oxygen Tension In Vitro. Cells 2024, 13, 954. https://doi.org/10.3390/cells13110954
Predheepan D, Salian SR, Uppangala S, Lakshmi R V, Kalthur G, Kovačič B, Adiga SK. Embryos from Prepubertal Hyperglycemic Female Mice Respond Differentially to Oxygen Tension In Vitro. Cells. 2024; 13(11):954. https://doi.org/10.3390/cells13110954
Chicago/Turabian StylePredheepan, Dhakshanya, Sujith Raj Salian, Shubhashree Uppangala, Vani Lakshmi R, Guruprasad Kalthur, Borut Kovačič, and Satish Kumar Adiga. 2024. "Embryos from Prepubertal Hyperglycemic Female Mice Respond Differentially to Oxygen Tension In Vitro" Cells 13, no. 11: 954. https://doi.org/10.3390/cells13110954
APA StylePredheepan, D., Salian, S. R., Uppangala, S., Lakshmi R, V., Kalthur, G., Kovačič, B., & Adiga, S. K. (2024). Embryos from Prepubertal Hyperglycemic Female Mice Respond Differentially to Oxygen Tension In Vitro. Cells, 13(11), 954. https://doi.org/10.3390/cells13110954






