Increasing GSH-Px Activity and Activating Wnt Pathway Promote Fine Wool Growth in FGF5-Edited Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. RNA Extraction and Quantitative RT-PCR Analysis
2.4. ELISA and Western Blot
2.5. Wool Phenotype Analysis
2.6. Histology Staining of Skin Tissues
2.7. Measurement of Skin Antioxidant Enzyme Activity
2.8. Isolation and Analysis of DPCs
2.9. DPCs Proliferation
2.10. Statistics
3. Results
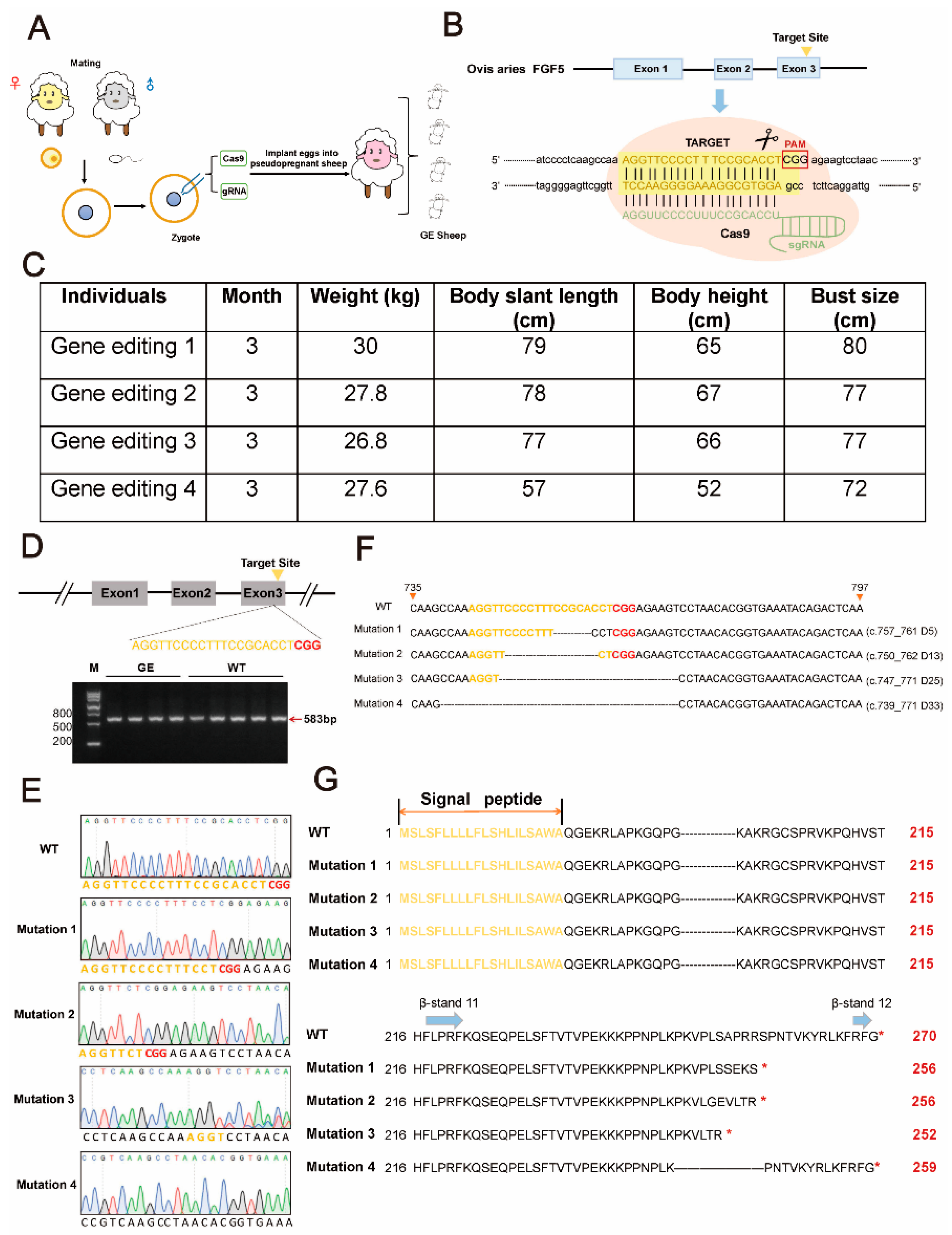
3.1. Generation of FGF5 Gene-Edited Sheep
3.2. The Expression of FGF5 mRNA and Protein Was Decreased in GE Sheep
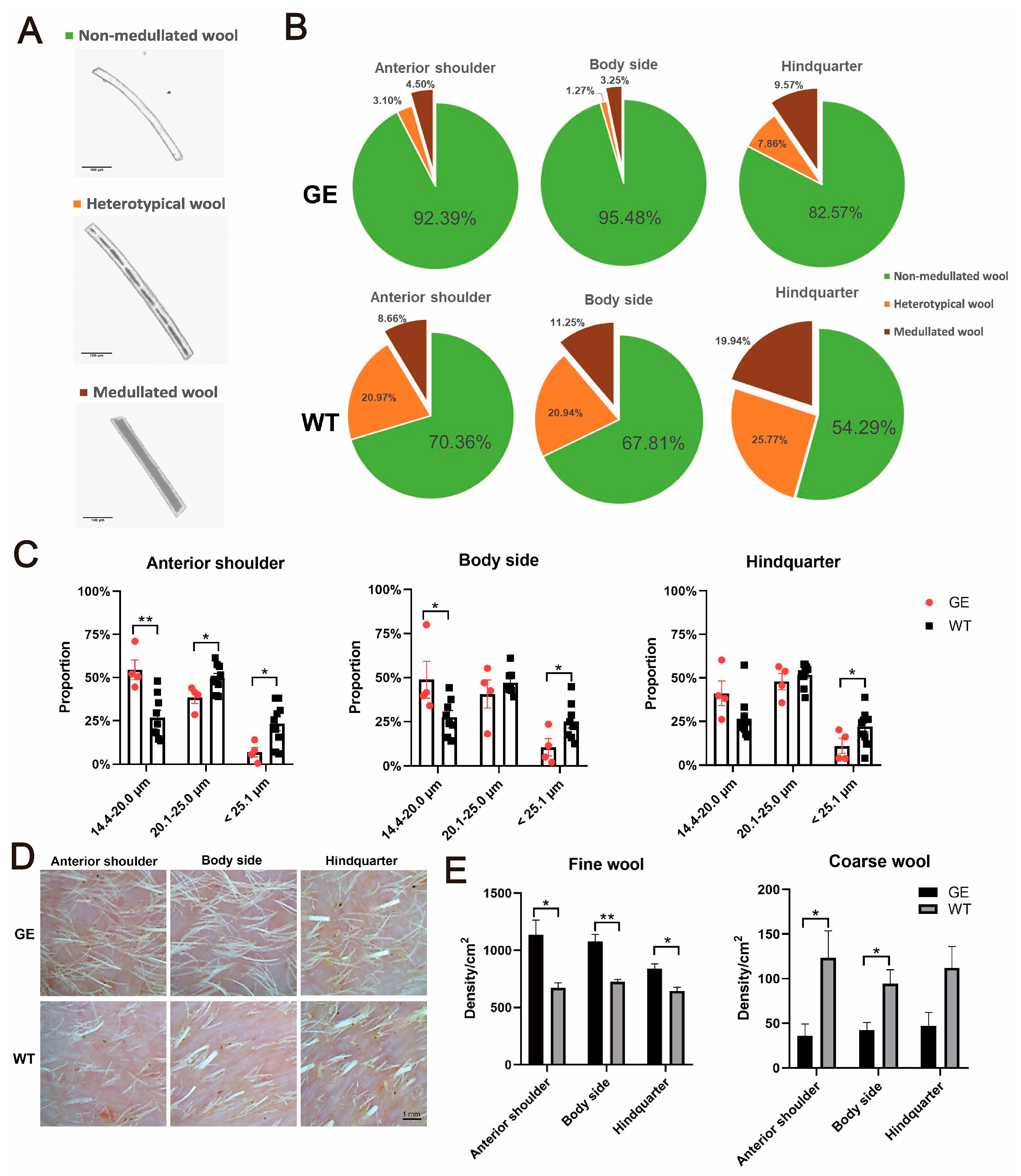
3.3. Effect of FGF5 Editing on Wool Traits in Sheep
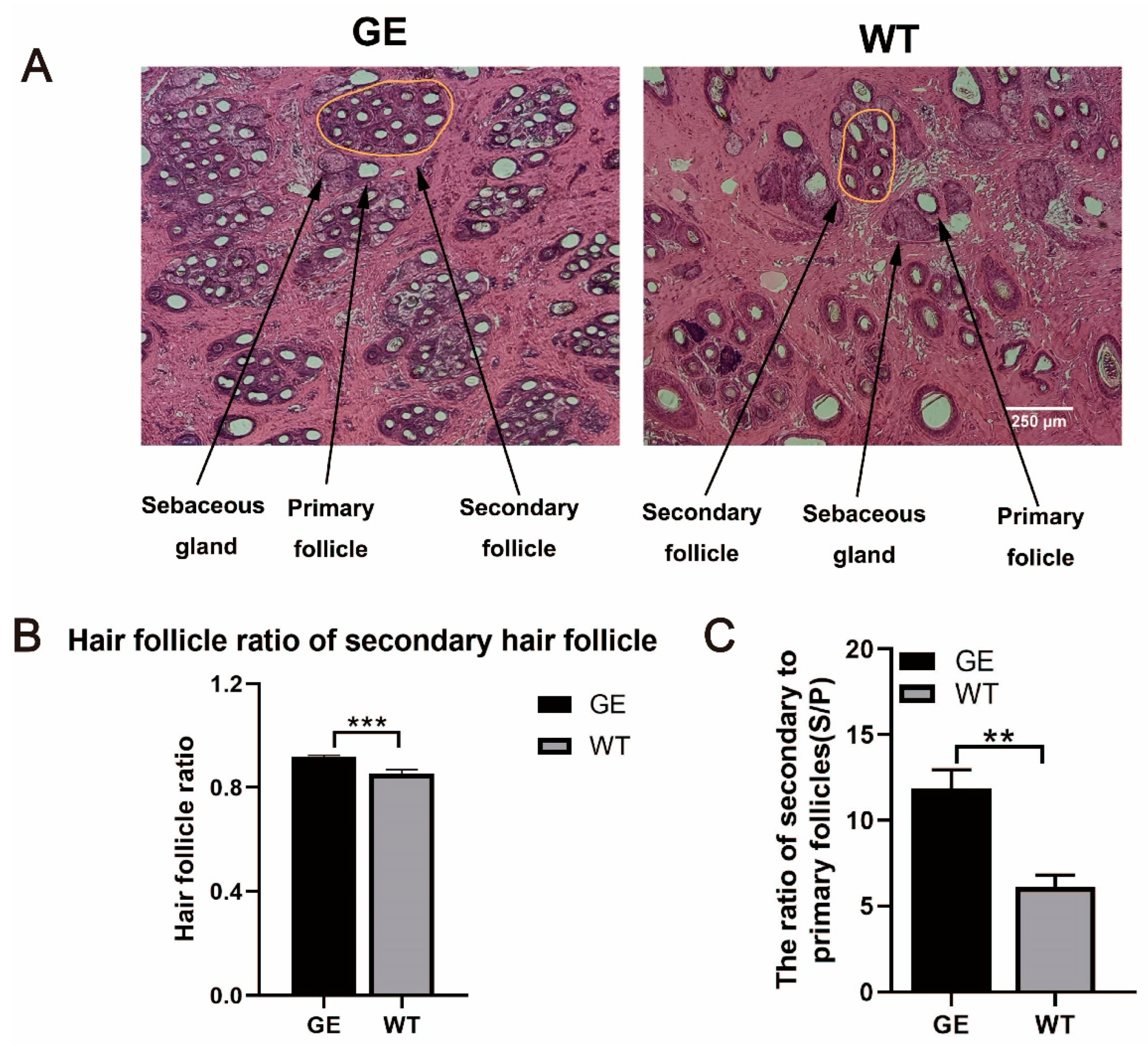
3.4. Effect of FGF5 Editing on Hair Follicle Development in Sheep
3.5. FGF5 Editing Increases Anti-Apoptotic Signaling in the Skin
3.6. FGF5 Editing Reduces Cortisol Concentration in the Sheep Skin
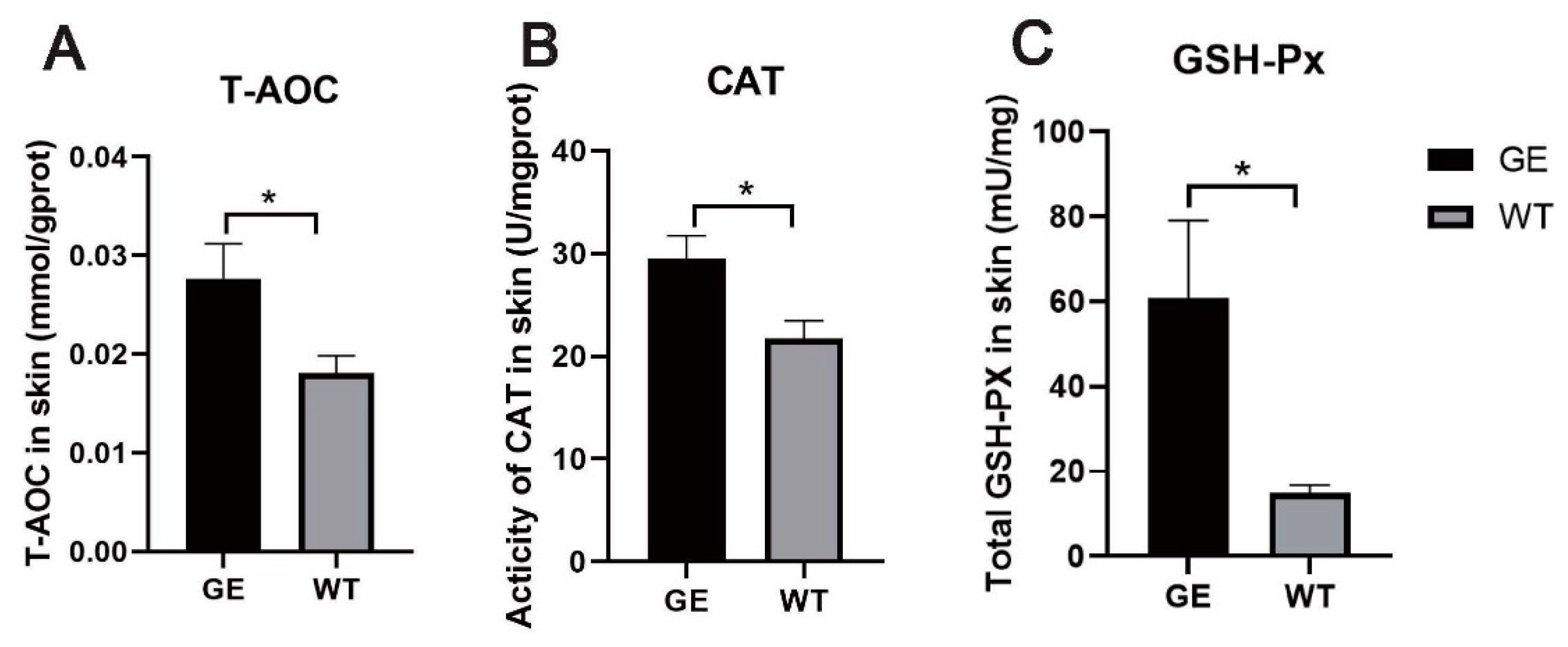
3.7. FGF5 Editing Increases the Activity of Antioxidant Enzymes
3.8. The Rspondin Protein Family Is Activated by FGF5 Editing
3.9. FGF5 Editing Significantly Promotes Dermal Papilla Cell Proliferation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. CB 2009, 19, R132–R142. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.W.; Kloepper, J.; Langan, E.A.; Kim, Y.; Yeo, J.; Kim, M.J.; Hsi, T.C.; Rose, C.; Yoon, G.S.; Lee, S.J.; et al. A Guide to Studying Human Hair Follicle Cycling In Vivo. J. Investig. Dermatol. 2016, 136, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Belov, A.A.; Mohammadi, M. Molecular mechanisms of fibroblast growth factor signaling in physiology and pathology. Cold Spring Harb. Perspect. Biol. 2013, 5, a015958. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Ota, Y.; Ozawa, K.; Imamura, T. Dual-mode regulation of hair growth cycle by two Fgf-5 gene products. J. Investig. Dermatol. 2000, 114, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Hébert, J.M.; Rosenquist, T.; Götz, J.; Martin, G.R. FGF5 as a regulator of the hair growth cycle: Evidence from targeted and spontaneous mutations. Cell 1994, 78, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Haub, O.; Goldfarb, M. Expression of the fibroblast growth factor-5 gene in the mouse embryo. Development 1991, 112, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, J.P.; Rourk, M.H.; Boggess, D.; Hogan, M.E.; Sundberg, B.A.; Bertolino, A.P. Angora mouse mutation: Altered hair cycle, follicular dystrophy, phenotypic maintenance of skin grafts, and changes in keratin expression. Vet. Pathol. 1997, 34, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Drögemüller, C.; Rüfenacht, S.; Wichert, B.; Leeb, T. Mutations within the FGF5 gene are associated with hair length in cats. Anim. Genet. 2007, 38, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Cadieu, E.; Neff, M.W.; Quignon, P.; Walsh, K.; Chase, K.; Parker, H.G.; Vonholdt, B.M.; Rhue, A.; Boyko, A.; Byers, A.; et al. Coat variation in the domestic dog is governed by variants in three genes. Science 2009, 326, 150–153. [Google Scholar] [CrossRef]
- Legrand, R.; Tiret, L.; Abitbol, M. Two recessive mutations in FGF5 are associated with the long-hair phenotype in donkeys. Genet. Sel. Evol. GSE 2014, 46, 65. [Google Scholar] [CrossRef]
- Higgins, C.A.; Petukhova, L.; Harel, S.; Ho, Y.Y.; Drill, E.; Shapiro, L.; Wajid, M.; Christiano, A.M. FGF5 is a crucial regulator of hair length in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 10648–10653. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, S.; Li, C.; Cai, B.; Yu, H.; Ma, B.; Huang, Y.; Ding, Y.; Liu, Y.; Ding, Q.; et al. Base pair editing in goat: Nonsense codon introgression into FGF5 results in longer hair. FEBS J. 2019, 286, 4675–4692. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Cidlowski, J.A. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Cascallana, J.L.; Bravo, A.; Donet, E.; Leis, H.; Lara, M.F.; Paramio, J.M.; Jorcano, J.L.; Pérez, P. Ectoderm-targeted overexpression of the glucocorticoid receptor induces hypohidrotic ectodermal dysplasia. Endocrinology 2005, 146, 2629–2638. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Handjiski, B.; Czarnetzki, B.M.; Eichmüller, S. A murine model for inducing and manipulating hair follicle regression (catagen): Effects of dexamethasone and cyclosporin A. J. Investig. Dermatol. 1994, 103, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, Y.; Jia, K.; Xu, X.; Li, Y.; Zhao, Y.; Zhang, X.; Zhang, J.; Liu, G.; Deng, S.; et al. Crosstalk between androgen and Wnt/β-catenin leads to changes of wool density in FGF5-knockout sheep. Cell Death Dis. 2020, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Fan, Z.Y.; Wang, B.Y.; Deng, S.L.; Zhang, X.S.; Zhang, J.L.; Han, H.B.; Lian, Z.X. RAPID COMMUNICATION: Generation of FGF5 knockout sheep via the CRISPR/Cas9 system. J. Anim. Sci. 2017, 95, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- Ota, Y.; Saitoh, Y.; Suzuki, S.; Ozawa, K.; Kawano, M.; Imamura, T. Fibroblast growth factor 5 inhibits hair growth by blocking dermal papilla cell activation. Biochem. Biophys. Res. Commun. 2002, 290, 169–176. [Google Scholar] [CrossRef]
- Harshuk-Shabso, S.; Dressler, H.; Niehrs, C.; Aamar, E.; Enshell-Seijffers, D. Fgf and Wnt signaling interaction in the mesenchymal niche regulates the murine hair cycle clock. Nat. Commun. 2020, 11, 5114. [Google Scholar] [CrossRef]
- Hu, X.; Hao, F.; Li, X.; Xun, Z.; Gao, Y.; Ren, B.; Cang, M.; Liang, H.; Liu, D. Generation of VEGF knock-in Cashmere goat via the CRISPR/Cas9 system. Int. J. Biol. Sci. 2021, 17, 1026–1040. [Google Scholar] [CrossRef]
- Wang, X.; Cai, B.; Zhou, J.; Zhu, H.; Niu, Y.; Ma, B.; Yu, H.; Lei, A.; Yan, H.; Shen, Q.; et al. Disruption of FGF5 in Cashmere Goats Using CRISPR/Cas9 Results in More Secondary Hair Follicles and Longer Fibers. PLoS ONE 2016, 11, e0164640. [Google Scholar] [CrossRef] [PubMed]
- Rosenquist, T.A.; Martin, G.R. Fibroblast growth factor signalling in the hair growth cycle: Expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1996, 205, 379–386. [Google Scholar] [CrossRef]
- Stenn, K.S.; Paus, R. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef]
- Myung, P.S.; Takeo, M.; Ito, M.; Atit, R.P. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J. Investig. Dermatol. 2013, 133, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.J.; Müller-Röver, S.; Patel, S.V.; Chronnell, C.M.; McKay, I.A.; Philpott, M.P. Contrasting localization of c-Myc with other Myc superfamily transcription factors in the human hair follicle and during the hair growth cycle. J. Investig. Dermatol. 2001, 116, 617–622. [Google Scholar] [CrossRef]
- Rahmani, W.; Abbasi, S.; Hagner, A.; Raharjo, E.; Kumar, R.; Hotta, A.; Magness, S.; Metzger, D.; Biernaskie, J. Hair follicle dermal stem cells regenerate the dermal sheath, repopulate the dermal papilla, and modulate hair type. Dev. Cell 2014, 31, 543–558. [Google Scholar] [CrossRef]
- Kulessa, H.; Turk, G.; Hogan, B.L. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J. 2000, 19, 6664–6674. [Google Scholar] [CrossRef] [PubMed]
- Ito, T. Hair follicle is a target of stress hormone and autoimmune reactions. J. Dermatol. Sci. 2010, 60, 67–73. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Qi, Q.; Lu, L.; Gan, W.; Loos, R.J.; Lin, X. Common variants in or near FGF5, CYP17A1 and MTHFR genes are associated with blood pressure and hypertension in Chinese Hans. J. Hypertens. 2011, 29, 70–75. [Google Scholar] [CrossRef]
- Newton-Cheh, C.; Johnson, T.; Gateva, V.; Tobin, M.D.; Bochud, M.; Coin, L.; Najjar, S.S.; Zhao, J.H.; Heath, S.C.; Eyheramendy, S.; et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat. Genet. 2009, 41, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, F.; Isono, M.; Katsuya, T.; Yamamoto, K.; Yokota, M.; Sugiyama, T.; Nabika, T.; Fujioka, A.; Ohnaka, K.; Asano, H.; et al. Blood pressure and hypertension are associated with 7 loci in the Japanese population. Circulation 2010, 121, 2302–2309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, M.; Yu, W.; Weinberg, J.; Shapiro, J.; McElwee, K.J. Development of alopecia areata is associated with higher central and peripheral hypothalamic-pituitary-adrenal tone in the skin graft induced C3H/HeJ mouse model. J. Investig. Dermatol. 2009, 129, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Arck, P.C.; Handjiski, B.; Hagen, E.; Joachim, R.; Klapp, B.F.; Paus, R. Indications for a ‘brain-hair follicle axis (BHA)’: Inhibition of keratinocyte proliferation and up-regulation of keratinocyte apoptosis in telogen hair follicles by stress and substance P. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2001, 15, 2536–2538. [Google Scholar] [CrossRef] [PubMed]
- Demerjian, M.; Choi, E.H.; Man, M.Q.; Chang, S.; Elias, P.M.; Feingold, K.R. Activators of PPARs and LXR decrease the adverse effects of exogenous glucocorticoids on the epidermis. Exp. Dermatol. 2009, 18, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Budunova, I.V.; Kowalczyk, D.; Pérez, P.; Yao, Y.J.; Jorcano, J.L.; Slaga, T.J. Glucocorticoid receptor functions as a potent suppressor of mouse skin carcinogenesis. Oncogene 2003, 22, 3279–3287. [Google Scholar] [CrossRef]
- Choi, S.; Zhang, B.; Ma, S.; Gonzalez-Celeiro, M.; Stein, D.; Jin, X.; Kim, S.T.; Kang, Y.L.; Besnard, A.; Rezza, A.; et al. Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature 2021, 592, 428–432. [Google Scholar] [CrossRef]
- Zhen, Y.F.; Wang, G.D.; Zhu, L.Q.; Tan, S.P.; Zhang, F.Y.; Zhou, X.Z.; Wang, X.D. P53 dependent mitochondrial permeability transition pore opening is required for dexamethasone-induced death of osteoblasts. J. Cell. Physiol. 2014, 229, 1475–1483. [Google Scholar] [CrossRef]
- Chen, J.; Liang, J.Q.; Zhen, Y.F.; Chang, L.; Zhou, Z.T.; Shen, X.J. DCAF1-targeting microRNA-3175 activates Nrf2 signaling and inhibits dexamethasone-induced oxidative injury in human osteoblasts. Cell Death Dis. 2021, 12, 1024. [Google Scholar] [CrossRef]
- Yang, C.H.; Xu, J.H.; Ren, Q.C.; Duan, T.; Mo, F.; Zhang, W. Melatonin promotes secondary hair follicle development of early postnatal cashmere goat and improves cashmere quantity and quality by enhancing antioxidant capacity and suppressing apoptosis. J. Pineal Res. 2019, 67, e12569. [Google Scholar] [CrossRef]
- Haslam, I.S.; Jadkauskaite, L.; Szabó, I.L.; Staege, S.; Hesebeck-Brinckmann, J.; Jenkins, G.; Bhogal, R.K.; Lim, F.L.; Farjo, N.; Farjo, B.; et al. Oxidative Damage Control in a Human (Mini-) Organ: Nrf2 Activation Protects against Oxidative Stress-Induced Hair Growth Inhibition. J. Investig. Dermatol. 2017, 137, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Saceda-Corralo, D.; Pindado-Ortega, C.; Moreno-Arrones, O.M.; Ortega-Quijano, D.; Fernández-Nieto, D.; Jiménez-Cauhe, J.; Vañó-Galván, S. Association of Inflammation With Progression of Hair Loss in Women With Frontal Fibrosing Alopecia. JAMA Dermatol. 2020, 156, 700–702. [Google Scholar] [CrossRef] [PubMed]
- Naito, A.; Midorikawa, T.; Yoshino, T.; Ohdera, M. Lipid peroxides induce early onset of catagen phase in murine hair cycles. Int. J. Mol. Med. 2008, 22, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yang, Z.; Andl, T.; Cui, C.; Kim, N.; Millar, S.E.; Cotsarelis, G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 2007, 447, 316–320. [Google Scholar] [CrossRef] [PubMed]
- DasGupta, R.; Fuchs, E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 1999, 126, 4557–4568. [Google Scholar] [CrossRef] [PubMed]
- Hawkshaw, N.J.; Hardman, J.A.; Alam, M.; Jimenez, F.; Paus, R. Deciphering the molecular morphology of the human hair cycle: Wnt signalling during the telogen-anagen transformation. Br. J. Dermatol. 2020, 182, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, J.; Burgeson, R.E.; Morgan, B.A. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000, 14, 1181–1185. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Yan, H.; Zeng, J.; Ma, S.; Niu, Y.; Zhou, G.; Jiang, Y.; Chen, Y. Comparative Transcriptome Analysis of Fetal Skin Reveals Key Genes Related to Hair Follicle Morphogenesis in Cashmere Goats. PLoS ONE 2016, 11, e0151118. [Google Scholar] [CrossRef]
- Hao, H.X.; Xie, Y.; Zhang, Y.; Charlat, O.; Oster, E.; Avello, M.; Lei, H.; Mickanin, C.; Liu, D.; Ruffner, H.; et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 2012, 485, 195–200. [Google Scholar] [CrossRef]
- Jiang, X.; Charlat, O.; Zamponi, R.; Yang, Y.; Cong, F. Dishevelled promotes Wnt receptor degradation through recruitment of ZNRF3/RNF43 E3 ubiquitin ligases. Mol. Cell 2015, 58, 522–533. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.-L.; Wu, S.-J.; Qi, S.-Y.; Chen, M.-M.; Liu, Z.-M.; Zhang, R.; Zhao, Y.; Liu, S.-Q.; Zhou, W.-D.; Zhang, J.-L.; et al. Increasing GSH-Px Activity and Activating Wnt Pathway Promote Fine Wool Growth in FGF5-Edited Sheep. Cells 2024, 13, 985. https://doi.org/10.3390/cells13110985
Xu X-L, Wu S-J, Qi S-Y, Chen M-M, Liu Z-M, Zhang R, Zhao Y, Liu S-Q, Zhou W-D, Zhang J-L, et al. Increasing GSH-Px Activity and Activating Wnt Pathway Promote Fine Wool Growth in FGF5-Edited Sheep. Cells. 2024; 13(11):985. https://doi.org/10.3390/cells13110985
Chicago/Turabian StyleXu, Xue-Ling, Su-Jun Wu, Shi-Yu Qi, Ming-Ming Chen, Zhi-Mei Liu, Rui Zhang, Yue Zhao, Shun-Qi Liu, Wen-Di Zhou, Jin-Long Zhang, and et al. 2024. "Increasing GSH-Px Activity and Activating Wnt Pathway Promote Fine Wool Growth in FGF5-Edited Sheep" Cells 13, no. 11: 985. https://doi.org/10.3390/cells13110985
APA StyleXu, X.-L., Wu, S.-J., Qi, S.-Y., Chen, M.-M., Liu, Z.-M., Zhang, R., Zhao, Y., Liu, S.-Q., Zhou, W.-D., Zhang, J.-L., Zhang, X.-S., Deng, S.-L., Yu, K., Li, Y., & Lian, Z.-X. (2024). Increasing GSH-Px Activity and Activating Wnt Pathway Promote Fine Wool Growth in FGF5-Edited Sheep. Cells, 13(11), 985. https://doi.org/10.3390/cells13110985







