In Vivo Imaging of Immune Rejection of MIN6 Cells Transplanted in C3H Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culturing MIN6 Cells
2.2. Subcutaneous Transplantation of Matrigel-Embedded MIN6 Cells in Nude and C3H Mice
2.3. In Vivo Imaging of Subcutaneous Matrigel-Embedded MIN6 Cells in Nude and C3H Mice
2.4. Histological Analysis of Matrigel-Embedded MIN6 Cell Grafts in Nude and C3H Mice
2.5. Statistical Analysis
3. Results
3.1. Evolution of Recipients’ Blood Glucose and Body Weight Measurements after Subcutaneous Transplantation of Matrigel-Embedded MIN6 Cells in Nude and C3H Mice
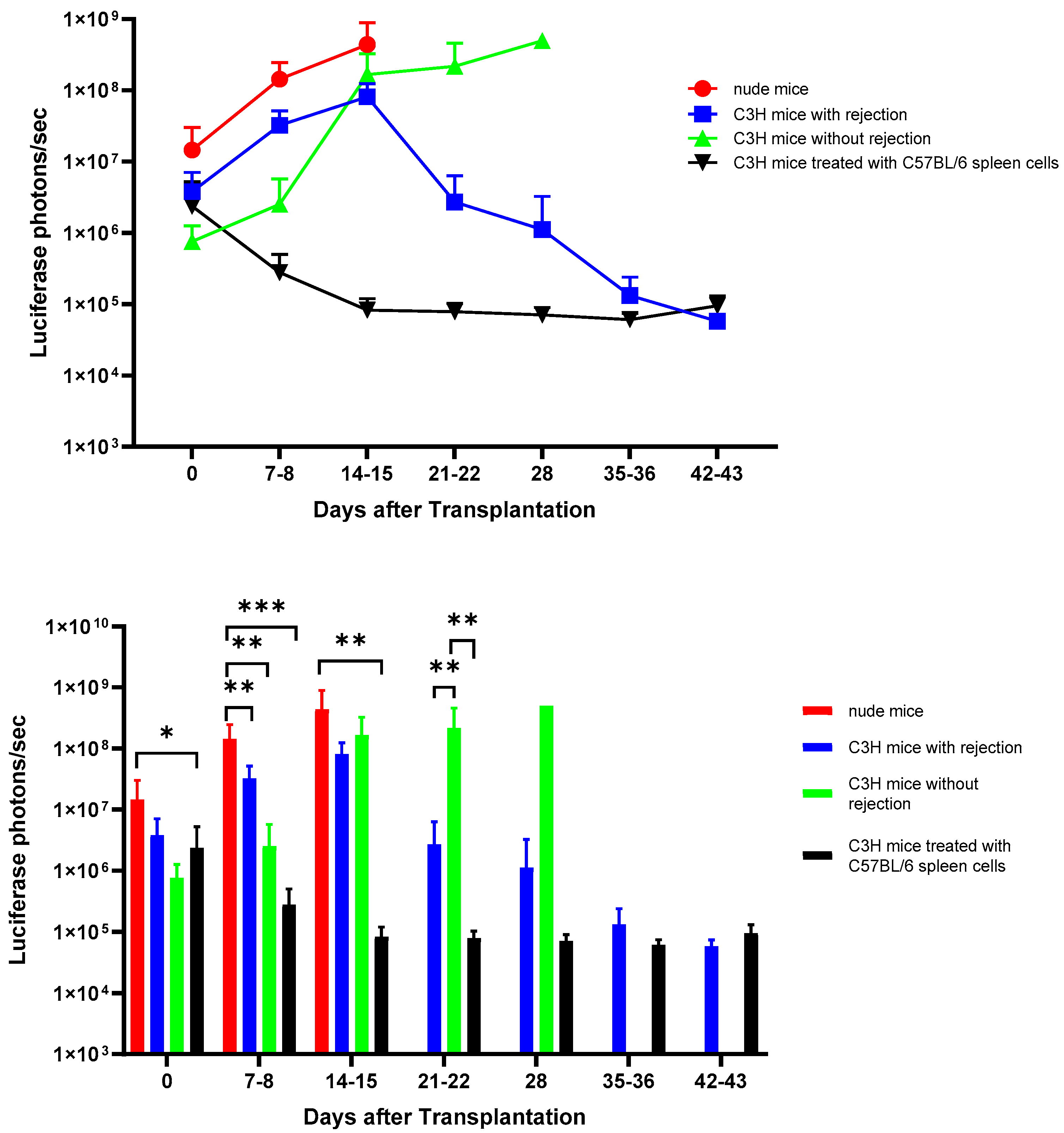
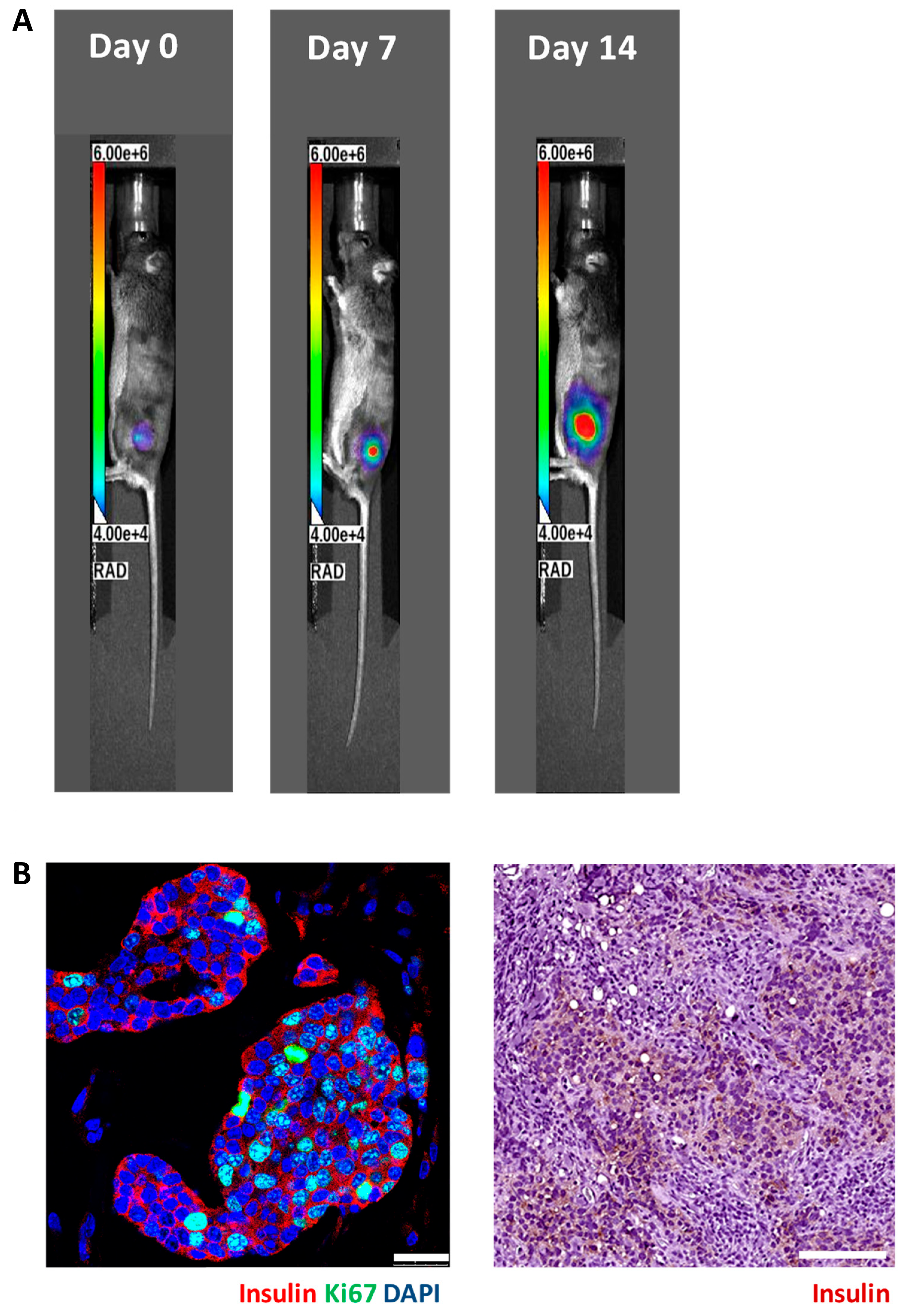
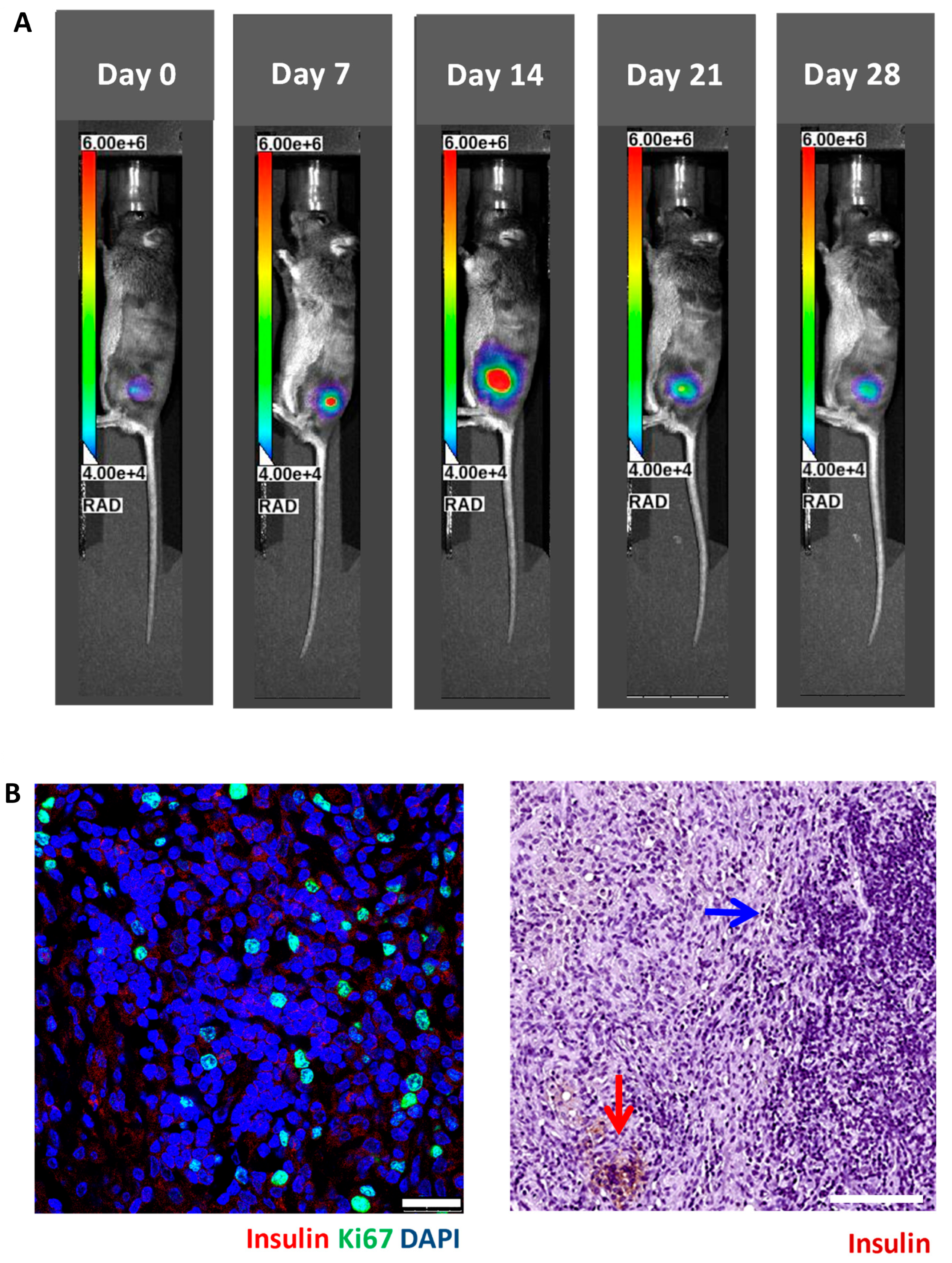
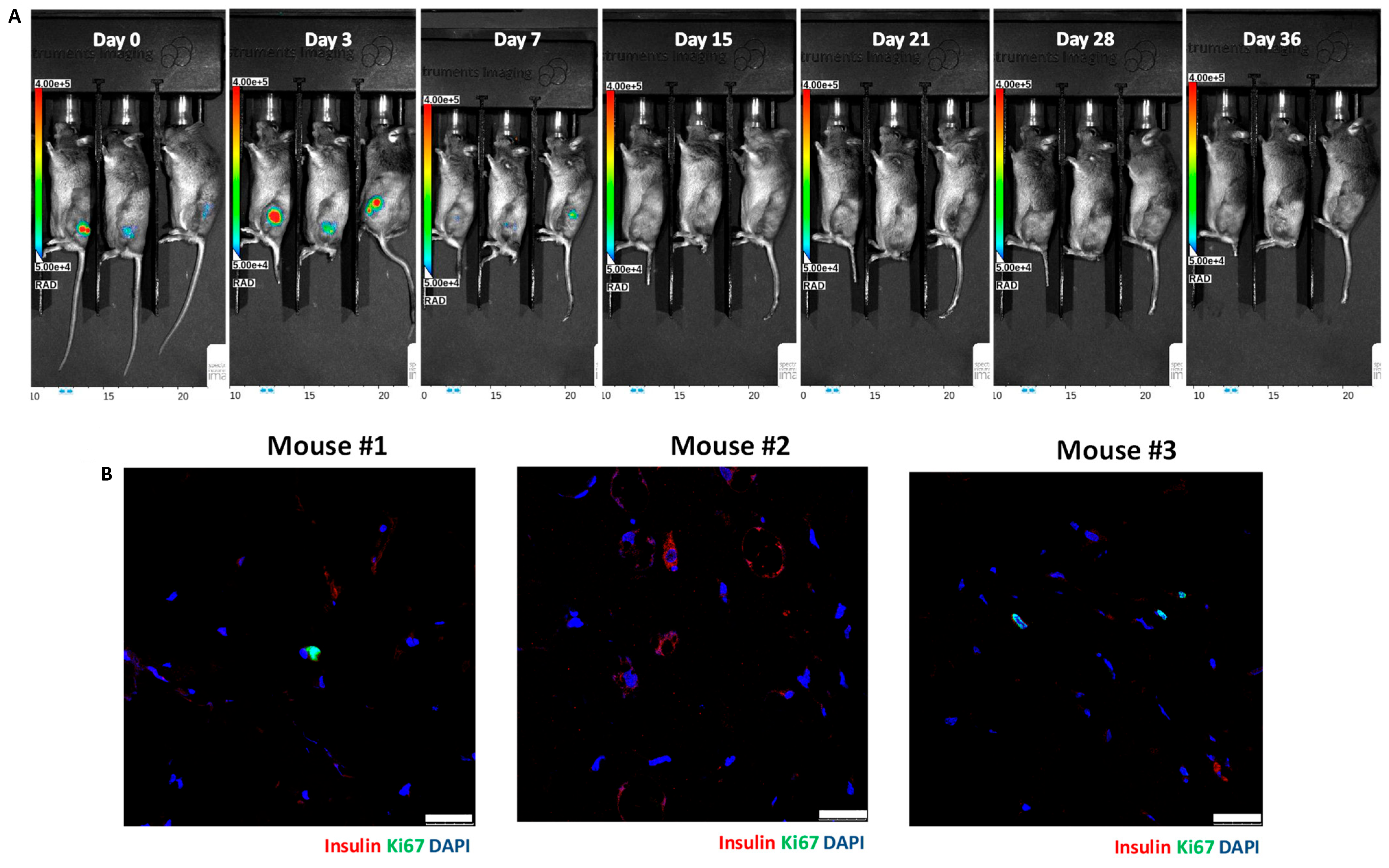
3.2. In Vivo Bioluminescence Imaging of Matrigel-Embedded MIN6 Cells Subcutaneously Transplanted in Nude and C3H Mice
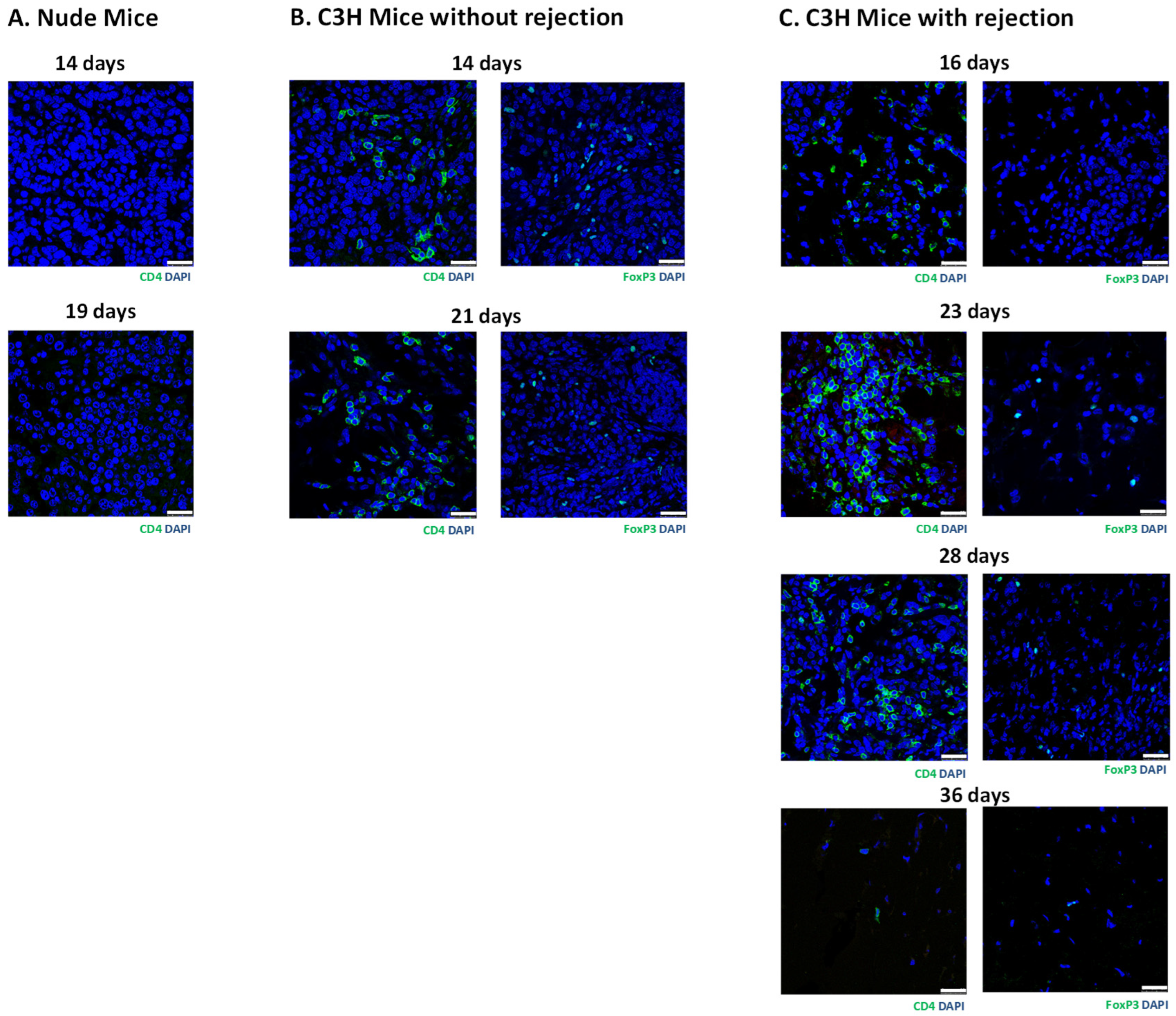
3.3. Histology of Subcutaneous Matrigel-Embedded MIN6 Cell Grafts in Nude and C3H Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisenbarth, G.S. Type I diabetes mellitus. A chronic autoimmune disease. N. Engl. J. Med. 1986, 314, 1360–1368. [Google Scholar]
- DeWitt, D.E.; Hirsch, I.B. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: Scientific review. JAMA 2003, 289, 2254–2264. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.D.; Dunn, T.B. Transplant strategies for type 1 diabetes: Whole pancreas, islet and porcine beta cell therapies. Diabetologia 2020, 63, 2049–2056. [Google Scholar] [CrossRef]
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef]
- Ryan, E.A.; Paty, B.W.; Senior, P.A.; Bigam, D.; Alfadhli, E.; Kneteman, N.M.; Lakey, J.R.; Shapiro, A.M. Five-year follow-up after clinical islet transplantation. Diabetes 2005, 54, 2060–2069. [Google Scholar] [CrossRef]
- Shapiro, A.M.J.; Ricordi, C.; Hering, B.J.; Auchincloss, H.; Lindblad, R.; Robertson, R.P.; Secchi, A.; Brendel, M.D.; Berney, T.; Brennan, D.C.; et al. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 2006, 355, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Marfil-Garza, B.A.; Imes, S.; Verhoeff, K.; Hefler, J.; Lam, A.; Dajani, K.; Anderson, B.; O’Gorman, D.; Kin, T.; Bigam, D.; et al. Pancreatic islet transplantation in type 1 diabetes: 20-year experience from a single-centre cohort in Canada. Lancet Diabetes Endocrinol. 2022, 10, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Gamble, A.; Pepper, A.R.; Bruni, A.; Shapiro, A.M.J. The journey of islet cell transplantation and future development. Islets 2018, 10, 80–94. [Google Scholar] [CrossRef]
- Rajab, A. Islet Transplantation: Alternative Sites. Curr. Diab. Rep. 2010, 10, 332–337. [Google Scholar] [CrossRef]
- Brusko, T.M.; Russ, H.A.; Stabler, C.L. Strategies for durable β cell replacement in type 1 diabetes. Science 2021, 373, 516–522. [Google Scholar] [CrossRef]
- Sakata, N.; Aoki, T.; Yoshimatsu, G.; Tsuchiya, H.; Hata, T.; Katayose, Y.; Egawa, S.; Unno, M. Strategy for clinical setting in intramuscular and subcutaneous islet transplantation. Diabetes Metab. Res. Rev. 2014, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.H.; Bonner-Weir, S.; Vacanti, J.P.; Ogawa, Y.; Weir, G.C. Outcome of subcutaneous islet transplantation improved by polymer device. Transplantation 1996, 61, 1557–1561. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.H.; Hsu, B.R.; Kuo, C.H. Islet transplantation at subcutaneous and intramuscular sites. Transplant. Proc. 2005, 37, 3479–3481. [Google Scholar] [CrossRef] [PubMed]
- Hunckler, M.D.; García, A.J. Engineered biomaterials for enhanced function of insulin-secreting β-cell organoids. Adv. Funct. Mater. 2020, 30, 2000134. [Google Scholar] [CrossRef]
- Deng, C.; Vulesevic, B.; Ellis, C.; Korbutt, G.S.; Suuronen, E.J. Vascularization of collagen-chitosan scaffolds with circulating progenitor cells as potential site for islet transplantation. J. Control Release 2011, 152 (Suppl. S1), e196–e198. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Araki, K.; Yamato, E.; Ikegami, H.; Asano, T.; Shibasaki, Y.; Oka, Y.; Yamamura, K. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: Special reference to expression of glucose transporter isoforms. Endocrinology 1990, 127, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Ohgawara, H.; Miyazaki, J.; Karibe, S.; Tashiro, F.; Akaike, T.; Hashimoto, Y. Embedded-culture of pancreatic beta-cells derived from transgenic mouse insulinoma as a potential source for xenotransplantation using a diffusion chamber. Cell Transplant. 1995, 4, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Kimura, F.; Gotoh, M.; Tanaka, T.; Luo, Z.; Miyazaki, J.; Uede, T.; Monden, M.; Miyasaka, M. Locally expressed CTLA4-Ig in a pancreatic beta-cell line suppresses accelerated graft rejection response induced by donor-specific transfusion. Diabetologia 2002, 45, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Sobel, D.O.; Ramasubramanian, B.; Mitnaul, L. Characterization of a mouse model of islet transplantation using MIN-6 cells. Islets 2020, 12, 71–86. [Google Scholar] [CrossRef]
- Yu, C.P.; Juang, J.H.; Lin, Y.J.; Kuo, C.W.; Hsieh, L.H.; Huang, C.C. Enhancement of subcutaneously transplanted β cell survival using 3d stem cell spheroids with proangiogenic and prosurvival potential. Adv. Biosyst. 2020, 4, e1900254. [Google Scholar] [CrossRef]
- Lin, H.-C.; Chen, C.-Y.; Kao, C.-W.; Wu, S.-T.; Chen, C.-L.; Shen, C.-R.; Juang, J.-H.; Chu, I.-M. In situ gelling-polypeptide hydrogel systems for the subcutaneous transplantation of MIN6 cells. J. Polym. Res. 2020, 27, 64. [Google Scholar] [CrossRef]
- Juang, J.-H.; Lin, H.-C.; Chen, C.-Y.; Kao, C.-W.; Chen, C.-L.; Wu, S.-T.; Lin, S.-H.; Shen, C.-R.; Wang, J.-J.; Tsai, Z.-T.; et al. Noninvasive tracking of mPEG-poly(Ala) hydrogel-embedded min6 cells after subcutaneous transplantation in mice. Polymers 2021, 13, 885. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.-H.; Chen, C.-L.; Kao, C.-W.; Chen, C.-Y.; Shen, C.-R.; Wang, J.-J.; Tsai, Z.-T.; Chu, I.-M. The image-histology correlation of subcutaneous mpeg-poly(ala) hydrogel-embedded min6 cell grafts in nude mice. Polymers 2023, 15, 2584. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jun, H.S. In Vivo Imaging of Transplanted Pancreatic Islets. Front. Endocrinol. 2018, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, Y.; Yang, B.; Zhang, B.; Wu, Y. Islet Transplantation Imaging in vivo. Diabetes Metab. Syndr. Obes. 2020, 13, 3301–3311. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.H.; Wang, J.J.; Shen, C.R.; Kuo, C.H.; Chien, Y.W.; Kuo, H.Y.; Tsai, Z.T.; Yen, T.C. Magnetic resonance imaging of transplanted mouse islets labeled with chitosan-coated superparamagnetic iron oxide nanoparticles. Transplant. Proc. 2010, 42, 2104–2108. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.H.; Shen, C.R.; Wang, J.J.; Kuo, C.H.; Chien, Y.W.; Kuo, H.Y.; Chen, F.R.; Chen, M.H.; Yen, T.C.; Tsai, Z.T. Magnetic resonance imaging of mouse islet grafts labeled with novel chitosan-coated superparamagnetic iron oxide nanoparticles. PLoS ONE 2013, 8, e62626. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.H.; Shen, C.R.; Wang, J.J.; Kuo, C.H.; Lin, M.Y.; Wu, S.T.; Tsai, Z.T.; Yen, T.C. Magnetic resonance imaging study of mouse islet allotransplantation. Transplant. Proc. 2010, 42, 4217–4220. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.-H.; Wang, J.-J.; Shen, C.-R.; Chen, C.-Y.; Kao, C.-W.; Chen, C.-L.; Lin, S.-H.; Wu, S.-T.; Li, W.-C.; Tsai, Z.-T. Magnetic resonance imaging of transplanted porcine neonatal pancreatic cell clusters labeled with chitosan-coated superparamagnetic iron oxide nanoparticles in mice. Polymers 2021, 13, 1238. [Google Scholar] [CrossRef]
- Harkness, J.E.; Wagner, J.E. (Eds.) Biology and Medicine of Rabbits and Rodents, 2nd ed.; Lea & Febige: Philadelphia, PA, USA, 1983. [Google Scholar]
- Evgenov, N.V.; Medarova, Z.; Pratt, J.; Pantazopoulos, P.; Leyting, S.; Bonner-Weir, S.; Moore, A. In vivo imaging of immune rejection in transplanted pancreatic islets. Diabetes 2006, 55, 2419–2428. [Google Scholar] [CrossRef]
- Fowler, M.; Virostko, J.; Chen, Z.; Poffenberger, G.; Radhika, A.; Brissova, M.; Shiota, M.; Nicholson, W.E.; Shi, Y.; Hirshberg, B.; et al. Assessment of pancreatic islet mass after islet transplantation using in vivo bioluminescence imaging. Transplantation 2005, 79, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Borot, S.; Crowe, L.A.; Toso, C.; Vallée, J.P.; Berney, T. Noninvasive Imaging Techniques in Islet Transplantation. Curr. Diab. Rep. 2011, 11, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, M.; Kusamori, K.; Oda, C.; Miyazaki, A.; Katsumi, H.; Sakane, T.; Nishikawa, M.; Yamamoto, A. Regulation of proliferation and functioning of transplanted cells by using herpes simplex virus thymidine kinase gene in mice. J. Control Release 2018, 275, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Krieger, N.R.; Yin, D.P.; Fathman, C.G. CD4+ but not CD8+ cells are essential for allorejection. J. Exp. Med. 1996, 184, 2013–2018. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.J. Regulatory T cells in transplantation. Transplant. Proc. 2011, 43, 2135–2136. [Google Scholar] [CrossRef]
- Kawakami, Y.; Inoue, K.; Hayashi, H.; Wang, W.J.; Setoyama, H.; Gu, Y.J.; Imamura, M.; Iwata, H.; Ikada, Y.; Nozawa, M.; et al. Subcutaneous xenotransplantation of hybrid artificial pancreas encapsulating pancreatic B cell line (MIN6): Functional and histological study. Cell Transplant. 1997, 6, 541–545. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juang, J.-H.; Chen, C.-L.; Kao, C.-W.; Wu, S.-T.; Shen, C.-R. In Vivo Imaging of Immune Rejection of MIN6 Cells Transplanted in C3H Mice. Cells 2024, 13, 1044. https://doi.org/10.3390/cells13121044
Juang J-H, Chen C-L, Kao C-W, Wu S-T, Shen C-R. In Vivo Imaging of Immune Rejection of MIN6 Cells Transplanted in C3H Mice. Cells. 2024; 13(12):1044. https://doi.org/10.3390/cells13121044
Chicago/Turabian StyleJuang, Jyuhn-Huarng, Chen-Ling Chen, Chen-Wei Kao, Shu-Ting Wu, and Chia-Rui Shen. 2024. "In Vivo Imaging of Immune Rejection of MIN6 Cells Transplanted in C3H Mice" Cells 13, no. 12: 1044. https://doi.org/10.3390/cells13121044






