Emerging Treatments Targeting the Tumor Microenvironment for Advanced Chondrosarcoma
Abstract
1. Introduction
1.1. Incidence and Histopathology
1.2. Clinical Features and Therapeutic Management
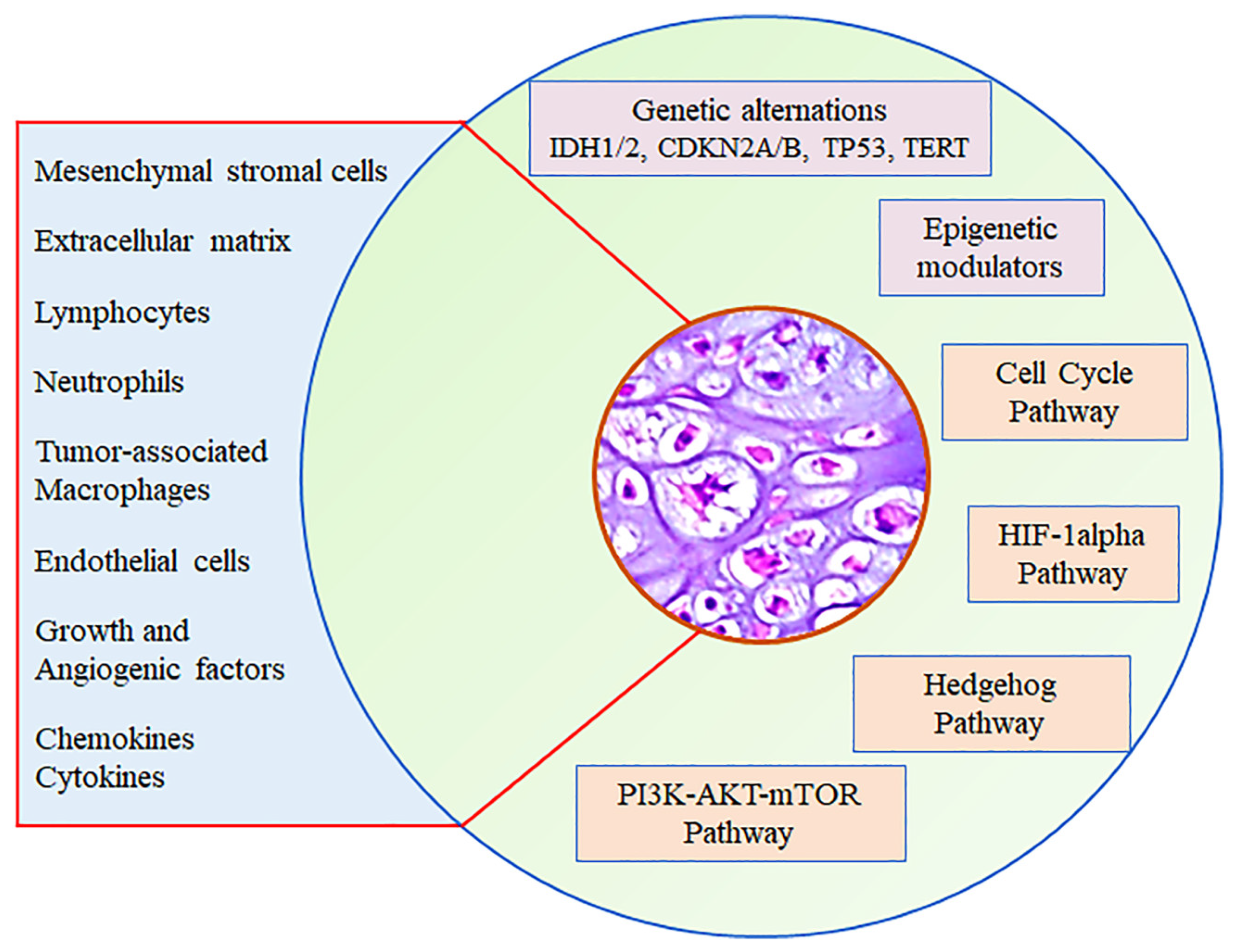
2. Tumor Microenvironment
2.1. Extracellular Matrix
2.2. Hypoxia
2.3. Adipose Tissue
2.4. Angiogenesis
2.5. Immune Environment
3. Immunotherapy
| NCT Number | Clinical Trial | Phase | Drug Class | Drug | Patients (n. ChSs) | Outcomes | Status | Ref. |
|---|---|---|---|---|---|---|---|---|
| NCT00928525 | Open-label trial of imatinib in patients with desmoid tumors and ChSs | 2 | Antiangiogenic MTKI | Imatinib– Mesylate | 34 (16 ChSs) | Efficacy ORR | Completed | [84] |
| NCT02982486 | Phase II of nivolumab plus ipilimumab in non-resectable sarcoma and endometrial carcinoma | 2 | Anti-PD-L1 Anti-CTLA-4 | Nivolumab Ipilimumab | Soft and bone sarcomas | Efficacy Safety PFS OS | Unknown status | [123] |
| NCT00464620 | Trial of dasatinib in advanced Sarcomas | 2 | Antiangiogenic MTKI | Dasatinib | 109 (33 ChSs) | ORR CBR RR PFSR OS | Completed | [124] |
| NCT02301039 | SARC028: A Phase II study of the anti-PD1 antibody pembrolizumab (MK-3475) in patients with advanced sarcomas | 2 | Anti-PD-1 | Pembrolizumab | Soft tissue and bone sarcoma (n. 42 bone sarcomas) | OR CR PR PFS RR OS | Completed | [119] |
| NCT03449108 | LN-145 or LN-145-S1 in treating patients with relapsed or refractory ovarian cancer, triple-negative breast cancer (TNBC), anaplastic thyroid cancer, osteosarcoma, or other bone and soft tissue sarcomas | 2 | Recombinant IL-2 BCL2 Inhibitor Anti-PD-1 Anti-CTLA-4 chemotherapy | Aldesleukin ALN-145 LN-145-S1 Cyclophosphamide Fludarabine Ipilimumab Nivolumab | 30 (bone sarcoma, ddChS, giant cell tumor of bone, and 13 more) | Efficacy Safety ORR DCR PFS PR CR, SD DoR CR PR PD, PFS OS | Active, not recruiting | [125] |
| NCT03474640 | Safety, tolerability, and pharmacokinetics of an anti-PD-1 monoclonal antibody in subjects with advanced malignancies | 1/2 | Humanized anti-PD-1 | Toripalimab | Bone sarcoma | Safety Toxicity PFS PFSR OS | Closed for enrollment | [126] |
| NCT01330966 | Study of pazopanib in the treatment of surgically unresectable or metastatic ChSs | 1/2 | Antiangiogenic MTKI | Pazopanib | 47 ChSs | Safety Efficacy DCR PFS CR SD PD PR OS | Completed | [85] |
| NCT03277924 | Trial of sunitinib and/or nivolumab plus chemotherapy in advanced soft tissue and bone sarcomas (ImmunoSarc) | 1/2 | Antiangiogenic MTKI Anti-PD-L1 chemotherapy | Sunitinib Nivolumab Epirubicin | 200 (1 clear cell ChS, 6 dd-ChSs) | Safety Toxicity PFS OR CR PR OS | Recruiting | [127] |
| NCT02888665 | Pembrolizumab and doxorubicin hydrochloride in treating patients with sarcomas that are metastatic or cannot be removed by surgery | 1/2 | Anti-PD-1 chemotherapy | Pembrolizumab Doxorubicin | Bone sarcomas (1 clear cell ChS, 3 Conventional, and 4 ddChSs) | ORR CR PR PFS MTD OS | Completed | [121] |
| NCT02389244 | A Phase II study evaluating the efficacy and safety of regorafenib in patients with metastatic bone sarcomas (REGOBONE) | 2 | Antiangiogenic MTKI | Regorafenib | Metastatic bone sarcomas (46 ChSs) | PFS | Recruiting | [86] |
| NCT05193188 | A multicenter clinically controlled study of anlotinib combined with PD-1 antibody on unresectable high-grade ChS with different IDH denotypes | 2 | Antiangiogenic MTKI PD-1 Ab | Anlotinib PD-1 Ab | high-grade, conventional, and ddChSs | PFSR ORR DCR | Recruiting | [87] |
| NCT04055220 | Efficacy and safety of regorafenib as maintenance therapy after first-line treatment in patients with bone sarcomas (REGOSTA) | 2 | Antiangiogenic MTKI | Regorafenib | Bone sarcomas | Efficacy Safety RFS OS | Recruiting | [128] |
| NCT04553692 | Phase I study of IGM-8444 as a single agent and in combination with subjects with relapsed and/or refractory solid cancers | 1 | Anti-DR5 Antiangiogenic chemotherapies BCL2 inhibitor | IGM-8444 Folfiri Bevacizumab Birinapant Venetoclax | Relapsed, refractory, or newly diagnosed solid cancers, including ChSs | ORR DoR PFS | Recruiting | [129,130] |
| NCT03282344 | A study of NKTR-214 in combination with nivolumab in patients with metastatic and/or locally advanced sarcomas | 2 | IL2 pathway agonist, ICI | NKTR-214 Nivolumab | Metastatic and/or locally advanced soft and bone sarcoma | PR CR PD SD | Active, not recruiting | [131] |
| NCT04458922 | Testing atezolizumab in people 2-17 years old with clear cell sarcoma or advanced ChS | 2 | Anti-PD-L1 | Atezolizumab | 27 ChSs | ORR CR PR, PD PFS RR OS | Active, not recruiting | [132] |
| NCT03414229 | A study of epacadostat, an IDO1 inhibitor, in combination with pembrolizumab in patients with metastatic and/or locally advanced sarcomas | 2 | IDO1 Inhibitor Anti-PD-1 | Epacadostat Pembrolizumab | Metastatic and/or locally advanced sarcoma | Efficacy Safety ORR PFS RFS OS | Active, not recruiting | [133] |
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACT | Atypical cartilaginous tumor |
| CBR | clinical benefit rate |
| ChS | Chondrosarcoma |
| CR | Complete response |
| CSC | Cancer stem cell |
| CSF1R | Colony stimulating factor 1 receptor |
| CSPG4 | ProteoGlycan 4 |
| DCR | Disease control rate |
| ddChS Dedifferentiated ChS | |
| DFS | Disease-free survival |
| DoR | Duration of response |
| ECM | Extracellular matrix |
| HDAC | Histone deacetylase |
| HIF | Hypoxia-inducible factor |
| ICI | Immune checkpoint inhibitor |
| IHC | Immunohistochemistry |
| IL | Interleukin |
| LOX | Lysyl oxidase |
| Matrix metalloproteinases | |
| MTD | Maximum tolerated dose |
| mTOR | Mammalian target of rapamycin |
| OR | Objective response |
| ORR | Overall response rate |
| OS | Overall survival |
| PD | Progressive disease |
| PDGF | Platelet-derived growth factor |
| PFS | Progression-free survival |
| PFSR | Progression-free survival rate |
| PR | Partial response |
| RFS | Relapse-free survival |
| RR | Response rate |
| SD | Stable disease |
| TAMs | Tumor-associated macrophages |
| TANs | Tumor-associated neutrophils |
| TME | Tumor microenvironment |
| Human umbilical vein endothelial cells | |
| VEGF | Vascular endothelial growth factor |
References
- Rozeman, L.B.; Cleton-Jansen, A.M.; Hogendoorn, P.C.W. Pathology of Primary Malignant Bone and Cartilage Tumours. Int. Orthop. 2006, 30, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Hogendoorn, P.C.W.; Bovée, J.; Nielsen, G.P.; Fletcher, C.D.M.; Bridge, J.A.; Hogendoorn, P.C.W.; Mertens, F. Chondrosarcoma (grades I–III), including primary and secondary variants and periosteal chondrosarcoma. In WHO Classification of Tumours of Soft Tissue and Bone, 4th ed.; IARC: Lyon, France, 2013; pp. 264–268. [Google Scholar]
- Giuffrida, A.Y.; Burgueno, J.E.; Koniaris, L.G.; Gutierrez, J.C.; Duncan, R.; Scully, S.P. Chondrosarcoma in the United States (1973 to 2003): An Analysis of 2890 Cases from the SEER Database. J. Bone Jt. Surg. Am. 2009, 91, 1063–1072. [Google Scholar] [CrossRef]
- Van Praag Veroniek, V.M.; Rueten-Budde, A.J.; Ho, V.; Dijkstra, P.D.S.; Study Group Bone and Soft Tissue Tumours (WeBot); Fiocco, M.; van de Sande, M.A.J. Incidence, Outcomes and Prognostic Factors during 25 Years of Treatment of Chondrosarcomas. Surg. Oncol. 2018, 27, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.E.; Childs, B.R.; Eckhoff, M.D.; Rajani, R.; Potter, B.K.; Polfer, E.M. Atypical Cartilaginous Tumors: Trends in Management. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2021, 5, e21.00277. [Google Scholar] [CrossRef]
- Thorkildsen, J.; Taksdal, I.; Bjerkehagen, B.; Haugland, H.K.; Børge Johannesen, T.; Viset, T.; Norum, O.-J.; Bruland, Ø.; Zaikova, O. Chondrosarcoma in Norway 1990–2013; an Epidemiological and Prognostic Observational Study of a Complete National Cohort. Acta Oncol. 2019, 58, 273–282. [Google Scholar] [CrossRef]
- Damron, T.A.; Ward, W.G.; Stewart, A. Osteosarcoma, Chondrosarcoma, and Ewing’s Sarcoma: National Cancer Data Base Report. Clin. Orthop. Relat. Res. 2007, 459, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.E.; Kopeć, S.; Szostakowski, B.; Spałek, M.J.; Fiedorowicz, M.; Bylina, E.; Filipowicz, P.; Szumera-Ciećkiewicz, A.; Tysarowski, A.; Czarnecka, A.M.; et al. Chondrosarcoma-from Molecular Pathology to Novel Therapies. Cancers 2021, 13, 2390. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, S.K. Classification of Chondrosarcoma: From Characteristic to Challenging Imaging Findings. Cancers 2023, 15, 1703. [Google Scholar] [CrossRef] [PubMed]
- Tosun, I.; Naderi, S. Approach to Primary Vertebral Tumors in the Light of the 2020 Updated World Health Organization Classification of Bone Tumors. Turk. Neurosurg. 2023, 33, 1–9. [Google Scholar] [CrossRef]
- Gilbert, A.; Tudor, M.; Montanari, J.; Commenchail, K.; Savu, D.I.; Lesueur, P.; Chevalier, F. Chondrosarcoma Resistance to Radiation Therapy: Origins and Potential Therapeutic Solutions. Cancers 2023, 15, 1962. [Google Scholar] [CrossRef]
- Limaiem, F.; Davis, D.D.; Sticco, K.L. Chondrosarcoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours; IARC: Lyon, France, 2013; ISBN 978-92-832-4502-5. [Google Scholar]
- Choi, J.H.; Ro, J.Y. The 2020 WHO Classification of Tumors of Bone: An Updated Review. Adv. Anat. Pathol. 2021, 28, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.; Król, S.K.; Rutkowski, P.; Czarnecka, A.M. Biological Heterogeneity of Chondrosarcoma: From (Epi) Genetics through Stemness and Deregulated Signaling to Immunophenotype. Cancers 2021, 13, 1317. [Google Scholar] [CrossRef] [PubMed]
- Douis, H.; Saifuddin, A. The Imaging of Cartilaginous Bone Tumours. II. Chondrosarcoma. Skelet. Radiol. 2013, 42, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Righi, A.; Pacheco, M.; Cocchi, S.; Asioli, S.; Gambarotti, M.; Donati, D.M.; Evangelista, A.; Gnoli, M.; Locatelli, M.; Mordenti, M.; et al. Secondary Peripheral Chondrosarcoma Arising in Solitary Osteochondroma: Variables Influencing Prognosis and Survival. Orphanet J. Rare Dis. 2022, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Endo, M.; Susuki, Y.; Yokoyama, N.; Maekawa, A.; Nabeshima, A.; Iida, K.; Fujiwara, T.; Setsu, N.; Matsunobu, T.; et al. Clinical, Radiological, and Histopathological Characteristics of Periosteal Chondrosarcoma with a Focus on the Frequency of Medullary Invasion. J. Clin. Med. 2022, 11, 2062. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Choy, E.; Raskin, K.A.; Schwab, J.H.; Nielsen, G.P.; Deshpande, V.; Chebib, I.; DeLaney, T.F.; Hornicek, F.J.; Cote, G.M.; et al. Prognostic Factors in Dedifferentiated Chondrosarcoma: A Retrospective Analysis of a Large Series Treated at a Single Institution. Sarcoma 2019, 2019, 9069272. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, D.C.; Beabout, J.W. Dedifferentiation of Low-Grade Chondrosarcomas. Cancer 1971, 28, 461–466. [Google Scholar] [CrossRef]
- Murphey, M.D.; Walker, E.A.; Wilson, A.J.; Kransdorf, M.J.; Temple, H.T.; Gannon, F.H. From the Archives of the AFIP: Imaging of Primary Chondrosarcoma: Radiologic-Pathologic Correlation. Radiographics 2003, 23, 1245–1278. [Google Scholar] [CrossRef]
- Swanson, P.E.; Lillemoe, T.J.; Manivel, J.C.; Wick, M.R. Mesenchymal Chondrosarcoma. An Immunohistochemical Study. Arch. Pathol. Lab. Med. 1990, 114, 943–948. [Google Scholar] [PubMed]
- Kumar, R.; David, R.; Cierney, G. Clear Cell Chondrosarcoma. Radiology 1985, 154, 45–48. [Google Scholar] [CrossRef]
- Amer, K.M.; Munn, M.; Congiusta, D.; Abraham, J.A.; Basu Mallick, A. Survival and Prognosis of Chondrosarcoma Subtypes: SEER Database Analysis. J. Orthop. Res. 2020, 38, 311–319. [Google Scholar] [CrossRef]
- Lin, P.P.; Moussallem, C.D.; Deavers, M.T. Secondary Chondrosarcoma. J. Am. Acad. Orthop. Surg. 2010, 18, 608–615. [Google Scholar] [CrossRef]
- Gazendam, A.; Popovic, S.; Parasu, N.; Ghert, M. Chondrosarcoma: A Clinical Review. J. Clin. Med. 2023, 12, 2506. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, G.; Chen, X.; Huang, X.; Liu, M.; Pan, W.; Yan, X.; Lin, N.; Ye, Z. Predictors of the Survival of Patients with Chondrosarcoma of Bone and Metastatic Disease at Diagnosis. J. Cancer 2019, 10, 2457–2463. [Google Scholar] [CrossRef]
- Catanzano, A.A.; Kerr, D.L.; Lazarides, A.L.; Dial, B.L.; Lane, W.O.; Blazer, D.G.; Larrier, N.A.; Kirsch, D.G.; Brigman, B.E.; Eward, W.C. Revisiting the Role of Radiation Therapy in Chondrosarcoma: A National Cancer Database Study. Sarcoma 2019, 2019, 4878512. [Google Scholar] [CrossRef] [PubMed]
- Italiano, A.; Mir, O.; Cioffi, A.; Palmerini, E.; Piperno-Neumann, S.; Perrin, C.; Chaigneau, L.; Penel, N.; Duffaud, F.; Kurtz, J.E.; et al. Advanced Chondrosarcomas: Role of Chemotherapy and Survival. Ann. Oncol. 2013, 24, 2916–2922. [Google Scholar] [CrossRef]
- Walter, S.G.; Knöll, P.; Eysel, P.; Quaas, A.; Gaisendrees, C.; Nißler, R.; Hieggelke, L. Molecular In-Depth Characterization of Chondrosarcoma for Current and Future Targeted Therapies. Cancers 2023, 15, 2556. [Google Scholar] [CrossRef] [PubMed]
- Boeuf, S.; Kunz, P.; Hennig, T.; Lehner, B.; Hogendoorn, P.; Bovée, J.; Richter, W. A Chondrogenic Gene Expression Signature in Mesenchymal Stem Cells Is a Classifier of Conventional Central Chondrosarcoma. J. Pathol. 2008, 216, 158–166. [Google Scholar] [CrossRef]
- Martínez-Delgado, P.; Lacerenza, S.; Obrador-Hevia, A.; Lopez-Alvarez, M.; Mondaza-Hernandez, J.L.; Blanco-Alcaina, E.; Sanchez-Bustos, P.; Hindi, N.; Moura, D.S.; Martin-Broto, J. Cancer Stem Cells in Soft-Tissue Sarcomas. Cells 2020, 9, 1449. [Google Scholar] [CrossRef]
- Terek, R.M.; Schwartz, G.K.; Devaney, K.; Glantz, L.; Mak, S.; Healey, J.H.; Albino, A.P. Chemotherapy and P-Glycoprotein Expression in Chondrosarcoma. J. Orthop. Res. 1998, 16, 585–590. [Google Scholar] [CrossRef]
- Jeong, W.; Kim, H.-J. Biomarkers of Chondrosarcoma. J. Clin. Pathol. 2018, 71, 579–583. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, H.; Van De Gucht, M.; De Ridder, M. Hypoxic Radioresistance: Can ROS Be the Key to Overcome It? Cancers 2019, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Bereza, M.; Dembiński, M.; Zając, A.E.; Piątkowski, J.; Dudzisz-Śledź, M.; Rutkowski, P.; Czarnecka, A.M. Epigenetic Abnormalities in Chondrosarcoma. Int. J. Mol. Sci. 2023, 24, 4539. [Google Scholar] [CrossRef]
- Miwa, S.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tsuchiya, H. Therapeutic Targets and Emerging Treatments in Advanced Chondrosarcoma. Int. J. Mol. Sci. 2022, 23, 1096. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.E.; Graves, L.A.; Rubin, E.M.; Reed, D.R.; Riedel, R.F.; Strauss, S.J. Bad to the Bone: Emerging Approaches to Aggressive Bone Sarcomas. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e390306. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.H.; Wenger, D.; Unni, K.; Sim, F.H. Does Local Recurrence Impact Survival in Low-Grade Chondrosarcoma of the Long Bones? Clin. Orthop. Relat. Res. 2007, 462, 175–180. [Google Scholar] [CrossRef]
- Shemesh, S.S.; Pretell-Mazzini, J.; Quartin, P.A.J.; Rutenberg, T.F.; Conway, S.A. Surgical Treatment of Low-Grade Chondrosarcoma Involving the Appendicular Skeleton: Long-Term Functional and Oncological Outcomes. Arch. Orthop. Trauma. Surg. 2019, 139, 1659–1666. [Google Scholar] [CrossRef]
- Görgün, B.; Özşahin, M.K.; Tok, O.; Davulcu, C.D.; Karaismailoğlu, B.; Hız, M. Intralesional Curettage and Cementation of Low-Grade Chondrosarcomas of the Appendicular Skeleton: Long-Term Results from a Single Center. Acta Orthop. Traumatol. Turc. 2022, 56, 402–407. [Google Scholar] [CrossRef]
- Erstad, D.J.; Ready, J.; Abraham, J.; Ferrone, M.L.; Bertagnolli, M.M.; Baldini, E.H.; Raut, C.P. Amputation for Extremity Sarcoma: Contemporary Indications and Outcomes. Ann. Surg. Oncol. 2018, 25, 394–403. [Google Scholar] [CrossRef]
- Mayer, S.; Milo, T.; Isaacson, A.; Halperin, C.; Miyara, S.; Stein, Y.; Lior, C.; Pevsner-Fischer, M.; Tzahor, E.; Mayo, A.; et al. The Tumor Microenvironment Shows a Hierarchy of Cell-Cell Interactions Dominated by Fibroblasts. Nat. Commun. 2023, 14, 5810. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Li, M.; Luo, T.; Yin, Y.; Jiang, Y. Matrix Metalloproteinases in Tumorigenesis: An Evolving Paradigm. Cell Mol. Life Sci. 2011, 68, 3853–3868. [Google Scholar] [CrossRef]
- Malcherczyk, D.; Heyse, T.J.; El-Zayat, B.F.; Kunzke, V.; Moll, R.; Fuchs-Winkelmann, S.; Paletta, J.R.J. Expression of MMP-9 Decreases Metastatic Potential of Chondrosarcoma: An Immunohistochemical Study. BMC Musculoskelet. Disord. 2018, 19, 9. [Google Scholar] [CrossRef]
- Yao, M.; Wang, X.; Zhao, Y.; Wang, X.; Gao, F. Expression of MMPs Is Dependent on the Activity of Mitogen-Activated Protein Kinase in Chondrosarcoma. Mol. Med. Rep. 2017, 15, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Takeuchi, A.; Munesue, S.; Yamamoto, N.; Hayashi, K.; Kimura, H.; Miwa, S.; Inatani, H.; Shimozaki, S.; Kato, T.; et al. Anti-tumor Effects of a Nonsteroidal Anti-inflammatory Drug Zaltoprofen on Chondrosarcoma via Activating Peroxisome Proliferator-activated Receptor Gamma and Suppressing Matrix Metalloproteinase-2 Expression. Cancer Med. 2018, 7, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Söderström, M.; Böhling, T.; Ekfors, T.; Nelimarkka, L.; Aro, H.T.; Vuorio, E. Molecular Profiling of Human Chondrosarcomas for Matrix Production and Cancer Markers. Int. J. Cancer 2002, 100, 144–151. [Google Scholar] [CrossRef]
- Fede, C.; Stecco, C.; Angelini, A.; Fan, C.; Belluzzi, E.; Pozzuoli, A.; Ruggieri, P.; De Caro, R. Variations in Contents of Hyaluronan in the Peritumoral Micro-Environment of Human Chondrosarcoma. J. Orthop. Res. 2019, 37, 503–509. [Google Scholar] [CrossRef]
- Hamada, S.; Nishida, Y.; Zhuo, L.; Shinomura, T.; Ikuta, K.; Arai, E.; Koike, H.; Kimata, K.; Ushida, T.; Ishiguro, N. Suppression of Hyaluronan Synthesis Attenuates the Tumorigenicity of Low-Grade Chondrosarcoma. J. Orthop. Res. 2018, 36, 1573–1580. [Google Scholar] [CrossRef]
- Nota, S.P.F.T.; Osei-Hwedieh, D.O.; Drum, D.L.; Wang, X.; Sabbatino, F.; Ferrone, S.; Schwab, J.H. Chondroitin Sulfate Proteoglycan 4 Expression in Chondrosarcoma: A Potential Target for Antibody-Based Immunotherapy. Front. Oncol. 2022, 12, 939166. [Google Scholar] [CrossRef]
- Johnston, K.A.; Lopez, K.M. Lysyl Oxidase in Cancer Inhibition and Metastasis. Cancer Lett. 2018, 417, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, H.-E.; Lin, S.-L.; Thadevoos, L.A.; Lien, M.-Y.; Yang, W.-H.; Ko, C.-Y.; Lin, C.-Y.; Huang, Y.-W.; Liu, J.-F.; Fong, Y.-C.; et al. Nerve Growth Factor Promotes Lysyl Oxidase-Dependent Chondrosarcoma Cell Metastasis by Suppressing miR-149-5p Synthesis. Cell Death Dis. 2021, 12, 1101. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, T. Inflammation as molecular target in chondrosarcoma. Pathologe 2014, 35 (Suppl. S2), 249–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-T.; Huang, Y.-L.; Tzeng, H.-E.; Tsai, C.-H.; Wang, S.-W.; Tang, C.-H. CCL5 Promotes Vascular Endothelial Growth Factor Expression and Induces Angiogenesis by Down-Regulating miR-199a in Human Chondrosarcoma Cells. Cancer Lett. 2015, 357, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Höckel, M.; Vaupel, P. Tumor Hypoxia: Definitions and Current Clinical, Biologic, and Molecular Aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Di Pompo, G.; Cortini, M.; Baldini, N.; Avnet, S. Acid Microenvironment in Bone Sarcomas. Cancers 2021, 13, 3848. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Sugita, T.; Shimose, S.; Matsuo, T.; Arihiro, K.; Ochi, M. Expression of Hypoxia-Inducible Factor-1alpha and Its Relationship to Tumour Angiogenesis and Cell Proliferation in Cartilage Tumours. J. Bone Jt. Surg. Br. 2008, 90, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lv, X.; Yan, Y.; Zhao, Y.; Ma, R.; He, M.; Wei, M. Hypoxia-Mediated Cancer Stem Cell Resistance and Targeted Therapy. Biomed. Pharmacother. 2020, 130, 110623. [Google Scholar] [CrossRef] [PubMed]
- Marhold, M.; Tomasich, E.; El-Gazzar, A.; Heller, G.; Spittler, A.; Horvat, R.; Krainer, M.; Horak, P. HIF1α Regulates mTOR Signaling and Viability of Prostate Cancer Stem Cells. Mol. Cancer Res. 2015, 13, 556–564. [Google Scholar] [CrossRef]
- Wu, S.-L.; Li, Y.-J.; Liao, K.; Shi, L.; Zhang, N.; Liu, S.; Hu, Y.-Y.; Li, S.-L.; Wang, Y. 2-Methoxyestradiol Inhibits the Proliferation and Migration and Reduces the Radioresistance of Nasopharyngeal Carcinoma CNE-2 Stem Cells via NF-κB/HIF-1 Signaling Pathway Inactivation and EMT Reversal. Oncol. Rep. 2017, 37, 793–802. [Google Scholar] [CrossRef]
- Sun, X.; Wei, L.; Chen, Q.; Terek, R.M. CXCR4/SDF1 Mediate Hypoxia Induced Chondrosarcoma Cell Invasion through ERK Signaling and Increased MMP1 Expression. Mol. Cancer 2010, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Ayala, G.; Liu, C.; Nicosia, R.; Horowitz, S.; Lackman, R. Microvasculature and VEGF Expression in Cartilaginous Tumors. Hum. Pathol. 2000, 31, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; McGough, R.; Aswad, B.; Block, J.A.; Terek, R. Hypoxia Induces HIF-1alpha and VEGF Expression in Chondrosarcoma Cells and Chondrocytes. J. Orthop. Res. 2004, 22, 1175–1181. [Google Scholar] [CrossRef]
- Kim, H.; Cho, Y.; Kim, H.-S.; Kang, D.; Cheon, D.; Kim, Y.-J.; Chang, M.J.; Lee, K.M.; Chang, C.B.; Kang, S.-B.; et al. A System-Level Approach Identifies HIF-2α as a Critical Regulator of Chondrosarcoma Progression. Nat. Commun. 2020, 11, 5023. [Google Scholar] [CrossRef]
- Hou, S.-M.; Lin, C.-Y.; Fong, Y.-C.; Tang, C.-H. Hypoxia-Regulated Exosomes Mediate M2 Macrophage Polarization and Promote Metastasis in Chondrosarcoma. Aging 2023, 15, 13163–13175. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-P.; Lin, C.-Y.; Shih, J.-S.; Fong, Y.-C.; Wang, S.-W.; Li, T.-M.; Tang, C.-H. Adiponectin Promotes VEGF-A-Dependent Angiogenesis in Human Chondrosarcoma through PI3K, Akt, mTOR, and HIF-α Pathway. Oncotarget 2015, 6, 36746–36761. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-C.; Shieh, D.-C.; Tong, K.-M.; Chen, C.-P.; Huang, K.-C.; Chen, P.-C.; Fong, Y.-C.; Hsu, H.-C.; Tang, C.-H. Involvement of AdipoR Receptor in Adiponectin-Induced Motility and Alpha2beta1 Integrin Upregulation in Human Chondrosarcoma Cells. Carcinogenesis 2009, 30, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-N.; Chen, H.-T.; Tsou, H.-K.; Huang, C.-Y.; Yang, W.-H.; Su, C.-M.; Fong, Y.-C.; Tseng, W.-P.; Tang, C.-H. Leptin Enhances Cell Migration in Human Chondrosarcoma Cells through OBRl Leptin Receptor. Carcinogenesis 2009, 30, 566–574. [Google Scholar] [CrossRef]
- Yang, W.-H.; Chen, J.-C.; Hsu, K.-H.; Lin, C.-Y.; Wang, S.-W.; Wang, S.-J.; Chang, Y.-S.; Tang, C.-H. Leptin Increases VEGF Expression and Enhances Angiogenesis in Human Chondrosarcoma Cells. Biochim. Biophys. Acta (BBA)–Gen. Subj. 2014, 1840, 3483–3493. [Google Scholar] [CrossRef]
- Yang, W.-H.; Chang, A.-C.; Wang, S.-W.; Wang, S.-J.; Chang, Y.-S.; Chang, T.-M.; Hsu, S.-K.; Fong, Y.-C.; Tang, C.-H. Leptin Promotes VEGF-C Production and Induces Lymphangiogenesis by Suppressing miR-27b in Human Chondrosarcoma Cells. Sci. Rep. 2016, 6, 28647. [Google Scholar] [CrossRef]
- McGough, R.L.; Aswad, B.I.; Terek, R.M. Pathologic Neovascularization in Cartilage Tumors. Clin. Orthop. Relat. Res. 2002, 397, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Minopoli, M.; Sarno, S.; Di Carluccio, G.; Azzaro, R.; Costantini, S.; Fazioli, F.; Gallo, M.; Apice, G.; Cannella, L.; Rea, D.; et al. Inhibiting Monocyte Recruitment to Prevent the Pro-Tumoral Activity of Tumor-Associated Macrophages in Chondrosarcoma. Cells 2020, 9, 1062. [Google Scholar] [CrossRef] [PubMed]
- Ingangi, V.; Bifulco, K.; Yousif, A.M.; Ragone, C.; Motti, M.L.; Rea, D.; Minopoli, M.; Botti, G.; Scognamiglio, G.; Fazioli, F.; et al. The Urokinase Receptor-Derived Cyclic Peptide [SRSRY] Suppresses Neovascularization and Intravasation of Osteosarcoma and Chondrosarcoma Cells. Oncotarget 2016, 7, 54474–54487. [Google Scholar] [CrossRef]
- Carriero, M.V.; Bifulco, K.; Ingangi, V.; Costantini, S.; Botti, G.; Ragone, C.; Minopoli, M.; Motti, M.L.; Rea, D.; Scognamiglio, G.; et al. Retro-Inverso Urokinase Receptor Antagonists for the Treatment of Metastatic Sarcomas. Sci. Rep. 2017, 7, 1312. [Google Scholar] [CrossRef]
- Bifulco, K.; Longanesi-Cattani, I.; Gala, M.; DI Carluccio, G.; Masucci, M.T.; Pavone, V.; Lista, L.; Arra, C.; Stoppelli, M.P.; Carriero, M.V. The Soluble Form of Urokinase Receptor Promotes Angiogenesis through Its Ser88-Arg-Ser-Arg-Tyr92 Chemotactic Sequence. J. Thromb. Haemost. 2010, 8, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Minopoli, M.; Polo, A.; Ragone, C.; Ingangi, V.; Ciliberto, G.; Pessi, A.; Sarno, S.; Budillon, A.; Costantini, S.; Carriero, M.V. Structure-Function Relationship of an Urokinase Receptor-Derived Peptide Which Inhibits the Formyl Peptide Receptor Type 1 Activity. Sci. Rep. 2019, 9, 12169. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, Z.; Cheng, F.; Shao, Z. Exosomal lncRNA RAMP2-AS1 Derived from Chondrosarcoma Cells Promotes Angiogenesis Through miR-2355-5p/VEGFR2 Axis. OncoTargets Ther. 2020, 13, 3291–3301. [Google Scholar] [CrossRef] [PubMed]
- Sulzbacher, I.; Birner, P.; Trieb, K.; Mühlbauer, M.; Lang, S.; Chott, A. Platelet-Derived Growth Factor-Alpha Receptor Expression Supports the Growth of Conventional Chondrosarcoma and Is Associated with Adverse Outcome. Am. J. Surg. Pathol. 2001, 25, 1520–1527. [Google Scholar] [CrossRef]
- Wang, C.-Q.; Lin, C.-Y.; Huang, Y.-L.; Wang, S.-W.; Wang, Y.; Huang, B.-F.; Lai, Y.-W.; Weng, S.-L.; Fong, Y.-C.; Tang, C.-H.; et al. Sphingosine-1-Phosphate Promotes PDGF-Dependent Endothelial Progenitor Cell Angiogenesis in Human Chondrosarcoma Cells. Aging 2019, 11, 11040–11053. [Google Scholar] [CrossRef]
- Zafar, S.Y.; Malin, J.L.; Grambow, S.C.; Abbott, D.H.; Schrag, D.; Kolimaga, J.T.; Zullig, L.L.; Weeks, J.C.; Fouad, M.N.; Ayanian, J.Z.; et al. Early Dissemination of Bevacizumab for Advanced Colorectal Cancer: A Prospective Cohort Study. BMC Cancer 2011, 11, 354. [Google Scholar] [CrossRef]
- Grignani, G.; Palmerini, E.; Stacchiotti, S.; Boglione, A.; Ferraresi, V.; Frustaci, S.; Comandone, A.; Casali, P.G.; Ferrari, S.; Aglietta, M. A Phase 2 Trial of Imatinib Mesylate in Patients with Recurrent Nonresectable Chondrosarcomas Expressing Platelet-Derived Growth Factor Receptor-α or -β: An Italian Sarcoma Group Study. Cancer 2011, 117, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.; Frankel, P.; Ruel, C.; Araujo, D.M.; Milhem, M.; Okuno, S.; Hartner, L.; Undevia, S.; Staddon, A. Results of a Prospective Phase 2 Study of Pazopanib in Patients with Surgically Unresectable or Metastatic Chondrosarcoma. Cancer 2020, 126, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Duffaud, F.; Italiano, A.; Bompas, E.; Rios, M.; Penel, N.; Mir, O.; Piperno-Neumann, S.; Chevreau, C.; Delcambre, C.; Bertucci, F.; et al. Efficacy and Safety of Regorafenib in Patients with Metastatic or Locally Advanced Chondrosarcoma: Results of a Non-Comparative, Randomised, Double-Blind, Placebo Controlled, Multicentre Phase II Study. Eur. J. Cancer 2021, 150, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, S.; Zhu, L.; Wang, J.; Zhang, P.; Li, P.; Zhang, F.; Yao, W. Efficacy and Safety of Anlotinib in Patients with Unresectable or Metastatic Bone Sarcoma: A Retrospective Multiple Institution Study. Cancer Med. 2021, 10, 7593–7600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The History and Advances in Cancer Immunotherapy: Understanding the Characteristics of Tumor-Infiltrating Immune Cells and Their Therapeutic Implications. Cell Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, G.; Yan, X.; Zhu, D.; Lin, P.P.; Wang, Z.; Qu, H.; He, X.; Fu, Y.; Zhu, X.; et al. Fresh Tissue Multi-Omics Profiling Reveals Immune Classification and Suggests Immunotherapy Candidates for Conventional Chondrosarcoma. Clin. Cancer Res. 2021, 27, 6543–6558. [Google Scholar] [CrossRef] [PubMed]
- Simard, F.A.; Richert, I.; Vandermoeten, A.; Decouvelaere, A.-V.; Michot, J.-P.; Caux, C.; Blay, J.-Y.; Dutour, A. Description of the Immune Microenvironment of Chondrosarcoma and Contribution to Progression. Oncoimmunology 2017, 6, e1265716. [Google Scholar] [CrossRef] [PubMed]
- Iseulys, R.; Anne, G.-B.; Corinne, B.; Gonzague, D.B.D.P.; Marie, K.; Jean-Yves, B.; Aurélie, D. The Immune Landscape of Chondrosarcoma Reveals an Immunosuppressive Environment in the Dedifferentiated Subtypes and Exposes CSFR1+ Macrophages as a Promising Therapeutic Target. J. Bone Oncol. 2020, 20, 100271. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as Tools and Targets in Cancer Therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef] [PubMed]
- Kostine, M.; Cleven, A.H.; de Miranda, N.F.C.C.; Italiano, A.; Cleton-Jansen, A.-M.; Bovée, J.V.M.G. Analysis of PD-L1, T-Cell Infiltrate and HLA Expression in Chondrosarcoma Indicates Potential for Response to Immunotherapy Specifically in the Dedifferentiated Subtype. Mod. Pathol. 2016, 29, 1028–1037. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific Recruitment of Regulatory T Cells in Ovarian Carcinoma Fosters Immune Privilege and Predicts Reduced Survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Nota, S.P.F.T.; Al-Sukaini, A.; Patel, S.S.; Sabbatino, F.; Nielsen, G.P.; Deshpande, V.; Yearley, J.H.; Ferrone, S.; Wang, X.; Schwab, J.H. High TIL, HLA, and Immune Checkpoint Expression in Conventional High-Grade and Dedifferentiated Chondrosarcoma and Poor Clinical Course of the Disease. Front. Oncol. 2021, 11, 598001. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Tang, F.; Wei, Y.-Q.; Wei, X.-W. Immunosuppressive Cells in Cancer: Mechanisms and Potential Therapeutic Targets. J. Hematol. Oncol. 2022, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Cannarile, M.A.; Weisser, M.; Jacob, W.; Jegg, A.-M.; Ries, C.H.; Rüttinger, D. Colony-Stimulating Factor 1 Receptor (CSF1R) Inhibitors in Cancer Therapy. J. Immunother. Cancer 2017, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Wang, S.; Guo, R.; Liu, D. CSF1R Inhibitors Are Emerging Immunotherapeutic Drugs for Cancer Treatment. Eur. J. Med. Chem. 2023, 245, 114884. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.; Berdugo, Y.A.; Tree, T. IL-2-Based Approaches to Treg Enhancement. Clin. Exp. Immunol. 2023, 211, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.E.; Czarnecka, A.M.; Rutkowski, P. The Role of Macrophages in Sarcoma Tumor Microenvironment and Treatment. Cancers 2023, 15, 5294. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-Beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. Tumour-Associated Neutrophils in Patients with Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 601–620. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Albelda, S.M. Tumor-Associated Neutrophils: Friend or Foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef]
- Jeon, H.; Han, S.R.; Lee, S.; Park, S.J.; Kim, J.H.; Yoo, S.-M.; Lee, M.-S. Activation of the Complement System in an Osteosarcoma Cell Line Promotes Angiogenesis through Enhanced Production of Growth Factors. Sci. Rep. 2018, 8, 5415. [Google Scholar] [CrossRef] [PubMed]
- Nabizadeh, J.A.; Manthey, H.D.; Steyn, F.J.; Chen, W.; Widiapradja, A.; Md Akhir, F.N.; Boyle, G.M.; Taylor, S.M.; Woodruff, T.M.; Rolfe, B.E. The Complement C3a Receptor Contributes to Melanoma Tumorigenesis by Inhibiting Neutrophil and CD4+ T Cell Responses. J. Immunol. 2016, 196, 4783–4792. [Google Scholar] [CrossRef] [PubMed]
- Guglietta, S.; Chiavelli, A.; Zagato, E.; Krieg, C.; Gandini, S.; Ravenda, P.S.; Bazolli, B.; Lu, B.; Penna, G.; Rescigno, M. Coagulation Induced by C3aR-Dependent NETosis Drives Protumorigenic Neutrophils during Small Intestinal Tumorigenesis. Nat. Commun. 2016, 7, 11037. [Google Scholar] [CrossRef]
- Magrini, E.; Di Marco, S.; Mapelli, S.N.; Perucchini, C.; Pasqualini, F.; Donato, A.; Guevara Lopez, M.d.l.L.; Carriero, R.; Ponzetta, A.; Colombo, P.; et al. Complement Activation Promoted by the Lectin Pathway Mediates C3aR-Dependent Sarcoma Progression and Immunosuppression. Nat. Cancer 2021, 2, 218–232. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 Is a Second Ligand for PD-1 and Inhibits T Cell Activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Taefehshokr, N.; Baradaran, B.; Baghbanzadeh, A.; Taefehshokr, S. Promising Approaches in Cancer Immunotherapy. Immunobiology 2020, 225, 151875. [Google Scholar] [CrossRef]
- Ko, B.; Takebe, N.; Andrews, O.; Makena, M.R.; Chen, A.P. Rethinking Oncologic Treatment Strategies with Interleukin-2. Cells 2023, 12, 1316. [Google Scholar] [CrossRef]
- Thanindratarn, P.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Advances in Immune Checkpoint Inhibitors for Bone Sarcoma Therapy. J. Bone Oncol. 2019, 15, 100221. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, G.; Yang, Z.; Zeng, K.; Liu, F.; Sun, J. Expression of PD-L1/PD-L2 Is Associated with High Proliferation Index of Ki-67 but not with TP53 Overexpression in Chondrosarcoma. Int. J. Biol. Markers 2018, 33, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Jin, Z.; Zhang, M.; Tang, Y.; Yang, G.; Yuan, X.; Yao, J.; Sun, D. Prognostic Value of Programmed Death-Ligand 1 in Sarcoma: A Meta-Analysis. Oncotarget 2017, 8, 59570–59580. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, T.; Ma, C.; Yuan, H.; Zhang, H.; Zhang, Z. Prognostic Value of Programmed Cell Death 1 Ligand-1 in Patients with Bone and Soft Tissue Sarcomas: A Systemic and Comprehensive Meta-Analysis Based on 3,680 Patients. Front. Oncol. 2020, 10, 749. [Google Scholar] [CrossRef] [PubMed]
- Paoluzzi, L.; Cacavio, A.; Ghesani, M.; Karambelkar, A.; Rapkiewicz, A.; Weber, J.; Rosen, G. Response to Anti-PD1 Therapy with Nivolumab in Metastatic Sarcomas. Clin. Sarcoma Res. 2016, 6, 24. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in Advanced Soft-Tissue Sarcoma and Bone Sarcoma (SARC028): A Multicentre, Two-Cohort, Single-Arm, Open-Label, Phase 2 Trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Keung, E.Z.; Burgess, M.; Salazar, R.; Parra, E.R.; Rodrigues-Canales, J.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Attia, S.; Riedel, R.F.; et al. Correlative Analyses of the SARC028 Trial Reveal an Association Between Sarcoma-Associated Immune Infiltrate and Response to Pembrolizumab. Clin. Cancer Res. 2020, 26, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Pollack, S.M.; Redman, M.W.; Baker, K.K.; Wagner, M.J.; Schroeder, B.A.; Loggers, E.T.; Trieselmann, K.; Copeland, V.C.; Zhang, S.; Black, G.; et al. Assessment of Doxorubicin and Pembrolizumab in Patients with Advanced Anthracycline-Naive Sarcoma: A Phase 1/2 Nonrandomized Clinical Trial. JAMA Oncol. 2020, 6, 1778–1782. [Google Scholar] [CrossRef]
- Singh, A.; Thorpe, S.W.; Darrow, M.; Carr-Ascher, J.R. Case Report: Treatment of Metastatic Dedifferentiated Chondrosarcoma with Pembrolizumab Yields Sustained Complete Response. Front. Oncol. 2022, 12, 991724. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Schuetze, S.M.; Wathen, J.K.; Lucas, D.R.; Choy, E.; Samuels, B.L.; Staddon, A.P.; Ganjoo, K.N.; von Mehren, M.; Chow, W.A.; Loeb, D.M.; et al. SARC009: Phase 2 Study of Dasatinib in Patients with Previously Treated, High-Grade, Advanced Sarcoma. Cancer 2016, 122, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Amaria, R.N.; Bernatchez, C.; Forget, M.-A.; Haymaker, C.L.; Conley, A.P.; Livingston, J.A.; Varadhachary, G.R.; Javle, M.M.; Maitra, A.; Tzeng, C.-W.D.; et al. Adoptive Transfer of Tumor-Infiltrating Lymphocytes in Patients with Sarcomas, Ovarian, and Pancreatic Cancers. JCO 2019, 37, TPS2650. [Google Scholar] [CrossRef]
- Yang, J.; Dong, L.; Yang, S.; Han, X.; Han, Y.; Jiang, S.; Yao, J.; Zhang, Z.; Zhang, S.; Liu, P.; et al. Safety and Clinical Efficacy of Toripalimab, a PD-1 mAb, in Patients with Advanced or Recurrent Malignancies in a Phase I Study. Eur. J. Cancer 2020, 130, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Martin-Broto, J.; Hindi, N.; Grignani, G.; Martinez-Trufero, J.; Redondo, A.; Valverde, C.; Stacchiotti, S.; Lopez-Pousa, A.; D’Ambrosio, L.; Gutierrez, A.; et al. Nivolumab and Sunitinib Combination in Advanced Soft Tissue Sarcomas: A Multicenter, Single-Arm, Phase Ib/II Trial. J. Immunother. Cancer 2020, 8, e001561. [Google Scholar] [CrossRef] [PubMed]
- Duffaud, F.; Chabaud, S.; Gautier, J.; Ferlay, C.; Vizoso, S.; Brahmi, M.; Benezech, S.; Dufresne, A.; Marec-Berard, P.; Ray-Coquard, I.L.; et al. REGOSTA: A Randomized, Placebo-Controlled, Double-Blinded, Multicenter Study Evaluating the Efficacy and Safety of Regorafenib (REGO) as Maintenance Therapy after First-Line Treatment in Patients (Pts) with Osteosarcoma (OS) and Non-Osteosarcomas (Non-OS) of Bone (Non-Ewing, Non-Chondrosarcomas and Non-Chordomas). JCO 2021, 39, TPS11576. [Google Scholar] [CrossRef]
- Wang, B.T.; Kothambawala, T.; Wang, L.; Matthew, T.J.; Calhoun, S.E.; Saini, A.K.; Kotturi, M.F.; Hernandez, G.; Humke, E.W.; Peterson, M.S.; et al. Multimeric Anti-DR5 IgM Agonist Antibody IGM-8444 Is a Potent Inducer of Cancer Cell Apoptosis and Synergizes with Chemotherapy and BCL-2 Inhibitor ABT-199. Mol. Cancer Ther. 2021, 20, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Desbois, M.; Calhoun, S.E.; Hart, K.C.; Denson, C.; Yakkundi, P.; Matthew, T.J.; Leabman, M.K.; Billic, S.; Hernandez, G.; Humke, E.W.; et al. Novel Combinations of Aplitabart, a DR5 Agonist IgM Antibody, with ADCs or Chemotherapeutic Agents Lead to Robust Anti-Tumor Responses in Solid Tumor Models. In Proceedings of the 115th Annual Meeting of the American Association for Cancer Research 2024, San Diego, CA, USA, 5–10 April 2024. [Google Scholar]
- D’Angelo, S.P.; Richards, A.L.; Conley, A.P.; Woo, H.J.; Dickson, M.A.; Gounder, M.; Kelly, C.; Keohan, M.L.; Movva, S.; Thornton, K.; et al. Pilot Study of Bempegaldesleukin in Combination with Nivolumab in Patients with Metastatic Sarcoma. Nat. Commun. 2022, 13, 3477. [Google Scholar] [CrossRef] [PubMed]
- Salkeni, M.A.; Ko, B.; Van Tine, B.A.; Conley, A.P.; Davis, E.J.; Burgess, M.A.; Close, J.L.; Foster, J.C.; O’Sullivan Coyne, G.H.; Moore, N.; et al. A Phase 2 Study of an Anti–PD-L1 Antibody (Atezolizumab) in Dedifferentiated Chondrosarcoma. JCO 2023, 41, 11533. [Google Scholar] [CrossRef]
- Kelly, C.M.; Qin, L.X.; Whiting, K.A.; Richards, A.L.; Avutu, V.; Chan, J.E.; Chi, P.; Dickson, M.A.; Gounder, M.M.; Keohan, M.L.; et al. A Phase II Study of Epacadostat and Pembrolizumab in Patients with Advanced Sarcoma. Clin. Cancer Res. 2023, 29, 2043–2051. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingangi, V.; De Chiara, A.; Ferrara, G.; Gallo, M.; Catapano, A.; Fazioli, F.; Di Carluccio, G.; Peranzoni, E.; Marigo, I.; Carriero, M.V.; et al. Emerging Treatments Targeting the Tumor Microenvironment for Advanced Chondrosarcoma. Cells 2024, 13, 977. https://doi.org/10.3390/cells13110977
Ingangi V, De Chiara A, Ferrara G, Gallo M, Catapano A, Fazioli F, Di Carluccio G, Peranzoni E, Marigo I, Carriero MV, et al. Emerging Treatments Targeting the Tumor Microenvironment for Advanced Chondrosarcoma. Cells. 2024; 13(11):977. https://doi.org/10.3390/cells13110977
Chicago/Turabian StyleIngangi, Vincenzo, Annarosaria De Chiara, Gerardo Ferrara, Michele Gallo, Antonio Catapano, Flavio Fazioli, Gioconda Di Carluccio, Elisa Peranzoni, Ilaria Marigo, Maria Vincenza Carriero, and et al. 2024. "Emerging Treatments Targeting the Tumor Microenvironment for Advanced Chondrosarcoma" Cells 13, no. 11: 977. https://doi.org/10.3390/cells13110977
APA StyleIngangi, V., De Chiara, A., Ferrara, G., Gallo, M., Catapano, A., Fazioli, F., Di Carluccio, G., Peranzoni, E., Marigo, I., Carriero, M. V., & Minopoli, M. (2024). Emerging Treatments Targeting the Tumor Microenvironment for Advanced Chondrosarcoma. Cells, 13(11), 977. https://doi.org/10.3390/cells13110977







