The Expression of a Subset of Aging and Antiaging Markers Following the Chondrogenic and Osteogenic Differentiation of Mesenchymal Stem Cells of Placental Origin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Culture MSCs
2.2. Flow Cytometry
2.3. Colony-Forming Unit (CFU) Assay
2.4. Osteogenic, Chondrogenic, and Adipogenic Induction
2.5. RT-qPCR
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic Differentiation of Mesenchymal Stem Cells: Challenges and Unfulfilled Expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, C.; Choi, Y.S.; Kim, M.; Park, C.; Suh, Y. Age-related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: Implication to age-associated bone diseases and defects. Mech. Ageing Dev. 2012, 133, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Wong, X.; Melendez-Perez, A.J.; Reddy, K.L. The Nuclear Lamina. Cold Spring Harb. Perspect. Biol. 2022, 14, a040113. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, D.; Gray, H.L.; Sammak, P.J.; Schatten, G.P.; Csoka, A.B. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells. Stem Cells 2006, 24, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.H.; Kang Sm Lee, S.J.; Woo, T.G.; Oh, A.Y.; Park, S.; Ha, N.-C.; Park, B.-J. p53 induces senescence through Lamin A/C stabilization-mediated nuclear deformation. Cell Death Dis. 2019, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Malashicheva, A.; Perepelina, K. Diversity of Nuclear Lamin A/C Action as a Key to Tissue-Specific Regulation of Cellular Identity in Health and Disease. Front. Cell Dev. Biol. 2021, 9, 761469. [Google Scholar] [CrossRef] [PubMed]
- Malashicheva, A.; Bogdanova, M.; Zabirnyk, A.; Smolina, N.; Ignatieva, E.; Freilikhman, O.; Fedorov, A.; Dmitrieva, R.; Sjöberg, G.; Sejersen, T.; et al. Various lamin A/C mutations alter expression profile of mesenchymal stem cells in mutation specific manner. Mol. Genet. Metab. 2015, 115, 118–127. [Google Scholar] [CrossRef]
- Sehgal, P.; Chaturvedi, P.; Kumaran, R.I.; Kumar, S.; Parnaik, V.K. Lamin A/C Haploinsufficiency Modulates the Differentiation Potential of Mouse Embryonic Stem Cells. PLoS ONE 2013, 8, e57891. [Google Scholar] [CrossRef]
- Naito, M.; Omoteyama, K.; Mikami, Y.; Takagi, M.; Takahashi, T. Suppression of lamin A/C by short hairpin RNAs promotes adipocyte lineage commitment in mesenchymal progenitor cell line, ROB-C26. Histochem. Cell Biol. 2012, 137, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, Y.; Keyimu, R.; Hao, J.; Zhao, Z.; Ye, R. The role of lamin A/C in mesenchymal stem cell differentiation. J. Physiol. Biochem. 2019, 75, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Busch, F.; Shayan, P.; Shakibaei, M. Sirtuin-1 (SIRT1) Is Required for Promoting Chondrogenic Differentiation of Mesenchymal Stem Cells. J. Biol. Chem. 2014, 289, 22048–22062. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Li, N.T.; Cheng, H.S.; Yen, M.L. Oxidative stress induces imbalance of adipogenic/osteoblastic lineage commitment in mesenchymal stem cells through decreasing SIRT1 functions. J. Cell. Mol. Med. 2018, 22, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Denu, R.A. SIRT3 Enhances Mesenchymal Stem Cell Longevity and Differentiation. Oxidative Med. Cell. Longev. 2017, 2017, 5841716. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A. SIRT7 in the aging process. Cell. Mol. Life Sci. 2022, 79, 297. [Google Scholar] [CrossRef] [PubMed]
- Raza, U.; Tang, X.; Liu, Z.; Liu, B. SIRT7: The seventh key to unlocking the mystery of aging. Physiol. Rev. 2023, 104, 253–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.E.M.; Zhang, W.; Ye, C.C.Y.; Gao, X.; Jiang, L.L.J.; Zhao, T.T.F.; Pan, Z.Z.J.; Xue, D.D.T. Knockdown of SIRT7 enhances the osteogenic differentiation of human bone marrow mesenchymal stem cells partly via activation of the Wnt/b-catenin signaling pathway. Cell Death Dis. 2017, 8, e3042. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.E.; Rai, R.; Khan, S.S.; Eren, M.; Ghosh, A.K. Plasminogen Activator Inhibitor-1 Is a Marker and a Mediator of Senescence. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1446–1452. [Google Scholar] [CrossRef]
- Takafuji, Y.; Tatsumi, K.; Ishida, M.; Kawao, N.; Okada, K.; Matsuo, O.; Kaji, H. Plasminogen activator inhibitor-1 deficiency suppresses osteoblastic differentiation of mesenchymal stem cells in mice. J. Cell. Physiol. 2019, 234, 9687–9697. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Incalzi, R.A. Plasminogen Activator Inhibitor-1 (PAI-1): A Key Factor Linking Fibrinolysis and Age-Related Subclinical and Clinical Conditions. Cardiovasc. Ther. 2010, 28, e72–e91. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Takeshita, K.; Shimokawa, T.; Yi, H.; Isobe, K.I.; Loskutoff, D.J.; Saito, H. Plasminogen activator inhibitor-1 is a major stress-regulated gene: Implications for stress-induced thrombosis in aged individuals. Proc. Natl. Acad. Sci. USA 2002, 99, 890–895. [Google Scholar] [CrossRef]
- Copland, I.B.; Lord-Dufour, S.; Cuerquis, J.; Coutu, D.L.; Annabi, B.; Wang, E.; Galipeau, J. Improved autograft survival of mesenchymal stromal cells by plasminogen activator inhibitor 1 inhibition. Stem Cells 2009, 27, 467–477. [Google Scholar] [CrossRef]
- Miao, S.B.; Xie, X.L.; Yin, Y.J.; Zhao, L.L.; Zhang, F.; Shu, Y.-N.; Chen, R.; Chen, P.; Dong, L.-H.; Lin, Y.-L.; et al. Accumulation of Smooth Muscle 22a Protein Accelerates Senescence of Vascular Smooth Muscle Cells via Stabilization of p53 In Vitro and In Vivo. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1849–1859. [Google Scholar] [CrossRef]
- Kim, T.R.; Lee, H.M.; Lee, S.Y.; Kim, E.J.; Kim, K.C.; Paik, S.G.; Cho, E.W.; Kim, I.G. SM22a-induced activation of p16INK4a/retinoblastoma pathway promotes cellular senescence caused by a subclinical dose of g-radiation and doxorubicin in HepG2 cells. Biochem. Biophys. Res. Commun. 2010, 400, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Abomaray, F.M.; Al Jumah, M.A.; Alsaad, K.O.; Jawdat, D.; Al Khaldi, A.; AlAskar, A.; Al Harthy, S.; Al Subayyil, A.; Khatlani, T.; Alawad, A.; et al. Phenotypic and Functional Characterization of Mesenchymal Stem/Multipotent Stromal Cells from Decidua Basalis of Human Term Placenta. Stem Cells Int. 2016, 2016, 5184601. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ventura Ferreira, M.S.; Bienert, M.; Müller, K.; Rath, B.; Goecke, T.; Opländer, C.; Braunschweig, T.; Mela, P.; Brümmendorf, T.H.; Beier, F.; et al. Comprehensive characterization of chorionic villi-derived mesenchymal stromal cells from human placenta. Stem Cell Res. Ther. 2018, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Barlow, S.; Brooke, G.; Chatterjee, K.; Price, G.; Pelekanos, R.; Rossetti, T.; Doody, M.; Venter, D.; Pain, S.; Gilshenan, K.; et al. Comparison of Human Placenta- and Bone Marrow-Derived Multipotent Mesenchymal Stem Cells. Stem Cells Dev. 2008, 17, 1095–1108. [Google Scholar] [CrossRef]
- Brooke, G.; Tong, H.; Levesque, J.P.; Atkinson, K. Molecular Trafficking Mechanisms of Multipotent Mesenchymal Stem Cells Derived from Human Bone Marrow and Placenta. Stem Cells Dev. 2008, 17, 929–940. [Google Scholar] [CrossRef]
- Mariotti, E.; Mirabelli, P.; Abate, G.; Schiattarella, M.; Martinelli, P.; Fortunato, G.; Di Noto, R.; Del Vecchio, L. Comparative Characteristics of Mesenchymal Stem Cells from Human Bone Marrow and Placenta: CD10, CD49d, and CD56 Make a Difference. Stem Cells Dev. 2008, 17, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, F.; Song, L.; Shu, Y.; Lin, Y.; Dong, L.; Nie, X.; Zhang, D.; Chen, P.; Han, M. Transcriptome profiling reveals that the SM22a-regulated molecular pathways contribute to vascular pathology. J. Mol. Cell. Cardiol. 2014, 72, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yang, M.; Jiang, H.; Ju, D.; Zheng, J.P.; Xu, Z.; Liao, T.-D.; Li, L. Arterial injury promotes medial chondrogenesis in Sm22 knockout mice. Cardiovasc. Res. 2011, 90, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Yang, L.; Chang, H.; Dai, G.; Wang, F.; Duan, X.; Guo, L.; Zhang, Y.; Chen, G. Wnt/beta-catenin signaling regulates the proliferation and differentiation of mesenchymal progenitor cells through the p53 pathway. PLoS ONE 2014, 9, e97283. [Google Scholar]
- Hashimoto, S.; Nishiyama, T.; Hayashi, S.; Fujishiro, T.; Takebe, K.; Kanzaki, N.; Kuroda, R.; Kurosaka, M. Role of p53 in human chondrocyte apoptosis in response to shear strain. Arthritis Rheum 2009, 60, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Tan, X.; Xie, Z.; Yu, J.; Li, P.; Lin, X.; Ouyang, S.; Liu, Z.; Hou, Q.; Xie, N.; et al. p53: A Key Target in the Development of Osteoarthritis. Mol. Biotechnol. 2024, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Diekman, B.O.; Sessions, G.A.; Collins, J.A.; Knecht, A.K.; Strum, S.L.; Mitin, N.K.; Carlson, C.S.; Loeser, R.F.; Sharpless, N.E. Expression of p16INK4a is a biomarker of chondrocyte aging but does not cause osteoarthritis. Aging Cell 2018, 17, e12771. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; de Castro, L.F.; Shin, M.H.; Dubois, W.; Yang, H.H.; Jiang, S.; Mishra, P.J.; Ren, L.; Gou, H.; Lal, A.; et al. p53 Loss Increases the Osteogenic Differentiation of Bone Marrow Stromal Cells. Stem Cells 2015, 33, 1304–1319. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kua, H.-Y.; Hu, Y.; Guo, K.; Zeng, Q.; Wu, Q.; Ng, H.-H.; Karsenty, G.; de Crombrugghe, B.; Yeh, J.; et al. p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J. Cell Biol. 2005, 172, 115–125. [Google Scholar] [CrossRef]
- González-Cruz, R.D.; Dahl, K.N.; Darling, E.M. The Emerging Role of Lamin C as an Important LMNA Isoform in Mechanophenotype. Front. Cell Dev. Biol. 2018, 6, 151. [Google Scholar] [CrossRef]
- Al-Saaidi, R.; Bross, P. Do lamin A and lamin C have unique roles? Chromosoma 2015, 124, 1–12. [Google Scholar] [CrossRef]
- González-Cruz, R.D.; Sadick, J.S.; Fonseca, V.C.; Darling, E.M. Nuclear Lamin Protein C Is Linked to Lineage-Specific, Whole-Cell Mechanical Properties. Cell. Mol. Bioeng. 2018, 11, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Cenni, V.; Capanni, C.; Mattioli, E.; Schena, E.; Squarzoni, S.; Bacalini, M.G.; Garagnani, P.; Salvioli, S.; Franceschi, C.; Lattanzi, G. Lamin A involvement in ageing processes. Ageing Res. Rev. 2020, 62, 101073. [Google Scholar] [CrossRef]
- Akter, R.; Rivas, D.; Geneau, G.; Drissi, H.; Duque, G. Effect of Lamin A/C Knockdown on Osteoblast Differentiation and Function. J. Bone Miner. Res. 2009, 24, 283–293. [Google Scholar] [CrossRef]
- Khan, H.; Mafi, P.; Mafi, R.; Khan, W. The Effects of Ageing on Differentiation and Characterisation of Human Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2018, 13, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Infante, A.; Rodríguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Law, S.F.; Chandra, A. Bone Aging, Cellular Senescence, and Osteoporosis. JBMR Plus 2021, 5, e10488. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, J.C.; Yi, N.; Roy, M.; Hendrickson, D.G.; Kelley, D.R. Differentiation reveals latent features of aging and an energy barrier in murine myogenesis. Cell Rep. 2021, 35, 109046. [Google Scholar] [CrossRef]
- Remark, L.H.; Leclerc, K.; Ramsukh, M.; Lin, Z.; Lee, S.; Dharmalingam, B.; Gillinov, L.; Nayak, V.V.; El Parente, P.; Sambon, M.; et al. Loss of Notch signaling in skeletal stem cells enhances bone formation with aging. Bone Res. 2023, 11, 50. [Google Scholar] [CrossRef]
- Kim, H.N.; Ponte, F.; Warren, A.; Ring, R.; Iyer, S.; Han, L.; Almeida, M. A decrease in NAD+ contributes to the loss of osteoprogenitors and bone mass with aging. NPJ Aging Mech. Dis. 2021, 7, 8. [Google Scholar] [CrossRef]
- Moerman, E.J.; Teng, K.; Lipschitz, D.A.; Lecka-Czernik, B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 2004, 3, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Bermeo, S.; Vidal, C.; Zhou, H.; Duque, G. Lamin A/C Acts as an Essential Factor in Mesenchymal Stem Cell Differentiation Through the Regulation of the Dynamics of the Wnt/β-Catenin Pathway. J. Cell. Biochem. 2015, 116, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Hadadeh, O.; Barruet, E.; Peiretti, F.; Verdier, M.; Bernot, D.; Hadjal, Y.; El Yazidi, C.; Robaglia-Schlupp, A.; De Paula, A.M.; Nègre, D.; et al. The plasminogen activation system modulates differently adipogenesis and myogenesis of embryonic stem cells. PLoS ONE 2012, 7, e49065. [Google Scholar] [CrossRef] [PubMed]


| Primer | Sense (5′→3′) | Antisense (5′→3′) | Probe (5′→3′) | Accession Number | Amplicon Length (bp) | Melting Temperature (°C) |
|---|---|---|---|---|---|---|
| SIRT7 | ACTGCTTCAGAAAGGGAGA | CACAGTTCTGAGACACCACA | ACTGCTTCAGAAAGGGAGA | NM_016538.2 | 128 | 92.5 |
| LMNA | TGACTGTGGTTGAGGACGAC | GACACTGGAGGCAGAAGAGC | CGCTGAGTACAACCT | NM_170707.3 | 221 | 98.5 |
| LMNC | GTGGAAGGCACAGAACACCT | GCGGCGGCTACCACTCAC | AGATGACCTGCTCCATCACC | NM_005572.3 | 178 | 94.0 |
| LMNAΔ10 | AACTCCACTGGGGAAGGCTCC | GCTCCTGAGCCGCTGGCAGA | AGTACAACCTGCGCTCGCGC | NM_170708.3 | 131 | 98.0 |
| p16INK4a | GGGGGCACCAGAGGCAGT | GGTTGTGGCGGGGGCAGTT | NM_000077.4 | 159 | 92 | |
| p53 (TP53) | CCGGCGCACAGAGGAAGAGA | TGGGGAGAGGAGCTGGTGTTGT | NM_000546.5 | 108 | 92.5 | |
| PAI-1 | GTGTTTCAGCAGGTGGCGC | CCGGAACAGCCTGAAGAAGTG | NM_001386460.1 | 300 | 94.0 | |
| SM22α | TGGCGTGATTCTGAGCAA | CTGCCAAGCTGCCCAAGG | NM_001001522.2 | 239 | 92.5 | |
| Ubiquitin C | ACTACAACATCCAGAAAGAGTCCA | CCAGTCAGGGTCTTCACGAAG | NM_021009.6 | 85 | 88.0 | |
| RPL13 | AACAAGTTGAAGTACCTGGCTTTC | TGGTTTTGTGGGGCAGCATA | NM_012423.4 | 130 | 95.3 | |
| Cyclophilin A | CCCACCGTGTTCTTCGACAT | TTTCTGCTGTCTTTGGGACCTT | NM_021130.5 | 94 | 92.0 | |
| GAPDH | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA | NM_002046.7 | 452 | 95.5 |
| Chondrogenic Lineage | Osteogenic Lineage | Adipogenic Lineage | |
|---|---|---|---|
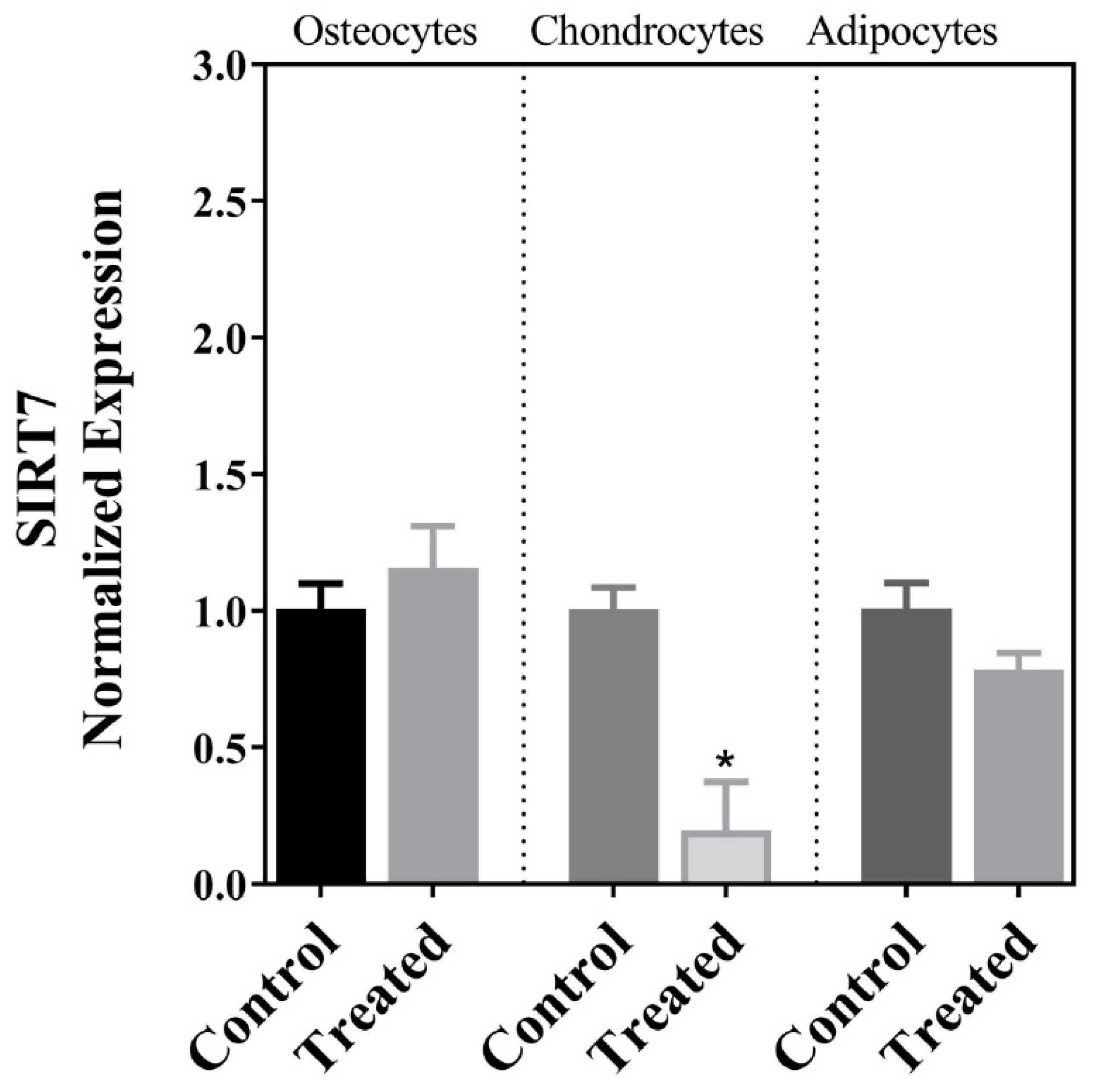
| SIRT7 | ↓ | ↔ | ↔ |
| PAI-1 | ↓ | ↔ | ↓ |
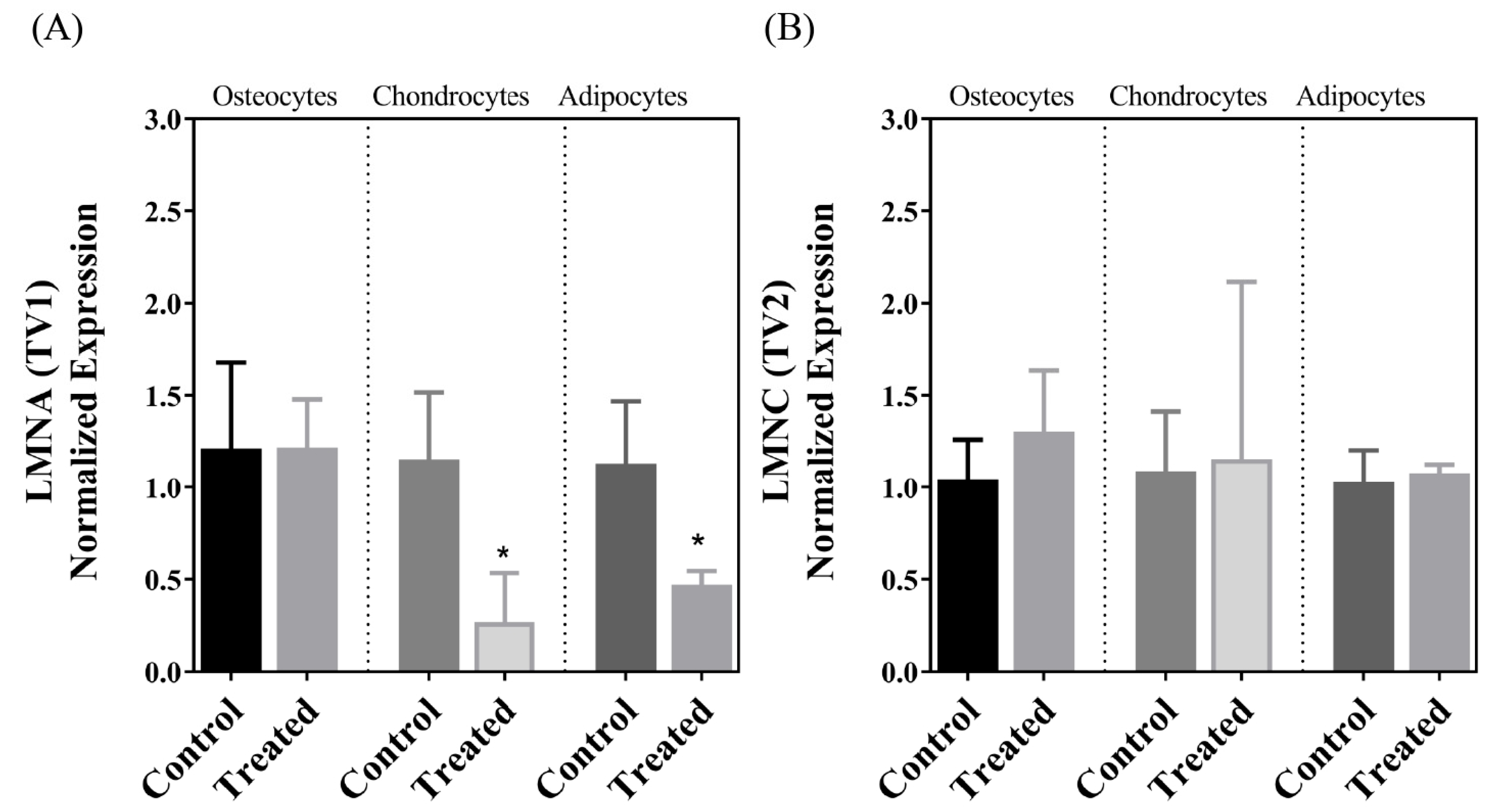
| LMNA | ↓ | ↔ | ↓ |
| LMNC | ↔ | ↔ | ↔ |
| LMNAΔ10 | ND | ND | ND |
| p16INK4a | ↑ | ↔ | ↔ |
| p53 (TP53) | ↑ | ↔ | ↔ |
| SM22α | ↓ | ↔ | ↔ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhra, M.; Magableh, A.M.; Samhan, L.M.; Fatani, L.M.; Qasem, R.J.; Aljada, A. The Expression of a Subset of Aging and Antiaging Markers Following the Chondrogenic and Osteogenic Differentiation of Mesenchymal Stem Cells of Placental Origin. Cells 2024, 13, 1022. https://doi.org/10.3390/cells13121022
Zhra M, Magableh AM, Samhan LM, Fatani LM, Qasem RJ, Aljada A. The Expression of a Subset of Aging and Antiaging Markers Following the Chondrogenic and Osteogenic Differentiation of Mesenchymal Stem Cells of Placental Origin. Cells. 2024; 13(12):1022. https://doi.org/10.3390/cells13121022
Chicago/Turabian StyleZhra, Mahmoud, Ahmad M. Magableh, Lara M. Samhan, Lein M. Fatani, Rani J. Qasem, and Ahmad Aljada. 2024. "The Expression of a Subset of Aging and Antiaging Markers Following the Chondrogenic and Osteogenic Differentiation of Mesenchymal Stem Cells of Placental Origin" Cells 13, no. 12: 1022. https://doi.org/10.3390/cells13121022






