Modulation of Redox and Inflammatory Signaling in Human Skin Cells Using Phytocannabinoids Applied after UVA Irradiation: In Vitro Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Cells Cultures
2.2. Methods
2.2.1. Cells Cultures
2.2.2. Cells Treatment
- Control—keratinocytes/fibroblasts incubated for 24 h in medium only under standard conditions;
- CBG—keratinocytes/fibroblasts incubated for 24 h in medium with CBG (1 µM);
- CBD—keratinocytes/fibroblasts incubated for 24 h in medium with CBD (5 µM);
- CBG+CBD—keratinocytes/fibroblasts incubated for 24 h in medium with CBG-1 µM+CBD-5 µM;
- UVA—keratinocytes exposed to UVA(30 J/cm2)/fibroblasts exposed to UVA(20 J/cm2);
- UVA+CBG—keratinocytes exposed to UVA(30 J/cm2)/fibroblasts exposed to UVA(20 J/cm2) and, after that, all cells were incubated for 24 h in medium with CBG(1 µM);
- UVA+CBD—keratinocytes exposed to UVA(30 J/cm2)/fibroblasts exposed to UVA(20 J/cm2) and, after that, all cells were incubated for 24 h in medium with CBD(5 µM);
- UVA+CBG+CBD—keratinocytes exposed to UVA(30 J/cm2)/fibroblasts exposed to UVA(20 J/cm2) and, after that, all cells were incubated for 24 h in a medium with CBG-1 µM+CBD-5 µM.
2.2.3. Prooxidative Parameters
2.2.4. Antioxidant Enzymes Activity
2.2.5. Non-Enzymatic Antioxidant Levels
2.2.6. Protein Expression
2.2.7. Lipid Peroxidation
2.2.8. Protein modifications
2.2.9. Immunofluorescence Staining and Confocal Microscopy
2.2.10. Statistical Analysis
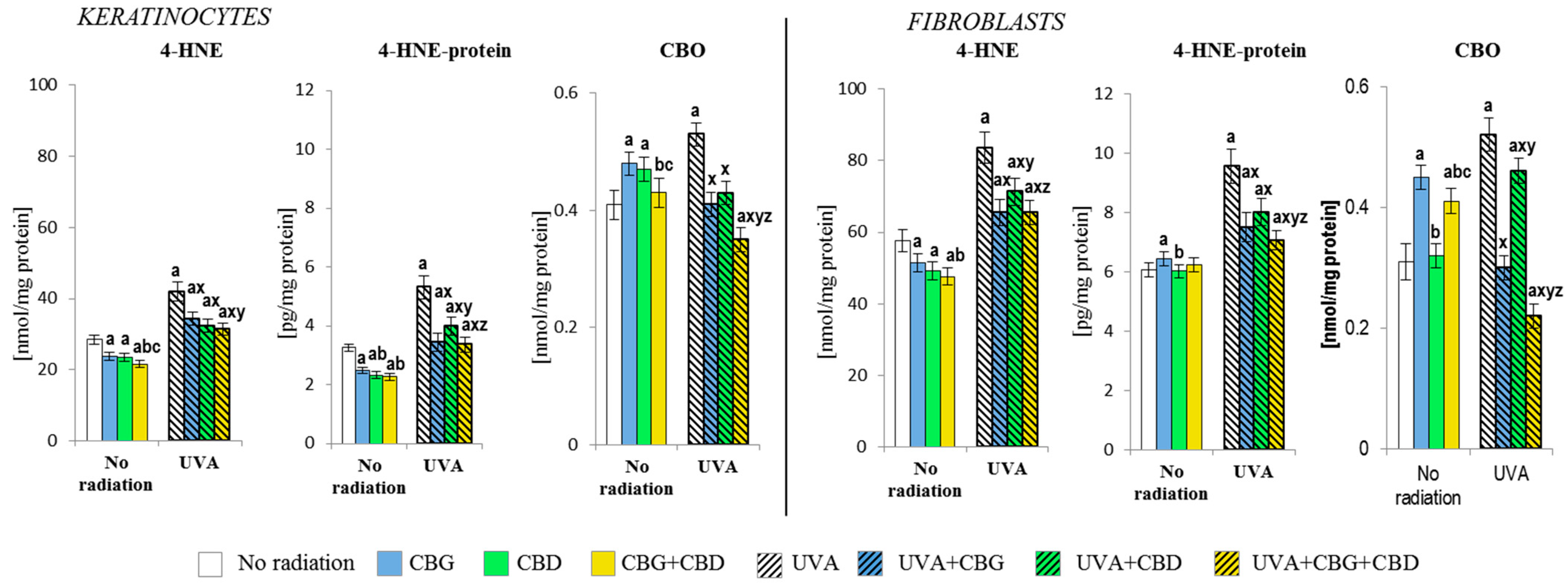
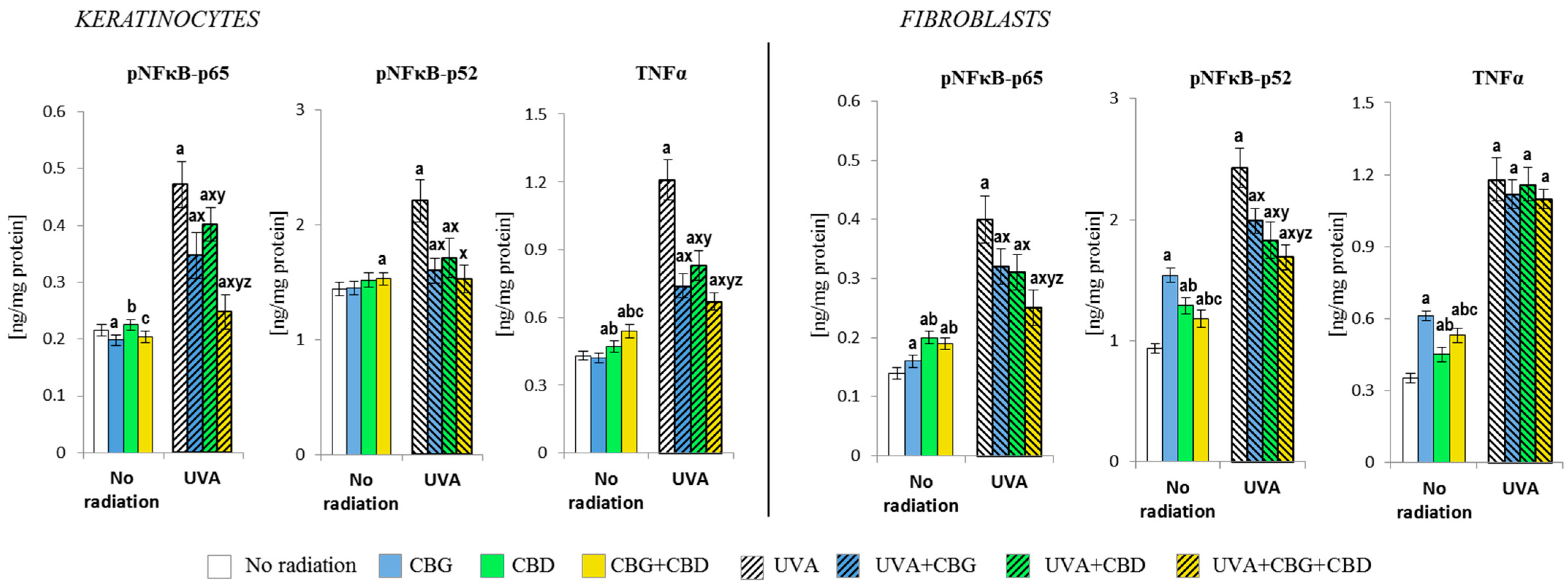
3. Results
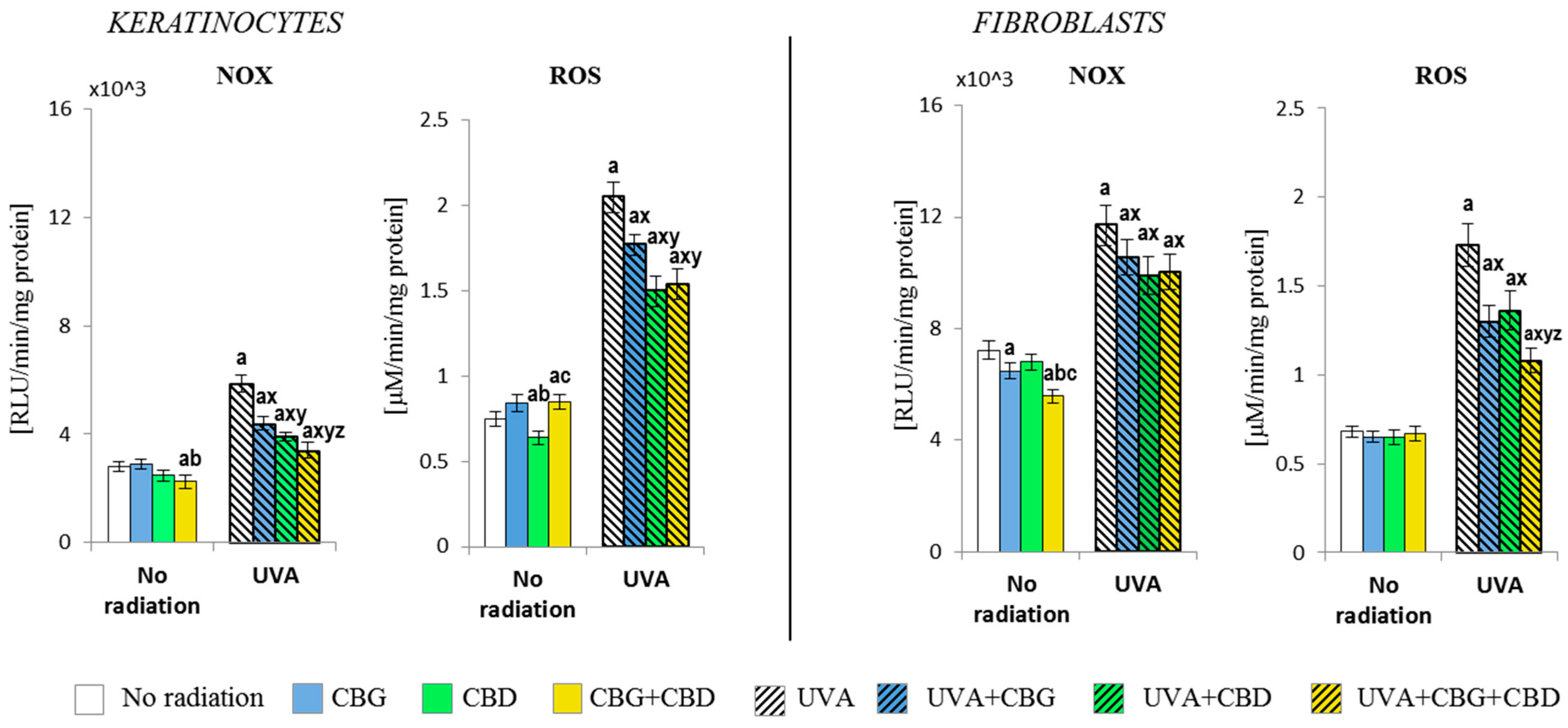
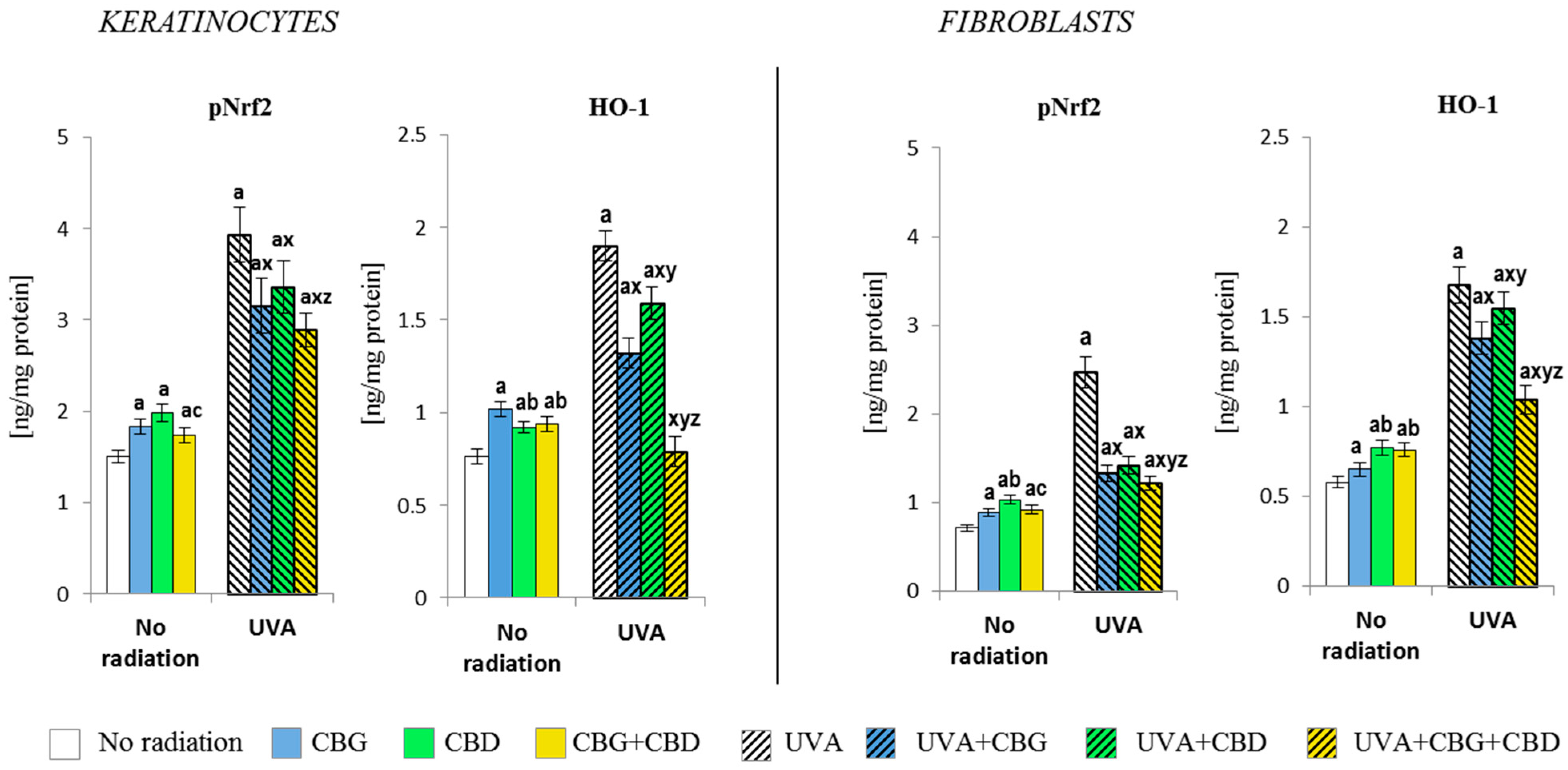
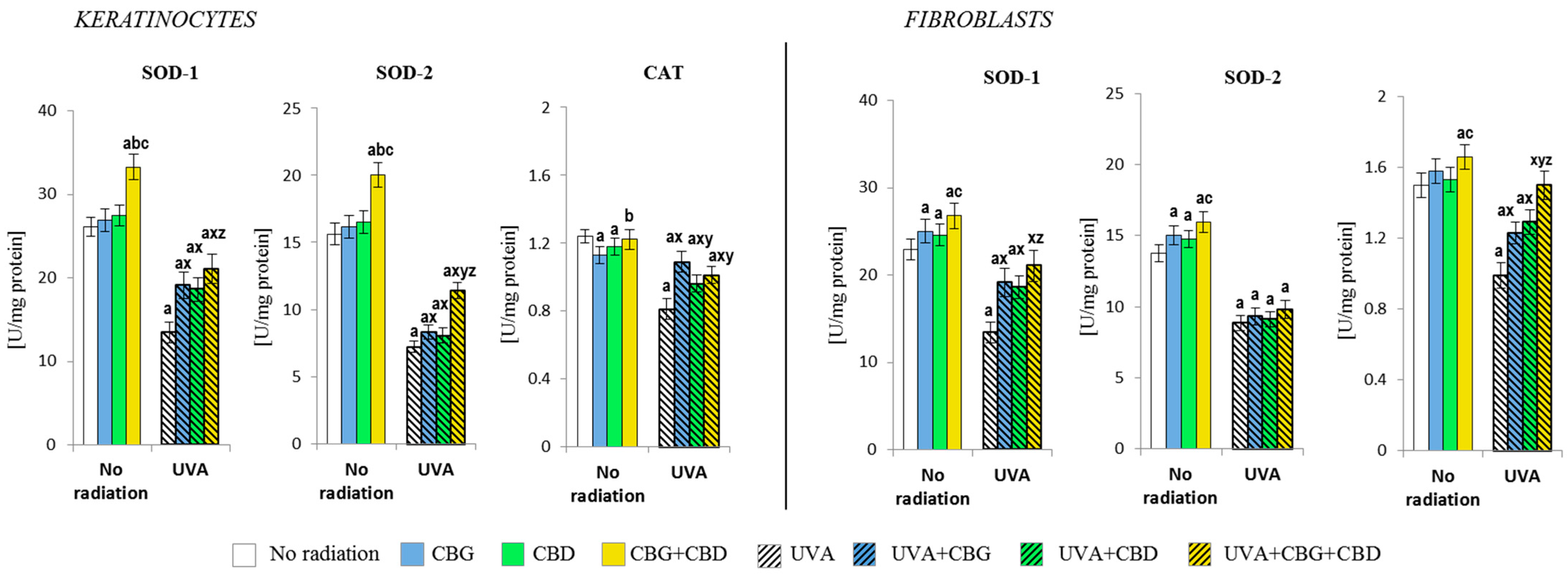
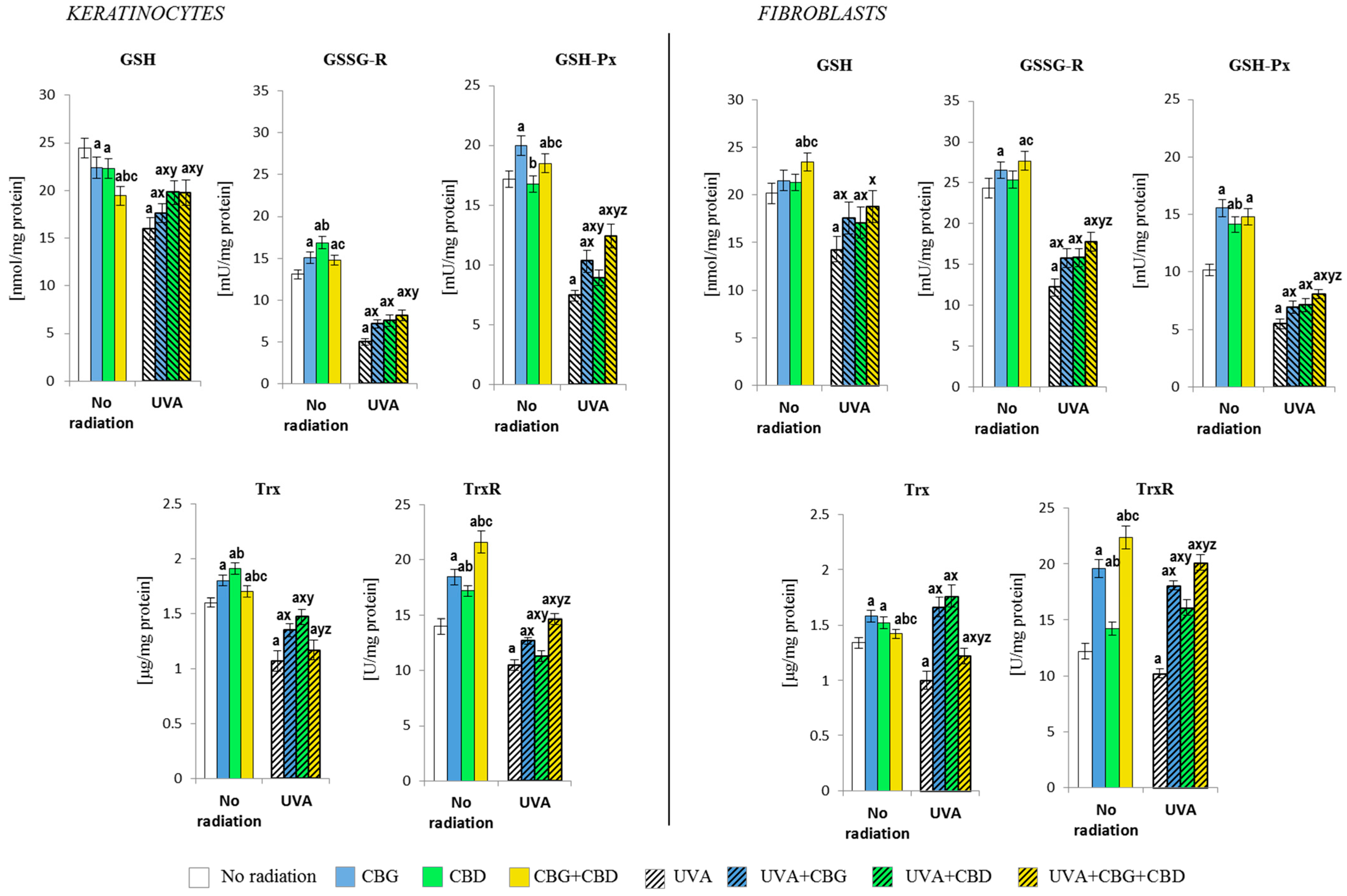
3.1. The Effect of CBG, CBD, and CBG+CBD on The Pro-Oxidative Parameters of Control and UVA-Irradiated Keratinocytes and Fibroblasts
3.2. The Effects of CBG, CBD, and CBG+CBD on the Antioxidant Efficiency of Control and UVA-Irradiated Keratinocytes and Fibroblasts
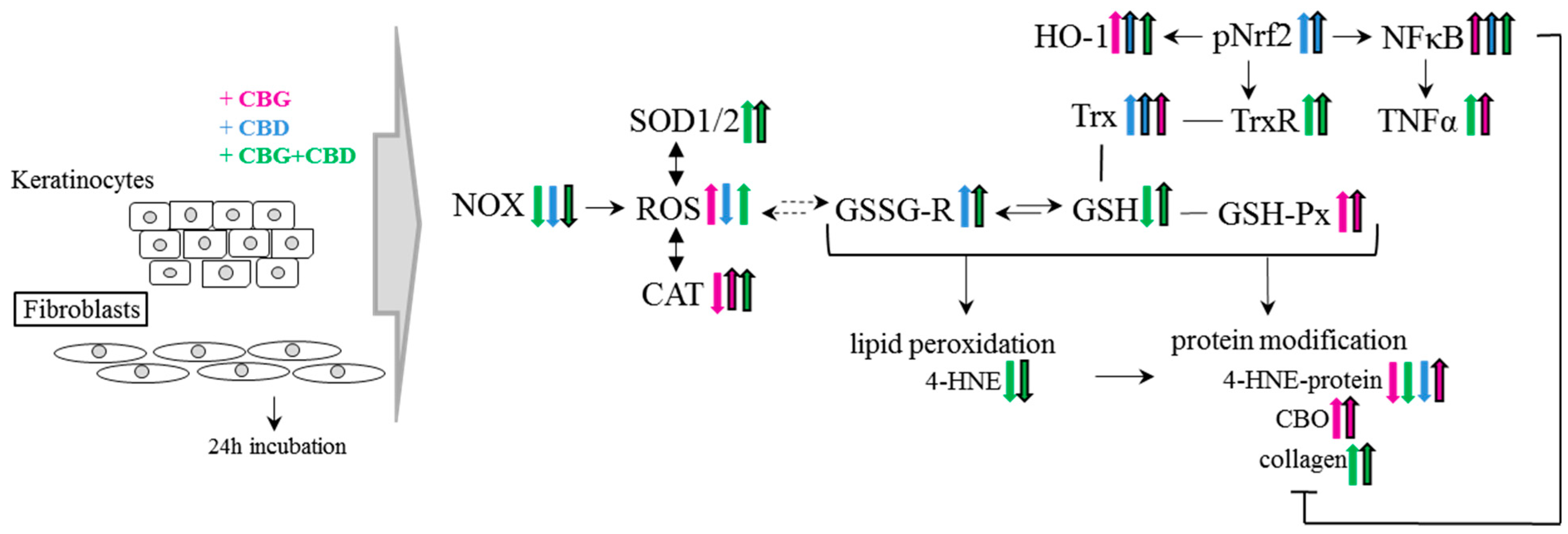
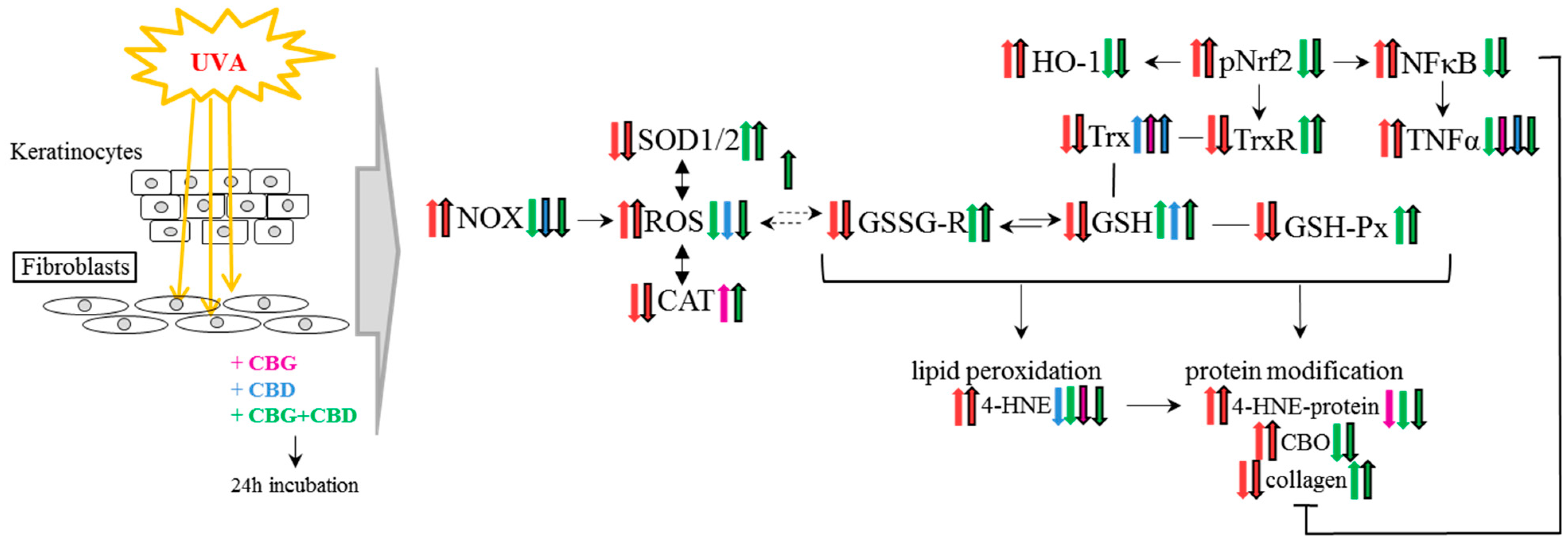
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohd Zaid, N.A.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Mat Rani, N.N.I.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V.; et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug. Des. Devel. Ther. 2022, 16, 23–66. [Google Scholar] [CrossRef] [PubMed]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The Role of Plant-Derived Natural Antioxidants in Reduction of Oxidative Stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, H.-Y.; Li, S.-H.; Ma, W.; Wu, D.-T.; Li, H.-B.; Xiao, A.-P.; Liu, L.-L.; Zhu, F.; Gan, R.-Y. Cannabis Sativa Bioactive Compounds and Their Extraction, Separation, Purification, and Identification Technologies: An Updated Review. TrAC Trends Anal. Chem. 2022, 149, 116554. [Google Scholar] [CrossRef]
- Jarocka-Karpowicz, I.; Biernacki, M.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Effects on Phospholipid Metabolism in Keratinocytes from Patients with Psoriasis Vulgaris. Biomolecules 2020, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Reggio, P.H.; Jagerovic, N. An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol. Front. Pharmacol. 2017, 8, 422. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Varani, K.; Lillo, A.; Vincenzi, F.; Rivas-Santisteban, R.; Raïch, I.; Reyes-Resina, I.; Ferreiro-Vera, C.; Borea, P.A.; Sánchez de Medina, V.; et al. Pharmacological Data of Cannabidiol- and Cannabigerol-Type Phytocannabinoids Acting on Cannabinoid CB1, CB2 and CB1/CB2 Heteromer Receptors. Pharmacol. Res. 2020, 159, 104940. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, T.; Lin, M.J.; Dubin, D.; Khorasani, H. The Potential Role of Cannabinoids in Dermatology. J. Dermatol. Treat. 2020, 31, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. Anticancer Effects of Phytocannabinoids Used with Chemotherapy in Leukaemia Cells Can Be Improved by Altering the Sequence of Their Administration. Int. J. Oncol. 2017, 51, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Lah, T.; Novak, M.; Almidon, M.; Marinelli, O.; Baškovič, B.; Majc, B.; Mlinar, M.; Bošnjak, R.; Breznik, B.; Zomer, R.; et al. Cannabigerol Is a Potential Therapeutic Agent in a Novel Combined Therapy for Glioblastoma. Cells 2021, 10, 340. [Google Scholar] [CrossRef]
- Mazuz, M.; Tiroler, A.; Moyal, L.; Hodak, E.; Nadarajan, S.; Vinayaka, A.C.; Gorovitz-Haris, B.; Lubin, I.; Drori, A.; Drori, G.; et al. Synergistic Cytotoxic Activity of Cannabinoids from Cannabis Sativa against Cutaneous T-Cell Lymphoma (CTCL) in-Vitro and Ex-Vivo. Oncotarget 2020, 11, 1141–1156. [Google Scholar] [CrossRef]
- Watt, G.; Karl, T. In Vivo Evidence for Therapeutic Properties of Cannabidiol (CBD) for Alzheimer’s Disease. Front. Pharmacol. 2017, 8, 234828. [Google Scholar] [CrossRef] [PubMed]
- Zagzoog, A.; Mohamed, K.A.; Kim, H.J.J.; Kim, E.D.; Frank, C.S.; Black, T.; Jadhav, P.D.; Holbrook, L.A.; Laprairie, R.B. In Vitro and in Vivo Pharmacological Activity of Minor Cannabinoids Isolated from Cannabis Sativa. Sci. Rep. 2020, 10, 20405. [Google Scholar] [CrossRef] [PubMed]
- Casares, L.; García, V.; Garrido-Rodríguez, M.; Millán, E.; Collado, J.A.; García-Martín, A.; Peñarando, J.; Calzado, M.A.; de la Vega, L.; Muñoz, E. Cannabidiol Induces Antioxidant Pathways in Keratinocytes by Targeting BACH1. Redox Biol. 2020, 28, 101321. [Google Scholar] [CrossRef] [PubMed]
- Polanska, H.H.; Petrlakova, K.; Papouskova, B.; Hendrych, M.; Samadian, A.; Storch, J.; Babula, P.; Masarik, M.; Vacek, J. Safety Assessment and Redox Status in Rats after Chronic Exposure to Cannabidiol and Cannabigerol. Toxicology 2023, 488, 153460. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Olszowy-Tomczyk, M.; Typek, R. Synergistic and Antagonistic Antioxidant Effects in the Binary Cannabinoids Mixtures. Fitoterapia 2021, 153, 104992. [Google Scholar] [CrossRef] [PubMed]
- Caprioglio, D.; Mattoteia, D.; Pollastro, F.; Negri, R.; Lopatriello, A.; Chianese, G.; Minassi, A.; Collado, J.A.; Munoz, E.; Taglialatela-Scafati, O.; et al. The Oxidation of Phytocannabinoids to Cannabinoquinoids. J. Nat. Prod. 2020, 83, 1711–1715. [Google Scholar] [CrossRef]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The Impact of Ultraviolet Radiation on Skin Photoaging—Review of in Vitro Studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef] [PubMed]
- Egambaram, O.P.; Kesavan Pillai, S.; Ray, S.S. Materials Science Challenges in Skin UV Protection: A Review. Photochem. Photobiol. 2020, 96, 779–797. [Google Scholar] [CrossRef]
- Valacchi, G.; Sticozzi, C.; Pecorelli, A.; Cervellati, F.; Cervellati, C.; Maioli, E. Cutaneous Responses to Environmental Stressors. Ann. N. Y. Acad. Sci. 2012, 1271, 75–81. [Google Scholar] [CrossRef]
- Calapai, F.; Cardia, L.; Esposito, E.; Ammendolia, I.; Mondello, C.; Lo Giudice, R.; Gangemi, S.; Calapai, G.; Mannucci, C. Pharmacological Aspects and Biological Effects of Cannabigerol and Its Synthetic Derivatives. Evid.-Based Complement. Altern. Med. 2022, 2022, 3336516. [Google Scholar] [CrossRef]
- Marabini, L.; Melzi, G.; Lolli, F.; Dell’Agli, M.; Piazza, S.; Sangiovanni, E.; Marinovich, M. Effects of Vitis Vinifera L. Leaves Extract on UV Radiation Damage in Human Keratinocytes (HaCaT). J. Photochem. Photobiol. B Biol. 2020, 204, 111810. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.; Fernandez, J.R.; Fitzgerald, C.; Rouzard, K.; Tamura, M.; Savile, C. In Vitro and Clinical Evaluation of Cannabigerol (CBG) Produced via Yeast Biosynthesis: A Cannabinoid with a Broad Range of Anti-Inflammatory and Skin Health-Boosting Properties. Molecules 2022, 27, 491. [Google Scholar] [CrossRef] [PubMed]
- Abidi, A.H.; Abhyankar, V.; Alghamdi, S.S.; Tipton, D.A.; Dabbous, M. Phytocannabinoids Regulate Inflammation in IL-1β-Stimulated Human Gingival Fibroblasts. J. Periodontal. Res. 2022, 57, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Viereckl, M.J.; Krutsinger, K.; Apawu, A.; Gu, J.; Cardona, B.; Barratt, D.; Han, Y. Cannabidiol and Cannabigerol Inhibit Cholangiocarcinoma Growth In Vitro via Divergent Cell Death Pathways. Biomolecules 2022, 12, 854. [Google Scholar] [CrossRef] [PubMed]
- Wroński, A.; Dobrzyńska, I.; Sękowski, S.; Łuczaj, W.; Olchowik-Grabarek, E.; Skrzydlewska, E. Cannabidiol and Cannabigerol Modify the Composition and Physicochemical Properties of Keratinocyte Membranes Exposed to UVA. Int. J. Mol. Sci. 2023, 24, 12424. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, G.; Timbrell, J.A. In Vitro Cytotoxicity Assays: Comparison of LDH, Neutral Red, MTT and Protein Assay in Hepatoma Cell Lines Following Exposure to Cadmium Chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, R.W. Angiotensin II Stimulates NADH and NADPH Oxidase Activity in Cultured Vascular Smooth Muscle Cells. Circ. Res. 1994, 74, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Kuzkaya, N.; Weissmann, N.; Harrison, D.G.; Dikalov, S. Interactions of Peroxynitrite, Tetrahydrobiopterin, Ascorbic Acid, and Thiols: Implications for Uncoupling Endothelial Nitric-Oxide Synthase. J. Biol. Chem. 2003, 278, 22546–22554. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W.; et al. Therapeutic Targeting of the NRF2 and KEAP1 Partnership in Chronic Diseases. Nat. Rev. Drug. Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. [13] Catalase in Vitro. In Methods in Enzymology; Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the Quantitative and Qualitative Characterization of Erythrocyte Glutathione Peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Mize, C.E.; Langdon, R.G. Hepatic Glutathione Reductase. I. Purification and General Kinetic Properties. J. Biol. Chem. 1962, 237, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A.; Björnstedt, M. Thioredoxin and Thioredoxin Reductase. Methods Enzym. 1995, 252, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Xie, C.; Gabbita, S.P.; Markesbery, W.R. Decreased Thioredoxin and Increased Thioredoxin Reductase Levels in Alzheimer’s Disease Brain. Free Radic. Biol. Med. 2000, 28, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Maeso, N.; García-Martínez, D.; Rupérez, F.J.; Cifuentes, A.; Barbas, C. Capillary Electrophoresis of Glutathione to Monitor Oxidative Stress and Response to Antioxidant Treatments in an Animal Model. J. Chromatogr. B 2005, 822, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hnasko, R.; Lin, A.; McGarvey, J.A.; Stanker, L.H. A Rapid Method to Improve Protein Detection by Indirect ELISA. Biochem. Biophys. Res. Commun. 2011, 410, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Rothmann, S.; Schneider, J.Y.; Gutzki, F.-M.; Beckmann, B.; Frölich, J.C. Simultaneous GC-MS/MS Measurement of Malondialdehyde and 4-Hydroxy-2-Nonenal in Human Plasma: Effects of Long-Term L-Arginine Administration. Anal. Biochem. 2017, 524, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Milkovic, L.; Bennett, S.J.; Griffiths, H.R.; Zarkovic, N.; Grune, T. Measurement of HNE-Protein Adducts in Human Plasma and Serum by ELISA-Comparison of Two Primary Antibodies. Redox Biol. 2013, 1, 226–233. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of Carbonyl Content in Oxidatively Modified Proteins. Methods Enzym. 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Luz-Veiga, M.; Amorim, M.; Pinto-Ribeiro, I.; Oliveira, A.L.S.; Silva, S.; Pimentel, L.L.; Rodríguez-Alcalá, L.M.; Madureira, R.; Pintado, M.; Azevedo-Silva, J.; et al. Cannabidiol and Cannabigerol Exert Antimicrobial Activity without Compromising Skin Microbiota. Int. J. Mol. Sci. 2023, 24, 2389. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Atalay, S.; Domingues, P.; Skrzydlewska, E. The Differences in the Proteome Profile of Cannabidiol-Treated Skin Fibroblasts Following UVA or UVB Irradiation in 2D and 3D Cell Cultures. Cells 2019, 8, 995. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, B.; Laurino, C.; Vadalà, M. A therapeutic effect of cbd-enriched ointment in inflammatory skin diseases and cutaneous scars. La Clin. Ter. 2019, 170, e93–e99. [Google Scholar] [CrossRef] [PubMed]
- Couch, D.G.; Maudslay, H.; Doleman, B.; Lund, J.N.; O’Sullivan, S.E. The Use of Cannabinoids in Colitis: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2018, 24, 680–697. [Google Scholar] [CrossRef] [PubMed]
- Mammana, S.; Cavalli, E.; Gugliandolo, A.; Silvestro, S.; Pollastro, F.; Bramanti, P.; Mazzon, E. Could the Combination of Two Non-Psychotropic Cannabinoids Counteract Neuroinflammation? Effectiveness of Cannabidiol Associated with Cannabigerol. Medicina 2019, 55, 747. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Verde, R.; Vaia, M.; Allarà, M.; Iuvone, T.; Di Marzo, V. Anti-Inflammatory Properties of Cannabidiol, a Nonpsychotropic Cannabinoid, in Experimental Allergic Contact Dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Nisimoto, Y.; Ogawa, H.; Kadokawa, Y.; Qiao, S. NADPH Oxidase 4 Function as a Hydrogen Peroxide Sensor. J. Biochem. 2018, 163, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacological Case for Cannabigerol. J. Pharmacol. Exp. Ther. 2021, 376, 204–212. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy-Tomczyk, M.; Typek, R. CBG, CBD, Δ9-THC, CBN, CBGA, CBDA and Δ9-THCA as Antioxidant Agents and Their Intervention Abilities in Antioxidant Action. Fitoterapia 2021, 152, 104915. [Google Scholar] [CrossRef]
- Navarro, G.; Varani, K.; Reyes-Resina, I.; Sánchez de Medina, V.; Rivas-Santisteban, R.; Sánchez-Carnerero Callado, C.; Vincenzi, F.; Casano, S.; Ferreiro-Vera, C.; Canela, E.I.; et al. Cannabigerol Action at Cannabinoid CB1 and CB2 Receptors and at CB1-CB2 Heteroreceptor Complexes. Front. Pharmacol. 2018, 9, 632. [Google Scholar] [CrossRef]
- Oláh, A.; Alam, M.; Chéret, J.; Kis, N.G.; Hegyi, Z.; Szöllősi, A.G.; Vidali, S.; Bíró, T.; Paus, R. Mitochondrial Energy Metabolism Is Negatively Regulated by Cannabinoid Receptor 1 in Intact Human Epidermis. Exp. Dermatol. 2020, 29, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Jarocka-Karpowicz, I.; Markowska, A.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Antioxidant and Anti-Inflammatory Effect of Cannabidiol Contributes to the Decreased Lipid Peroxidation of Keratinocytes of Rat Skin Exposed to UV Radiation. Oxidative Med. Cell. Longev. 2021, 2021, e6647222. [Google Scholar] [CrossRef]
- Atalay Ekiner, S.; Gęgotek, A.; Skrzydlewska, E. The Molecular Activity of Cannabidiol in the Regulation of Nrf2 System Interacting with NF-κB Pathway under Oxidative Stress. Redox Biol. 2022, 57, 102489. [Google Scholar] [CrossRef]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of Inflammation by the Antioxidant Haem Oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-H.; Jeong, G.-S. Fisetin Inhibits TNF-α-Induced Inflammatory Action and Hydrogen Peroxide-Induced Oxidative Damage in Human Keratinocyte HaCaT Cells through PI3K/AKT/Nrf-2-Mediated Heme Oxygenase-1 Expression. Int. Immunopharmacol. 2015, 29, 246–253. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Nisar, M.F.; Lin, M.; Zhong, J.L. Heme Oxygenases: Cellular Multifunctional and Protective Molecules against UV-Induced Oxidative Stress. Oxidative Med. Cell. Longev. 2019, 2019, e5416728. [Google Scholar] [CrossRef]
- Ryšavá, A.; Čížková, K.; Franková, J.; Roubalová, L.; Ulrichová, J.; Vostálová, J.; Vrba, J.; Zálešák, B.; Rajnochová Svobodová, A. Effect of UVA Radiation on the Nrf2 Signalling Pathway in Human Skin Cells. J. Photochem. Photobiol. B Biol. 2020, 209, 111948. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.A.; Kalinina, E.; Tatarskiy, V.; Shtil, A. The Thioredoxin System of Mammalian Cells and Its Modulators. Biomedicines 2022, 10, 1757. [Google Scholar] [CrossRef]
- Didier, C.; Pouget, J.-P.; Cadet, J.; Favier, A.; Béani, J.-C.; Richard, M.-J. Modulation of Exogenous and Endogenous Levels of Thioredoxin in Human Skin Fibroblasts Prevents DNA Damaging Effect of Ultraviolet A Radiation. Free Radic. Biol. Med. 2001, 30, 537–546. [Google Scholar] [CrossRef]
- Telorack, M.; Meyer, M.; Ingold, I.; Conrad, M.; Bloch, W.; Werner, S. A Glutathione-Nrf2-Thioredoxin Cross-Talk Ensures Keratinocyte Survival and Efficient Wound Repair. PLoS Genet. 2016, 12, e1005800. [Google Scholar] [CrossRef]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef]
- Gugliandolo, A.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. In Vitro Model of Neuroinflammation: Efficacy of Cannabigerol, a Non-Psychoactive Cannabinoid. Int. J. Mol. Sci. 2018, 19, 1992. [Google Scholar] [CrossRef]
- Giacoppo, S.; Gugliandolo, A.; Trubiani, O.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Cannabinoid CB2 Receptors Are Involved in the Protection of RAW264.7 Macrophages against the Oxidative Stress: An in Vitro Study. Eur. J. Histochem. 2017, 61, 2749. [Google Scholar] [CrossRef]
- Wang, P.-W.; Hung, Y.-C.; Lin, T.-Y.; Fang, J.-Y.; Yang, P.-M.; Chen, M.-H.; Pan, T.-L. Comparison of the Biological Impact of UVA and UVB upon the Skin with Functional Proteomics and Immunohistochemistry. Antioxidants 2019, 8, 569. [Google Scholar] [CrossRef]
- Liang, J.; Lian, L.; Wang, X.; Li, L. Thymoquinone, Extract from Nigella Sativa Seeds, Protects Human Skin Keratinocytes against UVA-Irradiated Oxidative Stress, Inflammation and Mitochondrial Dysfunction. Mol. Immunol. 2021, 135, 21–27. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV Radiation-Induced Inflammation and Immunosuppression Accelerate the Aging Process in the Skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef]
- Valencia, A.; Kochevar, I.E. Nox1-Based NADPH Oxidase Is the Major Source of UVA-Induced Reactive Oxygen Species in Human Keratinocytes. J. Investig. Dermatol. 2008, 128, 214–222. [Google Scholar] [CrossRef]
- Vacek, J.; Vostalova, J.; Papouskova, B.; Skarupova, D.; Kos, M.; Kabelac, M.; Storch, J. Antioxidant Function of Phytocannabinoids: Molecular Basis of Their Stability and Cytoprotective Properties under UV-Irradiation. Free Radic. Biol. Med. 2021, 164, 258–270. [Google Scholar] [CrossRef]
- di Giacomo, V.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Leone, S.; Brunetti, L.; Menghini, L.; Recinella, L.; et al. Neuroprotective and Neuromodulatory Effects Induced by Cannabidiol and Cannabigerol in Rat Hypo-E22 Cells and Isolated Hypothalamus. Antioxidants 2020, 9, 71. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Liu, Y.; Xu, Y.; Yang, B.; Li, H.; Chen, L. An Overview on Synthetic and Biological Activities of Cannabidiol (CBD) and Its Derivatives. Bioorganic Chem. 2023, 140, 106810. [Google Scholar] [CrossRef]
- Wilson, J.T.; Fief, C.A.; Jackson, K.D.; Mercer, S.L.; Deweese, J.E. HU-331 and Oxidized Cannabidiol Act as Inhibitors of Human Topoisomerase IIα and β. Chem. Res. Toxicol. 2018, 31, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.G.; Carrillo-Salinas, F.; Pagani, A.; Gómez-Cañas, M.; Negri, R.; Navarrete, C.; Mecha, M.; Mestre, L.; Fiebich, B.L.; Cantarero, I.; et al. A Cannabigerol Quinone Alleviates Neuroinflammation in a Chronic Model of Multiple Sclerosis. J. Neuroimmune Pharmacol. 2012, 7, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of Cannabinoids and Cannabinoid-Enriched Cannabis Extracts on TRP Channels and Endocannabinoid Metabolic Enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.-C.; Vudhya Gowrisankar, Y.; Chen, X.-Z.; Yang, Y.-C.; Yang, H.-L. The Antiaging Activity of Ergothioneine in UVA-Irradiated Human Dermal Fibroblasts via the Inhibition of the AP-1 Pathway and the Activation of Nrf2-Mediated Antioxidant Genes. Oxidative Med. Cell. Longev. 2020, 2020, e2576823. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Hinz, B. Cannabinoid Compounds as a Pharmacotherapeutic Option for the Treatment of Non-Cancer Skin Diseases. Cells 2022, 11, 4102. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Arcaro, A.; Pizzimenti, S.; Daga, M.; Cetrangolo, G.P.; Dianzani, C.; Lepore, A.; Graf, M.; Ames, P.R.J.; Barrera, G. DNA Damage by Lipid Peroxidation Products: Implications in Cancer, Inflammation and Autoimmunity. AIMS Genet. 2017, 4, 103–137. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hao, D.; Wei, D.; Xiao, Y.; Liu, L.; Li, X.; Wang, L.; Gan, Y.; Yan, W.; Ke, B.; et al. Photoprotective Effects of Cannabidiol against Ultraviolet-B-Induced DNA Damage and Autophagy in Human Keratinocyte Cells and Mouse Skin Tissue. Molecules 2022, 27, 6740. [Google Scholar] [CrossRef] [PubMed]
- Timucin, A.C.; Basaga, H. Pro-Apoptotic Effects of Lipid Oxidation Products: HNE at the Crossroads of NF-κB Pathway and Anti-Apoptotic Bcl-2. Free Radic. Biol. Med. 2017, 111, 209–218. [Google Scholar] [CrossRef]
- Mohania, D.; Chandel, S.; Kumar, P.; Verma, V.; Digvijay, K.; Tripathi, D.; Choudhury, K.; Mitten, S.K.; Shah, D. Ultraviolet Radiations: Skin Defense-Damage Mechanism. Adv. Exp. Med. Biol. 2017, 996, 71–87. [Google Scholar] [CrossRef]
- Goldminz, A.M.; Au, S.C.; Kim, N.; Gottlieb, A.B.; Lizzul, P.F. NF-κB: An Essential Transcription Factor in Psoriasis. J. Dermatol. Sci. 2013, 69, 89–94. [Google Scholar] [CrossRef]
- Tang, L.; Wang, K. Chronic Inflammation in Skin Malignancies. J. Mol. Signal. 2016, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Sztolsztener, K.; Konstantynowicz-Nowicka, K.; Pędzińska-Betiuk, A.; Chabowski, A. Concentration-Dependent Attenuation of Pro-Fibrotic Responses after Cannabigerol Exposure in Primary Rat Hepatocytes Cultured in Palmitate and Fructose Media. Cells 2023, 12, 2243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Hood, M.; Zhang, I.X.; Chen, C.L.; Zhang, L.L.; Du, J. Collagen and Soy Peptides Attenuate Contractile Loss from UVA Damage and Enhance the Antioxidant Capacity of Dermal Fibroblasts. J. Cosmet. Dermatol. 2021, 20, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, G.; Magnavacca, A.; Fumagalli, M.; Dell’Agli, M.; Piazza, S.; Sangiovanni, E. Cannabis Sativa and Skin Health: Dissecting the Role of Phytocannabinoids. Planta Med. 2022, 88, 492–506. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wroński, A.; Jarocka-Karpowicz, I.; Surażyński, A.; Gęgotek, A.; Zarkovic, N.; Skrzydlewska, E. Modulation of Redox and Inflammatory Signaling in Human Skin Cells Using Phytocannabinoids Applied after UVA Irradiation: In Vitro Studies. Cells 2024, 13, 965. https://doi.org/10.3390/cells13110965
Wroński A, Jarocka-Karpowicz I, Surażyński A, Gęgotek A, Zarkovic N, Skrzydlewska E. Modulation of Redox and Inflammatory Signaling in Human Skin Cells Using Phytocannabinoids Applied after UVA Irradiation: In Vitro Studies. Cells. 2024; 13(11):965. https://doi.org/10.3390/cells13110965
Chicago/Turabian StyleWroński, Adam, Iwona Jarocka-Karpowicz, Arkadiusz Surażyński, Agnieszka Gęgotek, Neven Zarkovic, and Elżbieta Skrzydlewska. 2024. "Modulation of Redox and Inflammatory Signaling in Human Skin Cells Using Phytocannabinoids Applied after UVA Irradiation: In Vitro Studies" Cells 13, no. 11: 965. https://doi.org/10.3390/cells13110965






