Regulator of Lipid Metabolism NHR-49 Mediates Pathogen Avoidance through Precise Control of Neuronal Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. C. elegans Growth and Maintenance
2.2. PA14 Survival and Lawn Avoidance Assay
2.3. Oleic Acid Supplementation
2.4. Real-Time Quantitative PCR
2.5. Plasmid Constructs and the Generation of Transgenic Lines
2.6. Calcium Imaging and Analysis
3. Results
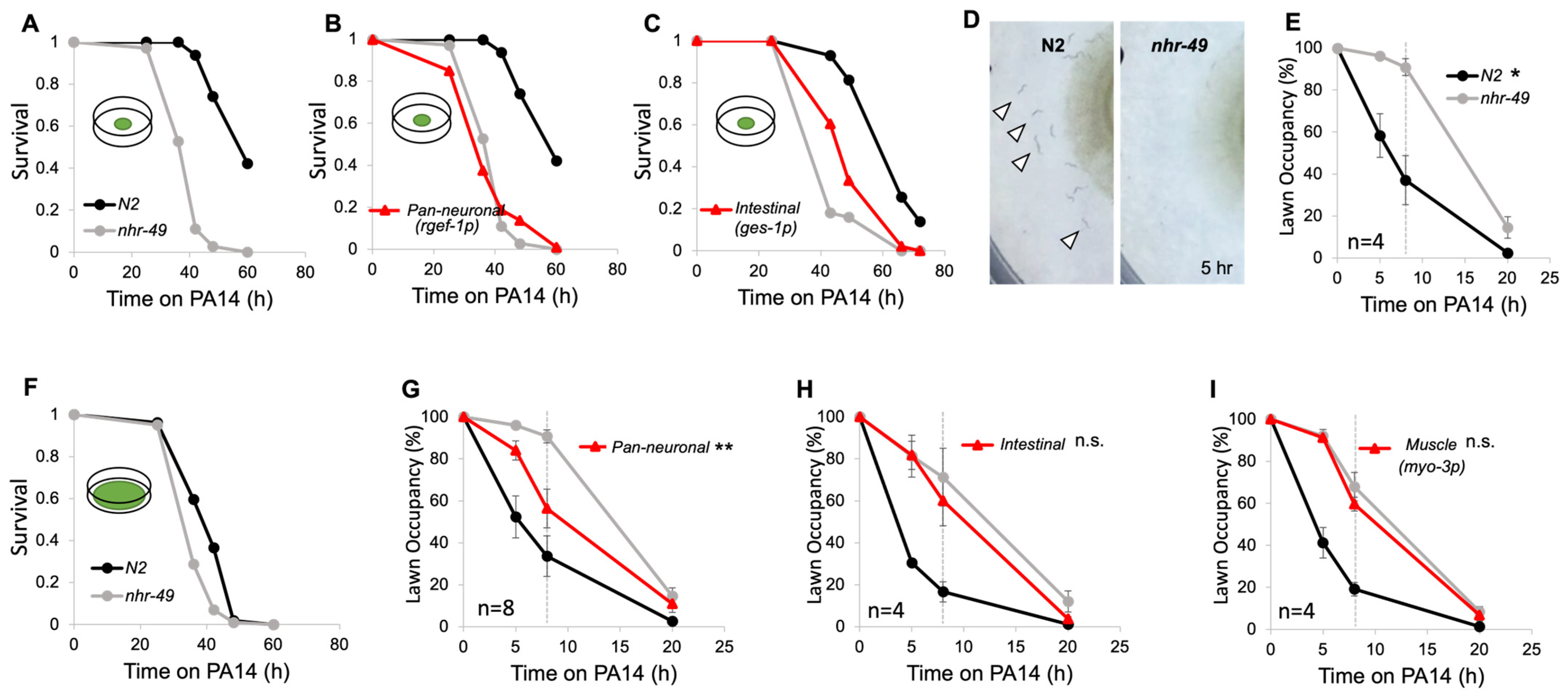
3.1. Neuronal NHR-49 Promotes Pathogen Avoidance to PA14
3.2. NHR-49 Acts Downstream of the TGFβ/DAF-7 Pathway
3.3. NHR-49 Acts Downstream of NPR-1 Ligand Upregulation
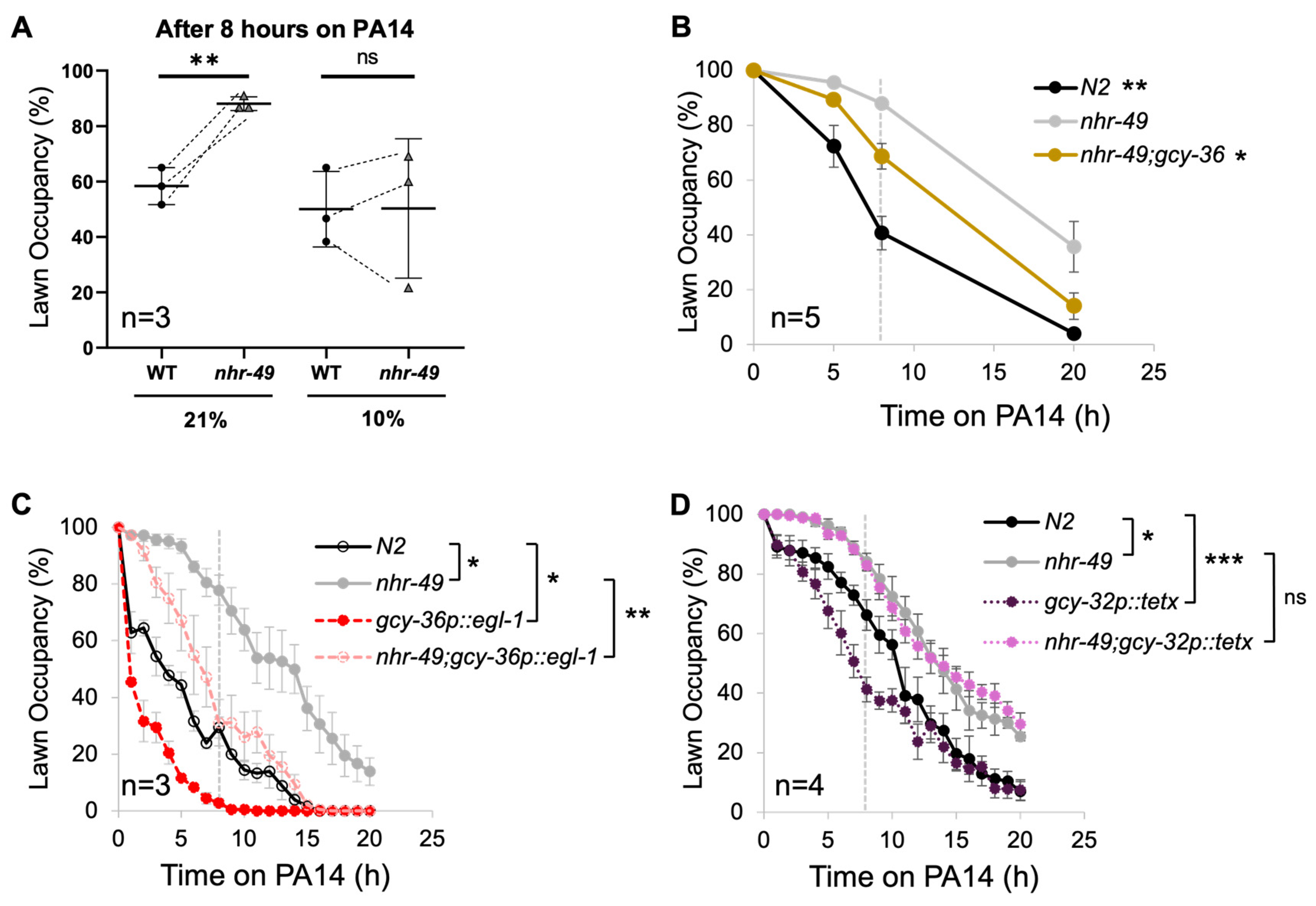
3.4. NHR-49 in URX, AQR, and PQR Neurons Are Sufficient for Pathogen Avoidance
3.5. Body Cavity Neuron Activity Negatively Contributes to Pathogenic Lawn Avoidance
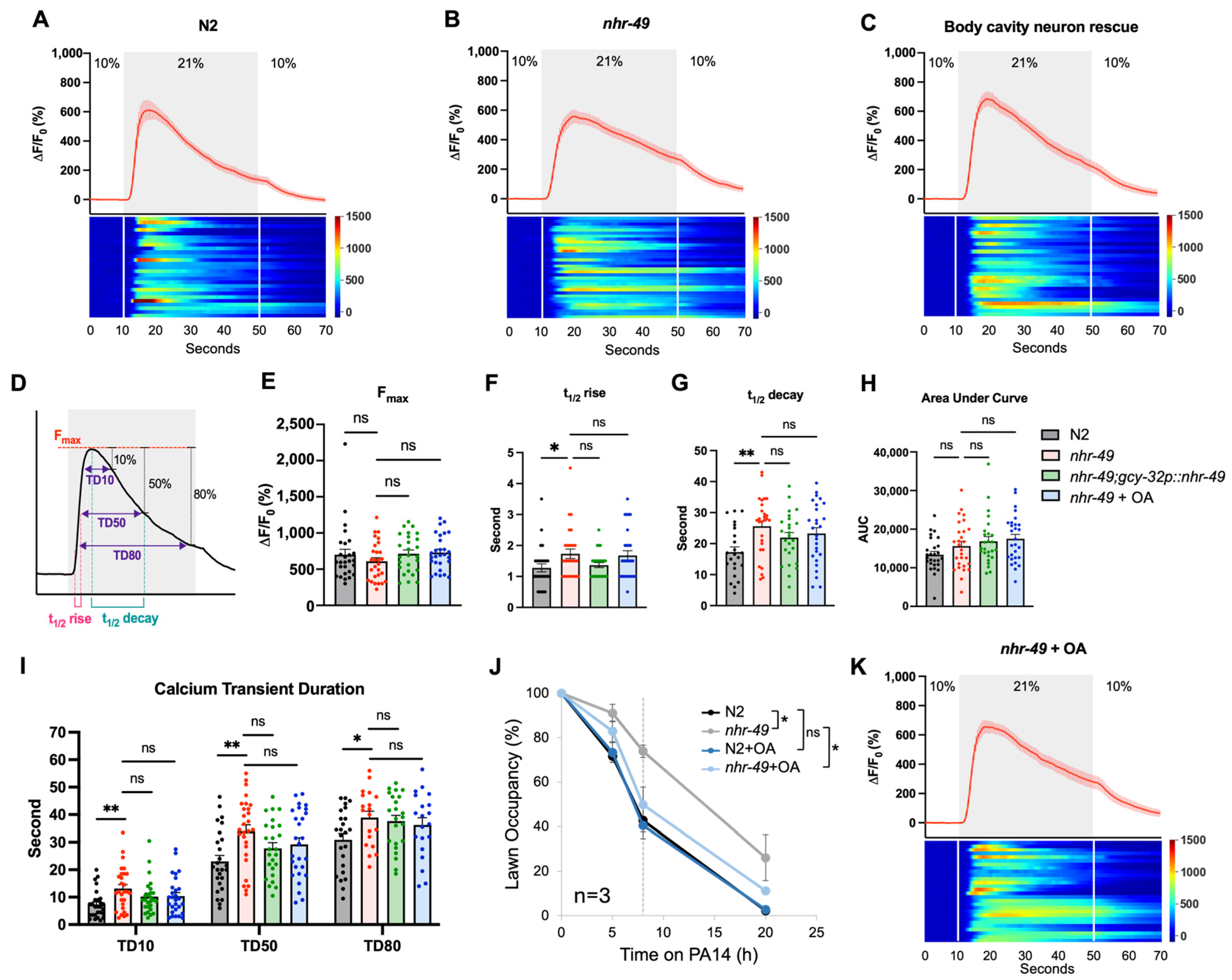
3.6. NHR-49 Affects URX Calcium Kinetics
3.7. Restoring Lipid Homeostasis Is Sufficient to Improve Pathogen Avoidance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turrigiano, G. Homeostatic synaptic plasticity: Local and global mechanisms for stabilizing neuronal function. Cold Spring Harb. Perspect. Biol. 2012, 4, a005736. [Google Scholar] [CrossRef] [PubMed]
- Giachello, C.N.; Baines, R.A. Regulation of motoneuron excitability and the setting of homeostatic limits. Curr. Opin. Neurobiol. 2017, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Targa Dias Anastacio, H.; Matosin, N.; Ooi, L. Neuronal hyperexcitability in Alzheimer’s disease: What are the drivers behind this aberrant phenotype? Transl. Psychiatry 2022, 12, 257. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Wong, R.O.; Ghosh, A. Activity-dependent regulation of dendritic growth and patterning. Nat. Rev. Neurosci. 2002, 3, 803–812. [Google Scholar] [CrossRef]
- Tracey, T.J.; Kirk, S.E.; Steyn, F.J.; Ngo, S.T. The role of lipids in the central nervous system and their pathological implications in amyotrophic lateral sclerosis. Semin. Cell Dev. Biol. 2021, 112, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Cong, W.N.; Ji, S.; Rothman, S.; Maudsley, S.; Martin, B. Metabolic dysfunction in Alzheimer’s disease and related neurodegenerative disorders. Curr. Alzheimer Res. 2012, 9, 5–17. [Google Scholar] [CrossRef]
- Duarte, J.M.; Schuck, P.F.; Wenk, G.L.; Ferreira, G.C. Metabolic disturbances in diseases with neurological involvement. Aging Dis. 2014, 5, 238–255. [Google Scholar] [CrossRef]
- Yoon, J.H.; Seo, Y.; Jo, Y.S.; Lee, S.; Cho, E.; Cazenave-Gassiot, A.; Shin, Y.S.; Moon, M.H.; An, H.J.; Wenk, M.R.; et al. Brain lipidomics: From functional landscape to clinical significance. Sci. Adv. 2022, 8, eadc9317. [Google Scholar] [CrossRef]
- Zhou, Y.; Sanchez, V.B.; Xu, P.; Flores-Mendez, M.; Ciesielski, B.; Yoo, D.; Teshome, H.; Henne, M.; O’Brien, T.; Mesaros, C.; et al. Altered lipid homeostasis underlies selective neurodegeneration in SNX14 deficiency. bioRxiv 2022. [Google Scholar] [CrossRef]
- Agrawal, I.; Lim, Y.S.; Ng, S.Y.; Ling, S.C. Deciphering lipid dysregulation in ALS: From mechanisms to translational medicine. Transl. Neurodegener. 2022, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Ralhan, I.; Chang, C.L.; Lippincott-Schwartz, J.; Ioannou, M.S. Lipid droplets in the nervous system. J. Cell Biol. 2021, 220, e202102136. [Google Scholar] [CrossRef]
- Taubert, S.; Ward, J.D.; Yamamoto, K.R. Nuclear hormone receptors in nematodes: Evolution and function. Mol. Cell. Endocrinol. 2011, 334, 49–55. [Google Scholar] [CrossRef]
- Van Gilst, M.R.; Hadjivassiliou, H.; Jolly, A.; Yamamoto, K.R. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol. 2005, 3, e53. [Google Scholar] [CrossRef]
- Van Gilst, M.R.; Hadjivassiliou, H.; Yamamoto, K.R. A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc. Natl. Acad. Sci. USA 2005, 102, 13496–13501. [Google Scholar] [CrossRef] [PubMed]
- Ratnappan, R.; Amrit, F.R.; Chen, S.W.; Gill, H.; Holden, K.; Ward, J.; Yamamoto, K.R.; Olsen, C.P.; Ghazi, A. Germline signals deploy NHR-49 to modulate fatty-acid beta-oxidation and desaturation in somatic tissues of C. elegans. PLoS Genet. 2014, 10, e1004829. [Google Scholar] [CrossRef]
- Goh, G.Y.S.; Winter, J.J.; Bhanshali, F.; Doering, K.R.S.; Lai, R.; Lee, K.; Veal, E.A.; Taubert, S. NHR-49/HNF4 integrates regulation of fatty acid metabolism with a protective transcriptional response to oxidative stress and fasting. Aging Cell 2018, 17, e12743. [Google Scholar] [CrossRef]
- Doering, K.R.S.; Cheng, X.; Milburn, L.; Ratnappan, R.; Ghazi, A.; Miller, D.L.; Taubert, S. Nuclear hormone receptor NHR-49 acts in parallel with HIF-1 to promote hypoxia adaptation in Caenorhabditis elegans. Elife 2022, 11, e67911. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, M.; Shashikanth, M.; Gupta, A.; Sandhu, A.; De, A.; Javed, S.; Singh, V. NHR-49 Transcription Factor Regulates Immunometabolic Response and Survival of Caenorhabditis elegans during Enterococcus faecalis Infection. Infect. Immun. 2020, 88, e00130-20. [Google Scholar] [CrossRef]
- Naim, N.; Amrit, F.R.G.; Ratnappan, R.; DelBuono, N.; Loose, J.A.; Ghazi, A. Cell nonautonomous roles of NHR-49 in promoting longevity and innate immunity. Aging Cell 2021, 20, e13413. [Google Scholar] [CrossRef]
- Wani, K.A.; Goswamy, D.; Taubert, S.; Ratnappan, R.; Ghazi, A.; Irazoqui, J.E. NHR-49/PPAR-alpha and HLH-30/TFEB cooperate for C. elegans host defense via a flavin-containing monooxygenase. Elife 2021, 10, e62775. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.R.; Santpere, G.; Weinreb, A.; Barrett, A.; Reilly, M.B.; Xu, C.; Varol, E.; Oikonomou, P.; Glenwinkel, L.; McWhirter, R.; et al. Molecular topography of an entire nervous system. Cell 2021, 184, 4329–4347.e23. [Google Scholar] [CrossRef] [PubMed]
- Stiernagle, T. Maintenance of C. elegans. In WormBook; Oxford University Press: Oxford, UK, 2006; pp. 1–11. [Google Scholar] [CrossRef]
- Meisel, J.D.; Panda, O.; Mahanti, P.; Schroeder, F.C.; Kim, D.H. Chemosensation of bacterial secondary metabolites modulates neuroendocrine signaling and behavior of C. elegans. Cell 2014, 159, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Lee, J.I.; Yoon, K.H. A Smartphone-Based Imaging Method for C. elegans Lawn Avoidance Assay. J. Vis. Exp. 2023, 192, e65197. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Park, K.S.; Yoon, K.H. Dissecting the Neuronal Contributions of the Lipid Regulator NHR-49 Function in Lifespan and Behavior in C. elegans. Life 2023, 13, 2346. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Avery, L.; Baude, E.; Garbers, D.L. Guanylyl cyclase expression in specific sensory neurons: A new family of chemosensory receptors. Proc. Natl. Acad. Sci. USA 1997, 94, 3384–3387. [Google Scholar] [CrossRef]
- Zimmer, M.; Gray, J.M.; Pokala, N.; Chang, A.J.; Karow, D.S.; Marletta, M.A.; Hudson, M.L.; Morton, D.B.; Chronis, N.; Bargmann, C.I. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron 2009, 61, 865–879. [Google Scholar] [CrossRef]
- Burkewitz, K.; Morantte, I.; Weir, H.J.M.; Yeo, R.; Zhang, Y.; Huynh, F.K.; Ilkayeva, O.R.; Hirschey, M.D.; Grant, A.R.; Mair, W.B. Neuronal CRTC-1 governs systemic mitochondrial metabolism and lifespan via a catecholamine signal. Cell 2015, 160, 842–855. [Google Scholar] [CrossRef]
- Reddy, K.C.; Andersen, E.C.; Kruglyak, L.; Kim, D.H. A polymorphism in npr-1 is a behavioral determinant of pathogen susceptibility in C. elegans. Science 2009, 323, 382–384. [Google Scholar] [CrossRef]
- Singh, J.; Aballay, A. Intestinal infection regulates behavior and learning via neuroendocrine signaling. Elife 2019, 8, e50033. [Google Scholar] [CrossRef]
- Filipowicz, A.; Lalsiamthara, J.; Aballay, A. TRPM channels mediate learned pathogen avoidance following intestinal distention. Elife 2021, 10, e65935. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Aballay, A. Microbial Colonization Activates an Immune Fight-and-Flight Response via Neuroendocrine Signaling. Dev. Cell 2019, 49, 89–99.e4. [Google Scholar] [CrossRef] [PubMed]
- Styer, K.L.; Singh, V.; Macosko, E.; Steele, S.E.; Bargmann, C.I.; Aballay, A. Innate immunity in Caenorhabditis elegans is regulated by neurons expressing NPR-1/GPCR. Science 2008, 322, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Goh, G.Y.; Wong, M.A.; Klassen, T.L.; Taubert, S. Gain-of-Function Alleles in Caenorhabditis elegans Nuclear Hormone Receptor nhr-49 Are Functionally Distinct. PLoS ONE 2016, 11, e0162708. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.M.; Hill, J.J.; Bargmann, C.I. A circuit for navigation in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2005, 102, 3184–3191. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.C.; Hunter, R.C.; Bhatla, N.; Newman, D.K.; Kim, D.H. Caenorhabditis elegans NPR-1-mediated behaviors are suppressed in the presence of mucoid bacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 12887–12892. [Google Scholar] [CrossRef] [PubMed]
- Pellizzari, R.; Rossetto, O.; Schiavo, G.; Montecucco, C. Tetanus and botulinum neurotoxins: Mechanism of action and therapeutic uses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Busch, K.E.; Laurent, P.; Soltesz, Z.; Murphy, R.J.; Faivre, O.; Hedwig, B.; Thomas, M.; Smith, H.L.; de Bono, M. Tonic signaling from O(2) sensors sets neural circuit activity and behavioral state. Nat. Neurosci. 2012, 15, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; An, S.W.A.; Jung, Y.; Yamaoka, Y.; Ryu, Y.; Goh, G.Y.S.; Beigi, A.; Yang, J.S.; Jung, G.Y.; Ma, D.K.; et al. MDT-15/MED15 permits longevity at low temperature via enhancing lipidostasis and proteostasis. PLoS Biol. 2019, 17, e3000415. [Google Scholar] [CrossRef]
- Jeong, J.H.; Han, J.S.; Jung, Y.; Lee, S.M.; Park, S.H.; Park, M.; Shin, M.G.; Kim, N.; Kang, M.S.; Kim, S.; et al. A new AMPK isoform mediates glucose-restriction induced longevity non-cell autonomously by promoting membrane fluidity. Nat. Commun. 2023, 14, 288. [Google Scholar] [CrossRef]
- Seah, N.E.; de Magalhaes Filho, C.D.; Petrashen, A.P.; Henderson, H.R.; Laguer, J.; Gonzalez, J.; Dillin, A.; Hansen, M.; Lapierre, L.R. Autophagy-mediated longevity is modulated by lipoprotein biogenesis. Autophagy 2016, 12, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Folick, A.; Oakley, H.D.; Yu, Y.; Armstrong, E.H.; Kumari, M.; Sanor, L.; Moore, D.D.; Ortlund, E.A.; Zechner, R.; Wang, M.C. Aging. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans. Science 2015, 347, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Chamoli, M.; Singh, A.; Malik, Y.; Mukhopadhyay, A. A novel kinase regulates dietary restriction-mediated longevity in Caenorhabditis elegans. Aging Cell 2014, 13, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Savini, M.; Folick, A.; Lee, Y.T.; Jin, F.; Cuevas, A.; Tillman, M.C.; Duffy, J.D.; Zhao, Q.; Neve, I.A.; Hu, P.W.; et al. Lysosome lipid signalling from the periphery to neurons regulates longevity. Nat. Cell Biol. 2022, 24, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.J.; Cheesman, H.K.; Liu, P.; Peterson, N.D.; Anderson, S.M.; Pukkila-Worley, R. Innate Immunity in the C. elegans Intestine Is Programmed by a Neuronal Regulator of AWC Olfactory Neuron Development. Cell Rep. 2020, 31, 107478. [Google Scholar] [CrossRef]
- Cao, X.; Aballay, A. Neural Inhibition of Dopaminergic Signaling Enhances Immunity in a Cell-Non-autonomous Manner. Curr. Biol. 2016, 26, 2398. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Cali, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef]
- Conrard, L.; Tyteca, D. Regulation of Membrane Calcium Transport Proteins by the Surrounding Lipid Environment. Biomolecules 2019, 9, 513. [Google Scholar] [CrossRef]
- Enyedi, A.; Vorherr, T.; James, P.; McCormick, D.J.; Filoteo, A.G.; Carafoli, E.; Penniston, J.T. The calmodulin binding domain of the plasma membrane Ca2+ pump interacts both with calmodulin and with another part of the pump. J. Biol. Chem. 1989, 264, 12313–12321. [Google Scholar] [CrossRef]
- Watts, J.L. Using Caenorhabditis elegans to Uncover Conserved Functions of Omega-3 and Omega-6 Fatty Acids. J. Clin. Med. 2016, 5, 19. [Google Scholar] [CrossRef]
- Kahn-Kirby, A.H.; Dantzker, J.L.; Apicella, A.J.; Schafer, W.R.; Browse, J.; Bargmann, C.I.; Watts, J.L. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell 2004, 119, 889–900. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, D.M.; Altshuler-Keylin, S.; Lee, J.I.; L’Etoile, N.D. Regulators of AWC-mediated olfactory plasticity in Caenorhabditis elegans. PLoS Genet. 2009, 5, e1000761. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, V.; Krieg, M.; Lockhead, D.; Goodman, M.B. Phospholipids that contain polyunsaturated fatty acids enhance neuronal cell mechanics and touch sensation. Cell Rep. 2014, 6, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Lesa, G.M.; Palfreyman, M.; Hall, D.H.; Clandinin, M.T.; Rudolph, C.; Jorgensen, E.M.; Schiavo, G. Long chain polyunsaturated fatty acids are required for efficient neurotransmission in C. elegans. J. Cell Sci. 2003, 116, 4965–4975. [Google Scholar] [CrossRef]
- McGhee, J.D. The C. elegans intestine. In WormBook; Oxford University Press: Oxford, UK, 2007; pp. 1–36. [Google Scholar] [CrossRef]
- Pathare, P.P.; Lin, A.; Bornfeldt, K.E.; Taubert, S.; Van Gilst, M.R. Coordinate regulation of lipid metabolism by novel nuclear receptor partnerships. PLoS Genet. 2012, 8, e1002645. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.B.; Thiele, C.; Tenedini, F.; Richard, M.; Leyendecker, P.; Hoermann, A.; Soba, P.; Tavosanis, G. Cell-Autonomous Control of Neuronal Dendrite Expansion via the Fatty Acid Synthesis Regulator SREBP. Cell Rep. 2017, 21, 3346–3353. [Google Scholar] [CrossRef]
- Warden, A.; Truitt, J.; Merriman, M.; Ponomareva, O.; Jameson, K.; Ferguson, L.B.; Mayfield, R.D.; Harris, R.A. Localization of PPAR isotypes in the adult mouse and human brain. Sci. Rep. 2016, 6, 27618. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Aparicio, R.; Flores, J.A.; Tasset, I.; Tunez, I.; Fernandez-Espejo, E. Mice lacking the peroxisome proliferator-activated receptor alpha gene present reduced number of dopamine neurons in the substantia nigra without altering motor behavior or dopamine neuron decline over life. Neuroscience 2011, 186, 161–169. [Google Scholar] [CrossRef]
- D’Agostino, G.; Cristiano, C.; Lyons, D.J.; Citraro, R.; Russo, E.; Avagliano, C.; Russo, R.; Raso, G.M.; Meli, R.; De Sarro, G.; et al. Peroxisome proliferator-activated receptor alpha plays a crucial role in behavioral repetition and cognitive flexibility in mice. Mol. Metab. 2015, 4, 528–536. [Google Scholar] [CrossRef]
- Roy, A.; Pahan, K. PPARalpha signaling in the hippocampus: Crosstalk between fat and memory. J. Neuroimmune Pharmacol. 2015, 10, 30–34. [Google Scholar] [CrossRef]
- Pierrot, N.; Ris, L.; Stancu, I.C.; Doshina, A.; Ribeiro, F.; Tyteca, D.; Bauge, E.; Lalloyer, F.; Malong, L.; Schakman, O.; et al. Sex-regulated gene dosage effect of PPARalpha on synaptic plasticity. Life Sci. Alliance 2019, 2, e201800262. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, S.; Strosznajder, A.K.; Jezyna, M.; Strosznajder, J.B. The Novel Role of PPAR Alpha in the Brain: Promising Target in Therapy of Alzheimer’s Disease and Other Neurodegenerative Disorders. Neurochem. Res. 2020, 45, 972–988. [Google Scholar] [CrossRef] [PubMed]
- Saez-Orellana, F.; Leroy, T.; Ribeiro, F.; Kreis, A.; Leroy, K.; Lalloyer, F.; Bauge, E.; Staels, B.; Duyckaerts, C.; Brion, J.P.; et al. Regulation of PPARalpha by APP in Alzheimer disease affects the pharmacological modulation of synaptic activity. JCI Insight 2021, 6, e150099. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Landreth, G.E. PPARs in the brain. Biochim. Biophys. Acta 2007, 1771, 1031–1045. [Google Scholar] [CrossRef]
- Marcellino, B.K.; Ekasumara, N.; Mobbs, C.V. Dietary Restriction and Glycolytic Inhibition Reduce Proteotoxicity and Extend Lifespan via NHR-49. Curr. Neurobiol. 2018, 9, 1–7. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, S.; Park, K.-S.; Yoon, K.-h. Regulator of Lipid Metabolism NHR-49 Mediates Pathogen Avoidance through Precise Control of Neuronal Activity. Cells 2024, 13, 978. https://doi.org/10.3390/cells13110978
Kwon S, Park K-S, Yoon K-h. Regulator of Lipid Metabolism NHR-49 Mediates Pathogen Avoidance through Precise Control of Neuronal Activity. Cells. 2024; 13(11):978. https://doi.org/10.3390/cells13110978
Chicago/Turabian StyleKwon, Saebom, Kyu-Sang Park, and Kyoung-hye Yoon. 2024. "Regulator of Lipid Metabolism NHR-49 Mediates Pathogen Avoidance through Precise Control of Neuronal Activity" Cells 13, no. 11: 978. https://doi.org/10.3390/cells13110978





